Abstract
Background
Pembrolizumab is a new drug approved in several countries for second-line therapy in non-small cell lung cancer (NSCLC) being programmed cell death ligand (PD-L1) positive. This drug has a high cost, and the cost-effectiveness ratio has been debated.
Patients and methods
The budget impact to the Northern Norwegian Regional Health Authority trust of implementing pembrolizumab in second-line therapy in patients with PD-L1-positive NSCLC was calculated. A model was developed employing data from the Cancer Registry of Norway, the KEYNOTE-010 study, the price list from The Hospital Pharmacy of North Norway, the cost of analysing PD-L1 expression and the cost of travelling. Today’s cost of second-line therapy was compared with the new standard employing pembrolizumab. The sale price of pembrolizumab in Norway was not published due to price confidentiality. Norwegian krone (NKr) was converted into Euros (€) at a rate of 1€=Nkr 8.8138. (Bank of Norway, 21 February 2017).
Results
105 new patients were identified available for pembrolizumab per year. The annual cost of pembrolizumab was €5.2 million, hospital pharmacy administration costs €0.1 million, PD-L1 testing €0.3 million, oncologist/pulmonologist/nurses €0.2 million, radiology €0.06 million and transportation €0.4 million. Savings due to avoided present second-line therapy was calculated €0.4 million. Consequently, the cost of implementing pembrolizumab was €5.5 million and the annual budget impact was €5.0 million. A mean gain of at least 9 months per patient treated was necessary to make pembrolizumab cost-effective.
Conclusions
The net budget impact of pembrolizumab was €5.0 million. The expenditure could not be indicated cost-effective. Price confidentiality is a growing problem in health economics and it has become a ‘menu without prices’ setting.
Keywords: non-small cell lung cancer, pembrolizumab, cost, effectiveness
Key questions.
What is already known about this subject?
This is a new drug in NSCLC being PD-L1 positive. It is costly. The cost-effectiveness has been heavily debated.
What does this study add?
This study documents the budget impact in northern Norway and illustrates the costs and savings that have to be considered. It also documents the necessary gain needed to make this drug cost-effective.
How might this impact on clinical practice?
The possible impact may be an awareness of the costs and the importance of price negotiations. Hopefully, price secrecy may be abandoned.
Introduction
In Norway, about 3000 patients are diagnosed with lung cancer each year and the figure is expected to rise to 3700 cases in 2025.1–4 Most cases are non-small cell lung cancer (NSCLC), and the median age at diagnosis is 70 years. A total of 85% of all patients are initially diagnosed with or develop advanced stage of disease (stage III or IV) during follow-up.
First-line treatment of metastatic NSCLC has traditionally been the use of a platinum doublet therapy. The combination of carboplatin and vinorelbin has shown less toxicity.5 Patients with epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) mutations have also been offered specific targeting treatments.1 Tyrosine kinase (TK) inhibitors (erlotinib, gefitinib and afatinib) have been employed in EGFR mutations and crizotinib in ALK translocations. Patients progressing on first-line therapy experiencing good performance status (Eastern Cooperative Oncology Group (ECOG) status 0–1) have traditionally been offered single-drug regimens consisting of docetaxel, pemetrexed, erlotinib or gemcitabine. The median overall survival has been reported between 5.7–9.3 months1 and 1-year overall survival around 30%.5 6
Pembrolizumab (Keytruda) is a new drug, recently approved for second-line treatment of patients with advanced or metastatic NSCLC with programmed cell death ligand (PD-L1) expression.7–9 Due to the significant cost of pembrolizumab, this therapy will obviously have a significant impact on the hospitals’ budgets. In this study, we aimed to clarify this impact and discuss whether this therapy can be considered cost-effective.
