Abstract
Background
The purposes of this study were to assess the usefulness of myocardial 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) for evaluating myocardial metabolic status in hypertrophic cardiomyopathy (HCM) and the therapeutic efficacy of alcohol septal ablation (ASA) in hypertrophic obstructive cardiomyopathy (HOCM).
Methods
Thirty HCM patients (64.4±10.5 years, 14 male, 12 hypertrophic non-obstructive cardiomyopathy [HNCM], 16 HOCM, and 2 dilated phase of HCM) underwent 18F-FDG-PET/CT. 18F-FDG uptake was semi-quantitatively evaluated using an uptake score in each 17 segment and the entire LV or regional standardized uptake value (SUV).
Results
18F-FDG uptake was observed mostly in a hypertrophied myocardium in HNCM patients, whereas 18F-FDG was extensively accumulated beyond the hypertrophied myocardium in HOCM patients. There was a positive correlation between the summed uptake score of 18F-FDG and high-sensitive troponin T level in HNCM patients (r = 0.603, p = 0.049), whereas the score was positively correlated with brain natriuretic peptide level (r = 0.614, p = 0.011) in HOCM patients. In 10 patients who received ASA, the maximum SUV of the entire LV was significantly reduced from 5.6±2.6 to 3.2±2.1 (p = 0.040) after ASA. Reduction of that maximum SUV was particularly significant in the lateral region (from 5.5±2.6 to 2.9 ±2.2, p = 0.024) but not significant in the anteroseptal region (from 4.5±2.6 to 2.9±1.6, p = 0.12).
Conclusion
Extensive 18F-FDG uptake beyond the hypertrophied myocardium was observed in HOCM. ASA attenuates 18F-FDG uptake in a remote lateral myocardium.
Introduction
Hypertrophic cardiomyopathy (HCM) is characterized by asymmetric left ventricular (LV) hypertrophy in the absence of other cardiac or systemic diseases that may cause cardiac hypertrophy [1]. The symptom and clinical course of HCM vary, regardless of the type and severity of HCM [2]. Although many patients stay asymptomatic and experience no serious cardiac event during their life time, some develop sudden death, refractory heart failure, repetitive syncope, and/or severe angina [1]. These various clinical presentations are closely associated with narrowing LV capacity, intra-LV obstruction, severe tissue degradation, extensive myocardial fibrosis, and diastolic dysfunction [1, 3]. Myocardial ischemia also plays an important role in those conditions [4, 5]. The pathohistological degradation involves not only the myocytes but also small coronary arteries, which cause microcirculation disturbance in some HCM patients [6]. Demand ischemia caused by supply/demand mismatch can also occur, particularly when the myocardium contracts against pressure overload [7]. It is important to understand whether myocardial ischemia is responsible for these symptoms when treating symptomatic HCM patients.
The significance of radionuclide imaging with a metabolic tracer in HCM has been reported [8, 9]. These methods are useful, particularly for evaluating myocardial ischemia in HCM, since fatty acid metabolism shifts to glucose metabolism in the setting of ischemia [10, 11]. We therefore focused on regional myocardial energy metabolism in various types of HCM patients using myocardial 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT). We sought to determine the relationship between the 18F-FDG uptake and type and severity of HCM or clinical parameters in HCM patients. Furthermore, we also assessed whether regional 18F-FDG uptake indicating energy metabolic shift or inflammatory response, can be altered by septal reduction therapy with alcohol septal ablation (ASA) in hypertrophic obstructive cardiomyopathy (HOCM) patients suffering from heart failure or angina symptoms refractory to medical treatment.
Material and methods
Patient population
This study has been approved by Institutional Review Board in Nippon Medical School, and has been conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from the participants. Between April 2013 and May 2016, 36 new patients with HCM were referred to the HCM clinic from other institutions for ASA or further medical treatment. Two patients with a dilated phase of HCM (DHCM) who used to have a typical HCM apparatus and currently had dilated cardiomyopathy-like echocardiographic findings were also included. Among them, 30 patients who agreed to receive 18F-FDG-PET/CT were included in this study. The inclusion criteria of this study were 1) the presence of a maximal LV wall thickness ≥15mm by transthoracic echocardiography (TTE) performed before initiation of medical treatment [12], and 2) the absence of other conditions that might explain left ventricular hypertrophy (LVH) during the clinical course. The diagnosis of HCM was based on electrocardiogram (ECG) and echocardiographic demonstrations. ECG and TTE showed a non-dilated, asymmetrical, hypertrophic LV in the absence of other cardiac or systemic diseases that could produce hypertrophy. All patients underwent either coronary angiography or coronary CT angiography and were confirmed to have no significant (>50%) coronary artery disease. Those who had significant valvular heart disease except mitral regurgitation, concomitant neoplasm, or poorly controlled diabetes mellitus (fasting blood sugar level ≥ 120 mg/dL) were not included in this study. HOCM was defined when intra-LV pressure gradient was > 30 mmHg at rest as seen on TTE [13]. A complete history was obtained and clinical examination was performed, along with assessments of New York Heart Association (NYHA) functional class, present drug therapy, and device therapy. We measured serum high-sensitive troponin T (HSTnT) and brain natriuretic peptide (BNP) levels with an AIA-2000ST analyzer (TOSOH, Tokyo, Japan) in accordance with the manufacturer’s instructions. The imaging protocol consisted of TTE, 18F-FDG-PET/CT, and cardiac magnetic resonance (CMR) imaging. They received those examinations within 3 weeks. The patients who received ASA underwent repeat imaging protocol approximately 6 months after ASA. Medication was kept constant during the study.
TTE study
We performed two-dimensional (2D), M-mode, and Doppler echocardiographic studies with PHILIPS IE 33 (Philips Healthcare, Best, The Netherlands) or GE Vivid E 9 ultrasound systems. We measured wall thickness (intraventricular septal thickness [IVST]; posterior wall thickness [PWT]), LV diastolic function (E/A [peak early transmitral filling velocity/peak late transmitral filling velocity], and E/e’ [peak early transmitral filling velocity/peak early diastolic mitral annulus velocity] of the septal side or lateral side on tissue Doppler imaging) and maximum pressure gradient in the LV at rest.
18F-FDG-PET/CT imaging
18F-FDG-PET/CT imaging proceeded as described [14]. Briefly, patients underwent dietary preparation with 24 hours of carbohydrate restriction (glucose < 10g) to suppress the physiological uptake of 18F-FDG. 60 min after intravenous administration of a 4MBq/kg dose of 18F-FDG, we acquired PET and CT images using a PET/CT scanner with 16-slice CT (GEMINI TF 16, Philips Healthcare, Best, The Netherlands). We checked the blood sugar level before the test to confirm that it is under 120mg/dl. Diabetic patients who received oral hypoglycemic agents or insulin were not included in the present study.
