Abstract
Introduction
Patient-reported pain severity and related impact in advanced/metastatic breast cancer (ABC/MBC) are not well documented. The objective of this study was to assess pain and general health status in hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) ABC/MBC.
Methods
Data were collected in the USA and Europe in a real-world, cross-sectional study. Patients were recruited at oncology practices and completed validated questionnaires; pain severity and interference were assessed using the Brief Pain Inventory (BPI) and general health status using the EuroQoL-5D (EQ-5D-3L). Descriptive statistics were generated for the overall cohort, and stratified by type of therapy and sites of metastases. Differences between patient groups were assessed via the Mann-Whitney Wilcoxon test. The relationship between pain scores and general health status was assessed using Kruskal-Wallis tests.
Results
Overall, 173 oncologists and 739 patients participated. The majority of patients rated their worst pain, average pain and pain interference as mild (59%, 77% and 70%, respectively). Most patients (>90%) reported no problems or moderate problems for all items of the EQ-5D-3L. Current treatment had no significant associations with pain severity or interference. Patients on chemotherapy reported significantly higher proportions of moderate/extreme levels of anxiety/depression (66.7%) and significantly lower general health status (60.7) compared with those on endocrine therapy (53.1% and 64.4, respectively). Pain severity and interference, all EQ-5D-3L items except self-care and the EQ-5D-3L health utility index were also significantly associated with sites of metastases, with greater impact in patients with visceral and bone metastases than those with bone only or visceral only metastases. Significant associations were observed between pain and health status, with increased pain severity and pain interference associated with worse health utility and general health status.
Conclusion
There is a clear unmet need for treatments that can reduce pain and preserve health status in patients with HR+/HER2− ABC/MBC.
Keywords: Advanced Breast Cancer, Metastatic Breast Cancer, Brief Pain Inventory, Eq-5d, Real-world
Key questions.
What is already known about this subject?
Endocrine therapy and chemotherapy are commonly used to treat patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) advanced or metastatic breast cancer (ABC/MBC). Both forms of treatment are known to be associated with some side effects. Most studies of patient-reported pain and health status in patients with HR+/HER2− ABC/MBC are within the context of clinical trials or involve very limited patient populations, and there is little information on the impact of HR+/HER2− ABC/MBC in real-world clinical practice or large patient populations across multiple countries.
What does this study add?
This study provides some insight from the patient perspective on pain severity, interference due to pain and health status in patients with HR+/HER2− ABC/MBC. It also explores the impact of the type of treatment and location of metastases on these patient-reported outcomes, as well as the relationship between pain and general health status.
How might this impact on clinical practice?
These findings suggest that that effective management of pain might help maintain general health status in patients with HR+/HER2− ABC/MBC. There is currently an unmet need for safe and effective treatments that help maintain general health status and delay deterioration of pain symptoms in these patients.
Introduction
Breast cancer is the most frequently occurring cancer among women globally, and the second most common cancer for the world’s population.1 2 An estimated 1.67 million new cases of breast cancer were diagnosed in 2012 worldwide, accounting for 25.2% of incident cancers.1 2 Together, advanced breast cancer (ABC) and metastatic breast cancer (MBC)—stages III and IV—have been reported to account for 8%–22% of all incident cases of breast cancer.3–5 Hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer forms the largest subtype (66%) of all patients with ABC/MBC.6
Endocrine therapy and chemotherapy are the most commonly used treatments in patients with HR+/HER2− ABC/MBC, with endocrine therapy being recommended in both US and European clinical guidelines, optionally in combination with a cyclin-dependent kinase inhibitor.7 8 Musculoskeletal problems, including joint pain and stiffness, are commonly experienced by patients receiving aromatase inhibitors9 and have been reported to lead to discontinuation of treatment in a high proportion of patients.10 However, there are a wide range of side effects of chemotherapy, including fatigue, gastrointestinal issues (loss of appetite, nausea and vomiting), hair loss and increased susceptibility to bruising and infections.11
Although there are several studies investigating patient pain and the treatment of pain in patients with breast cancer, the majority are limited to clinical trial settings and/or single country studies, and thus are not generalisable to the larger patient population.12 13 Additionally, while there is existing literature assessing patient pain in patients with ABC/MBC, there is limited focus on the relationship between patient pain and metastatic sites, with most papers focusing largely on patient quality of life.13–15 Therefore, studies of patient-reported pain severity and related impact in ABC/MBC in real-world settings across multiple countries have not been well documented.