Materials and methods
We calculated the budget impact to the Northern Norwegian Regional Healthcare Authority (NNRHA) trust of implementing pembrolizumab (instead of docetaxel or pemetrexed) as standard second-line therapy in advanced (stage III) or metastatic (stage IV) NSCLC with PD-L1 expression. A model-based cost-minimising analysis was performed. The model included two alternatives: pembrolizumab 2 mg/kg or standard second-line therapy.1 Alternatives employed in second-line therapy in Norway are docetaxel, pemetrexed, navelbine, erlotinib and gefitinib. The majority of patients are treated with docetaxel and some with pemetrexed (non-squamous cell carcinoma). Two clinicians, working daily with patients with lung cancer at our two major hospitals, were included in the development of the model. The model is shown in figure 1.
Figure 1.
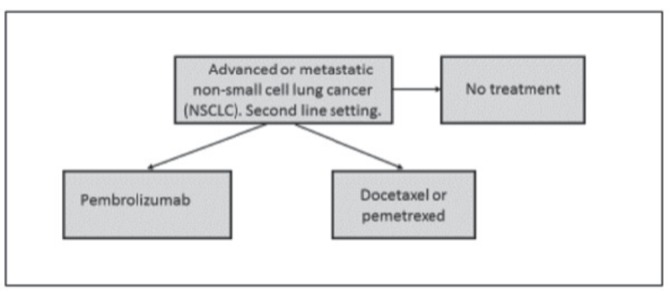
The model employed.
Population
Norwegian guidelines1 state that immunotherapy with pembrolizumab should be considered in patients with good performance status (ECOG 0–1) and PD-L1-expressing NSCLC. The PD-L1 expression was set to at least 1%.1 7 According to Norwegian data,3 northern Norway had 10.3% of all lung cancers in Norway. In 2015, there were 3035 cases, 84% of them were NSCLC and 70% of patients had an advanced stage of disease (stage III or IV) at the time of diagnosis.4 Half of the patients with localised disease developed distant metastasis during follow-up. Consequently, 85% of patients were potential candidates for pembrolizumab therapy. However, according to the clinicians’ experience, 30% of these patients would, due to poor performance status (ECOG >1) and/or short life expectancy (<3 months), not be candidates for second-line chemotherapy. Furthermore, two-thirds do have a PD-L1 expression in at least 1% of tumour cells.7
Costs
All costs and savings were calculated from the healthcare’s point of view, and Norwegian krone (NKr) was converted into Euros (€) at a rate of 1€=8.8138 NKr (Bank of Norway, 21 February 2017). Cost of the analysis of PD-L1 status was based on data from the Department of Pathology at the University Hospital of North Norway (UNN) and Nordland Hospital (NH), respectively. Together, they argued for one biomedical laboratory scientist (€79 421/year) and the total need for clinical pathologist resources was calculated a half position (€110 959/year). Costs connected to personnel included employers’ costs due to pension and social costs (30%). The cost per Dako-kit was €7148; 380 tests per year and 25 cases per kit was calculated. Consequently, the total number of kits was 25 annually.
MRI or CT is the most commonly employed imaging tool to document the disease status and evaluate treatment effects in NSCLC. On the basis of the clinicians’ advice, we calculated CT as the main tool, and 5% of patients in the pembrolizumab arm were assumed undergoing additionally MRI due to suspected cerebral metastasis. Furthermore, a total of 20% of patients were concluded undergoing CT-guided biopsy to achieve the necessary tissue to clarify PD-L1 status. The 2016 price list of the Norwegian Health Economics Administration (NHEA) was used.10 Norwegian hospital trusts are financed partly on activity and partly on basic funding, with equal shares. Consequently, the NHEA figures were doubled when calculating costs. Evaluation was performed every ninth week during treatment.
