Abstract
Commuters who spend long hours on roads are exposed to high levels of traffic related air pollutants (TRAPs). Despite some well-known multiple adverse effects of TRAPs on human health, limited studies have focused on mitigation strategies to reduce these effects. In this study, we measured fine particulate matter (PM2.5) and ultrafine particle (UFP) concentrations inside and outside 17 taxis simultaneously while they were driven on roadways. The drivers’ urinary monohydroxylated polycyclic aromatic hydrocarbons (OH-PAHs) and malondialdehyde (MDA) concentrations just before and right after the driving tests were also determined. Data were collected under three driving conditions (i.e. no mitigation (NM), window closed (WC), and window closed plus using high efficiency cabin air filters (WC+HECA)) for each taxi and driver. The results show that, compared to NM, the WC+HECA reduced in-cabin PM2.5 and UFP concentrations, by 37% and 47% respectively (p < 0.05), whereas the reductions on PAH exposures were insignificant. Although nonsignificant, a reduction of 17% was also observed in the drivers’ urinary MDA under WC+HECA. The MDA concentrations were found to be significantly associated with the in-cabin PM2.5 and UFP concentrations, suggesting the reduction of the drivers’ lipid peroxidation can be at least partially attributed to the PM2.5 and UFP reduction by WC+HECA. Overall, these results suggest HECA filters have potential to reduce particle levels inside taxis and protect drivers’ health.
Introduction
Air pollution is one of the leading risk factors of human premature death globally [1, 2]. As the most substantial combustion source in urban areas with dense population, vehicular emissions significantly increase carbon monoxide (CO), nitrogen oxides (NOx), fine particulate matter (PM2.5), ultrafine particles (UFPs), and polycyclic aromatic hydrocarbon (PAH) concentrations on/near roadways and inside vehicles [3–6]. Over 600 million people worldwide are exposed to these hazardous traffic related air pollutants (TRAPs) [7, 8], which have been associated with various adverse health effects [9–11].
Among the TRAPs, PM2.5, UFP and PAHs have been found to be associated with cardiovascular diseases, which are the primary contributors to the air pollution related premature deaths [12]. Because of the frequently observed association between PM2.5 and the oxidative damage, the oxidative stress has been suggested to play a role linking the exposures with the cardiovascular diseases, although the mechanism has not been fully understood. Specifically, previous studies have shown that PM2.5 and UFP from traffic emissions have high oxidative potential that induces elevated systematic oxidative stress in rats and human [13–15]. Malondialdehyde (MDA) is a lipid peroxidation end-product and is usually produced under body systematic oxidative stress. Some in vitro and in vivo studies also suggest that PAHs and their metabolites can join the redox-active cycle and produce reactive oxygen species (ROS), which can attack larger biological molecules including polyunsaturated lipids and generate MDA [16, 17]. In addition, MDA has the potential to react with nucleic acid bases to form DNA adducts, create DNA interstrand, and even DNA protein cross-links to induce more health risks [18, 19]. Thus, MDA is a widely used biomarker for evaluation of oxidative stress associated with exposures to combustion-related air pollutants [18, 20].
Vehicular emissions are known as the primary sources of ambient PM in the Los Angeles air basin [21]. About 33–45% of the total UFP exposure for Los Angeles residents occur due to time spent traveling in vehicles [22]. Some mitigation strategies have been explored in previous studies to reduce PM exposures for commuter, which include closing vehicle windows and using the high efficiency cabin air (HECA) filters. A passenger vehicle study found that keeping vehicle windows closed and using outside air ventilation mode reduced the on-road PM by up to 40% [23]. Furthermore, when the vehicle was equipped with a HECA filter, it reduced the on-road PM2.5 and UFP by 70% and 92%, respectively [24]. However, it remains unknown to what extent these mitigation strategies benefit the commuters’ health.
About 4,000 taxi drivers are working in the Greater Los Angeles area. They work 72 hours per week on average, and potentially have high TRAP exposures because of the long hours spent on roadways [25]. In this study, we measured real time PM2.5 and UFP concentrations simultaneously inside and outside 17 Los Angeles taxis while they were driven on roads, under different mitigation conditions. The drivers’ urinary monohydroxylated-PAHs (OH-PAHs) and MDA concentrations were also measured just before and right after each 6-hour monitored driving test. The aim of this study is to evaluate whether using HECA filters can effectively reduce the drivers’ PM and PAH exposures, as well as the lipid peroxidation levels in their body.
