ABSTRACT
Objective: To describe immunization attitudes and practices among family medicine providers across New York State. Methods: In this cross-sectional survey study, family medicine providers across New York State completed a questionnaire to assess vaccine beliefs and barriers and immunization practices. Statistical analysis: Descriptive statistical methods were used to define provider characteristics, knowledge and vaccine practices. Results: Completed questionnaires from 226 family medicine providers were included for analysis. As a group, 207/218 (95%) of providers who answered the question state they always recommend standard pediatric vaccines. Of the 209 providers who answered both questions, 47 (22%) state they always recommend standard pediatric vaccines but do not always recommend HPV vaccine to eligible 11–12 year-old patients. Only 75% of providers strongly disagreed with the statement ‘vaccinating adolescents against HPV increases the likelihood of unprotected sex'. Even though 178/190 (94%) and 164/188 (87%) of surveyed family medicine providers reported recommending that their pregnant patients receive influenza vaccine and Tdap vaccine, respectively, only 134/185 (72%) routinely do so in their office. Conclusion: Most family medicine providers self-report always recommending standard pediatric vaccines, however only a minority are following ACIP recommendations. Educational sessions to update family medicine providers on ACIP recommendations and address individual provider concerns may improve provider vaccine confidence and uptake of vaccines by their patients.
KEYWORDS: family medicine, family practice, vaccine attitudes, vaccine hesitancy
Introduction
Immunizations are among the most successful public health achievements for prevention of life-threatening diseases. The Advisory Committee on Immunization Practices (ACIP) currently recommends vaccines targeting 17 diseases. Yet, nationally, vaccine completion rates remain low, particularly for human papillomavirus (HPV) vaccine among adolescents, and for vaccines specifically recommended for adults, including pregnant women.1,2
Vaccine acceptance is affected by a variety of factors, with the single most commonly cited determinant of successful vaccination being provider recommendation.3-7 Vaccine recommendation practices are strongly influenced by provider vaccine attitudes. Specifically, provider knowledge and awareness of vaccine safety and efficacy is associated with improved provider vaccine confidence.8 Family medicine providers have the unique opportunity to care for patients of all ages and belonging to all risk groups, so it is particularly important for these providers to be vaccine confident, to clearly understand vaccine safety and efficacy, and to recommend vaccines according to authoritative guidelines. Despite specific guidance available across professional organizations, including the ACIP and the American Academy of Family Physicians (AAFP), significant variation in vaccine practices remains among family medicine providers.9-11
In this work, we aim to describe vaccine attitudes and immunization practices among family medicine providers across New York State to aid in guiding the development of interventions to improve provider vaccine confidence, vaccine recommendation practices, and overall vaccine coverage rates.
Results
Of the 544 questionnaires delivered to family medicine providers in New York State, 226 (42%) providers returned completed surveys and were included in the analysis. Of the 198 providers who answered the provider role and gender questions, 141 (71%) were physicians and 91 (46%) were male. Of the 211 providers who answered the community served question, the majority of providers cared for patients in suburban (128, 61%) and rural (144, 68%) communities. Of the 193 providers who reported the insurance accepted, 179 (93%) accepted patients with public insurance and 191 (99%) accepted patients with private insurance (Table 1).
Table 1.
Family medicine provider demographics.
| Demographic | N (%) |
|---|---|
| Total enrolled providers | 226 |
| Provider role answered | 198 (88) |
| Physician | 141 (71) |
| Mid-level provider | 57 (29) |
| Gender answered | 198 (88) |
| Male | 91 (46) |
| Years in practice answered | 212 (94) |
| < 5 | 41 (19) |
| 5 – 10 | 24 (11) |
| 10 – 19 | 62 (29) |
| 20 – 29 | 50 (24) |
| > 30 | 35 (17) |
| Community served answereda | 211 |
| Rural | 144 (68) |
| Suburban | 128 (61) |
| Urban | 65 (31) |
| Insurance accepted answereda | 193 |
| Private | 191 (99) |
| Public | 179 (93) |
| None | 33 (17) |
percentages add up to more than 100 because providers could give multiple answers for these questions.
As a group, 207/218 (95%) of providers who answered the question state they always recommend standard pediatric vaccines (Table 2). Providers in practice for less than 20 years were more likely to routinely recommend standard pediatric vaccines (121/123 (98%) vs 73/81 (90%)) (p < 0.05). Of the 213 providers who answered both questions, 14 (7%) state they always recommend standard pediatric vaccines but do not always recommend influenza vaccine to eligible patients. Of the 209 providers who answered both questions, 47 (22%) state they always recommend standard pediatric vaccines, but do not always recommend HPV vaccine to eligible 11–12 year-old patients.
