Abstract
Gastric cancer is the third leading cause of cancer-related death globally with approximately 723 000 deaths every year. Most patients present with advanced unresectable or metastatic disease, only amenable to palliative systemic treatment and a median survival uncommonly exceeding 12 months. Over the last years, the efficacy of chemotherapy combination has plateaued and the introduction of the anti-human epidermal growth factor receptor 2 trastuzumab has resulted in a limited survival gain in the upfront setting. After this positive experience, first-line treatment with new targeted therapies failed to improve the outcome of advanced gastric cancer. On the contrary, second-line options, including monochemotherapy with taxanes or irinotecan and the anti-vascular endothelial growth factor receptor 2 ramucirumab, either alone or combined with paclitaxel, opened new therapeutic rooms for an ever-increasing number of patients who maintain an acceptable performance status across multiple lines. This article provides an updated overview on the current management of advanced gastric cancer and discusses how the different treatment options available may be best combined to favourably impact the outcome of patients following the logic of a treatment strategy.
Keywords: advanced gastric cancer, targeted therapy, ramucirumab, treatment strategy
Introduction
Despite a steadily decline in its incidence and mortality over the 20th century, gastric cancer (GC) remains a global public health problem as it still ranks as the fifth most common malignancy and the third leading cause of cancer-related death worldwide.1 Furthermore, in Western countries approximately 50% of patients presents with metastatic disease at diagnosis and 40%–60% systemically relapse after radical surgery.2 Chemotherapy represents the standard treatment for advanced gastric cancer (AGC) based on its ability to prolong survival and improve quality of life compared with best supportive care (BSC). However, even the most effective regimens are unable to achieve a median overall survival (OS) longer than 9–11 months and a 5-year OS higher than 5%–10%.3 In addition, the significant survival benefit conferred by the anti-human epidermal growth factor receptor 2 (HER2) trastuzumab (14–16 months) is limited to the small subset of HER2-positive disease (15%–20%).4 Although several first-line trials with new targeted agents have carried out over recent years, none of them added a valuable benefit.5–12
Since it is increasingly unlikely to improve the survival of patients by a first-line treatment only, a potential way could be to expand the lines of treatment from the first- to the second-line and beyond. Indeed, currently roughly 50% of patients progressing after first-.line maintain acceptable general conditions and are still good candidates to receive further therapies. Also, the benefit of second-line chemotherapy has been convincingly established in randomised trials,13–15 and more recently, the anti-vascular endothelial growth factor receptor 2 (VEGFR-2) ramucirumab has shown to improve survival either as single agent over BSC16 or combined with paclitaxel over paclitaxel alone in pretreated patients.17
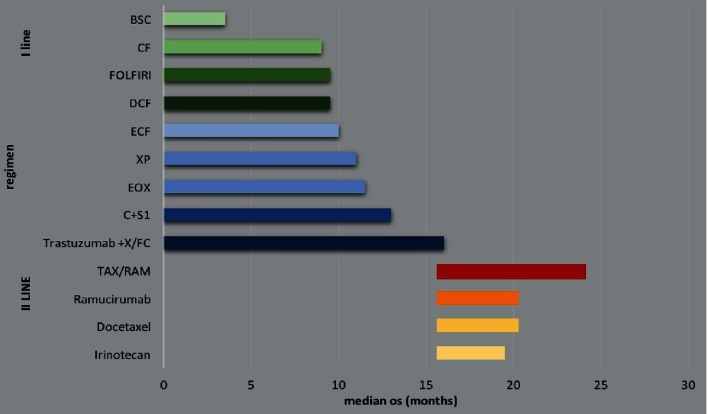
Therefore, the challenge is becoming how to incorporate the novel treatment options available to define a concept and a practice of a ‘continuum of care’ aimed at improving survival and quality of life of patients with AGC (Figure 1).
Figure 1.
Median overall survival by chemotherapy regimen in the first- and the second-line setting for advanced gastric cancer.
First-line treatment in AGC: successes and disappointments
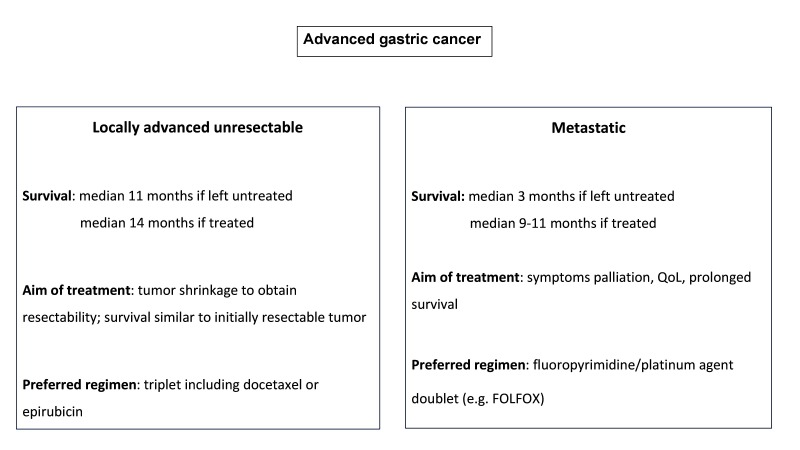
AGC is a heterogeneous entity that encompasses two different clinical situations: the locally advanced unresectable and the metastatic disease. Though historically coupled together within clinical trials, they may portend distinct prognosis and should require different therapeutic approaches (Figure 2). If chemotherapy is the mainstay of upfront treatment in both settings, more aggressive regimens may be of choice in patients without distant metastases since they might be deemed operable following a good response to systemic therapy, while less aggressive regimens may be more useful in patients with metastases in order to obtain palliation.
Figure 2.
Advanced gastric cancer: a tale of two diseases.68 BSC, best supportive care; ECOG, Eastern Cooperative Oncology Group.