Drug cost and drug administration cost were obtained from the Hospital Pharmacy of North Norway, as of 1 January 2017. The selling price of pembrolizumab is a secret between the manufacturer Merck, Sharp & Dohme (MSD) and the Norwegian Hospital Procurement trust. Consequently, due to price confidentiality, we cannot publish data making the price available to third parties. The mean treatment time in the docetaxel arm was 4.7 cycles and consequently 5.7 outpatient visits were calculated.7 In the pembrolizumab arm, several patients were under therapy when the study was reported. On the basis of the data on the Norwegian Medicines Agency (NMA),9 Huang and colleagues,11 results presented at 17th World Conference on Lung Cancer (WCLC) in Vienna in December 2016 and the qualified guess of our group of clinicians, we estimated the mean number of cycles. The number of cycles cannot be given due to price confidentiality. Furthermore, patients’ mean weight was calculated to 75 kg and the height was 179.6 cm, based on data from Statistics Norway (www.ssb.no). The Mosteller method12 was employed to calculate the body surface area (1.93 m2). We did not reveal any information about significant differences in treatments following progression in the docetaxel or pembrolizumab arm. We, therefore, did not include any differences in costs of third-line therapy or costs of end-of-life therapy.
The cost of present standard second-line therapy was calculated according to the selling price of docetaxel and pemetrexed at the Pharmacy of Northern Norway trust. A dose of 75 mg/m2 was employed and the pharmacy production/administration cost was included. Similarly, the cost of pemetrexed was calculated at a dose of 500 mg/m2. The selling price of pemetrexed and docetaxel was confidential between the manufacturers and the Norwegian Hospital Procurement trust. To avoid revealing trade secrets and after a careful comparison of the calculated costs and the refunds according to the diagnosis-related group (DRG) system (DRG 856D, €1227), we concluded to employ the latter in our calculation.13 This refund was meant to compensate the expenses, but negotiations over price had somewhat lowered the cost.
The extra resources of oncologist/pulmonologist/nurses needed, due to prolonged therapy, were estimated by the clinicians to €153 169.
Costs due to patient travelling were based on data from The Health Enterprises’ Centre for Patient Journeys. According to regional guidelines, all patients undergoing pembrolizumab therapy were referred to the two main hospitals, UNN in Tromsø and NH in Bodø. In the docetaxel or pemetrexed alternative, patients were treated at their local hospitals. Consequently, the distribution of the patients within the region and their nearest hospital offering the actual therapy was employed when calculating travelling expenses.
Patient shares were included according to the price list of outpatient clinics (€39/visit).10 Similarly, the patient’s share was calculated €28 per CT or MR examination.10
The northern Norwegian hospitals do also have incomes. Consequently, when measuring budget impact, these incomes have to be included. Our patient clinics are partly financed by patients’ shares and refunding based on the DRG system.13 In this setting, the DRG 856D was employed (€1227).
Willingness to pay and loss of prognosis
In Norway, the willingness to pay is based on the severity of the disease.14 The severity is measured employing the quality-adjusted life expectancy (QALE) method. The mean age, among patients diagnosed with NSCLC, was 70 years. We have no quality-of-life data for the general population in Norway and, therefore, we employed the Swedish data.15 On the basis of these data, the QALE of 70-year-old persons, in general, is 11 quality-adjusted life-years (QALYs). On the basis of the knowledge that NSCLC is more common among males, males having a shorter life expectancy and the life expectancy of patients undergoing second-line therapy for advanced or metastatic NSCLC is <1 year, we calculated a loss of 10 QALYs among patients diagnosed with advanced or metastatic NSCLC. In such a setting, the willingness to pay among Norwegian healthcare administrators is somewhere between €57 000 and €68 000 per QALY.14
Results
Costs
We calculated 105 patients available for pembrolizumab therapy in northern Norway each year. Furthermore, the total cost of documenting the PD-L1 status, including bioengineer, pathologist and Dako-kit was calculated to a total of €301.527. The corresponding costs of radiological examinations (CT, MR and CT-guided biopsy) were €60 536 and €32 398 in the pembrolizumab and docetaxel or pemetrexed arm, respectively. The annual drug cost of pembrolizumab was €5 178 026 and the administration/production cost at the pharmacy was €87 911. The corresponding cost of the pharmacy expenses in the docetaxel or pemetrexed arm was €605 551 or €37 906, respectively. The total increased need of nurse/oncologist/pulmonologist resources in the pembrolizumab arm was estimated €153 169. Travel expenses in the pembrolizumab arm and docetaxel or pemetrexed arm were calculated as €430 909 and €95 144, respectively.