18F-FDG-PET/CT imaging analysis
We analyzed all PET/CT data as described [14] with a computer workstation. For the visual analysis of PET, we defined the 18F-FDG uptake as positive if it was greater than that for a physiologically normal liver. We used the AHA 17-segment model of the LV myocardium to visually localize 18F-FDG accumulated myocardium [15] using a semi-quantitative uptake score. Scores were defined as the following; 0: no uptake, 1: slight uptake, 2: mild uptake 3: moderate uptake, and 4: dense uptake. Extent score was calculated as the number of segments with 18F-FDG uptake. To compare 18F-FDG uptake between pre- and post-ASA, The difference score was calculated by subtracting the post-value from the pre-value for each segment. We also performed another semi-quantitative analysis. 2D regions of interest (ROI) were drawn on the transaxial slices of the PET images to measure the standardized uptake value (SUV) of the entire or regional (anteroseptal, inferior, or lateral) LV myocardium; SUV = (peak kBq/mL in ROI)/(injected activity/g body weight). Three investigators (two radiologists and a cardiologist) separately interpreted 18F-FDG-PET/CT findings. Consensus was reached in case of discrepancy.
CMR imaging
The electrocardiography-gated CMR protocol proceeded with breath-holding as described [16] using an Achieva 1.5 and 3.0 T (Philips Healthcare, Best, The Netherlands). Patients who had a pacemaker, claustrophobia, or renal dysfunction were excluded. Twenty-four patients received the CMR imaging with gadolinium enhancement and one received that without enhancement.
CMR imaging analysis
We measured the LV myocardial mass index (g/cm2), LV ejection fraction using Simpson’s method, mitral regurgitation (MR) jet, left ventricular outflow tract (LVOT) jet, and left atrial diameter on cine steady state free precession using a workstation (View Forum, Philips, Best, The Netherlands). Additionally, experienced radiologists evaluated imaging of late gadolinium enhancement (LGE) to clarify its presence. We applied the American Heart Association (AHA) 17-segments method to directly compare imaging and 18F-FDG-PET/CT for the visual evaluation of the extent of LGE.
ASA procedure
We performed ASA in 10 hypertrophic obstructive cardiomyopathy (HOCM) patients out of 20 candidates. Indication for HOCM was determined according to the following criteria: 1) symptoms were life limiting after optimization of medication, 2) resting or provoked gradient > 50 mmHg that was confirmed by at least one method during simultaneous pressure recordings, and 3) appropriate target branch(es) leading to the septal myocardium were responsible for intra-LV obstruction. ASA was performed as previously described [17].
Statistical analysis
Data were expressed as a mean ± the standard deviation. We performed all statistical analyses with IBM SPSS statistics version 21 and compared continuous variables between HNCM and LVOT obstruction (LVOTO) or segments with and without LGE using a Student's t-test or the Mann-Whitney's U test when appropriate. We compared categorical variables using the chi-square test and assessed the relationships between the summed uptake score and clinical parameters using Pearson's correlation. Pre- and post-ASA values were compared using a paired t-test or the Wilcoxon signed-rank test. Statistical significance was determined when a P-value was less than 0.05.
Results
Participants
Thirty HCM patients underwent 18F-FDG-PET/CT (age 64.4±10.5 years, 14 male [46.6%]). Twelve patients had HNCM, 14 had HOCM with LVOTO, two had HOCM with isolated mid-ventricular obstruction (MVO), and two had DHCM. The clinical characteristics of the HNCM and LVOTO patients in our study are listed in Table 1 and that of the 30 HCM patients in S1 Table. NYHA functional class and BNP values were significantly higher in HOCM patients with LVOTO than HNCM patients (2.5±0.6 vs. 1.6±0.9, P = 0.016, and 406.3±335.3 vs. 176.0±196.6 pg/ml, P = 0.043, respectively). E/e’ of HOCM patients with LVOTO was significantly higher than that of HNCM patients (25.1±13.6 vs. 14.2±4.8, P = 0.040 at septal side and 19.3±11.5 vs. 8.8±2.8, P = 0.030 at lateral side). Intra-LV pressure gradients of HOCM patients with LVOTO were significantly higher than that of HNCM patients (72.0±42.5 vs. 15.7±12.9 mmHg, P<0.001).
Table 1. Clinical characteristics of the study population.
| HNCM | LVOTO | P-value | |
|---|---|---|---|
| (HNCM vs. HOCM) | |||
| (n = 12) | (n = 14) | ||
| Age (years) | 61.5±8.7 | 67.6±9.7 | 0.11 |
| Male | 5(41.7) | 6 (42.8) | 0.63 |
| NYHA | 1.6±0.9 | 2.5±0.6 | 0.016 |
| BNP (pg/ml) | 176.0±196.6 | 406.3±335.3 | 0.043 |
| HSTnT (ng/ml) | 0.02±0.01 | 0.03±0.04 | 0.40 |
| Hypertension | 7 (58.3) | 9 (64.2) | 0.76 |
| Dyslipidemia | 7 (58.3) | 11 (78.6) | 0.27 |
| Diabetes mellitus | 2 (16.7) | 0 (0) | 0.11 |
| Smoking | 4 (33.3) | 2 (14.2) | 0.25 |
| eGFR (mL/min/1.73m2) | 64.5±7.5 | 55.6±16.5 | 0.07 |
| Syncope | 0 (0) | 4 (28.6) | 0.04 |
| Family history | 4 (33.3) | 3 (21.4) | 0.50 |