The objective of this analysis was to assess pain severity, interference due to pain and general health status in patients with HR+/HER2− ABC/MBC in a real-world setting. Data were analysed for the total study population and for subgroups stratified by type of therapy (endocrine therapy vs. chemotherapy) and sites of metastases (bone only vs. visceral only vs. bone and visceral). The relationships between pain severity, pain interference and health status were also explored.
Methods
Data collection
Data were taken from the Adelphi Real World Advanced Breast Cancer Disease Specific Programme (DSP), a real-world, cross-sectional, patient record-based study.16 Data were collected in the USA, France, Germany, Italy, Spain and the UK between February and May 2015.
Oncologists were recruited from office and hospital practices including university/teaching hospitals, community hospitals, government hospitals, specialist cancer hospitals and nursing homes. Oncologists who participated in the DSP had to have qualified as oncologists between 1978 and 2011 and had to be actively involved in prescribing decisions for patients with ABC/MBC. Participating oncologists completed patient record forms for the first 8 to 10 consecutive patients they consulted after enrolling into the study. Patients whose records were abstracted had an oncologist-confirmed diagnosis of ABC/MBC and were currently receiving cancer treatment at the time of record abstraction. Oncologists completed a patient record form for each recruited patient, which included basic demographics, clinical characteristics (including HR and HER2 status) and treatment history.
Each patient was invited to complete a patient self-completion form (PSC), containing multiple validated questionnaires that assessed various aspects of patient-reported outcomes, such as quality of life, symptoms, therapy satisfaction and general health status. Within this paper, we focus on a modified version of the Brief Pain Inventory (BPI) and the EuroQoL-5D (EQ-5D-3L). Completion of the PSC was not mandatory; only patients who completed a PSC were included in this analysis.
The BPI is a widely used patient self-reported measure of pain severity and the degree to which their pain interferes with a patient’s daily life. The version included in the PSC comprised four items addressing pain severity (worst pain in last 24 hours, least pain in last 24 hours, average pain in last 24 hours, pain right now), and seven items addressing pain interference in the past 24 hours (general activity, mood, walking ability, normal walk, relations with other people, sleep and enjoyment of life). Each item was rated from 0, indicating no pain or no interference from pain, to 10, indicating pain as bad as the patient can imagine or pain that completely interferes.17
The EQ-5D-3L consists of five single-items and a 20 cm Visual Analogue Scale (VAS) describing the respondent’s general health status at time of completion. Each of the single items has three response options scored from 1 to 3, with a score of 1 indicating the absence of a symptom/problem, a score of 2 indicating a moderate symptom/problem and a score of 3 indicating an extreme symptom/problem.18 Application of country-specific scoring algorithms to the scores of the five items resulted in a single health utility index score ranging from −0.654 to 1, where 1 indicates perfect health, 0 death and <0 worse than death.19 The EQ-VAS assessing general health status provided a score of 0–100, where 0 indicated the worst imaginable health state and 100 indicated the best imaginable health state.18
Analyses
Data from all countries were pooled for this analysis and descriptive statistics were reported. Means and SD were calculated for continuous variables, while frequency counts and percentages were calculated for categorical variables. Analyses were conducted for the overall cohort, and stratified by type of current treatment (endocrine therapy only, chemotherapy only), and current sites of metastatic disease (bone only, bone and visceral, visceral only—for stage IV patients only). The statistical significance of differences across patient groups was assessed using Mann-Whitney Wilcoxon tests for current treatment and Kruskal-Wallis tests for metastatic sites. The relationship between the EQ-5D-3L health state utility and general health status and BPI items were also assessed using Kruskal-Wallis tests; BPI items were grouped as mild (1–4), moderate (5–6) and severe.7–10 20 All analyses were performed using Stata statistical software V. 14.2.21
Missing data were not unexpected, due to patients not wishing to answer some questions. Missing data were not imputed but remained missing (except when adhering to the scoring algorithm of the BPI); therefore, the base of patients for analysis was expected to vary from variable to variable. The number of patients included in each analysis is reported.