Income
Norwegian hospitals get half of their funding through the DRG system. However, the present DRG has not included the cost of pembrolizumab and, consequently, the income does not reflect this cost. In total, the DRG income was calculated as €702 181 and the patient shares were €48 909 in the pembrolizumab alternative. The corresponding figures in the docetaxel or pemetrexed arm was €302 775 and €23 427, respectively.
Budget impact
The total cost implementing pembrolizumab in the specialised healthcare in northern Norway was €5.5 million and the annual net budget influencing €5.0 million. The mean increased cost per patient treated was €48 000. An overview is shown in table 1.
Table 1.
An overview of the budget impact of implementing pembrolizumab in second-line therapy of non-small cell lung cancer
| Cost | Pembrolizumab | Docetaxel or pemetrexed | Budget impact |
| PD-L1 testing | €301 527 | €0 | €301 527 |
| Radiology (CT, MR) | €60 536 | €32 398 | €28 138 |
| Drug costs | €5 178 026 | €605 551∗ | €4 572 475 |
| Pulmonologist/oncologist/nurse | €153 169 | €0 | €153 169 |
| Pharmacy | €87 911 | €37 906† | €50 005 |
| Travelling expenses | €430 909 | €95 144 | €335 765 |
| Income | |||
| DRG refunding (50%) | €702 181 | €302 775 | €399 406 |
| Patient shares | €48 909 | €23 427 | €25 482 |
| Budget impact | €5 460 988 | €444 797 | € 5 016 191 |
*Costs based on DRG refunding (100%).
†The pharmacy-related costs were included in the DRG refunding and consequently excluded when summarising the budget impact.
DRG, diagnosis-related group.
Cost-effectiveness
On the basis of the budget impact of € 5.0 million and the willingness to pay between €57 000 and 68,000, about 80 life-years have to be gained per year to reach the level of recommended use. Consequently, each patient treated (105 patients/year) with pembrolizumab in northern Norway should gain a mean life expectancy of 9 months to make this therapy cost-effective.
Sensitivity analysis
A one-way sensitivity analysis was done to clarify the robustness of the model. The following variables were included in the analysis and varied by +/−20%: patient share, DRG income, travelling expenses, pharmacy administration cost, drug costs, radiology and PD-L1 testing. Details are shown in the tornado diagram in figure 2. Except for drug costs, the other factors had only minor impact on the budget. Consequently, variations in the price of pembrolizumab due to currency fluctuations and negotiations over price will have significant impact on Norwegian hospitals’ budgets.
Figure 2.
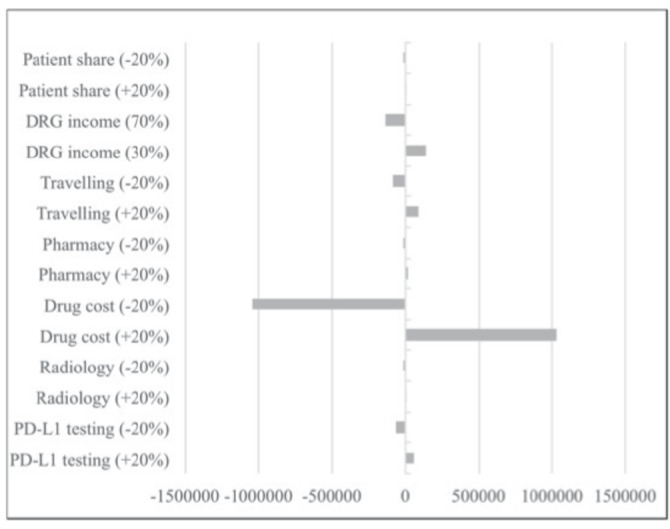
Tornado diagram showing a one-way sensitivity analysis. 0=€5.0 million. DRG, diagnosis-related group; PD-L1, programmed cell death ligand.