| AF | 1 (8.3) | 4 (28.6) | 0.19 |
| VT/VF | 1 (8.3) | 1 (7.1) | 0.91 |
| Medication | |||
| β-blocker | 12 (100) | 14 (100) | 1.00 |
| CCB | 6 (50) | 6 (42.9) | 0.51 |
| Class Ia | 5 (41.7) | 12 (85.7) | 0.025 |
| ICD/ pacemaker | 1 (8.3) | 1 (7.1) | 0.72 |
| TTE | |||
| IVST (mm) | 14.3±2.7 | 14.9±4.1 | 0.89 |
| LVPWT (mm) | 8.9±1.7 | 11.3±4.0 | 0.046 |
| Max. LV wall thickness (mm) | 15.9±2.0 | 16.6±3.0 | 0.53 |
| LAD (mm) | 39.2±6.3 | 43.7±8.2 | 0.12 |
| E/A | 1.1±0.5 | 1.2±0.6 | 0.69 |
| E/e’ (sep) | 14.2±4.8 | 25.1±13.6 | 0.040 |
| E/e’ (lat) | 8.8±2.8 | 19.3±11.5 | 0.030 |
| Intra-LVPG (mmHg) | 15.7±12.9 | 72.0±42.5 | <0.001 |
| SAM | 4 (33.3) | 9 (64.2) | 0.12 |
| 18F-FDG-PET/CT | |||
| Summed uptake score | 11.6±10.8 | 22.0±16.3 | 0.064 |
| Extent score | 5.2±3.8 | 7.3± 4.5 | 0.14 |
| Mean SUV (LV) | 1.5±0.4 | 2.0± 1.1 | 0.18 |
| Maximum SUV (LV) | 3.4±2.0 | 4.3± 2.8 | 0.35 |
| CMR imaging | |||
| Performed | 9 (75) | 14 (100) | 0.084 |
| performed with gadolinium enhancement | 9 (75) | 13 (92.9) | 0.24 |
| LV mass index (g/cm2) | 78.6±21.2 | 100.5±39.9 | 0.11 |
| LVEF (%) | 55.8±10.5 | 61.7±8.0 | 0.17 |
| The presence of LGE | 8 (66.7) | 7(50) | 0.46 |
| LVOT jet | 4(33.3) | 13 (92.9) | 0.007 |
| MR jet | 1 (8.3) | 10 (71.4) | 0.001 |
Data are expressed as mean ± standard deviation or number of the patients (percentage). P-value compares HNCM and LVOTO for continuous variables using Student's t-test and categorical and ordinal variables using chi-square test. P-value compares between two groups for NYHA using Mann-Whitney's U test. HNCM: hypertrophic non-obstructive cardiomyopathy, LVOTO: left ventricular outflow tract obstruction, NYHA: New York Heart Association functional class, BNP: brain natriuretic peptide, HSTnT: high-sensitive troponin T, Smoking: previous or current smoker, eGFR: estimated glomerular filtration rate, Syncope: past history of syncope, Family history: family history of HCM, AF: atrial fibrillation, VT: ventricular tachycardia, VF: ventricular fibrillation, CCB: calcium channel blocker, Class Ia: class Ia antiarrhythmic agents, TTE: transthoracic echocardiography, IVST: interventricular septum thickness, LVPWT: left ventricular posterior wall thickness, LAD: left atrium demension, E/A: peak early diastolic LV filling velocity/peak atrial filling velocity ratio, E/e’ (sep) and E/e’ (lat): the ratio between standard Doppler derived transmitral early diastolic velocity (E) and pulsed Doppler derived early diastolic velocity of the mitral annulus (e’) measured at the septal site and at the lateral site of the mitral annulus, Intra-LVPG: intra-left ventricular pressure gradient, SAM: systolic anterior motion of the mitral valve, 18F-FDG-PET/CT: myocardial 18F-fluorodeoxyglucose positron emission tomography/computed tomography, SUV: standardized uptake value, CMR: cardiac magnetic resonance, LV mass Index: left ventricular mass index, LVEF: left ventricular ejection fraction, LGE: late gadolinium enhancement, LVOT: left ventricular outflow tract, MR: mitral regurgitation.
18F-FDG and CMR analyses
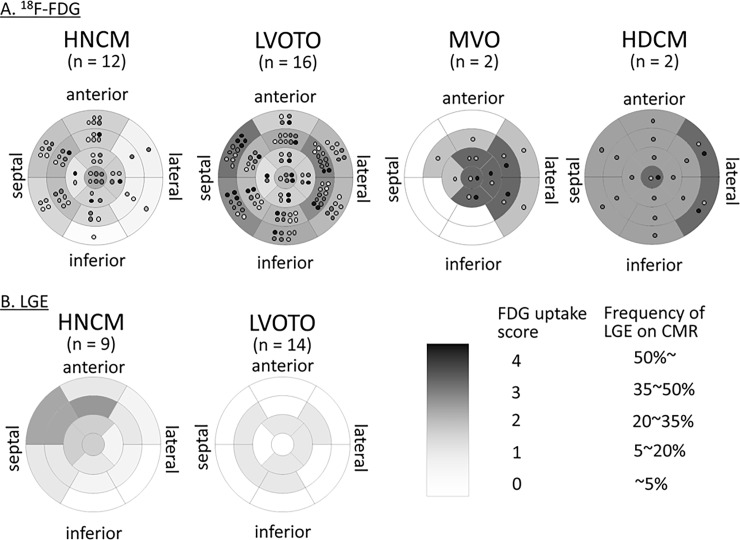
The 18F-FDG uptake in each type of HCM is shown in Fig 1A. Patients with HNCM had 18F-FDG uptake mostly in the basal and mid-anteroseptal segments, corresponding to a hypertrophied myocardium. On the other hand, HOCM patients with LVOTO had strong 18F-FDG uptake not only in the basal septal segments but also in the mid-lateral segments. HOCM patients with isolated MVO had 18F-FDG uptake mainly in the segments close to the apical or mid-free wall. The detailed degree of 18F-FDG uptake in each segment is shown in S2 Table and S1 Fig.
Fig 1. The degree of 18F-fluorodeoxyglucose (18F-FDG) uptake and the frequency of late gadolinium enhancement (LGE) at each LV 17 segment.
(A) The 18F-FDG uptake in each type of HCM. The degree of 18F-FDG uptake was visually evaluated and semi-quantitatively scored using an uptake score (0: no uptake, 1: slight uptake, 2: mild uptake 3: moderate uptake, and 4: dense uptake). Light gray dot indicates the individual with slight uptake, middle gray dot mild uptake, dark gray dot moderate uptake, and black dot dense uptake. The mean uptake score was calculated in each segment and expressed by the density of gray color. (B) The frequency of LGE in HNCM and HOCM with LVOTO. The frequency of LGE was calculated in each LV segment and expressed by the density of gray color. The data of MVO and DHCM are not shown because only one patient per type received CMR.
The frequency of LGE in each 17 segment in the HNCM and LVOTO types of HOCM is shown in Fig 1B. In HNCM patients, LGE was frequently observed in the anteroseptal segments. On the other hand, LGE was randomly observed in HOCM with LVOTO. The segments with LGE had a higher 18F-FDG uptake score compared to those without (1.4 ± 1.3 vs. 0.7 ± 1.2, P = 0.02) in HNCM patients, whereas the difference was not significant (2.0 ± 1.5 vs. 1.7 ± 1.5, P = 0.29) in HOCM patents. The frequency of LGE on each segment is shown in S3 Table.