Results
Study population
In total, 173 oncologists participated in the study and recruited 739 HR+/HER2− patients who completed a PSC form. The mean (SD) age of the patients was 65.2 (10.6) years, and most (88%) were postmenopausal (table 1). The majority of patients (83%) had MBC (stage IV breast cancer) at diagnosis, and of these, a greater proportion of patients had visceral metastases than bone only or bone and visceral metastases (table 1). Similar proportions of patients (≈40%) were receiving chemotherapy and endocrine therapy at the time of completion of the PSC (table 1). Patients receiving chemotherapy only were on average younger compared with patients receiving endocrine therapy only (means: 63.0 years vs 68.7 years) (data not shown). Patients with different metastatic sites also differed in terms of age (means: bone only—67.4 years; visceral only—65.9 years; bone and visceral—64.3 years) (data not shown). No other differences in demographics were observed between treatment groups or metastatic sites.
Table 1.
Key patient characteristics and treatment distribution
| Patient characteristic | |
| Country, n (%) | |
| France | 151 (20.4) |
| Germany | 114 (15.4) |
| Italy | 144 (19.5) |
| Spain | 143 (19.4) |
| UK | 61 (8.3) |
| USA | 126 (17.1) |
| Age,* years; mean (SD) | 65.2 (10.6) |
| Ethnicity, n (%) | |
| Black/African-American/Afro-Caribbean | 34 (4.6) |
| Asian | 13 (1.8) |
| Hispanic/Latino | 31 (4.2) |
| White/Caucasian | 630 (85.3) |
| Other | 31 (4.2) |
| Current stage, n (%) | |
| Stage IIIB | 67 (9.1) |
| Stage IIIC | 61 (8.3) |
| Stage IV | 611 (82.7) |
| Stage at diagnosis, n (%) | |
| De novo metastatic | 414 (56.0) |
| Recurring from earlier stage | 325 (44.0) |
| Patient’s menopausal status, n (%) | |
| Premenopausal | 52 (7.0) |
| Perimenopausal | 24 (3.2) |
| Postmenopausal | 652 (88.2) |
| Ovaries removed | 11 (1.5) |
| Sites of metastases,† n (%) | |
| Bone only | 174 (28.5) |
| Visceral only | 254 (41.6) |
| Bone and visceral | 119 (19.5) |
| Other‡ | 64 (10.5) |
| Current treatment, n (%) | |
| Chemotherapy only | 305 (41.3) |
| Endocrine therapy only | 293 (39.6) |
| Other§ | 141 (19.1) |
*Four patients reported to be 90+ were assumed to be 90 for the purposes of this calculation.
†Among 611 patients with stage IV disease.
‡Lymph nodes with or without bone metastases or unspecified sites.
§Receiving chemotherapy and/or endocrine combination therapies with or without targeted agents.
Patient-reported pain severity and pain interference
Overall study population
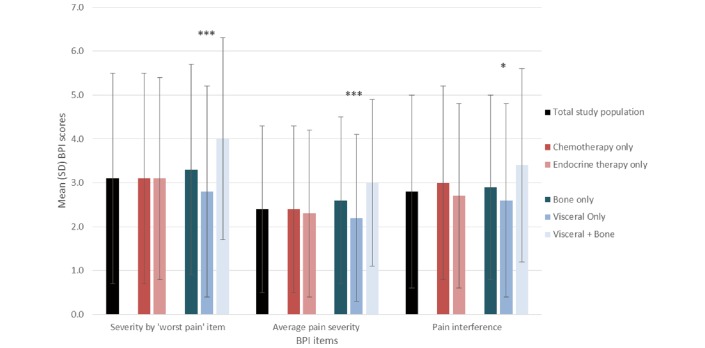
Mean (SD) scores for the ‘worst pain’ item, the average of the four pain severity items and the average of the seven pain interference items on the BPI are shown in figure 1. When asked to rate their worst pain on the BPI, 71% of all participating patients indicated it was mild (1–4) and 10% that it was severe (7–10). In rating their average pain on the BPI, only 4% rated it as severe, while 82% of patients considered it to be mild. The majority (76%) of patients rated pain interference on the BPI as mild, with 6% rating it as severe.