Nowadays, regimens containing platinum agents and fluoropyrimidines, with or without epirubicin or docetaxel are regarded as the standard first-line treatment. Epirubicin, cisplatin and 5-FU (ECF regimen) have become one of most adopted chemotherapy combinations, particularly in the UK.18 Subsequently, docetaxel replaced epirubicin in the three-drug regimen.
Notably, the taxane-based regimen resulted in higher response rates (RR) but at the same time in a significantly higher incidence of grade 3–4 adverse events (AEs), with half of patients who came off study as a result.19 Recently, Shah et al proposed a modified docetaxel, cisplatin and fluorouracil (DCF) regimen demonstrating a comparable efficacy and a better safety profile than parent DCF, also when supported with growth factors.20 Nonetheless, the relevant rate of toxicity-related hospitalisation (22%) in the modified DCF arm underscores the importance to reserve taxane-based triplet for younger medically fit patients. A further development aimed at increasing tolerability without compromising efficacy was represented by the incorporation of oxaliplatin instead of cisplatin within the biweekly TEF (docetaxel, oxaliplatin, 5-FU) and FLOT regimens (5-FU, oxaliplatin, docetaxel) that showed to be active and well tolerated in the GATE and the FLOT-1 trial, respectively.21 22
Remarkably, the FLOT regimen has yielded higher pathological complete RR than ECF/ECX (epirubicin, cisplatin and capecitabine) (16% vs 6%; p=0.02) as perioperative therapy in resectable gastric and gastro-oesophageal adenocarcinoma.23 These findings, while certainly expand the available options in the perioperative setting, also further strengthen the rationale for docetaxel-based triplet in the locally advanced unresectable disease.
Other contemporary trials have established the non-inferiority of oxaliplatin and capecitabine over cisplatin and 5-FU, respectively, with a more tolerable safety profile making more patients candidates to receive these combinations.24–28 A valuable first-line alternative to platinum/fluoropyrimidine-based chemotherapy is represented by the FOLFIRI (5-FU, leucovorin, and irinotecan) combination which was shown to be at least as effective as and better tolerable than CF29 and ECF.30
In Eastern countries, the novel oral fluoropyrimidine S-1 is a widely accepted standard first-line treatment option either as single agent or combined with other cytotoxics.31–33
Table 1 summarises key clinical trials investigating chemotherapy doublets and triplets as first-line treatment of AGC.
Table 1.
Major randomised phase III trials of first-line chemotherapy in advanced gastric cancer.
| Author (year) | Study intervention | Number of patients | Primary endpoint | ORR (%) | Median PFS/FFS/TTP (months) | Median OS (months) |
| Webb et al
(1997)18 |
ECF vs FAMTX | 274 | OS | 45 vs 21 (p=0.0002) |
FFS 7.4 vs 3.4 (p=0.00006) |
8.9 vs 5.7 (p=0.0009) |
| Van Cutsem et al (2006)19 | DCF vs CF | 445 | TTP | 37 vs 25 (p=0.01) |
TTP 5.6 vs 3.7 (HR 1.47, p<0.001) |
9.2 vs 8.6 (HR 1.29, p=0.02) |
| Cunningham et al (2008)24 | ECF vs ECX vs EOF vs EOX |
1002 | OS* | 40.7 vs 46.4 vs 42.4 vs 47.9 (NS) | PFS 6.2 vs 6.7 6.5 vs 7.0 (NS) |
9.9 vs 9.9 vs 9.3 vs 11.2 (HR 0.80, p=0.02)† |
| Dank et al (2008)29 | IF vs CF | 333 | TTP | 31.8 vs 25.8 (NS) | TTP 5.0 vs 4.2 (NS) |
9 vs 8.7 (NS) |
| Al-Batran et al (2008)27 | FLO vs FLC | 220 | PFS | 24.5 vs 34.8 (NS) |
PFS 5.8 vs 3.9 (NS) |
10.7 vs 8.8 (NS) |
| Kang et al (2009)25 | CX vs CF | 316 | PFS‡ | 46 vs 32 (p=0.020) |
PFS 5.6 vs 5.0 (HR 0.81, p<0.001)‡ |
10.5 vs 9.3 (HR 0.85, p<0.008)‡ |
| Koizumi et al (2008)31 | CS1 vs S1 | 305 | OS | 54 vs 31 (p=0.002) |
PFS 6.0 vs 4.0 (HR, p<0.0001) |
13 vs 11 (HR 0.77, p=0.04) |
| Ajani et al (2010)33 | CS1 vs CF | 1053 | OS | 29.1 vs 31.9 (NS) |
PFS 4.8 vs 5.5 (NS) |
8.6 vs 7.9 (NS) |
| Yamada et al (2014)69 | OXS1 vs CS1 | 685 | PFS, OS§ | 55.7 vs 52.2 (HR NR) |
PFS 5.5 vs 5.4 (HR 1.004, p=0.0044) |
14.1 vs 13.1 (HR 0.958, p = NR) |
| Guimbaud et al (2014)30 | FOLFIRI vs ECF | 416 | TTF¶ | 39.2 vs 37.8 (NS) |
TTF 5.3 vs 5.8 (NS) |
9.5 vs 9.7 (NS) |
| Wang et al (2016)70 | mDCF vs CF | 243 | PFS | 48.7 vs 33.9 (p=0.0244) |
PFS 7.2 vs 4.9 (HR 0.58, p=0.0008) |
10.2 vs 8.5 (HR 0.71, p=0.0319) |
*Noninferiority for the triplet therapies containing capecitabine as compared with fluorouracil and for those containing oxaliplatin as compared with cisplatin.
†EOX vs ECF.
‡Noninferiority of CX versus CF.
§Noninferiority in PFS for OXS1 compared with CS1; relative efficacy in OS between OXS1 and CS1.
¶TTF was significantly longer with FOLFIRI than with ECX (5.1 v 4.2 months; P= 0.008).