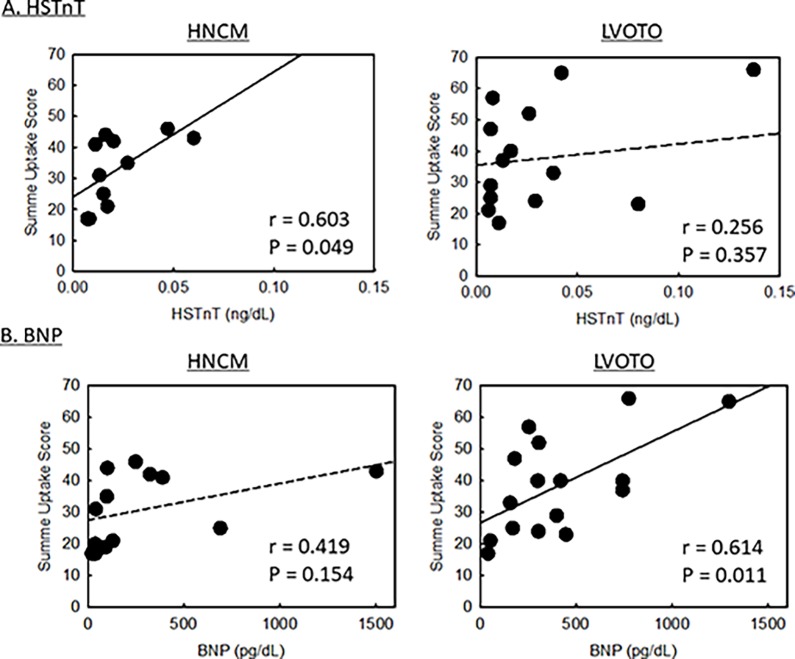
The relationships between the summed uptake score of 18F-FDG and HSTnT and BNP are shown in Fig 2. In HNCM patients, there was a positive correlation between the uptake score and HSTnT level (r = 0.603, P = 0.049) but not between the uptake score and BNP level (r = 0.419, P = 0.154). On the other hand, in HOCM patients, there as a positive correlation between the uptake score and BNP level (r = 0.614, P = 0.011) but not between the uptake score and HSTnT level (r = 0.256, P = 0.357).
Fig 2. The relationships between summed uptake score of 18F-FDG and clinical parameters.
(A) The relationships between the summed uptake score and high-sensitive troponin T (HSTnT) level in HNCM patients (the left panel) and in HOCM patients (the right panel). (B) The relationships between the summed uptake score and brain natriuretic peptide (BNP) level in HNCM patients (the left panel) and in HOCM patients (the right panel).
ASA procedure
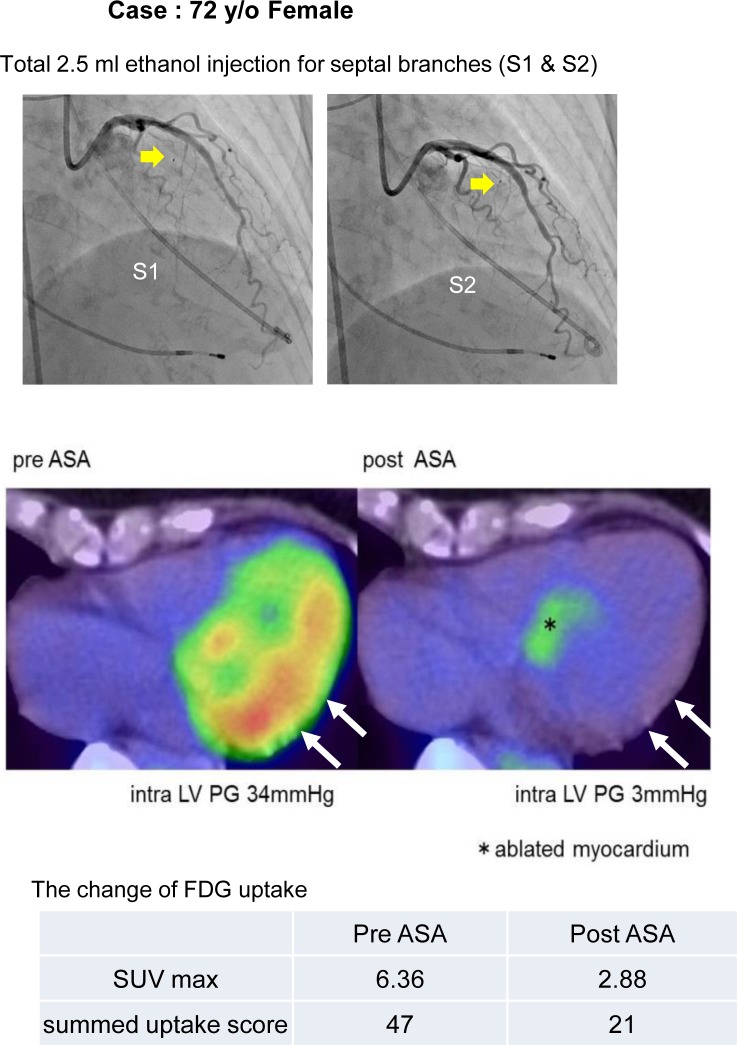
Ten HOCM cases received ASA. A representative case of a 72-year-old woman with HOCM who received ASA is described in Fig 3. As well as the significant reduction of the intra-LV pressure gradient, 18F-FDG uptake in the lateral LV wall was attenuated (Fig 3).
Fig 3. A representative case of ASA.
Images of serial 18F-FDG-positron emission tomography (PET)/computed tomography (CT) in a representative case of an HOCM patient who received alcohol septal ablation therapy (ASA). Ablated septal branches are indicated with yellow arrows in the upper panels. In the middle panels, long axis trans-vertical LV views of 18F-FDG-PET /CT before and after ASA are shown. An ablated myocardium is indicated with a star symbol, and the portion with a significant reduction of 18F-FDG uptake in the lateral and posterior LV wall is indicated with white arrows. In the lower table, the changes of the maximum standardized uptake value (SUV max) and summed uptake score are shown.