Figure 1.
BPI scores for total study population and stratified by type of therapy and metastatic site.*p<0.05, ***p<0.0001. BPI, Brief Pain Inventory.
Relationship of current treatment to pain
There were no significant differences in worst pain severity level, average pain severity level or pain interference reported on the BPI between patient groups stratified according to the type of treatment they were receiving (figure 1). The mean (SD) worst pain severity, average pain severity and pain interference were 3.1 (2.4), 2.4 (1.9) and 3.0 (2.2), respectively, for patients receiving chemotherapy only (figure 1). In comparison, the corresponding scores for patients receiving endocrine therapy only were 3.1 (2.3), 2.3 (1.9) and 2.7 (2.1) (figure 1). Proportions of patients reporting severity levels or pain interference of 1–4 (mild), 5–6 (moderate) and 7–10 (severe) were similar in both patient groups.
Relationship of current metastatic sites to pain
All three measures from the BPI differed significantly across patient groups as defined by current metastatic site(s), with patients suffering from bone and visceral metastases reporting the worst scores on each measure (figure 1). The mean (SD) worst pain severity level was 3.3 (2.4), 2.8 (2.4) and 4.0 (2.3) for patients with bone only, visceral only, and bone and visceral metastases, respectively (p<0.0001). The mean (SD) average pain severity level for patients with bone only, visceral only and bone and visceral metastases was 2.6 (1.9), 2.2 (1.9) and 3.0 (1.9), respectively (p<0.0001). The mean (SD) pain interference for patients with bone only, visceral only and bone and visceral metastases was 2.9 (2.1), 2.6 (2.2) and 3.4 (2.2), respectively (p<0.05).
General health status
Overall study population
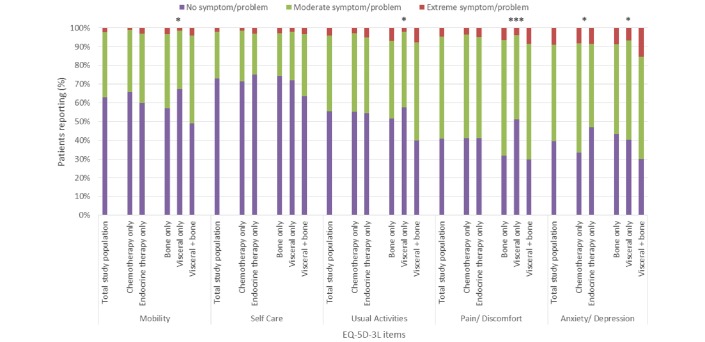
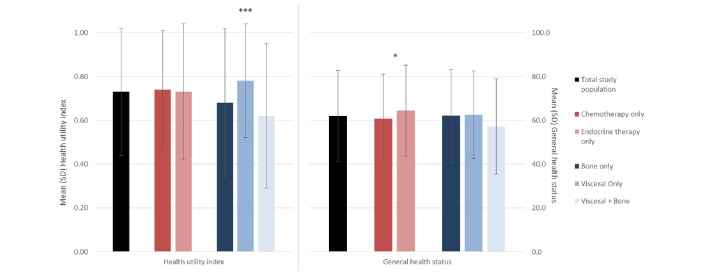
The EQ-5D-3L item with the highest proportion of patients reporting extreme problems was anxiety/depression (9%; figure 2). Only 41% and 40% of patients had no problems with pain/discomfort and anxiety/depression, respectively, but more than half the total patients included reported no problems with mobility, self-care and usual activities (figure 2). The mean (SD) health utility index was 0.73 (0.29) for the total population while the mean (SD) self-reported general health status was 62.0 (20.7) (figure 3).
Figure 2.
EQ-5D-3L item scores for total study population and stratified by type of therapy and metastatic site.*p<0.05, ***p<0.0001. EQ-5D-3L, EuroQoL-5D.