Methods
Subjects
The detailed description on the taxi driver recruitment can be found elsewhere [26]. Briefly, in March 2013, 2449 recruitment forms were hand distributed to taxi drivers passing through the Los Angeles International Airport (LAX) taxi holding lot. A total of 316 completed forms were obtained. Taxi drivers who smoked or drove Ford Crown Victoria taxis were excluded from this study, because cigarette smoking interferes with the PAH measurements, and the Ford Crown Victoria taxis don’t have a cabin air filter holder. Finally, 17 subjects were random picked out of the 90 qualified taxi drivers. Their demographic characteristics, body mass index (BMI), years as taxi drivers, and taxi vehicle make/models are summarized in Table 1. The study design and experimental protocol have been approved by the UCLA Institutional Review Board (IRB). Informed consents were obtained from all participants.
Table 1. Characteristics of the studied taxi drivers*.
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Age (year) | 47 ± 13 | 28 | 67 |
| Year as Taxi Driver | 10 ± 6 | 2 | 20 |
| BMI (kg / m2) | 26.8 ± 4.6 | 19.5 | 38.7 |
| Ethnicity, n (%) | Taxi Model, n (%) | ||
| Black | 2 (11.8%) | Chevy Uplander | 1 (5.9%) |
| Hispanic/Latino | 0 (0%) | Dodge Caravan | 3 (17.6%) |
| White | 6 (35.3%) | Toyota Prius | 10 (58.8%) |
| Asian | 5 (29.4%) | Toyota Camry | 3 (17.6%) |
| Other | (23.5%) | ||
* Of seventeen total, sixteen were males (94.1%) and one female.
Monitoring of driving test
Each driver was monitored for six hours a day and for three consecutive days while driving in the Greater Los Angeles area. The starting time and driving routes of each test day were kept consistent for each driver. During the tests the drivers were requested to mimic their actual work for driving, parking/waiting, and taking breaks during the monitored six hours.
On the first test day, the taxi drivers were allowed to control the window positions and use their originally equipped manufacturer (OEM) cabin filter to simulate their regular working conditions without any mitigation (No mitigation; NM). On the second test day, the taxi drivers were requested to keep their vehicle windows closed, but their OEM cabin filter remained unchanged (Window closed; WC). The third test day was designed to have the most strict mitigation strategy in place by keeping the taxi windows closed and using a HECA filter simultaneously (WC+HECA). The HECA filters were provided by an industrial partner and technical details of the filters are described elsewhere [24]. Throughout the period of measurements, the taxi ventilation system was set to outdoor air mode to avoid CO2 build-up. The fan was set to medium level when the windows were closed. The total mileage driven by the tested taxi drivers was approximately 11,000 kilometers and the total amount of time of field measurements was approximately 300 hours.
PM monitoring and urine collection
During the 6-hr monitored driving tests, two sets of portable condensation particle counter (CPC) (Model 3007, TSI Inc., St. Paul, MN) and DustTrak (Model 8520, TSI Inc., St. Paul, MN) were deployed to measure UFP and PM2.5 concentrations concurrently inside and outside of each taxi, respectively. The CPC measures the number concentration for particles sizes from 0.01 to 1.0 μm. The DustTrak was connected with a 2.5 μm impactor to measure the PM2.5 mass concentration. For in-cabin measurements, the inlets of the two pieces of instrument were connected with the same length of tubing extending to the driver’s breathing zone. For on-road measurements, similar tubing of the same length was mounted on the taxi window to compensate any diffusion loss in the sampling lines. Both CPC and DustTrak are real-time direct reading instrument with data logging function. UFP and PM2.5 concentrations were logged with 1-sec interval during the experiments. Daily 6-hr averages were used for subsequent analysis. Urine samples were collected in 90 ml sterile screw cap urine containers just before and right after the 6-hr monitored driving test (pre- and post-test) on each test day for each taxi driver. All collected urine samples were stored in a -20°C freezer until analysis.
Analysis of urinary OH-PAHs and MDA
The OH-PAH analysis method was based on enzymatic deconjugation, liquid-liquid extraction, trimethylsilylation of the OH-PAHs, followed by gas chromatography/isotope dilution high-resolution mass spectrometry [27]. Nine OH-PAHs quantified were: 1- and 2-hydroxynaphthalene (1- and 2-OH-NAP), 2-, 3- and 9-hydroxyfluorine (2-, 3-, and 9-OH-FLU), 1-, 2-, and 3-hydroxyphenanthrene (1-, 2-, and 3-OH-PHE), and 1-hydroxypyrene (1-OH-PYR). Explained in a previous study [28], briefly, the urinary MDA was analyzed by a high performance liquid chromatography (HPLC) system with a fluorescent detector. Each urine sample was added with a mixture of phosphoric acid and thiobarbituric acid (TBA, 42 mM), and incubated for 1 hour at 80°C. Then the solution with MDA-TBA derivatives was injected into the HPLC system with a fluorescence detector at 532 nm. Urinary creatinine was analyzed with Jaffe method which generates stable creatinine results for urine samples kept at a temperature of -20°C and thawed only once [29, 30]. The urinary OH-PAH and MDA concentrations were adjusted with creatinine.