Table 2.
Vaccine practices reported by family medicine providers across New York State.
| Vaccine practices | N/number of providers who answered (%) |
|---|---|
| Always recommend standard childhood vaccines | 207/218 (95) |
| Always recommend influenza vaccine to eligible pregnant women | 178/190 (94) |
| Always recommend Tdap vaccine to eligible pregnant women | 164/188 (87) |
| Always provide pediatric vaccine information to pregnant women | 71/176 (40) |
| Always administer vaccine to pregnant women in the office | 134/185 (72) |
| Always recommend HPVa vaccine to eligible patients at their 11–12 year old visits | 162/219 (74) |
| Always recommend HPV vaccine to eligible 13–18 year old patients | 193/222 (87) |
| Always recommend HPV vaccine to eligible 19–26 year old patients | 152/222 (68) |
| Always recommend influenza vaccine to eligible patients | 204/225 (91) |
| Always recommend Tdap vaccine to adults with infant contact | 189/223 (85) |
| Always recommend PCV-13b to adults older than 65 years | 200/222 (90) |
| Always recommend zoster vaccine to adults older than 60 years | 181/224 (81) |
| Always receive influenza vaccine annually | 213/222 (96) |
human papillomavirus.
pneumococcal conjugate vaccine-13.
More providers reported that they always recommended HPV vaccine to eligible 13–18 year-old patients (193/222, 87%) than eligible 11–12 year-old patients (162/219, 74%) or eligible 19–26 year-old patients (152/222, 68%). Mid-level providers (54/57, 95%) were more likely to routinely recommend HPV vaccine to eligible 13–18 year olds than were physicians (115/138, 83%) (p < 0.05). Providers who reported always recommending HPV vaccine to eligible 11–12 year olds were also more likely to recommend HPV vaccine to eligible 13–18 year olds (161/161, 100%) and eligible 19–26 year olds (126/158, 80%) than providers who reported not routinely recommending vaccine to the younger cohort (29/57 (51%) and 21/57 (37%), respectively) (p < 0.05).
While 178/190 (94%) and 164/188 (87%) of surveyed family medicine providers reported that they recommend that their pregnant patients receive influenza vaccine and tetanus-diphtheria-acellular pertussis (Tdap) vaccine, respectively, only 134/185 (72%) routinely administer vaccines to pregnant women in their office. Providers who routinely administer vaccines to pregnant women in the office are more likely to report always recommending influenza vaccine to eligible pregnant women (130/133, 98%) compared to providers who refer their pregnant patients elsewhere for vaccine (43/51, 84%) (p < 0.05). Providers who report always recommending influenza vaccine to eligible pregnant patients are much more likely to report always recommending Tdap vaccine to this patient population (162/172 (94%) vs 2/10 (20%)) (p < 0.05). Similarly, providers who always recommend Tdap vaccine to their eligible pregnant patients are more likely to also always recommend Tdap vaccine to adults with infant contact (147/163 (90%) vs 13/24 (54%)) (p < 0.05).
Of the surveyed providers who answered the question, 213/222 (96%) state they always receive an annual influenza vaccine, 204/225 (91%) always recommend influenza vaccine to all eligible patients, and 178/190 (94%) always recommend influenza vaccine to pregnant women. Providers who always receive the annual influenza vaccine are more likely to routinely recommend influenza vaccine to eligible patients (195/212 (92%) vs 6/9 (67%)), including eligible pregnant women (168/178 (94%) vs 8/12 (67%)) (p < 0.05).
Ninety percent (200/222) of providers stated they always recommend pneumococcal conjugate vaccine -13 (PCV13) to adults 65 years and older. Similarly, 81% (181/224) of providers routinely recommended zoster vaccine to adults 60 years and older. Providers who strongly agreed that zoster vaccine was effective in this population were more likely to routinely recommend it (99/109 (91%) vs 81/113 (72%)) (p < 0.05).