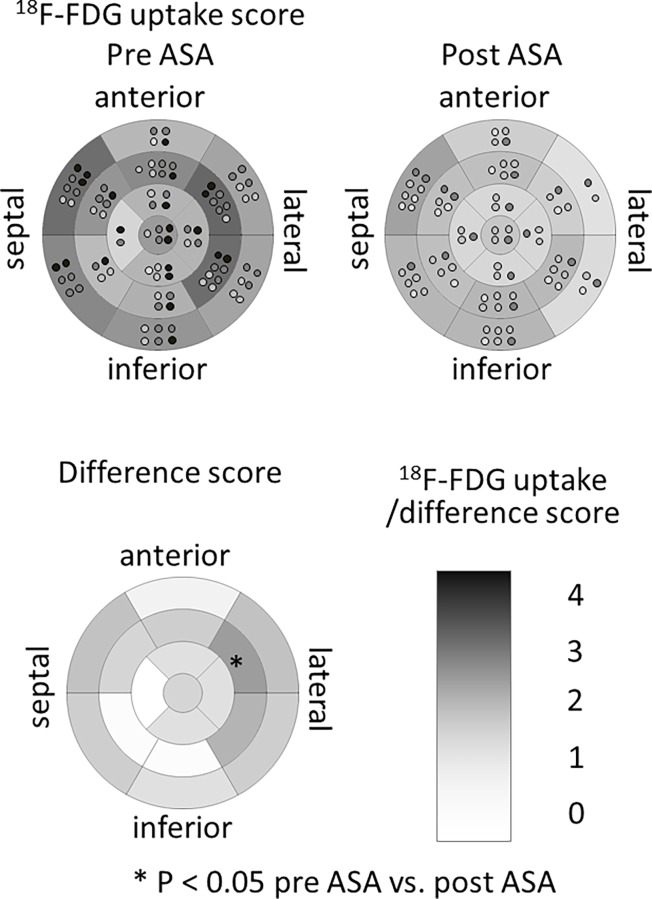
Table 2 shows the details of the procedures and the changes of clinical parameters after ASA. ASA reduced the intra-LV pressure gradient from 60.2±39.9 to 21.7±22.7 mmHg (P<0.001) and improved NYHA functional class from 2.5±0.5 to 1.1±0.3 (P<0.001). As well as the improvement of several clinical parameters such as BNP (from 496.1±361.7 to 238.2±155.5, P = 0.004) and E/e’ at both septal and lateral sides (from 25.1±9.7 to 19.1±5.9, P = 0.010, and from 17.6±8.6 to 9.8±3.1, P = 0.022, respectively), the summed uptake score of 18F-FDG uptake significantly reduced (from 26.9±14.5 to 15.6±16.1, P = 0.022). The semi-quantitative analysis of 18F-FDG uptake in the LV myocardium, expressed as maximum SUV of the entire LV, was significantly reduced (from 5.6±2.6 to 3.2±2.1, P = 0.040). The reduction was particularly significant in the lateral region (from 5.5±2.6 to 2.9±2.2, P = 0.024), not in the anteroseptal or inferior regions (from 4.5±2.6 to 2.9±1.6, P = 0.12, or from 4.4±2.9 to 2.5±1.5, P = 0.085, respectively). The semi-quantitative scoring method also revealed the significant reduction of 18F-FDG uptake in the mid-anterolateral segment after ASA (Fig 4). The detailed 18F-FDG uptake score before and after ASA and difference of uptake score at each segment are shown in S4 Table and S2 Fig.
Table 2. Changes of clinical parameters after ASA therapy.
| HOCM patients (n = 10) | Pre-ASA | Post-ASA | P-value | ||
|---|---|---|---|---|---|
| Age (years) | 64.7±12.6 | - | |||
| Male | 4 (40) | - | |||
| ASA procedure | |||||
| The amount of injected alcohol (ml) | 3.3±1.3 | - | |||
| The number of treated septal branches | 2.0±0.7 | - | |||
| Peak CPK (IU/L) | 1740.5±635.7 | - | |||
| Peak CPK-MB (IU/L) | 257.1±122.0 | - | |||
| NYHA functional class | 2.5±0.5 | 1.1±0.3 | <0.001 | ||
| BNP (pg/ml) | 496.1±361.7 | 238.2±155.5 | 0.004 | ||
| HSTnT (ng/ml) | 0.01±0.03 | 0.03±0.04 | 0.64 | ||
| TTE | |||||
| IVST (mm) | 15.6±4.3 | 13.1±2.7 | 0.072 | ||
| LVPWT (mm) | 11.5±4.3 | 11.3±4.4 | 0.38 | ||
| LAD (mm) | 46.4±7.5 | 40.2±7.8 | 0.004 | ||
| E/A | 1.3±0.5 | 1.1±0.7 | 0.16 | ||
| E/e' (sep) | 25.1±9.7 | 19.1±5.9 | 0.010 | ||
| E/e' (lat) | 17.6±8.6 | 9.8±3.1 | 0.022 | ||
| Intra-LVPG (mmHg) | 60.2±39.9 | 21.7±22.7 | <0.001 | ||
| SAM | 5 (50) | 2 (20) | 0.17 | ||
| Intra-LVPG by catheter measurement (mmHg) | 81.0±63.0 | 26.6±31.2 | 0.002 | ||
| 18F-FDG-PET/CT imaging | |||||
| Summed uptake score | 26.9±14.5 | 15.6±16.1 | 0.022 | ||
| Extent score | 8.1±3.9 | 6.0±3.5 | 0.26 | ||
| Mean SUV | Entire LV | 2.5±1.1 | 1.7±0.8 | 0.077 | |
| Anteroseptal | 2.3±1.2 | 1.7±0.6 | 0.14 | ||
| Inferior | 2.5±1.1 | 1.6±0.8 | 0.062 | ||
| Lateral | 2.6±1.3 | 1.6±0.9 | 0.053 | ||
| Maximum SUV | Entire LV | 5.6±2.6 | 3.2±2.1 | 0.040 | |
| Anteroseptal | 4.5±2.6 | 2.9±1.6 | 0.12 | ||
| Inferior | 4.4±2.9 | 2.5±1.5 | 0.085 | ||
| Lateral | 5.5±2.6 | 2.9±2.2 | 0.024 | ||
| CMR imaging | |||||
| LV mass index (g/cm2) | 102.3±43.9 | 101.8±51.4 | 0.47 | ||
| LVEF (%) | 59.7±7.3 | 60.4±10.2 | 0.56 | ||
| Presence of LGE | 5 (50) | 5 (50) | 0.67 | ||
| LVOT jet | 10 (100) | 7 (70) | 0.10 | ||
| MR jet | 7 (70) | 3 (30) | 0.086 | ||
Data are expressed as mean ± standard deviation or number of the patients (percentage). P-value compares pre- and post-ASA for continuous variables using paired Student's t-test and for categorical variables by using chi-square test.