Figure 3.
EQ-5D-3L health utility index and general health status for total study population and stratified by type of therapy and metastatic site.*p<0.05, ***p<0.0001. EQ-5D-3L, EuroQoL-5D.
Relationship of current treatment to health status
Mean (SD) health state scores as indicated by health utility index did not differ significantly between patients receiving chemotherapy only (0.74 (0.27)) and patients receiving endocrine therapy only (0.73 (0.31)) (figure 3). Current treatment also had little effect on the level of problems patients reported for each of the five items of the EQ-5D-3L, with the exception of anxiety/depression (figure 2). There was a significant difference in the level of anxiety/depression reported depending on current treatment (p=0.0040), with a greater proportion of patients currently receiving endocrine therapy only (47%) reporting no problems with anxiety/depression than those receiving chemotherapy only (33%), while a greater proportion of patients currently receiving chemotherapy only (58%) reported moderate problems than those receiving endocrine therapy only (45%) (figure 2). General health status as measured by the VAS was significantly lower among patients receiving chemotherapy only of (60.7 (20.4)) compared with endocrine therapy only (64.4 (20.9)) (p=0.0237) (figure 3).
Relationship of current metastatic sites to health status
There was a significant difference in the mean health utility index related to metastatic sites, with mean (SD) index for patients with visceral and bone, bone only and visceral only metastases reported as 0.62 (0.33), 0.68 (0.34) and 0.78 (0.26), respectively (p<0.0001; figure 3). The level of problems patients reported for four of the five items of the EQ-5D-3L also differed by current metastatic sites, with differences being significant for mobility (p=0.0017), usual activities (p=0.0025), pain/discomfort (p<0.0001) and anxiety/depression (p=0.0171) (figure 2). The group of patients with visceral and bone metastases had the highest proportion of patients reporting extreme problems, and the lowest proportion of patients reporting no problems, for all items of the EQ-5D-3L (figure 2). No differences in self-reported general health status were observed across patient groups (figure 3).
Relationship of pain severity and pain interference with health utility index and general health status
Mean health utility index and general health status scores were significantly associated with pain severity as indicated by the ‘worst pain’ item from the BPI (p<0.0001 for both), with lower mean scores reported by patients with greater pain severity (table 2). Similarly, both health utility index and general health status scores were significantly associated with pain severity as indicated by the ‘average pain’ item from the BPI (p<0.0001 for both; table 2).
Table 2.
Relationship of pain severity and pain interference with health utility index and general health status
| Level of pain severity or interference, mean (SD) | ||||
| Mild (1–4) | Moderate (5–6) | Severe (7–10) | p Value | |
| Worst pain severity | ||||
| Health utility index | 0.81 (0.21) | 0.63 (0.27) | 0.40 (0.45) | <0.0001 |
| General health status | 67.1 (18.6) | 51.7 (18.7) | 43.9 (21.6) | <0.0001 |
| Average pain severity | ||||
| Health utility index | 0.78 (0.23) | 0.57 (0.37) | 0.23 (0.41) | <0.0001 |
| General health status | 65.3 (18.9) | 49.2 (20.6) | 35.9 (22.8) | <0.0001 |
| Pain interference | ||||
| Health utility index | 0.81 (0.20) | 0.59 (0.31) | 0.19 (0.45) | <0.0001 |
| General health status | 66.8 (18.0) | 49.8 (19.3) | 36.0 (24.1) | <0.0001 |
Higher levels of ‘pain interference’ reported with the BPI were also significantly associated with lower mean health utility index and general health status scores (p<0.0001 for both; table 2).
Discussion
This paper reports pain and health utility data for a population of patients with HR+/HER2− ABC/MBC participating in a large real-world study, and presents analyses stratified by the type of therapy patients were receiving (endocrine therapy only vs chemotherapy only), and the current sites of metastases (visceral only vs bone only vs visceral and bone).