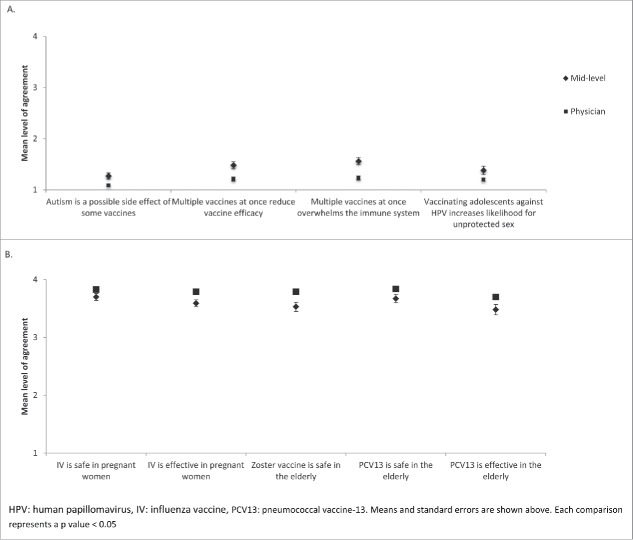
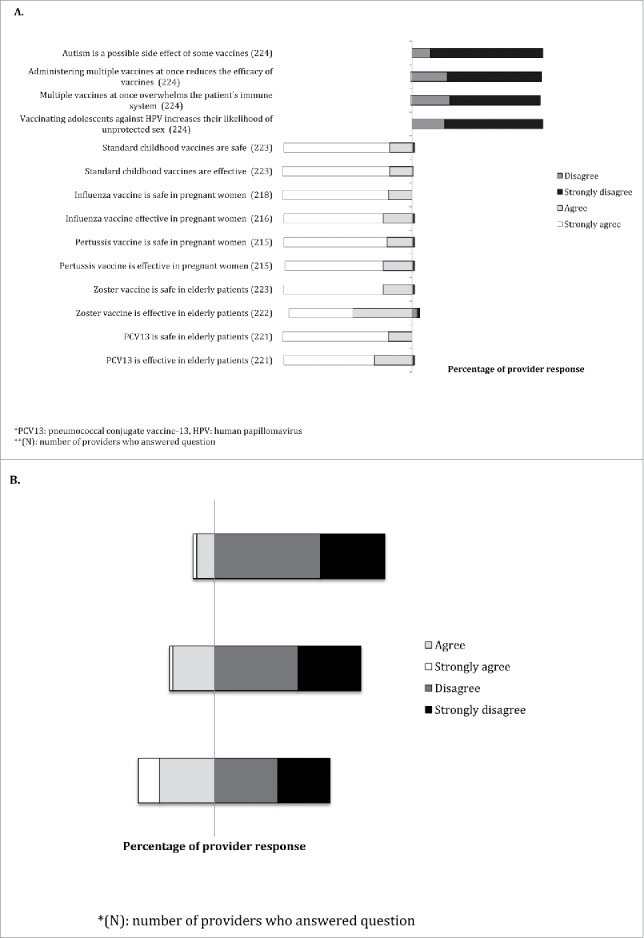
Regarding vaccine misperceptions, 86% (192/224) of providers strongly disagreed with the statement ‘autism is a possible side effect of some vaccines’, 72% (162/224) with the statement ‘administering multiple vaccines at once reduces the efficacy of vaccines’, 69% (155/224) with the statement ‘multiple vaccines at once overwhelms the patient's immune system’, and 75% (167/224) with the statement ‘vaccinating adolescents against HPV increases the likelihood of unprotected sex’ (Fig. 1). Mid-level providers had a higher mean level of agreement than physicians with each of these statements (Fig. 2) (p < 0.05).
Figure 1.
Vaccine beliefs about safety and efficacy (A) and barriers (B) reported by family medicine providers across New York State.
Figure 2.
Vaccine beliefs reported by family medicine physicians and mid-level providers across New York State regarding (A) pediatric vaccine misperceptions and (B) vaccine safety and efficacy.
81% (180/223) and 82% (182/223) of providers strongly agreed with the statements, ‘pediatric vaccines are safe’ and ‘pediatric vaccines are effective’, respectively. Only, 2% (4/223) and 1% (3/223) of providers disagreed with these same statements, respectively (Fig. 1). Physicians had a higher mean level of agreement than mid-level providers with the statements, ‘influenza vaccine is safe in pregnant women’, ‘influenza vaccine is effective in pregnant women’, ‘zoster vaccine is safe in the elderly’, ‘PCV13 is safe in the elderly’, and ‘PCV13 is effective in the elderly’ (p < 0.05). (Fig. 2). With the exception of zoster vaccine efficacy, there were no other associations between belief in vaccine safety or efficacy and providing routine vaccine recommendations (p > 0.05).
The combined mean for the four questions regarding vaccine misperceptions was significantly higher for mid-level providers (1.41, 95% CI [1.30, 1.52] than for physicians (1.18, 95% CI [1.13, 1.23], p < 0.001), which indicates higher level agreement with incorrect vaccine statements among the mid-level providers. The combined mean for the ten questions regarding correct perceptions of vaccine safety and efficacy in select populations among physicians (3.75, 95% CI [3.68, 3.81]) was marginally higher than for mid-level providers (3.62, 95% CI [3.50, 3.74], p = 0.088), suggesting a trend toward higher levels of agreement on vaccine safety and efficacy among physicians. There were no significant differences in the combined means for correct and incorrect perceptions about vaccine safety and efficacy for gender or the number of years in practice.