CF, cisplatin and fluorouracil; CS1, cisplatin and S1; CX, cisplatin and capecitabine; DCF, docetaxel, cisplatin and fluorouracil; ECF, epirubicin, cisplatin and fluorouracil; ECX, epirubicin, cisplatin, and capecitabine; EOF, epirubicin, oxaliplatin and fluorouracil; EOX, epirubicin, oxaliplatin and capecitabine; FAMTX, fluorouracil, doxorubicin and methotrexate; FFS, failure free survival; FLC, fluorouracil, leucovorin and cisplatin; FLO, fluorouracil, leucovorin and oxaliplatin; FOLFIRI, fluorouracil, leucovorin and irinotecan; IF, irinotecan and fluorouracil; mDCF, modified docetaxel, cisplatin and fluorouracil. ORR, overall response rate; NR, not reported; NS: not significant; OS, overall survival; OXS1, oxaliplatin and S1; PFS, progression free survival; TTP, time to progression.
Finally, the discovery that approximately 20% of AGC overexpressed HER2 has prompted the development of the anti-HER2 directed therapy in this disease. In the pivotal phase III TOGA trial, 594 patients with previously untreated HER2 positive (immunohistochemistry 3+ or HER2:CEP17 ratio ≥2 on FISH) AGC were randomised to receive a chemotherapy regimen consisting of fluoropyrimidine (capecitabine or 5-FU) plus cisplatin with or without trastuzumab every 3 weeks for six cycles.4 The overall response rate (ORR) (47% vs 35%, p=0.0017), progression free survival (PFS) (6.7 vs 5.5 months, p=0.0002) and OS (13.8 vs 11.1 months, p=0.0046) were all improved with the addition of trastuzumab, with the greatest benefit seen for strongly HER2-overexpressing tumours (16 vs 11.8 months, p=0.0046). The survival advantage of trastuzumab was maintained though reduced over time as shown by the decrease of HR for OS from 0.73 to 0.80 as well as the difference in median OS from 2.7 to 1.4 months on a longer follow-up.34
How to improve the results of first-line treatment
Regardless of the best first-line combination, more than half of patients with AGC are refractory to chemotherapy, and even in responders, disease progression invariably occurs within 6–7 months. Over the last years, the efficacy of chemotherapy seems to have achieved a plateau. Recent efforts have been made to improve patient outcomes that pointed towards two main directions: (1) addition of new biological agents to backbone chemotherapy and (2) administration of sequential lines of treatment in adequate patients.
New targeted agents
The improved knowledge of molecular underpinnings of GC has prompted the drug development process so that an unprecedented plethora of novel targeted therapeutics has been evaluated or is currently under evaluation. Epidermal growth factor receptor (EGFR), HER2, angiogenesis, MET and immune checkpoints are among the most attractive and actively investigated targets. The discovery that EGFR is overexpressed in 30%–50% of GC and associated with unfavourable prognosis35–37 has led to two large phase III randomised trials investigating anti-EGFR monoclonal antibody in unselected patient populations. However, neither the addition of cetuximab to cisplatin/capecitabine (EXPAND trial) nor panitumumab to EOC (epirubicin, oxaliplatin, and capecitabine) regimen (REAL-3 trial) resulted in any improvement in survival.5 6 Of note, panitumumab showed a detrimental effect on survival (OS 8.8 vs 11.3 months; p=0.013), probably due to dose reductions of the backbone cytotoxics. Among strategies to exploit HER2, lapatinib was evaluated in combination with capecitabine and oxaliplatin without showing any improvement in survival compared with chemotherapy alone in the overall HER2-amplified population. Notably, a benefit was seen in preplanned subgroup analyses of Asian and younger patients.7 Since up to 50% of GC stain positively for MET on immunohistochemistry and 2%–10% are MET amplified, being associated with depth of tumour invasion, lymph node metastasis and poor prognosis,38–40 several approaches have been directed against the MET/HGF axis. In the RILOMET-1 trial patients randomly assigned to the anti-HFG rilotumumab plus ECX experienced a worse survival compared with those receiving chemotherapy alone (OS 9.6 vs 11.5 months, HR 1.36; p=0.021), because of an increase in deaths in the rilotumumab arm.8 Similarly, the enrolment onto the phase III METGastric trial that investigated mFOLFOX6 (modified 5-FU, leucovorin, oxaliplatin) plus the anti-MET onartuzumab or placebo stopped early due to negative results from the phase II trial without showing any survival benefit.9
According to aforementioned results, also the targeting of angiogenesis proved unsuccessful in the first-line setting. The AVAGAST trial, which enrolled patients to receive chemotherapy with or without bevacizumab, failed to meet its primary endpoint (OS 12.1 vs 10.1 months, HR 0.87, p=0.1).10 However, the addition of the antiangiogenics resulted in significantly improved PFS and RR, with less benefit in Asians than non-Asians. Moreover, plasma VEGF-A and neuropilin 1 emerged as potential biomarkers since patients with plasma VEGF-A levels above the median or neuropilin 1 expression below the median have been suggested to live longer, though these findings were not applicable to the subgroup of Asians and have never been validated.41 Likewise, in the AVATAR trial which had a study design similar to that of AVAGAST, the addition of bevacizumab to capecitabine-cisplatin did not prolong OS in Chinese patients.42
Other antiangiogenic agents examined as first-line treatment in AGC include the anti-VEGFR-2 ramucirumab11 and aflibercept,12 both of which failed to improve the efficacy of the mFOLFOX regimen. The significant proportion of oesophageal cancers enrolled onto these trials has been advocated to explain the negative impact on survival of the antiangiogenic strategy. We should not consider oesophageal and gastric adenocarcinoma as they are the same disease, in fact, there are increasing data suggesting that they differ in terms of clinical features and in their genomic landscape.43
Notably, the RAINFALL is currently an ongoing phase III trial which further addresses the role of antiangiogenics in first line by adding ramucirumab to cisplatin/capecitabine chemotherapy (ClinicalTrials.gov Identifier: NCT02314117).