Discussion
The annual impact of pembrolizumab on the Northern Norwegian hospitals’ budget was €5.0 million. The estimated mean raised cost per patient treated was €50 209. The sensitivity analysis documented the price of pembrolizumab having the greatest impact on the result. A mean gain of 9 months per patient treated was necessary to make this treatment cost-effective, given the drug prices stay as of 1 January 2017.
The NMA9 concluded the cost of implementing pembrolizumab in second-line NSCLC in Norway to be €56.7 million. This national figure was based on the cost of pembrolizumab prior to negotiations. Knowing the selling price of pembrolizumab and the northern region having 10.3% (€5.8 million) of all patients with NSCLC, the true costs of implementing this drug was higher than these prior estimations. This was mainly due to higher travelling expenses and the hospitals’ membership cost of the Norwegian Hospital Procurement trust, included in the price of pembrolizumab. As we had to employ the refunds (according to the DRG system), and not the final results of negotiations over price for the comparators, the true difference is even greater.13
The main goal in the second-line treatment of NSCLC is prolonged survival/extended life and second improved quality of life.13 The duration of treatment and survival gain in the pembrolizumab alternative was difficult to estimate.7 In the KEYNOTE-010 study, no patients were treated for more than 24 months and the median follow-up was only 13.1 months. The 1-year survival was 57% and 65% in the two arms. Usually, cancer treatment is discontinued due to progressive disease, toxicity or patient’s choice. Whereas all patients had stopped therapy in the docetaxel arm, several patients were still on therapy at evaluation in the pembrolizumab arms.7 The median duration of response was 8 months in the docetaxel arm and not reached in the pembrolizumab arms. Our estimate was based on the fact that no patient achieved complete remission and, consequently, no cure could be anticipated. Some researchers have calculated a ‘tale of patients’ experiencing a prolonged survival.11
We have strongly indicated a significant budget impact by introducing pembrolizumab in second-line NSCLC therapy. On the basis of data from the Cancer Registry of Norway, it may be as long as 40 years before the incidence of lung cancer may start to drop, due to fewer smokers. The CRN has estimated that the total numbers of new lung cancers will increase to 3700 new cases per year in 2025.4 Consequently, the influence on hospitals’ budgets will obviously grow.
We did not report the treatment duration calculated in the pembrolizumab arm. This is a significant limitation and was due to secrecy. Trade secrets can be worth tens of hundreds of millions of dollars, and damage awards in trade secret litigation have been high.16 Our concern was due to the agreement between the manufacturer (MSD) and the Norwegian Hospital Procurement Trust, making it illegal to report the sale price of pembrolizumab in Norway. This has become a growing problem worldwide, making it difficult to undertake and publish health economic analysis on new drugs. The aggressive effort by manufacturers to enforce price confidentiality has been commented by several authors. Lerner and colleagues17 pointed on the fact that secrecy prevents hospitals from revealing prices to third parties that may help them negotiate prices. The price of the drug itself was the major cost in our analysis. Consequently, significant variations in cost may end up in various national conclusions with regard to cost-effectiveness. Unfortunately, this variation in pricing cannot be explored due to price confidentiality. In our neighbouring country, Sweden, significant differences have been revealed between the county councils.18 A Bloomberg report19 mentioned that health insurance companies buy prescription drugs the way US consumers buy cars: there is the sticker price (which few people actually pay) and there is the negotiated price. One of these reports19analysed 39 medicines with global sales of more than US$1 billion a year and showed that 30 of them logged price increases of more than double the rate of inflation from 2009 to 2015. One example was imatinib (Glivec), launched in 2001 at a price of US$31 930 per year and the corresponding cost in 2015 was US$118,000.20