40% (89/224) of providers agreed or strongly agreed that lack of reimbursements was a barrier to vaccinations in the office, while 24% (52/222) agreed or strongly agreed that lack of time to discuss vaccines was a barrier to vaccination, and 11% (24/217) agreed or strongly agreed that vaccine safety concerns were a barrier to vaccinations in the office (Fig. 1). There were no differences in attitudes towards barriers to vaccination in the office when stratified by demographics.
Only 6/226 (3%) of providers stated they were vaccine hesitant. The majority of providers reported caring for vaccine hesitant patients or families, with ∼40% of providers encountering vaccine hesitancy on a weekly basis (Fig. 3). The top three vaccines reported to be of concern to patients and parents include HPV vaccine, influenza vaccine, and measles-mumps-rubella vaccine (Fig. 3).
Figure 3.
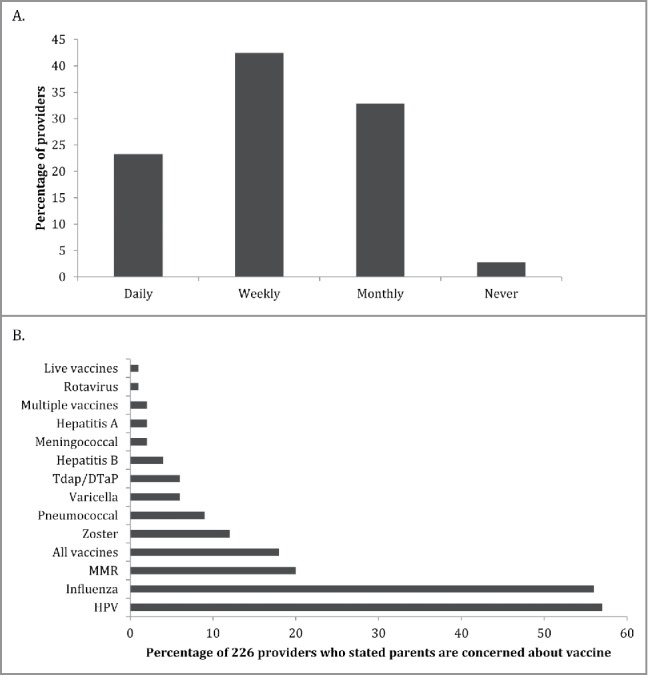
Frequency with which family medicine providers across New York State report encountering vaccine hesitant patients (A) and the specific vaccines parents reported to have the most concern about (B).
Discussion
In this work, we describe vaccine attitudes and practices among family medicine providers across 45 counties in New York State. We found that 5% of surveyed family medicine providers reported that they do not routinely recommend standard pediatric vaccines, 26% reported not routinely recommending HPV vaccine to eligible 11–12 year olds, 13% reported not routinely recommending Tdap vaccine to eligible pregnant women, and 19% reported not routinely recommending zoster vaccine to adults 60 years of age and older, yet only 3% of providers stated they themselves, were vaccine hesitant. These data suggest that organizational guidelines alone are insufficient to ensure provider vaccine recommendations or vaccine confidence. In fact, lack of guideline clarity has been described as a barrier for provider vaccine recommendations.12 Nationally, coverage rates for each of these immunizations remain under 50% [1, 2]. As provider recommendation is the most consistently cited factor associated with vaccine acceptance and uptake, even among parents and patients with negative vaccine attitudes, understanding the gaps in knowledge and factors associated with provider vaccine practices may aid in the development of interventions to improve vaccine recommendations and ultimately immunization coverage rates.3,4,13-16
Despite the vaccine education resources available, 69–86% of surveyed providers expressed strong disagreement with common pediatric vaccine misperceptions and only ∼80% strongly agreed that pediatric vaccines were safe and effective. Mid-level providers were more likely to have a higher mean level of agreement with vaccine misperceptions and a lower mean level of agreement with vaccine safety and efficacy than physicians, suggesting that further education needs to occur at all levels in the practice. Passive education alone has been shown to make little sustained effect in practice change.17,18 Instead, a multi-component program combining peer education regarding updated ACIP guidance, addressing vaccine concerns and misperceptions, and the safety and importance of vaccines in disease prevention may be more effective in improving provider vaccine confidence.19
The ACIP currently recommends universal HPV vaccination to begin at 11 or 12 years of age. A decade after the initial recommendation, over one-quarter of the surveyed family medicine providers report that they do not routinely recommend HPV vaccine to this cohort. Among the providers who stated they routinely recommend all standard pediatric vaccines, 22% went on to state that they do not routinely recommend HPV vaccine to eligible 11–12 year olds, suggesting that surveyed providers do not consider HPV vaccine to be a standard pediatric vaccine. Prior studies suggest that providers exhibit only moderate levels of adherence to professional guidelines regarding HPV vaccination and that many did not believe that guidance regarding vaccine was clear.12 Similarly, Gilkey et al found that providers endorsed and recommended HPV vaccine less strongly than the other routinely recommended adolescent vaccines.20 Providers may benefit from educational programs that convey the importance of HPV vaccination of their 11–12 year-old patients. Efforts might include instruction on the bundling of adolescent immunizations, reducing missed opportunities common in the adolescent age group, and observations related to the more robust antibody response to vaccines that occurs in younger adolescents.21-23 Efforts to include HPV vaccine along with current anticipatory guidance on cancer prevention also holds some promise.24