Based on a random sampling of the general UK population, normative values have been reported as 0.85 for the EQ-5D-3L health utility index (based on 1925 women), and 82.3 for general health status (based on 1915 women).22 In the current study, for the total study population, the mean EQ-5D-3L health utility index was 0.73, and the mean general health status score was 62.0, reflecting a reduced health status compared with UK population norms. The findings in the current analysis reflect those reported elsewhere; the mean general health status in a population of women with ABC has been reported as 64.7.23
The treatment currently being received by patients (chemotherapy only vs endocrine therapy only) had no significant associations with the mean EQ-5D-3L health utility index or with pain levels or pain interference reported on the BPI. However, general health status as assessed by EQ-VAS scores was significantly associated with type of treatment, with worse health status seen in those receiving chemotherapy compared with endocrine therapy. Patients receiving chemotherapy only reported significantly higher proportions of moderate/severe levels of anxiety/depression in the EQ-5D-3L, compared with patients receiving endocrine therapy only. This confirms the findings of other studies; an association of chemotherapy with depression and anxiety has been previously reported.24 25 There were significant associations between the site of metastases and the level of problems patients reported for all items of the EQ-5D-3L, except for self-care, and the mean EQ-5D-3L health utility index, with patients with visceral and bone metastases reporting greater problems than those with metastases in other sites.
Pain severity and interference were also significantly associated with current metastatic site, with patients with visceral and bone metastases generally reporting more severe pain levels and greater pain interference than those with bone only or visceral only metastases. Significant negative correlations were seen between both pain severity levels and pain interference on the BPI and the EQ-5D-3L health utility index and general health status, suggesting a link between health status and severity and impact of pain.
Several potential limitations of this study are acknowledged. While minimal exclusion criteria governed the selection of oncologists, inclusion was likely influenced by willingness to participate and practical considerations of geographical location, resulting in a convenience sample, which might not be fully representative of the broader population of clinicians managing patients with ABC/MBC. The sample of patients collected within the DSP is not a truly random sample, rather a quasirandom sample, because oncologists were asked to select the first 8–10 patients with ABC/MBC they consulted after enrolling into the study. As only patients who consulted an oncologist and agreed to participate were included, the sample might include over-representation of patients who consult more frequently, or those with more severe disease. This was a cross-sectional rather than a longitudinal study; thus, data are presented assessing the association between factors rather than an assessment of causality. Patients who did not complete the PSC were not included in this analysis, and as such might be different from those who did agree to complete the PSC. This could have introduced some bias into the results. It should also be noted that patients receiving chemotherapy only and patients receiving endocrine therapy only may have different underlying conditions that have not been adjusted for in this analysis. Some data were missing for each of the assessments; missing data are not unexpected owing to patients being unwilling to answer some questions.
A search of PubMed for similar studies identified no publications reporting the relationship of current treatment type or metastatic site with health status or pain severity and impact in patients with ABC/MBC. The study reported here therefore provides important information from a patient perspective on the association of several factors with health status and pain in patients with HR+/HER2− ABC/MBC in a real-world setting. These findings emphasise the need for treatments that help maintain general health status and delay deterioration of pain symptoms in these patients, without inducing pain-related side effects. Further research is needed to gain greater understanding of causal relationships between elements of patient management with health status and pain in this patient group, in order to optimise treatment outcomes in this patient group.
Acknowledgments
This study was sponsored by Pfizer. Editorial assistance was provided by Carole Evans of Plan X Consulting and was funded by Adelphi Real World.
Footnotes
Contributors: All authors contributed to conception and design of the study; drafting and reviewing the article; and approval of the final article. RW contributed to the analysis and interpretation of data. JdC contributed to acquisition of data.
Funding: This research was performed under a research contract between Adelphi Real World and Pfizer and was funded by Pfizer.
Disclaimer: DM and SI are employees of Pfizer, RW and JdC are employees of Adelphi Real World, who were paid contractors to Pfizer in the development of this manuscript.
Competing interests: None declared.
Patient consent: Patients provided consent to participate by checking a box confirming their willingness to participate after having read information about the research and the use to which the data will be put. We did not ask for a signature as this could reveal their name which would be classed as a personal identifier. Checking the box allows for consent in an anonymous fashion.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data that support the findings of this study are available from Adelphi Real World but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Adelphi Real World.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer and World Health Organization. GLOBOCAN. Estimated cancer incidence, mortality and prevalence worldwide 2012. http://globocan.iarc.fr/Default.aspx (accessed Nov 1 2016).