Expanding the lines of treatment: second-line chemotherapy and beyond
In the past, the lack of a robust evidence for salvage chemotherapy along with the rapid decline of patients’ performance status (PS) has precluded the widespread administration of further lines of treatment. Indeed, only about 20% of patients went on to receive second-line therapy in historical studies,44 whereas in more recent phase III trials the percentage of candidates has risen from 40% in Europe45 to as high as 75% in Japan.31 Recently, a growing evidence has accumulated on the effectiveness and the feasibility of second-line chemotherapy and a better delivery of cytotoxics together with the improvement in simultaneous care has allowed more patients to tolerate multiple lines of treatment.
Monochemotherapy versus BSC
Three are the landmark phase III randomised trials that successfully explored the role of second-line monochemotherapy in patients with AGC.
The German Arbeitsgemeinschaft Internistische Onkologie trial compared a 3-week schedule of irinotecan 250 mg/m² (escalated up to 350 mg/m² depending on toxicity) with BSC in patients with Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2 who had received prior fluoropyrimidine/platinum combination and whose disease progressed during or within 6 months following first line.13 Although the study was terminated prematurely due to poor accrual, among 40 enrolled patients the median OS was significantly longer in the irinotecan arm than in the BSC arm (4 vs 2.4 months, HR=0.48, p=0.023). The UK COUGAR-2 trial enrolled 168 patients to receive either docetaxel 75 mg/m² every 3 weeks plus BSC for a maximum of six cycles or BSC alone.15 The median OS was improved with docetaxel compared with BSC (5.2 vs 3.6 months, HR=0.67, p=0.01). Despite a higher incidence of grade 3–4 neutropenia, infection and febrile neutropenia, patients receiving docetaxel experienced less pain, nausea, vomiting and constipation and decreased dysphagia and abdominal pain.
A Korean trial tried to answer the question about the optimal cytotoxics to be used in second line. In this study, 202 patients with ECOG PS 0–1 and failing one or two prior chemotherapy lines were randomised in a 2:1 ratio to either salvage chemotherapy (docetaxel 60 mg/m² every 3 weeks or irinotecan 150 mg/m² every 2 weeks upon investigator’s choice) or BSC.14 The administration of second-line chemotherapy resulted in a significant improvement in OS compared with BSC (5.3 vs 3.8 months, HR=0.657, p=0.007), while no survival difference was recorded between docetaxel and irinotecan (5.2 vs 6.5 months, p=0.116). Even side effects were similar in both treatment arms.
A meta-analysis of patient-level data from the abovementioned trials including a total of 410 patients underscored the median OS gain of roughly 2 months for second-line monochemotherapy as compared with BSC, with a significant reduction in the risk of death by 37% (HR=0.63, p<0.0001). This benefit was conferred by both irinotecan and docetaxel and was of similar magnitude through patients of different ethnic origin.46 Of note, when we consider these results we have to remind that the docetaxel benefit is limited to a 3-week schedule at a higher dose, while the weekly lower dose regimen did not seem to yield a similar advantage.47
On the contrary, a weekly paclitaxel regimen provided an OS comparable to that achieved with irinotecan in 219 patients refractory to standard first-line treatment (9.5 vs 8.4 months, HR=1.13, p=0.38).48
Combination chemotherapy versus monochemotherapy
Unlike the first-line setting, combination chemotherapy failed to demonstrate a survival benefit over single.-agent in pretreated AGC. In a small Korean phase II trial, irinotecan monotherapy was as effective as FOLFIRI in terms of ORR (17.2% vs 20%, p=0.525), PFS (2.2 vs 3.0 months, p=0.481) and OS (5.8 vs 6.7 months, p=0.514); grade 3–4 toxicity was also superimposable between treatment arms.49 In another Japanese phase III study comparing biweekly irinotecan (60 mg/m²) plus cisplatin (30 mg/m²) to biweekly irinotecan alone (150 mg/m²) in 130 patients refractory to S1-based first-line chemotherapy, PFS was significantly prolonged in the combination arm (3.8 vs 2.8 months, HR 0.68, p=0.0398) but OS did not.50 A meta-analysis of 10 randomised trials confirmed that doublet chemotherapy does not significantly improve OS compared with single agent, while resulting in more grade 3–4 myelosuppression, diarrhoea and fatigue, suggesting monochemotherapy as standard of care in this setting.51
Ramucirumab: single agent and combinatorial approach
In spite of negative results coming from first-line trials, the therapeutic exploitation of angiogenesis turned out to be effective in second line. Ramucirumab, a fully human immunoglobulin IgG1 monoclonal antibody targeting VEGFR-2, has been shown to significantly improve survival in two pivotal international phase III double-blind, placebo-controlled trials. In the REGARD trial, 355 patients whose disease progressed within 4 months of fluoropyrimidine or platinum-containing first-line chemotherapy or within 6 months of completion of adjuvant therapy, and with an ECOG PS of 0–1, were randomised in a 2:1 ratio to either ramucirumab 8 mg/kg or placebo, intravenously every 2 weeks.16 Patients receiving ramucirumab had an improvement in both OS (5.2 vs 3.8 months, HR=0.776, p=0.047) and PFS (2.1 vs 1.3 months, HR=0.48, p<0.0001), with a reduction in the risk of death by 22% compared with placebo. Also, the disease control rate was significantly higher in the experimental arm (49% vs 23%), although objective responses were infrequent with ramucirumab. The survival benefit remained significant after adjusting for main prognostic variables such as PS, tumour location and peritoneal disease. The efficacy of ramucirumab alone was comparable to that reported in phase III trials of second-line chemotherapy, with a more favourable toxicity profile.