Consistent with prior research, a higher percentage of surveyed providers routinely reported recommending HPV vaccine to adolescents over 13 years of age.25 Providers who routinely recommended HPV vaccine to 11 and 12 year olds were also more likely to also routinely recommend vaccine to the older age groups. Common reasons for delaying HPV vaccine series initiation include concerns that parents might resist, lack of time to discuss vaccines in detail, and the belief that a discussion of sex needs to accompany a recommendation for HPV vaccine.12,26,27 Only 75% of surveyed providers strongly disagreed that vaccinating adolescents against HPV increases the likelihood of unprotected sex, despite published data showing no change in incidence of sexual activity or sexually transmitted infections after HPV vaccine series initiation. Furthermore, providers who emphasize the potential cancer prevention benefits of HPV vaccine have higher self-reported vaccination rates when compared to those who time vaccination around the initiation of sexual activity.28 Educational programs emphasizing provider over-estimation of parental vaccine hesitancy and linking HPV vaccine to a cancer prevention message are likely to improve provider vaccine recommendations to the 11 and 12 year-old cohort. Such a change may also have a trickle up effect leading to improvement in HPV vaccine uptake in all relevant age groups.
The ACIP recommends that influenza and Tdap vaccines be administered during each pregnancy to reduce the risk of morbidity from maternal and neonatal influenza and pertussis infection. We found that 95% of surveyed family medicine providers state they routinely recommended influenza vaccine to their pregnant patients, a higher rate than reported by others,29 yet, only 87% of surveyed providers routinely recommend Tdap vaccine to eligible pregnant women. One possible explanation for the discrepancy between the percentage of providers recommending these two vaccines to the same population is that Tdap administration during pregnancy is a newer recommendation. However, prior studies show that despite the ACIP providing category A vaccine recommendations, some physicians continue to prioritize some vaccines over others.9 Provider vaccine attitudes influence vaccine recommendation practices, therefore efforts aimed at improving vaccine confidence and a better understanding of organizational guidance is crucial to improve provider vaccine recommendations.
While the majority of the providers we surveyed reported routinely recommending vaccines to pregnant women, 28% state they refer patients elsewhere for vaccine administration. Providers who vaccinated pregnant women in the office were more likely to routinely recommend other vaccines to this group of patients. Family medicine providers are not the only group to refer patients outside of their practice for vaccination. In a previous study, we found that 40% of surveyed obstetricians recommended vaccines to their pregnant patients but referred them elsewhere to receive them.30 Referral outside of one's practice becomes an obstacle to vaccine completion unless the patient is particularly motivated. We found that surveyed obstetricians most commonly reported a lack of financial reimbursement as the reason for not vaccinating in the office.30 This is not a new concern posed by physicians. In a study published in 2014, 10% of pediatric and family medicine physicians seriously considered not providing routine pediatric vaccines due to high cost and dissatisfaction with insurance reimbursements for their purchase and administration.31 Almost a quarter of surveyed providers agreed that lack of time to discuss vaccines was a barrier to vaccine administration in the office. Similarly, Gilkey et al found that providers estimated that discussions regarding HPV vaccine are more time consuming than discussions focusing on the other standard adolescent vaccines.20 Developing interventions that incentivize and facilitate the logistics and cost to providers for purchasing, storing and administering vaccines in the office may improve patient vaccine uptake.
There are several limitations to our study. First, the results of this survey are self-reported and are based on recall of daily practice. Also, study recruitment was voluntary and may have led to a selection bias, thus leading to an over-representation of vaccine confident family medicine providers. Lastly, while we are able to describe the vaccines for which providers report parental concern, we do not know the reasons for these concerns and if they relate to vaccine safety or efficacy. While we recognize the limitations of survey methodology, this study allowed us to describe vaccine attitudes and practices among family medicine providers across New York State to determine areas for future interventions aimed at increasing provider vaccine recommendations and patient vaccine uptake.