- 3. Maclean R, Jeffreys M, Ives A, et al. Primary care characteristics and stage of cancer at diagnosis using data from the national cancer registration service, quality outcomes framework and general practice information. BMC Cancer 2015;15:500 10.1186/s12885-015-1497-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ginsburg OM, Fischer HD, Shah BR, et al. A population-based study of ethnicity and breast cancer stage at diagnosis in Ontario. Curr Oncol 2015;22:97–104. 10.3747/co.22.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walters S, Maringe C, Butler J, et al. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: a population-based study. Br J Cancer 2013;108:1195–208. 10.1038/bjc.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat 2013;141:507–14. 10.1007/s10549-013-2711-y [DOI] [PubMed] [Google Scholar]
- 7. Rugo HS, Rumble RB, Macrae E, et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016;34:3069–103. 10.1200/JCO.2016.67.1487 [DOI] [PubMed] [Google Scholar]
- 8. Cardoso F, Costa A, Senkus E, et al. European School of Oncology; European Society of Medical Oncology. ESO-ESMO 3rd international consensus guidelines for advanced breast cancer (ABC 3). Breast 2017;31:244–59. [DOI] [PubMed] [Google Scholar]
- 9. Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 2007;25:3877–83. 10.1200/JCO.2007.10.7573 [DOI] [PubMed] [Google Scholar]
- 10. Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 2012;30:936–42. 10.1200/JCO.2011.38.0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clinic M. Chemotherapy for breast cancer. 2016. http://www.mayoclinic.org/tests-procedures/chemotherapy-for-breast-cancer/details/risks/cmc-20253603 (accessed Nov 1 2016).
- 12. Anderson P, Benford M, Harris N, et al. Real-world physician and patient behaviour across countries: Disease-Specific Programmes - a means to understand. Curr Med Res Opin 2008;24:3063–72. 10.1185/03007990802457040 [DOI] [PubMed] [Google Scholar]
- 13. Smyth EN, Shen W, Bowman L, et al. Patient-reported pain and other quality of life domains as prognostic factors for survival in a phase III clinical trial of patients with advanced breast cancer. Health Qual Life Outcomes 2016;14:1–10. 10.1186/s12955-016-0449-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cleeland CS, Body J-J, Stopeck A, et al. Pain outcomes in patients with advanced breast cancer and bone metastases. Cancer 2013;119:832–8. 10.1002/cncr.27789 [DOI] [PubMed] [Google Scholar]
- 15. Spiegel D, Bloom JR. Pain in metastatic breast cancer. Cancer 1983;52:341–5. [DOI] [PubMed] [Google Scholar]
- 16. Chow E, Hird A, Velikova G, et al. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for patients with bone metastases: the EORTC QLQ-BM22. Eur J Cancer 2009;45:1146–52. 10.1016/j.ejca.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 17. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- 18. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 19. Szende A, Oppe M, Devlin N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. The Netherlands: Springer, 2007. [Google Scholar]
- 20. Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995;61:277–84. 10.1016/0304-3959(94)00178-H [DOI] [PubMed] [Google Scholar]
- 21. StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp, 2015. [Google Scholar]
- 22. Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D, 1999. The University of York Centre for Health Economics Discussion Paper 172. [Google Scholar]
- 23. Wallwiener M, Simoes E, Sokolov AN, et al. Health-related Quality of Life in Metastatic and Adjuvant Breast Cancer Patients. Geburtshilfe Frauenheilkd 2016;76:1065–73. 10.1055/s-0042-113188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Badger T, Segrin C, Dorros SM, et al. Depression and anxiety in women with breast cancer and their partners. Nurs Res 2007;56:44–53. 10.1097/00006199-200701000-00006 [DOI] [PubMed] [Google Scholar]
- 25. Hwang SY, Chang SJ, Park BW. Does chemotherapy really affect the quality of life of women with breast cancer? J Breast Cancer 2013;16:229–35. 10.4048/jbc.2013.16.2.229 [DOI] [PMC free article] [PubMed] [Google Scholar]