Similarly, in the RAINBOW trial, which is the largest second-line trial in AGC, the addition of ramucirumab to weekly paclitaxel significantly prolonged either median OS (9.6 vs 7.4 months, HR=0.807, p=0.017) or PFS (4.4 vs 2.9 months, HR=0.635, p<0.0001) when compared with paclitaxel monotherapy in 665 patients.17 A decrease in the risk of death by 19% was seen and a significantly greater proportion of patients attained an objective response in the combination group than in the single-agent group (28% vs 16%, p=0.0001). These results are noteworthy especially in the light of poor risk feature of patients enrolled as demonstrated by the rate of peritoneal metastases higher than 40% in both the experimental and control arms. In a preplanned subgroup analysis, Asian patients derived less survival benefit than non-Asian. A dilution effect by poststudy discontinuation treatment as well as difference in pharmacokinetics has been advocated to explain this discrepant outcome. Interestingly, the survival benefit was achieved while maintaining patient quality of life, delaying symptom worsening and functional status deterioration.52 The toxicity of ramucirumab was tolerable and, as expected, higher in the combination regimen. In the single-agent trial the most common AE was an increased risk of grade 3 or higher hypertension (8% vs 3%), while when combined with paclitaxel, ramucirumab resulted in significantly increased rates of grade 3–4 neutropenia (40.7% vs 18.8%), though this did not translate into higher incidence of febrile neutropenia. Antiangiogenic class side effects such as proteinuria, bleeding and gastrointestinal perforations were mainly infrequent, mild in grade and more commonly noted in the combination arm.
Table 2 summarises efficacy and safety results from major randomised phase III trials investigating second-line treatments in patients with AGC.
Table 2.
Major randomised phase III trials of second-line treatments in advanced gastric cancer.
| Trial | Study intervention | Number of patients | Median PFS (months) | Median OS (months) | Grade ≥3 AEs in the experimental arm |
| AIO* | Irinotecan + BSC vs BSC | 40 | 2.6 vs NR | 4.0 vs 2.4 HR 0.48, p=0.012 |
Diarrhoea 26%, leucopenia 21%, febrile neutropenia 16% |
| Kang et al 14 | Docetaxel or irinotecan vs BSC | 202 | NR | 5.3 vs 3.8 HR 0.65, p=0.007† |
Anaemia 31%, fatigue 18%, neutropenia 17% |
| WJOG 400748 | Paclitaxel vs irinotecan |
219 | 3.6 vs 2.3 | 9.5 vs 8.4 HR 1.13, p=0.38 |
Neutropenia 39.1%, anaemia 30%, anorexia 17.3% |
| COUGAR-0215 | Docetaxel + BSC vs BSC |
168 | NR | 5.2 vs 3.6 HR 0.67, p=0.01 |
Neutropenia 15%, infection 19%, febrile neutropenia 7% |
| REGARD16 | Ramucirumab + BSC vs BSC |
355 | 2.1 vs 1.3 | 5.2 vs 3.8 HR 0.77, p=0.047 |
Hypertension 8%, fatigue 6%, abdominal pain 6% |
| RAINBOW17 | Ramucirumab + paclitaxel vs paclitaxel | 665 | 4.4 vs 2.8 | 9.6 vs 7.4 HR 0.87, p=0.017 |
Neutropenia 41%, leucopenia 17%, hypertension 14% |
*Prematurely closed due to poor patients accrual.
†No OS difference between docetaxel (5.2 moths) and irinotecan (6.5 months).
AIO, Arbeitsgemeinschaft Internistische Onkologie; AE: adverse event; BSC: best supportive care; NR: not reported.
Third-line treatment
Although currently not supported by randomised data, third-line chemotherapy is increasingly administered to patients failing previous lines and maintaining an acceptable PS, particularly in Asian countries. As such, more than 70% and 27% of patients received a third-line chemotherapy in a Japanese48 and Korean14 phase III trial of second-line chemotherapy, respectively. Sparse data from small phase II and retrospective studies suggested an RR in the range of 15%–23% for single-agent taxanes and irinotecan53–55 but their impact on patients’ quality of life and survival is unknown. Since patients’ PS is expected to deteriorate with the advancement in lines of treatment and the absolute benefit of therapy reduced, it is mandatory to carefully select those candidates to further treatment. It has been reported that ECOG PS, serum albumin, histological type and PFS under second line are independent prognostic factors for survival in patients receiving third-line chemotherapy, thus serving as a useful tool to guide treatment decision.56 Lately, apatinib, a novel receptor tyrosine kinase inhibitor selectively targeting VEGFR-2, has shown to significantly prolong survival compared with placebo in Chinese patients who experienced disease progression after two or more lines of systemic therapy (6.5 vs 4.7 months, p=0.0156).57 This is the first agent that proved effective in heavily pretreated AGC. Likewise, a very recent abstract presented by Kang and colleagues at the 2017 Gastrointestinal Cancer Symposium reported extremely promising results on the anti-PD1 nivolumab. In the phase III randomised ONO-4538-12 study, 493 patients who failed two or more previous lines of chemotherapy were randomly allocated to nivolumab 3 mg/kg every 2 weeks or placebo. Nivolumab resulted in significantly improved ORR (11.2% vs 0%), PFS (1.61 vs 1.45 months) and OS (5.32 vs 4.14 months) and lower grade ≥3 AEs (11.5% vs 5.5%) compared with placebo.58
The nutritional issue of patients with AGC
Malnutrition is emerging as a highly prevalent comorbid condition in patients with cancer, including GC, characterised by progressive depletion of nutritional status and worsening of metabolic alterations. While weight loss >10% of usual weight within previous 6 months was reported in 15% of GC at diagnosis, malnutrition has been described in up to 80% of AGC.59 Factors known to deteriorate the nutritional status depend on both disease and treatment-related catabolic effects. An impaired nutritional status is associated with increased morbidity and mortality during anticancer treatment since patients who are malnourished commonly require dose reductions, delays or even treatment discontinuation, higher frequency of hospitalisation, reduced quality of life and ultimately decreased survival. In particular, malnutrition may be responsible for increased treatment-related toxicities through sarcopenia, a low level of muscle mass, which results in higher drug exposure as demonstrated by the higher area under the time–concentration curve in patients with sarcopenia versus patients without sarcopenia.60 Moreover, nutritional deterioration combined with energy and protein deficiencies has been suggested to have a significant impact on quality of life, even superior of cancer stage. This has been reported to occur early even before starting any oncological treatment and it is independent from oncological characteristics.61
There is accumulating evidence that for non-imminently dying patients with AGC nutritional support may be beneficial in terms of both quality of life and survival. When feasible, the oral and enteral routes are preferred to the parenteral one, although patients with AGC may often have an impaired gastrointestinal function depending on several factors: stenosis of the cardia or pylorus, peritoneal carcinosis, chemotherapy-related gastrointestinal side effects, short bowel syndrome, which contraindicate enteral feeding. In a study by Qiu and colleagues, the nutritional support has shown to improve the prognosis of high nutritional risk AGC (nutritional risk score ≥3), which obtained an NRS shift to <3 (median survival 14.3 vs 9.6 months, p=0.001).62 Similarly, short-term home parental nutrition is largely indicated based on the improved quality of life, and nutritional and functional status seen in patients who are malnourished and with AGC; however, this did not affect neither the toxicity nor the RR of treatment.59
Given the high prevalence and early onset of malnutrition, nutritional screening and assessment should become an integral part of care in AGC in order to establish an adequate and prompt intervention to maintain or even improve patients’ outcomes and quality of life.