We describe vaccine attitudes and practices among family medicine providers across New York State. We found that a substantial number of providers do not follow ACIP vaccine recommendations, despite self-reporting by most that they recommend all standard vaccines. This discrepancy was greatest with regards to HPV vaccine in young adolescents and influenza and Tdap vaccines during pregnancy. Educational efforts that target individual provider concerns and highlight the ACIP vaccine recommendations are needed to improve provider vaccine confidence and patient vaccine uptake.
Methods
The study team developed a one-page, self-administered questionnaire to assess provider vaccine attitudes and practices (Table 3). The survey was pilot tested with a convenience sample to ensure clarity of questions and ease of administration. The study team created a list of family medicine practices in New York State using an online search engine. The team then contacted 173 family medicine practices by telephone, explained the study goals, and faxed a cover letter and blank surveys to the practices that agreed to participate. After 2 months, surveys were again mailed to each of these practices in the event that they had not yet responded. Practice providers, including physicians, nurse practitioners, and physician assistants were asked to complete the survey, which was then returned, via fax, back to the study team. Completed surveys remained both anonymous and confidential. There were no incentives offered to the participants.
Table 3.
Questionnaire supplied to family medicine providers across New York State.
| Always | More than 50% | Less than 50% | None | |
|---|---|---|---|---|
| I recommend all standard childhood vaccines to my pediatric patients. | ||||
| I recommend influenza vaccine to my pregnant patients. | ||||
| I recommend pertussis vaccine to my pregnant patients. | ||||
| I provide pediatric vaccine information to my pregnant patients. | ||||
| We administer vaccines to pregnant women in the practice. | ||||
| I recommend HPVa vaccine to my patients at 11–12 year old visits. | ||||
| I recommend HPV vaccine to my adolescent patients (13–18 years). | ||||
| I recommend HPV vaccine to eligible patients 19–26 years old. | ||||
| I recommend influenza vaccine to all eligible patients. | ||||
| I recommend pertussis vaccine to adults with infant contact. | ||||
| I recommend PCV-13b to adults over 65 years of age. | ||||
| I recommend zoster vaccine to adults over 60 years of age. | ||||
| I get the influenza vaccine annually. | ||||
| Strongly agree | Agree | Disagree | Strongly disagree | |
| Autism is a possible side effect of some vaccines. | ||||
| Administering multiple vaccines at once reduces the efficacy of vaccines. | ||||
| Multiple vaccines at once overwhelms the patient's immune system. | ||||
| Vaccinating adolescents against HPV increases their likelihood of unprotected sexual activity. | ||||
| Standard childhood vaccines are safe. | ||||
| Standard childhood vaccines are effective. | ||||
| Influenza vaccine is safe in pregnant women. | ||||
| Influenza vaccine is effective in pregnant women. | ||||
| Pertussis vaccine is safe in pregnant women. | ||||
| Pertussis vaccine is effective in pregnant women. | ||||
| Zoster vaccine is safe in elderly patients. | ||||
| Zoster vaccine is effective in elderly patients. | ||||
| PCV-13* is safe in elderly patients. | ||||
| PCV-13* is effective in elderly patients. | ||||
| Lack of reimbursements inhibits vaccination in our office. | ||||
| Low patient uptake inhibits vaccination in our office. | ||||
| Lack of time with patients limits my time to address vaccine concerns | ||||
| Vaccine safety concerns affect my vaccine recommendations. | ||||
| Do you consider yourself to be vaccine hesitant? | Yes | No | ||
| How often do you encounter vaccine hesitant patients/parents? | Never | Daily | Weekly | Monthly |
| Which vaccines are your patients/parents concerned about? | ||||
| Do you feel comfortable educating vaccine hesitant patients/parents regarding vaccines? | Yes | No |
human papillomavirus.
pneumococcal conjugate vaccine-13.
Demographic information, including gender, provider role, years in practice, community served (suburban, rural, urban), patient insurance type accepted (public, private) were collected. Providers were able to identify more than one answer for community served and accepted insurance. Provider practices were assessed regarding recommendations of standard pediatric vaccines, including HPV vaccines for adolescents, influenza and Tdap vaccines for pregnant women, PCV13 to adults older than 65 years, and zoster vaccine to adults 60 years and older. Provider attitudes regarding vaccine safety and efficacy were assessed using an ordinal scale questionnaire, with an agreement level of 1 representing strongly disagree, 2 disagree, 3 agree, 4 strongly agree. The denominators presented in the results section represent the number of providers who answered the question(s) described. The study was determined to be exempt from approval by the SUNY Upstate Medical University institutional review board.