HOCM: obstructive hypertrophic cardiomyopathy, ASA: alcohol septal ablation, CPK: creatine phosphokinase, CPK-MB: creatine phosphokinase MB isoenzyme, NYHA: New York Heart Association, BNP: brain natriuretic peptide, TTE: transthoracic echocardiography, IVST: interventricular septum thickening, LVPWT: left ventricular posterior wall thickening, LAD: left atrium dimension, E/A: peak early diastolic LV filling velocity/peak atrial filling velocity ratio, E/e’ (sep) and E/e’ (lat): the ratio between standard Doppler-derived transmitral early diastolic velocity (E) and pulsed Doppler-derived early diastolic velocity of the mitral annulus (e’) measured at the septal site and at the lateral site of the mitral annulus, intra-LVPG: intra-LV pressure gradient SAM: systolic anterior motion of the mitral valve, 18F-FDG-PET/CT: myocardial 18F-fluorodeoxyglucose positron emission tomography/computed tomography, SUV: standardized uptake value, CMR: cardiac magnetic resonance, LV mass index: left ventricular mass index, LVEF: left ventricular ejection fraction, LGE: late gadolinium enhancement, LVOT: left ventricular outflow tract, MR: mitral regurgitation.
Fig 4. Change of 18F-FDG uptake score after ASA.
Mean uptake score of 18F-FDG before and after ASA and difference score at each LV 17-segment. The mean uptake score was calculated with a semi-quantitative scoring method for each segment. Light gray dot indicates the individual with slight uptake, middle gray dot mild uptake, dark gray dot moderate uptake, and black dot dense uptake. The difference score was calculated by subtracting the post-value from the pre-value for each segment. The values were expressed by the density of gray color. * P<0.05 between pre- and post-ASA uptake score.
Discussion
In the present study, we addressed regional myocardial 18F-FDG uptake which indicate energy metabolic shift or inflammatory response using 18F-FDG-PET/CT in each type of HCM. Uptake of 18F-FDG was limited in a hypertrophied myocardium in HNCM whereas extensive 18F-FDG uptake beyond the hypertrophied myocardium was observed in HOCM. The extent and degree of 18F-FDG uptake was closely related with the HSTnT level, as well as the parameters of diastolic LV function and BNP. Reduction of intra-LV obstruction using ASA can affect myocardial metabolic shift in the lateral myocardium of HOCM.
Methodological considerations
In order to more precisely evaluate myocardial metabolism, we contrived a new carbohydrate restriction diet protocol in addition to conventional 18F-FDG-PET. A major obstacle in diagnosing myocardial metabolism by using 18F-FDG-PET is the high physiological accumulation of 18F-FDG in the myocardium, which interferes with the recognition of abnormal 18F-FDG uptake [14]. Suppression of this unfavorable uptake is important to identify myocardium metabolism status. It is reported that carbohydrate restriction over 24 hours significantly suppresses physiological accumulation of 18F-FDG in the myocardium [14]. Therefore, all patients underwent over 24 hours carbohydrate restriction (glucose, <10g) before 18F-FDG-PET/CT study.
In addition to the semi-quantitative scoring method, we also measure SUV which is considered to be more objective evaluation. The summed uptake score was positively correlated with mean SUV of entire LV (R = 0.673, P<0.001, S3 Fig). This indicates the appropriateness of our uptake scoring evaluation and sufficient restriction of physiological 18F-FDG uptake to other organ.
The significance of 18F-FDG uptake in HCM
The energy source of a normal myocardium is mainly fatty acids (over 90%)[10]. In some pathologic conditions such as ischemia or inflammation, the energy source of the myocardium shifts to glucose metabolism from fatty acids[8]. 18F-FDG is an analogue of glucose, and 18F-FDG-PET is used to visualize glucose metabolism in vivo [18]. In HCM, we postulate that the following four mechanisms are involved, in which glucose metabolism is necessary: 1) increased energy demand due to myocardial hypertrophy[19], 2) inflammatory response caused by inflammatory cell infiltration[20], 3) myocardial ischemia due to microangiopathy [21, 22], and 4) demand myocardial ischemia due to supply/demand mismatch of blood flow [23]. We speculate that the accumulation of 18F-FDG that we observed at non-hypertrophied lesions in LVOTO patients mainly involves the demand myocardial ischemia, because blood supply decreases due to increased extravascular compressive forces and oxygen demand increases in order to contract against wall stress under the increased pressure overload condition.
The usefulness of 18F-FDG-PET in HCM has been reported in previous studies [24–26]. Uehara reported that 18F-FDG uptake is increased not only in hypertrophied but also in the non-hypertrophied myocardium in HCM with asymmetrical septal hypertrophy or a dilated phase of HCM, whereas it was limited in a hypertrophied myocardium in HNCM [26]. The present study, in agreement with Uehara’s report, identified extensive pathophysiological metabolism beyond the hypertrophied myocardium in HOCM using 18F-FDG-PET/CT although the PET protocol was different from that in the previous study. As novel findings, we demonstrated that abnormal metabolism at a non-hypertrophied myocardium can be reversed by the attenuation of intra-LV obstruction.
We found that18F-FDG uptake score was positively correlated with HSTnT level in HNCM patients. Because HSTnT is generally considered as the cardiac marker of ongoing myocardial injury, 18F-FDG uptake observed in hypertrophic myocardium may be reflecting inflammatory response or ischemia caused by microcirculation disturbance. On the other hand, we found the positive correlation between 18F-FDG uptake score and BNP level in HOCM patients, indicating that 8F-FDG uptake was more extensively observed in failing heart in this condition. Flow disturbance at LVOT due to obstruction causes metabolic abnormality in not only hypertrophied but also remote myocardium possibly due to demand ischemia, as well as increased secretion of BNP.
LGE and 18F-FDG uptake
Since LGE seen on CMR is known to reflect myocardial fibrosis, the existence of LGE in HCM indicates an advanced stage of this disease [27]. The pathohistological changes of HCM are characterized by myocyte disarray, enlarged cardiomyocytes, and deposition of interstitial fibrosis [27]. To develop dense myocardial fibrosis, detected as LGE on CMR, sustained pathological stress is considered to be necessary. In this process, microvascular ischemia or inflammatory response plays an important role. A previous study has reported a significant relationship between microvascular ischemia and myocardial fibrosis [28]. In the present study, by comparing CMR and 18F-FDG-PET/CT, we clarified the spatial relationship between LGE and 18F-FDG uptake in each type of HCM. In patients with HNCM, we found LGE as well as 18F-FDG uptake mostly in hypertrophied segments. This may indicate that the myocardium requiring glucose metabolism also has fibrotic changes. Therefore, it is possible that the 18F-FDG uptake may indicate the risk of the development of fibrosis in HNCM patients. On the other hand, in patients with LVOTO, 18F-FDG was widely distributed in both hypertrophied septal and remote lateral segments whereas LGE was rarely observed in those segments. The possible explanation of less frequent LGE segments compared with 18F-FDG uptake segments in LVOTO is that LGE reflects progressed myocardial cell damage, whereas 18F-FDG uptake reflects metabolic disorders which emerge from the early stage of myocardial cell damage. Since the pathological condition responsible for their symptom in patients with LVOTO who were referred for ASA was mainly due to mechanical intra LV obstruction, LVOTO patients enrolled in the present study might be still on the way to the development of myocardial fibrosis.