Discussion
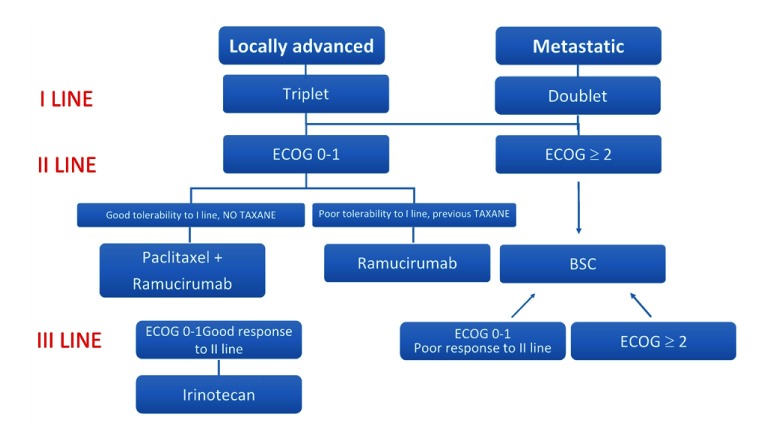
Akin to colorectal cancer, it seems that the management of AGC is witnessing a shift from a step-by-step approach to a tailored therapeutic strategy in which oncologists can plan to give the patients sequentially over the entire disease course all active agents available in order to improve their outcome. In this scenario, the challenge becomes how to best integrate and combine emerging therapies into a treatment paradigm that started looking beyond first line along a continuum of care. The pivotal driver of this decision-making process is the goal of treatment, which ranges from symptoms palliation and prolongation of survival for the vast majority of patients to rarely cure. Moreover, given the paucity of validated predictive and prognostic factors, the choice of the optimal treatment strategy should involve clinical factors pertaining to both the patient and the disease. Based on data from clinical trials and on the different treatment aims, we propose an algorithm as guidance for the management of patients with AGC (Figure 1).
As such, the higher RR yielded by taxane-containing triplets (ORR 37%–48%) makes them reasonable upfront treatment options for locally advanced unresectable GC to maximise the chance of resectability and ultimately cure, since salvage surgery is the single most important predictor of survival after neoadjuvant chemotherapy.63 Similarly, three-drug regimens may be offered to selected suitably fit patients suffering from tumour-related symptoms and/or bulky disease who therefore require rapid shrinkage. Current available literature suggests similar activity for epirubicin and docetaxel-based regimens in AGC but different safety profiles, with a decrease in the risk of diarrhoea, stomatitis, neutropenia and fatigue for the former and a decrease in the risk of thrombocytopenia, anaemia, nausea, vomiting, and hand and foot syndrome for the latter.64
On a different approach, for metastatic disease and for patients deemed unfit for triplets, the combination of a fluoropyrimidine and a platinum agent should represent the preferred backbone of first-line treatment palliation in HER2 negative AGC. Particularly, the mFOLFOX regimen is advocated over cisplatin-containing doublets based on its greater efficacy and safety.65 66 Second-line treatment has unquestionably become a standard of care for several patients with AGC. In this view, the adequate selection is a crucial step able to identify patients more likely to benefit from a treatment beyond first line while sparing them unacceptable toxicities. Several clinical factors have been associated with outcome in patients who receive second-line therapy. Catalano and colleagues found ECOG PS>2, haemoglobin ≤11.5 g/L, CEA>50 ng/mL, three more metastatic sites and time to progression on first line ≤6 months as independent predictors of poor survival in multivariate analysis.45 Accordingly, a more recent study based on individual data of 868 patients suggested that those with favourable ECOG PS (0–1), lower LDH level (≤480 U/L), lower neutrophils/lymphocytes ratio (2.7) and longer PFS in first line (≥6.8 months) achieve better outcomes.67 According to this, patients who maintain a good PS (0–1) and have a longer time to progression after first line are the optimal candidates to second-line therapy. For those receiving a taxane-free regimen (either a doublet or a triplet) in the upfront setting, the combination of ramucirumab and paclitaxel should be the preferred choice. Contrariwise, patients not amenable to the combination owing to poor tolerability to first line, residual toxicity or personal preference could receive single-agent ramucirumab. Other valuable options in adequate patients are taxanes or irinotecan monotherapy, which have similar activity but increased toxicity compared with the anti-VEGFR-2 alone and a lower activity and an equivalent toxicity in comparison with paclitaxel/ramucirumab combination.
Finally, the question of how to treat patients with impaired PS is still open since those with an ECOG PS of 2 or worse were under-represented in the trials of chemotherapy (total of 37 patients) and were not included neither in the REGARD nor in the RAINBOW trial. Therefore, BSC alone should be offered to patients with poor PS (≥2) and/or unwilling for further treatment.