Quality assurance and quality control
For both CPC and DustTrak, flow rate measurement and zero check with HEPA filters was performed before and after each monitored tests. The DustTrak monitor was calibrated against simultaneous gravimetric measurements of PM2.5. A factor of 2.4 was achieved and used for DustTrak data correction, which was consistent with data reported in previous studies [31, 32]. For OH-PAH analysis, quality control (QC) samples were analyzed along with the taxi driver samples to assure the precision and accuracy of the data. QC data were reviewed based on Westgard rule [33, 34]. Proficiency Test (PT) or inter-laboratory QC samples of unknown concentrations were performed twice a year for demonstration of OH-PAH analytical method performance.
Estimation of PAH exposure due to traffic
Urinary OH-PAHs are biomarkers of integrated body PAH intake through different exposure routes. The half-lives of the urinary OH-PAHs are usually several hours in human body, and it takes days to completely excrete OH-PAHs after the PAH exposures. Although we tried to minimize the PAH intake from other sources by prohibiting eating barbequed or fried food during the test days, taxi drivers’ urinary OH-PAH levels could still be influenced by the PAH exposures other than the traffic emissions during the monitored driving tests, such as previous TRAP exposures or dietary intake. Therefore, neither the pre- nor post-test urinary OH-PAHs can directly indicate the traffic-related PAH exposure levels during the monitored taxi driver tests. Instead, we used a well-established one-compartment pharmacokinetic model with continuous infusion to estimate the traffic-related PAH exposure during the monitored tests [35]. In this model, we assumed constant and continuous exposure to PAHs during the tested six hours. We also assumed PAHs underwent a first-order elimination in human body, which is widely supported by previous studies [36]. The average elimination rates of different PAH species were obtained from a previous study [37]. The details of the calculation method are showed in Section A of S1 File. The calculated OH-PAH increments, denoted as OH-PAHtrap, were used as the surrogates of the traffic-related PAH exposure during the driving tests.
Data and statistical analysis
The urinary OH-PAH concentrations below detection limits (BDL) were calculated as half of the detection limit values. Calculated urinary OH-PAH increments due to the TRAP exposure during the monitored tests (OH-PAHtrap) were used as the surrogates of traffic-related PAHs exposure during the monitored six hours. In order to normalize the variable distributions, all sampling results were log transformed. Shapiro-Wilk test was conducted to ensure the transformed data were normally distributed before running the subsequent statistical tests and regression models.
Paired t-test was used to detect differences for in-cabin and on-road UFP and PM2.5 levels as well as OH-PAHtrap between interventions and the NM condition. Pearson’s correlations and mixed effect linear regression models with random intercepts for each driver were used to test the associations between variables. The model can be expressed by the equation below:
Where i is the index of each driver, and j is the index of mitigation strategies. α and β are the fixed intercept and slope respectively, and μi is the random intercept of each driver under each mitigation condition. εij is the residual. PM2.5, UFP, and OH-PAHtrap were log transformed before fitting the model. The level of significance was taken as p < 0.05. SAS 9.4 software (SAS Institute, Cary, NC) was used for statistical analysis.
Results
On-road and in-cabin PM and PAH exposures
The PM2.5 and UFPs concentrations measured inside and outside the 17 taxis during the driving tests were summarized in Table 2. Because the start time, driving routes, and break times were well controlled, and the driving tests were conducted in three consecutive days to test the three mitigation conditions for each driver, the on-road PM2.5 and UFPs didn’t show significant differences based on the paired t-test among different test conditions.
Table 2. Summary of taxi drivers’ exposures under different test conditions (geometric mean, interquartile range, and percentage of change from NM).