Serial assessment of 18F-FDG-PET/CT before and after ASA
ASA is an optional treatment for symptomatic HOCM patients who are refractory to medical therapy [29]. Increasing evidence has supported the beneficial effect of ASA in improving symptoms associated with HOCM [30, 31]. Timmer et al. have reported that ASA has favorable effects on myocardial metabolism. 15O-H2O PET and 11C-acetate PET showed the changes of microvascular function and myocardial metabolism by relief of LVOTO [32]. In the present study, we found altered metabolism or myocardial inflammation detected as 18F-FDG uptake which observed in a non-hypertrophied myocardium can be reversed by ASA. We attributed this improvement to attenuation of demand myocardial ischemia, which is associated with wall stress from pressure overload. A previous study has reported the beneficial effect of ASA on the systolic movement of lateral (remote) wall using CMR. In the present study, we demonstrated the disappearance of 18F-FDG uptake in the remote myocardium as well as diastolic function in the lateral side. The metabolic improvement may explain, at least in part, the mechanism of the improvement of systolic function observed in the previous study [33].
Currently, indication for ASA is fundamentally determined by the degree of pressure gradient and assessment of life-limiting symptoms, assessed by NYHA grade [30, 31]. However, a discrepancy between these two factors is frequently observed [17]. We therefore postulate that the severity and extent of 18F-FDG uptake may be helpful for determining the indication for ASA, although further study is necessary to confirm the generalizability of our findings. 18F-FDG-PET/CT can be also useful to evaluate the therapeutic efficacy of ASA.
Limitations
The number of patients was small due to the limited capacity of PET imaging in our institution. Particularly, we only included two isolated MVO patients and two dilated HCM patients in this study. Therefore, we did not include their data in our analysis and avoided commenting on isolated MVO or dilated HCM, although data are shown in the figures and tables as references. Secondly, the study was basically cross-sectional and no long-term follow-up data were addressed. Thus, prognostic implication of 18F-FDG-PET/CT could not be assessed in this study. Finally, although we observed significant 18F-FDG uptake in hypertrophied myocardium in HNCM patients, it remains unclear whether the uptake is indeed pathophysiological or mostly due to the greater thickness of the hypertrophied septum. The increase of 18F-FDG uptake might be related to a relative, partial-volume dependent overestimation of the true 18F-FDG tissue concentration.
Conclusion
We addressed regional myocardial energy metabolic shift using 18F-FDG-PET/CT in HNCM and HOCM patients. Uptake of 18F-FDG is limited in the hypertrophied myocardium in HNCM whereas extensive 18F-FDG uptake beyond the hypertrophied myocardium was observed in HOCM. The fact that ASA can contribute to the improvement of myocardial metabolism or inflammation in the remote myocardium suggests the novel, beneficial effect of ASA besides the symptomatic improvement.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
Acknowledgments
Part of the data of the present study was previously presented in ACC. 16 Scientific Session and JCS2016 –The 80th Annual Scientific Meeting.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. Jama. 2002;287(10):1308–20. Epub 2002/03/12. . [DOI] [PubMed] [Google Scholar]
- 2.Wigle ED, Sasson Z, Henderson MA, Ruddy TD, Fulop J, Rakowski H, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1–83. Epub 1985/07/01. . [DOI] [PubMed] [Google Scholar]
- 3.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295–303. Epub 2003/01/24. doi: 10.1056/NEJMoa021332 . [DOI] [PubMed] [Google Scholar]
- 4.Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, et al. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2006;47(5):1043–8. Epub 2006/03/07. doi: 10.1016/j.jacc.2005.10.050 . [DOI] [PubMed] [Google Scholar]
- 5.Cecchi F, Sgalambro A, Baldi M, Sotgia B, Antoniucci D, Camici PG, et al. Microvascular dysfunction, myocardial ischemia, and progression to heart failure in patients with hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2(4):452–61. Epub 2010/06/19. doi: 10.1007/s12265-009-9142-5 . [DOI] [PubMed] [Google Scholar]
- 6.Kakoi H, Inoue T, Morooka S, Hayashi T, Takabatake Y. Relationship between regional abnormality of left ventricular rapid filling and coronary microcirculation disturbance in hypertrophic cardiomyopathy. Clinical cardiology. 1996;19(5):379–83. Epub 1996/05/01. . [DOI] [PubMed] [Google Scholar]
- 7.Cannon RO, 3rd, Rosing DR, Maron BJ, Leon MB, Bonow RO, Watson RM, et al. Myocardial ischemia in patients with hypertrophic cardiomyopathy: contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation. 1985;71(2):234–43. Epub 1985/02/01. . [DOI] [PubMed] [Google Scholar]
- 8.Kurata C, Tawarahara K, Taguchi T, Aoshima S, Kobayashi A, Yamazaki N, et al. Myocardial emission computed tomography with iodine-123-labeled beta-methyl-branched fatty acid in patients with hypertrophic cardiomyopathy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1992;33(1):6–13. Epub 1992/01/01. . [PubMed] [Google Scholar]
- 9.Chen SL, Uehara T, Morozumi T, Yamagami H, Kusuoka H, Nishimura T. Myocardial metabolism of 123I-BMIPP in patients with hypertrophic cardiomyopathy: assessment by radial long-axis SPET. Nuclear medicine communications. 1995;16(5):336–43. . [DOI] [PubMed] [Google Scholar]
- 10.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987;79(2):359–66. Epub 1987/02/01. doi: 10.1172/JCI112820 ; PubMed Central PMCID: PMCPMC424073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshinaga K, Tamaki N. Imaging myocardial metabolism. Curr Opin Biotechnol. 2007;18(1):52–9. Epub 2006/12/13. doi: 10.1016/j.copbio.2006.11.003 . [DOI] [PubMed] [Google Scholar]