There have been some advocates for disclosing costs. In the USA, Vermont was the first state to require drug makers to justify price hikes.21 Henrikson et al 22 argued that healthcare in the USA had come to resemble a ‘menu without prices’ for both physicians and patients, who systematically lacks access to the price of treatments, procedures and diagnostic tests. This ‘firewall’ may once have served an ethical purpose. However, they22 propose that complete price transparency for people with cancer should be an integral part of patient-centred care. Most people with cancer report wishing to discuss cancer care costs with their providers,23 and patient interest in price data is increasing.24 Trust is a crucial component of the entire physician patient relationship, including cost-related discussions.25
On the basis of our analysis, we cannot conclude whether pembrolizumab is cost-effective. This is due to the fact that we do not know the survival gain. However, the present data from the KEYNOTE-010 study7 were far from the needed 9 months level to make it cost-effective. In the total population, the median overall survival was 10.4 months in the pembrolizumab 2 mg/kg arm and 8.5 months in the docetaxel arm, respectively.7 However, as mentioned, several patients were still on therapy in the pembrolizumab arm when the study data were evaluated. In this study, we employed the recommended PD-L1 expression level (> 1% of tumour cells).7 Knowing the correlation between PD-L1 expression and differential activity of pembrolizumab, higher levels of expression (ie, >5%) should be explored to define the group making this therapy cost-effective.26
Conclusion
The introduction of pembrolizumab in the second-line treatment of advanced or metastatic NSCLC will have significant impact on healthcare budgets in northern Norway. A mean survival gain of 9 months per patient treated should be achieved to make it cost-effective. The lack of price transparency has made it impossible to run transparent health economic analyses. In the future, various PD-L1 expressions levels for initiation of therapy may be explored.
Acknowledgments
We appreciate the comments of several colleagues during this work and the service offered by the library at the UiT—The Arctic University of North Norway in Tromsø.
Footnotes
Contributors: The idea was initially developed by JN and MAA in cooperation with TT and NH. All authors took part in the development of the model. Data implemented were accessed by JN, MAA, TT, KA-S, GA and NH. All authors commented the model when the first version was made. The article was written by JN and partly by MAA. Comments were given during the writing process by all authors and the final version has been approved by all authors.
Funding: The publication charges for this article have been funded by a grant from the publication fund of UiT—The Arctic University of Norway.
Competing interests: None declared.
Patient consent: This is a model-based health economic analysis.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Norwegian Directorate of Health. The national treatment program and guidelines for the diagnosis, treatment and follow-up of lung cancer, mesothelioma and thymoma. Oslo: Norwegian Directorate of Health, 2016. [Google Scholar]
- 2. Cancer Registry of Norway. The 2014 annual report of the quality of care register for lung cancer. Oslo: Cancer Registry of Norway, 2015. [Google Scholar]
- 3. Cancer Registry of Norway. Cancer incidence, mortality, survival and prevalence in Norway. Oslo: Special issue. Cancer Registry of Norway, 2015. [Google Scholar]
- 4. Cancer Registry of Norway. The 2015 annual report of the quality of care register for lung cancer. Oslo: Cancer Registry of Norway, 2016. [Google Scholar]