Statistical analysis: Descriptive statistics were used to quantify demographics of the sample. Association between vaccine practices and categorical factors, such as provider role, were tested using Pearson's chi-square tests of independence or Fisher's exact tests, as indicated. Ordinal scale measures of vaccine attitudes were compared across demographic factors using Mann-Whitney and Kruskal-Wallis tests. The scores for the four questions that measured agreement with incorrect perceptions about vaccine safety and efficacy were summed, as were the ten questions that gauged agreement with correct vaccine perceptions. A combined mean level of agreement for each of these categories was calculated for each subject. The combined means and mean ranks in each category were compared by provider demographics using Mann Whitney and Kruskal-Wallis tests. All statistical testing was conducted using an a priori α = 0.05, with reporting of two-tailed p-values. P-values > 0.05 and ≤ 0.10 were interpreted as indicating a trend towards significance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.CDC Surveillance of vaccination coverage among adult populations – United States, 2014. Surveillance Summaries, MMWR. 2016;65:1-36. doi: 10.15585/mmwr.ss6501a1. [DOI] [PubMed] [Google Scholar]
- 2.CDC National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years – United States, 2015. MMWR. 2016;65:850-8. PMID:27561081 [DOI] [PubMed] [Google Scholar]
- 3.Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: A critical review. Soc Sci& Med. 2014;112:1-11. doi: 10.1016/j.socscimed.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Darden PM, Jacobsen RM. Impact of a physician recommendation. Hum Vaccin Immunother. 2014;10:2632-5. doi: 10.4161/hv.29020. PMID:25483503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omer SB, Salmon DA, Orenstein WA, deHart P, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360:1981-8. doi: 10.1056/NEJMsa0806477. PMID:19420367 [DOI] [PubMed] [Google Scholar]
- 6.Posfay-Barbe KM, Heininger U, Aebi C, Desgrandchamps D, Vaudaux B, Siegrist CA. How do physicians immunize their own children? Differences among pediatricians and nonpediatricians. Pediatrics. 2005;116:e623-32. doi: 10.1542/peds.2005-0885. PMID:16263976 [DOI] [PubMed] [Google Scholar]
- 7.Katz-Sidlow RJ, Sidlow R. A look at the pediatrician as parent: Experiences with the introduction of varicella vaccine. Clin Pediatr. 2003;42:635-40. doi: 10.1177/000992280304200710. [DOI] [PubMed] [Google Scholar]
- 8.Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6. doi: 10.1016/j.vaccine.2016.10.042. PMID:27810314 [DOI] [PubMed] [Google Scholar]
- 9.Hurley LP, Bridges CB, Harpaz R, Allison MA, O'Leary ST, Crane LE, Brtnikova M, Stokley S, Beaty BL, Jimenez-Zambrano A, et al.. Physician attitudes toward adult vaccines and other preventive practices, United States, 2012. Public Health Rep. 2016;131:320-30. doi: 10.1177/003335491613100216. PMID:26957667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freed GL, Clark SJ, Cowan AE, Coleman MS. Primary care physician perspectives on providing adult vaccines. Vaccine. 2011;29:1850-4. doi: 10.1016/j.vaccine.2010.12.097. PMID:21216314 [DOI] [PubMed] [Google Scholar]
- 11.Nichol KL, Zimmerman R. Generalist and subspecialist physicians' knowledge, attitudes, and practices regarding influenza and pneumococcal vaccinations for elderly and other high-risk patients. Arch Intern Med. 2001;161:2702-8. doi: 10.1001/archinte.161.22.2702. PMID:11732935 [DOI] [PubMed] [Google Scholar]
- 12.Kulczycki A, Qu H, Shewchuk R. Primary care physicians' adherence to guidelines and their likelihood to prescribe the human papillomavirus vaccine for 11- and 12-year old girls. Women Health Iss. 2016;26:34-9. doi: 10.1016/j.whi.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC Influenza vaccination coverage among pregnant women – United States, 2014–2015 Influenza Season. MMWR. 2015;64:1000-5. PMID:26390253 [DOI] [PubMed] [Google Scholar]
- 14.Gargano LM, Herbert NL, Painter JE, Sales JM, Morfaw C, Rask K, Murray D, DiClemente RJ, Hughes JM. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-33. doi: 10.4161/hv.25823. PMID:23883781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuen CY, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women – A systematic review. Vaccine. 2014;32:4602-13. doi: 10.1016/j.vaccine.2014.06.067. PMID:24996123 [DOI] [PubMed] [Google Scholar]