Search strategy and selection criteria
A search of the literature was done on PubMed using the keywords ‘advanced gastric cancer’ paired with ‘chemotherapy’, ‘first-line’, ‘second-line’, third-line’, ‘targeted agent’ and ‘immunotherapy’. Only articles published in English language up to 25 March 2017 were considered. Other articles were retrieved by searching manually and cross-referencing the bibliography of relevant studies.
Figure 3.
Algorithm for personalised allocation to treatments in patients with advanced gastric cancer. BSC, best supportive care; CF, cisplatin and 5-FU; DCF, docetaxel, cisplatin and fluorouracil; ECF, epirubicin, cisplatin and 5-FU; OS, overall survival; XP, capecitabine and cisplatin, epirubicin, oxaliplatin, and capecitabine, paclitaxel and ramucirumab.
Footnotes
Contributors: All authors searched the data and literature. MS drafted the first version of the text. All authors wrote the text, prepared the tables and figures, and agreed to the final version of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64. 10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- 3. Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;3:CD004064. [DOI] [PubMed] [Google Scholar]
- 4. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 5. Lordick F, Kang YK, Chung HC, et al. Arbeitsgemeinschaft Internistische Onkologie, EXPAND investigators. capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open label phase 3 trial. Lancet Oncol 2013;14:490–9. [DOI] [PubMed] [Google Scholar]
- 6. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481–9. 10.1016/S1470-2045(13)70096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC-A randomized phase III trial. J Clin Oncol 2016;34:443–51. 10.1200/JCO.2015.62.6598 [DOI] [PubMed] [Google Scholar]
- 8. Cunningham D, Tebbutt NC, Davidenko I, et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol 2015;33(suppl; abstr 4000). [Google Scholar]
- 9. Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–76. 10.1200/JCO.2011.36.2236 [DOI] [PubMed] [Google Scholar]
- 11. Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter phase II trial. Ann Oncol 2016. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enzinger PC, McCleary NJ, Zheng H, et al. Multicenter double-blind randomized phase II: FOLFOX + ziv-aflibercept/placebo for patients (pts) with chemo-naive metastatic esophagogastric adenocarcinoma (MEGA). J Clin Oncol 2016;34(suppl 4S; abstr 4). 10.1200/jco.2016.34.4_suppl.4 [DOI] [Google Scholar]
- 13. Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer-a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306–14. 10.1016/j.ejca.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 14. Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–8. 10.1200/JCO.2011.39.4585 [DOI] [PubMed] [Google Scholar]
- 15. Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78–86. 10.1016/S1470-2045(13)70549-7 [DOI] [PubMed] [Google Scholar]
- 16. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9. 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 17. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 18. Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric Cancer. J Clin Oncol 1997;15:261–7. 10.1200/JCO.1997.15.1.261 [DOI] [PubMed] [Google Scholar]
- 19. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–7. 10.1200/JCO.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- 20. Shah MA, Janjigian YY, Stoller R, et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US gastric cancer consortium. J Clin Oncol 2015;33:3874–9. 10.1200/JCO.2015.60.7465 [DOI] [PubMed] [Google Scholar]
- 21. Van Cutsem E, Boni C, Tabernero J, et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Ann Oncol 2015;26:149–56. 10.1093/annonc/mdu496 [DOI] [PubMed] [Google Scholar]
- 22. Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008;19:1882–7. 10.1093/annonc/mdn403 [DOI] [PubMed] [Google Scholar]
- 23. Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697–708. 10.1016/S1470-2045(16)30531-9 [DOI] [PubMed] [Google Scholar]
- 24. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36–46. 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 25. Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009;20:666–73. 10.1093/annonc/mdn717 [DOI] [PubMed] [Google Scholar]
- 26. Okines AF, Norman AR, McCloud P, et al. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 2009;20:1529–34. 10.1093/annonc/mdp047 [DOI] [PubMed] [Google Scholar]
- 27. Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435–42. 10.1200/JCO.2007.13.9378 [DOI] [PubMed] [Google Scholar]
- 28. Montagnani F, Turrisi G, Marinozzi C, et al. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2011;14:50–5. 10.1007/s10120-011-0007-7 [DOI] [PubMed] [Google Scholar]
- 29. Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 2008;19:1450–7. 10.1093/annonc/mdn166 [DOI] [PubMed] [Google Scholar]
- 30. Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French Intergroup Study. J Clin Oncol 2014;32:3520–6. [DOI] [PubMed] [Google Scholar]
- 31. Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215–21. 10.1016/S1470-2045(08)70035-4 [DOI] [PubMed] [Google Scholar]
- 32. Ajani JA, Buyse M, Lichinitser M, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer study. Eur J Cancer 2013;49:3616–24. 10.1016/j.ejca.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 33. Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 2010;28:1547–53. 10.1200/JCO.2009.25.4706 [DOI] [PubMed] [Google Scholar]
- 34. Food and Drug Administration. Transtuzumab. Office of medical products and tobacco [online]. 2010. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm230418.htm
- 35. Wang KL, Wu TT, Choi IS, et al. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer 2007;109:658–67. 10.1002/cncr.22445 [DOI] [PubMed] [Google Scholar]
- 36. Galizia G, Lieto E, Orditura M, et al. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg 2007;31:1458–68. 10.1007/s00268-007-9016-4 [DOI] [PubMed] [Google Scholar]
- 37. Atmaca A, Werner D, Pauligk C, et al. The prognostic impact of epidermal growth factor receptor in patients with metastatic gastric cancer. BMC Cancer 2012;12:524 10.1186/1471-2407-12-524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and More. Nat Rev Mol Cell Biol 2003;4:915–25. 10.1038/nrm1261 [DOI] [PubMed] [Google Scholar]