- 5. Helbekkmo N, Sundstrøm SH, Aasebø U, et al. Vinorelbine/carboplatin vs gemcitabine/carboplatin in advanced NSCLC shows similar efficacy, but different impact of toxicity. Br J Cancer 2007;97:283–9. 10.1038/sj.bjc.6603869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grønberg BH, Bremnes RM, Fløtten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:3217–24. 10.1200/JCO.2008.20.9114 [DOI] [PubMed] [Google Scholar]
- 7. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 8. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 9. Norwegian Medicines Agency. Fast track health technology assessment (HTA) of pembrolizumab (Keytruda®) in advanced or metastatic PD-L1 positive non-small cell lung cancer – second line treatment. Oslo: Norwegian Medicines Agency, 2016. [Google Scholar]
- 10. Ministry of Health and Care Services. Regulations about coverage of expenditures related to health care services performed in out-patient clinics at Norwegian public health care institutions and private institutions with a signed agreement with regional health care authorities. Oslo: Ministry of Health and Care Services, 2016. [Google Scholar]
- 11. Huang M, Lou Y, Pellissier J, et al. Cost-effectiveness of pembrolizumab versus docetaxel for the treatment of previously treated PD-L1 positive advanced NSCLC patients in the United States. J Med Econ 2017;20:140–50. 10.1080/13696998.2016.1230123 [DOI] [PubMed] [Google Scholar]
- 12. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317:1098 10.1056/NEJM198710223171717 [DOI] [PubMed] [Google Scholar]
- 13. Norwegian Directorate of Health. Activity based funding 2016. Oslo: Norwegian Directorate of Health, 2015. [Google Scholar]
- 14. Ministry of Health and Care Services. Report number 34 to the Norwegian Parliament (2015-2016). Values in patients’ health care. Oslo: Ministry of Health and Care Services, 2016. [Google Scholar]
- 15. Burström K, Rehnberg C. Health related quality of life in Stocholm County 2002-2006. Report 2006:1.. Stockholm: Unit of Social Medicine and Health Economy, Stockholm County Council, 2006. [Google Scholar]
- 16. Nealey T, Daignault RM, Cai Y. Trade secrets in life science and pharmaceutical companies. Cold Spring Harb Perspect Med 2014;5 10.1101/cshperspect.a020982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lerner JC, Fox DM, Nelson T, et al. The consequence of secret prices: the politics of physician preference items. Health Aff 2008;27:1560–5. 10.1377/hlthaff.27.6.1560 [DOI] [PubMed] [Google Scholar]
- 18. Jönsson B, Wilking N. New cancer drugs in Sweden: assessment, implementation and access. J Cancer Policy 2014;2:45–62. 10.1016/j.jcpo.2014.01.003 [DOI] [Google Scholar]
- 19. Langreth R, Keller M, Cannon C. Decoding big pharma’s secret drug pricing practices. Bloomberg, SSR Health, Connecture Inc. New York 2016. [Google Scholar]
- 20. Langreth R. Secret rebates: why patients pay $600 for drugs that cost $300. New York: Bloomberg, 2017. [Google Scholar]
- 21. Silverman E. Vermont becomes first state to require drug makers to justify price hikes. Boston: STAT Pharmalot, 2016. [Google Scholar]
- 22. Henrikson NB, Shankaran V. Improving price transparency in cancer care. J Oncol Pract 2016;12:44–7. 10.1200/JOP.2015.006171 [DOI] [PubMed] [Google Scholar]
- 23. Bullock AJ, Hofstatter EW, Yushak ML, et al. Understanding patients’ attitudes toward communication about the cost of cancer care. J Oncol Pract 2012;8:e50–e58. 10.1200/JOP.2011.000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schleifer D, Hagelskamp C, Rinehart C, et al. How much will it cost? How Americans use prices in health care. San Francisco, CA: Public Agenda, 2015. [Google Scholar]
- 25. Danis M, Sommers R, Logan J, et al. Exploring public attitudes towards approaches to discussing costs in the clinical encounter. J Gen Intern Med 2014;29:223–9. 10.1007/s11606-013-2543-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carbognin L, Pilotto S, Milella M, et al. Differential activity of Nivolumab, Pembrolizumab and MPDL3280A according to the tumor expression of Programmed Death-Ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One 2015;10:e0130142 e0130142. 1 10.1371/journal.pone.0130142 [DOI] [PMC free article] [PubMed] [Google Scholar]