- 16.Dempsey AF, Pyrzanowski JJ, Lockhart S, Campagna E, Barnard J, O'Leary ST. Parents' perceptions of provider communication regarding adolescent vaccines. Hum Vaccin & Immunother. 2016;12:1469-75. doi: 10.1080/21645515.2016.1147636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis D. Does CME work? An analysis of the effect of educational activities on physician performance or health care outcomes. Int J Psychiatry Med. 1998;28:21-39. doi: 10.2190/UA3R-JX9W-MHR5-RC81. PMID:9617647 [DOI] [PubMed] [Google Scholar]
- 18.Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA. 1995;274:700-5. doi: 10.1001/jama.1995.03530090032018. PMID:7650822 [DOI] [PubMed] [Google Scholar]
- 19.Boom JA, Nelson CS, Laufman LE, Kohrt AE, Kozinetz CA. Improvement in provider immunization knowledge and behaviors following a peer education intervention. Clin Pediatr. 2007;46:706-717. doi: 10.1177/0009922807301484. [DOI] [PubMed] [Google Scholar]
- 20.Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PD, Brewer NT. Physician communication about adolescent vaccination: How is human papillomavirus vaccine different? Prev Med. 2015;77:181-5. doi: 10.1016/j.ypmed.2015.05.024. PMID:26051197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rand CM, Shone LP, Albertin C, Auinger P, Klein JD, Szilagyi PG. National health care visit patterns of adolescents: Implications for delivery of new adolescent vaccines. Arch Pediatr Adolesc Med. 2007;161:252-9. doi: 10.1001/archpedi.161.3.252. PMID:17339506 [DOI] [PubMed] [Google Scholar]
- 22.Tsai Y, Zhou F, Wortley P, Shefer A, Stokley S. Trends and characteristics of preventive care visits among commercially insured adolescents, 2003–2010. J Pediatr. 2014;164:625-30. doi: 10.1016/j.jpeds.2013.10.042. PMID:24286572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meites E, KEmpe A, Markowitz LE. Use of a 2 dose schedule for Human Papillomavirus vaccination – Updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65:1405-8. PMID:27977643 [DOI] [PubMed] [Google Scholar]
- 24.Suryadevara M, Bonville CA, Domachowske JB. Cancer prevention bundle improves adolescent HPV vaccination rates. Accepted for poster presentation at Pediatric Academic Society, 2017, San Fransisco, CA [Google Scholar]
- 25.Daley MF, Crane LA, Markowitz LE, Black SR, Beaty BL, Barrow J, et al.. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics. 2010;126:425-33. doi: 10.1542/peds.2009-3500. PMID:20679306 [DOI] [PubMed] [Google Scholar]
- 26.Rutten LF, St. Sauver JL, Beebe TJ, Wilson PM, Jacobson DJ, Fan C, Breitkopf CR, Vadaparampil ST, Jacobson RM. Clinician knowledge, clinician barriers, and perceived parental barriers regarding human papillomavirus vaccination: Association with initiation and completion rates. Vaccine. 2017;35:164-9. doi: 10.1016/j.vaccine.2016.11.012. PMID:27887795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allison MA, Hurley LP, Markowitz L, Crane LA, Brtnikova M, Beaty BL, Snow M, Cory J, Stokley S, Roark J, Kempe A. Primary care physicians' perspectives about HPV vaccine. Pediatrics. 2016;137:e20152488. doi: 10.1542/peds.2015-2488. PMID:26729738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sussman AL, Helitzer D, Bennett A, Solares A, Lanoue M, Getrich CM. Catching up with the HPV vaccine: Challenges and opportunities in primary care. Ann Fam Med. 2015;13:354-60. doi: 10.1370/afm.1821. PMID:26195681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arao RF, Rosenberg KD, McWeeney S, Hedberg K. Influenza vaccination of pregnant women: Attitudes and behaviors of Oregon physician prenatal care providers. Matern Child Health J. 2015;19:783-9. doi: 10.1007/s10995-014-1569-x. PMID:25034358 [DOI] [PubMed] [Google Scholar]
- 30.Bonville CA, Cibula DA, Domachowske JB, Suryadevara M. Vaccine attitudes and practices among obstetric providers in New York State following the recommendation for pertussis vaccination during pregnancy. Hum Vaccin Immunother. 2015;11(3):713-8. doi: 10.1080/21645515.2015.1011999. PMID:25714987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Leary ST, Allison MA, Lindley MC, Crane LA, Hurley LP, Brtnikova M, Beaty BL, Babbel CI, Jimenez-Zambrano A, Berman S, Kempe A. Vaccine financing from the perspective of primary care physicians. Pediatrics. 2014;133(3):367-74. doi: 10.1542/peds.2013-2637. PMID:24567011 [DOI] [PMC free article] [PubMed] [Google Scholar]