- 39. Graziano F, Galluccio N, Lorenzini P, et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol 2011;29:4789–95. 10.1200/JCO.2011.36.7706 [DOI] [PubMed] [Google Scholar]
- 40. Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 2011;29:4803–10. 10.1200/JCO.2011.35.4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–27. 10.1200/JCO.2011.39.9824 [DOI] [PubMed] [Google Scholar]
- 42. Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2015;18:168–76. 10.1007/s10120-014-0351-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. The Cancer Genome Atlas Research Network, Analysis 924 Working Group. Integrated genomic characterization of 925 oesophageal carcinoma. Nature. 2017;541:169–75. 10.1038/nature20805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer-pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004;22:2395–403. 10.1200/JCO.2004.08.154 [DOI] [PubMed] [Google Scholar]
- 45. Catalano V, Graziano F, Santini D, et al. Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer 2008;99:1402–7. 10.1038/sj.bjc.6604732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Janowitz T, Thuss-Patience P, Marshall A, et al. Chemotherapy vs supportive care alone for relapsed gastric, gastroesophageal junction, and oesophageal adenocarcinoma: a meta-analysis of patient-level data. Br J Cancer 2016;114:381–7. 10.1038/bjc.2015.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Graziano F, Catalano V, Baldelli AM, et al. A phase II study of weekly docetaxel as salvage chemotherapy for advanced gastric cancer. Ann Oncol 2000;11:1263–6. 10.1023/A:1008373814453 [DOI] [PubMed] [Google Scholar]
- 48. Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438–44. 10.1200/JCO.2012.48.5805 [DOI] [PubMed] [Google Scholar]
- 49. Sym SJ, Hong J, Park J, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol 2013;71:481–8. 10.1007/s00280-012-2027-3 [DOI] [PubMed] [Google Scholar]
- 50. Higuchi K, Tanabe S, Shimada K, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer 2014;50:1437–45. 10.1016/j.ejca.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Ma B, Huang XT, et al. Doublet versus single agent as second-line treatment for advanced gastric cancer: a meta-analysis of 10 randomized controlled trials. Medicine 2016;95:e2792 10.1097/MD.0000000000002792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Al-Batran SE, Van Cutsem E, Oh SC, et al. Quality-of-life and performance status results from the phase III RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann Oncol 2016;27:673–9. 10.1093/annonc/mdv625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee MJ, Hwang IG, Jang JS, et al. Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res Treat 2012;44:235–41. 10.4143/crt.2012.44.4.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shimoyama R, Yasui H, Boku N, et al. Weekly paclitaxel for heavily treated advanced or recurrent gastric cancer refractory to fluorouracil, irinotecan, and cisplatin. Gastric Cancer 2009;12:206–11. 10.1007/s10120-009-0524-9 [DOI] [PubMed] [Google Scholar]
- 55. Lee JH, Kim SH, Oh SY, et al. Third-line docetaxel chemotherapy for recurrent and metastatic gastric cancer. Korean J Intern Med 2013;28:314–32. 10.3904/kjim.2013.28.3.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang DH, Bae WK, Hwang J, et al. Prognostic factor analysis of third-line chemotherapy in patients with advanced gastric cancer. J Clin Oncol 2011;29(15 suppl):e14613 10.1200/jco.2011.29.15_suppl.e14613 [DOI] [PubMed] [Google Scholar]
- 57. Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. 10.1200/JCO.2015.63.5995 [DOI] [PubMed] [Google Scholar]
- 58. Kang Y-K, Satoh T, Ryu M-H, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): a double-blinded, randomized, phase III trial. J Clin Oncol 2017;35(suppl 4S; abstract no. 2). 10.1200/JCO.2017.35.4_suppl.2 [DOI] [Google Scholar]
- 59. Rosania R, Chiapponi C, Malfertheiner P, et al. Nutrition in patients with gastric cancer: an update. Gastrointest Tumors 2016;2:178–87. 10.1159/000445188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One 2012;7:e37563 10.1371/journal.pone.0037563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gavazzi C, Colatruglio S, Sironi A, et al. Importance of early nutritional screening in patients with gastric cancer. Br J Nutr 2011;106:1773–8. 10.1017/S0007114511002509 [DOI] [PubMed] [Google Scholar]
- 62. Qiu M, Zhou YX, Jin Y, et al. Nutrition support can bring survival benefit to high nutrition risk gastric cancer patients who received chemotherapy. Support Care Cancer 2015;23:1933–9. 10.1007/s00520-014-2523-6 [DOI] [PubMed] [Google Scholar]
- 63. Yano M, Shiozaki H, Inoue M, et al. Neoadjuvant chemotherapy followed by salvage surgery: effect on survival of patients with primary noncurative gastric cancer. World J Surg 2002;26:1155–9. 10.1007/s00268-002-6362-0 [DOI] [PubMed] [Google Scholar]
- 64. Petrioli R, Roviello G, Zanotti L, et al. Epirubicin-based compared with docetaxel-based chemotherapy for advanced gastric carcinoma: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2016;102:82–8. 10.1016/j.critrevonc.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 65. Petrelli F, Barni S, Cascinu S, et al. Gastric cancer: toward a cisplatin-free disease? J Gastrointest Oncol 2014;5:318–22. 10.3978/j.issn.2078-6891.2014.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: a network meta-analysis. J Natl Cancer Inst 2016;108 10.1093/jnci/djw166 [DOI] [PubMed] [Google Scholar]
- 67. Fanotto V, Cordio S, Pasquini G, et al. Prognostic factors in 868 advanced gastric cancer patients treated with second-line chemotherapy in the real world. Gastric Cancer 2016;66 10.1007/s10120-016-0681-6 [DOI] [PubMed] [Google Scholar]
- 68. Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858–9. 10.1056/NEJMc0911925 [DOI] [PubMed] [Google Scholar]
- 69. Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015;26:141–8. 10.1093/annonc/mdu472 [DOI] [PubMed] [Google Scholar]
- 70. Wang J, Xu R, Li J, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer 2016;19:234–44. 10.1007/s10120-015-0457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]