- 12.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58(25):e212–60. doi: 10.1016/j.jacc.2011.06.011 . [DOI] [PubMed] [Google Scholar]
- 13.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–79. Epub 2014/09/01. doi: 10.1093/eurheartj/ehu284 . [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi Y, Kumita S-i, Fukushima Y, Ishihara K, Suda M, Sakurai M. Significant suppression of myocardial 18F-fluorodeoxyglucose uptake using 24-h carbohydrate restriction and a low-carbohydrate, high-fat diet. Journal of cardiology. 2013;62(5):314–9. doi: 10.1016/j.jjcc.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. The international journal of cardiovascular imaging. 2002;18(1):539–42. Epub 2002/07/24. . [PubMed] [Google Scholar]
- 16.Amano Y, Kitamura M, Tachi M, Takeda M, Mizuno K, Kumita S. Delayed enhancement magnetic resonance imaging in hypertrophic cardiomyopathy with Basal septal hypertrophy and preserved ejection fraction: relationship with ventricular tachyarrhythmia. J Comput Assist Tomogr. 2014;38(1):67–71. Epub 2014/01/01. doi: 10.1097/RCT.0b013e3182a2fb01 . [DOI] [PubMed] [Google Scholar]
- 17.Kitamura M, Takayama M, Matsuda J, Kubota Y, Nakamura S, Takano H, et al. Clinical Characteristics and Outcome of Alcohol Septal Ablation With Confirmation by Nitroglycerin Test for Drug-Refractory Hypertrophic Obstructive Cardiomyopathy With Labile Left Ventricular Outflow Obstruction. Am J Cardiol. 2015;116(6):945–51. Epub 2015/07/26. doi: 10.1016/j.amjcard.2015.06.023 . [DOI] [PubMed] [Google Scholar]
- 18.Handa N, Magata Y, Mukai T, Nishina T, Konishi J, Komeda M. Quantitative FDG-uptake by positron emission tomography in progressive hypertrophy of rat hearts in vivo. Annals of nuclear medicine. 2007;21(10):569–76. Epub 2007/12/20. doi: 10.1007/s12149-007-0067-2 . [DOI] [PubMed] [Google Scholar]
- 19.Stanley WC. Myocardial energy metabolism during ischemia and the mechanisms of metabolic therapies. J Cardiovasc Pharmacol Ther. 2004;9 Suppl 1:S31–45. Epub 2004/09/21. PubMed doi: 10.1177/107424840400900104 . [DOI] [PubMed] [Google Scholar]
- 20.Kuusisto J, Karja V, Sipola P, Kholova I, Peuhkurinen K, Jaaskelainen P, et al. Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart. 2012;98(13):1007–13. doi: 10.1136/heartjnl-2011-300960 ; PubMed Central PMCID: PMC3368494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grover-McKay M, Schwaiger M, Krivokapich J, Perloff JK, Phelps ME, Schelbert HR. Regional myocardial blood flow and metabolism at rest in mildly symptomatic patients with hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 1989;13(2):317–24. Epub 1989/02/01. . [DOI] [PubMed] [Google Scholar]
- 22.Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84(5):476–82. Epub 2000/10/20. PubMed doi: 10.1136/heart.84.5.476 ; PubMed Central PMCID: PMCPmc1729476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raphael CE, Cooper R, Parker KH, Collinson J, Vassiliou V, Pennell DJ, et al. Mechanisms of Myocardial Ischemia in Hypertrophic Cardiomyopathy: Insights From Wave Intensity Analysis and Magnetic Resonance. Journal of the American College of Cardiology. 2016;68(15):1651–60. doi: 10.1016/j.jacc.2016.07.751 ; PubMed Central PMCID: PMC5054113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagaya Y, Ishide N, Takeyama D, Kanno Y, Yamane Y, Shirato K, et al. Differences in myocardial fluoro-18 2-deoxyglucose uptake in young versus older patients with hypertrophic cardiomyopathy. Am J Cardiol. 1992;69(3):242–6. Epub 1992/01/15. . [DOI] [PubMed] [Google Scholar]
- 25.Tadamura E, Tamaki N, Matsumori A, Magata Y, Yonekura Y, Nohara R, et al. Myocardial metabolic changes in hypertrophic cardiomyopathy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1996;37(4):572–7. Epub 1996/04/01. . [PubMed] [Google Scholar]
- 26.Uehara T, Ishida Y, Hayashida K, Shimonagata T, Miyake Y, Sago M, et al. Myocardial glucose metabolism in patients with hypertrophic cardiomyopathy: assessment by F-18-FDG PET study. Annals of nuclear medicine. 1998;12(2):95–103. Epub 1998/06/24. . [DOI] [PubMed] [Google Scholar]
- 27.Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2004;43(12):2260–4. Epub 2004/06/15. doi: 10.1016/j.jacc.2004.03.035 . [DOI] [PubMed] [Google Scholar]
- 28.Villa AD, Sammut E, Zarinabad N, Carr-White G, Lee J, Bettencourt N, et al. Microvascular ischemia in hypertrophic cardiomyopathy: new insights from high-resolution combined quantification of perfusion and late gadolinium enhancement. J Cardiovasc Magn Reson. 2016;18:4 Epub 2016/01/16. doi: 10.1186/s12968-016-0223-8 ; PubMed Central PMCID: PMCPMC4714488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995;346(8969):211–4. Epub 1995/07/22. . [DOI] [PubMed] [Google Scholar]
- 30.Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006;19(4):319–27. Epub 2006/08/03. doi: 10.1111/j.1540-8183.2006.00153.x . [DOI] [PubMed] [Google Scholar]
- 31.Nagueh SF, Groves BM, Schwartz L, Smith KM, Wang A, Bach RG, et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy. A multicenter North American registry. Journal of the American College of Cardiology. 2011;58(22):2322–8. Epub 2011/11/19. doi: 10.1016/j.jacc.2011.06.073 . [DOI] [PubMed] [Google Scholar]
- 32.Timmer SA, Knaapen P, Germans T, Dijkmans PA, Lubberink M, Ten Berg JM, et al. Effects of alcohol septal ablation on coronary microvascular function and myocardial energetics in hypertrophic obstructive cardiomyopathy. Am J Physiol Heart Circ Physiol. 2011;301(1):H129–37. Epub 2011/04/15. doi: 10.1152/ajpheart.00077.2011 . [DOI] [PubMed] [Google Scholar]
- 33.van Dockum WG, Kuijer JP, Gotte MJ, Ten Cate FJ, Ten Berg JM, Beek AM, et al. Septal ablation in hypertrophic obstructive cardiomyopathy improves systolic myocardial function in the lateral (free) wall: a follow-up study using CMR tissue tagging and 3D strain analysis. Eur Heart J. 2006;27(23):2833–9. Epub 2006/11/14. doi: 10.1093/eurheartj/ehl358 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.