| Mitigation | NMa | WCb | WC+HECAc |
|---|---|---|---|
| On-road PM2.5 (μg/m3) | 31 (20, 53) | 28 (20, 40) (-10%)d | 29 (24, 33) (-6%) |
| In-cabin PM2.5 (μg/m3) | 19 (15, 22) | 20 (16, 20) (5%) | 12 (10, 16) * (-37%) |
|
On-road UFP (x104 cm-3) |
2.71 (2.44, 3.02) | 2.72 (2.29, 3.46) (0%) | 2.70 (2.34, 3.55) (-0%) |
|
In-cabin UFP (x104 cm-3) |
1.40 (1.13, 1.97) | 1.33 (1.07, 1.77) (-5%) | 0.74 (0.59, 1.02) * (-47%) |
|
∑OH-NAPtrap (μg/g cr) |
4.69 (2.30, 8.96) | 3.47 (1.99, 5.38) (-26%) | 4.40 (2.50, 6.47) (-6%) |
|
∑OH-FLUtrap (μg/g cr) |
0.51 (0.29, 0.71) | 0.40 (0.30, 0.74) (-22%) | 0.48 (0.33, 0.60) (-6%) |
|
∑OH-PHEtrap (μg/g cr) |
0.15 (0.08, 0.24) | 0.12 (0.07, 0.20) (-20%) | 0.18 (0.14, 0.20) (20%) |
|
1-OH-PYRtrap (μg/g cr) |
0.05 (0.02, 0.10) | 0.05 (0.02, 0.10) (0%) | 0.07 (0.05, 0.10) (40%) |
|
Pre-test MDA (μg/g cr) |
20.80 (20.53, 54.18) | 10.74 (9.23, 40.95) (-48%) | 27.14 (13.49, 56.39) (30%) |
|
Post-test MDA (μg/g cr) |
31.91 (22.78, 47.25) | 31.73 (22.87, 43.90) (-1%) | 23.68 (9.99, 55.37) (-17%) |
a. No mitigation
b. Window closed
c. High efficiency cabin air filter
d. Numbers in parenthesis indicate percentage of change from NM.
* indicate significance of paired t-test (p < 0.05) compared with NM.
Under the NM condition, the vehicle ventilation and window position were controlled by the drivers. The sampling results reflect their exposures to UFP, PM2.5, and PAHs under normal working conditions. The geometric mean PM2.5 and UFPs levels inside the tested 17 taxis were 19 μg/m3 and 1.40 x 104 particles/cm3, respectively. The model calculated increments due to traffic exposure for ∑OH-NAP, ∑OH-FLU, ∑OH-PHE and 1-OH-PYR were 4.69, 0.51, 0.15, and 0.05 μg/g creatinine (cr), respectively (Table 2).
Compared with the NM condition, WC condition changed the in-cabin geometric mean PM2.5 and UFP by -5% and 5%, respectively, but the differences were not significant (p > 0.05). WC did not reduce the taxi driver urinary OH-PAHtrap of any individual PAH species either (Table 2). However, under WC+HECA, compared with NM, the geometric mean PM2.5 and UFP levels were reduced by 37% and 47%, respectively. The geometric mean ∑OH-NAPtrap and ∑OH-FLUtrap levels were reduced by 6%, whereas the geometric mean ∑OH-PHEtrap and 1-OH-PYRtrap levels increased 20% and 40%, respectively. The reduction of PM2.5 and UFP concentrations were significant (p < 0.05) (Table 2 and Table A in S1 File). These results show that, as a mitigation strategy, WC+HECA is effective in reducing PM levels inside these tested 17 taxi vehicles, but not effective in reducing urinary PAH metabolize levels of taxi drivers.
Table 2 also shows that, the geometric mean post-test MDA levels were similar under NM and WC (1% difference), but was reduced by 17% under the WC+HECA condition. Although the reduction was not significant (p > 0.05), the trend among the three mitigation conditions was similar with the trends of in-cabin PM2.5 and UFP concentrations, suggesting the changes in MDA might be linked with PM2.5 and UFP reduction. This hypothesis is tested in the following section.
Associations among PM, OH-PAHs, and MDA
The in-cabin geometric mean PM2.5 concentrations were not significantly correlated with UFP or any calculated OH-PAHtrap levels. However, the geometric mean UFP concentrations were correlated significantly with 1-OH-PYRtrap levels (p < 0.05). For each OH-PAH species, significant correlations between pre- and post-test levels were detected (p < 0.05, Table B in S1 File), suggesting the impacts of pre-test OH-PAH levels on the post-test OH-PAHs. Therefore, the post-test OH-PAH levels were not good indicators of the taxi drivers’ in-cabin PAH exposures during the driving tests. Instead, the calculated OH-PAHtrap levels were used as the surrogate of traffic-related PAH exposures during the 6-hr driving test in this study.
Unlike the urinary OH-PAHs, the pre- and post-test MDA concentrations didn’t show significant correlation with each other. However, the post-test MDA levels showed a similar trend with PM and OH-PAHtrap, in that the lowest geometric mean was observed under WC+HECA (Table 2). Thus, the TRAP exposures might have some impacts on the post-test MDA levels.
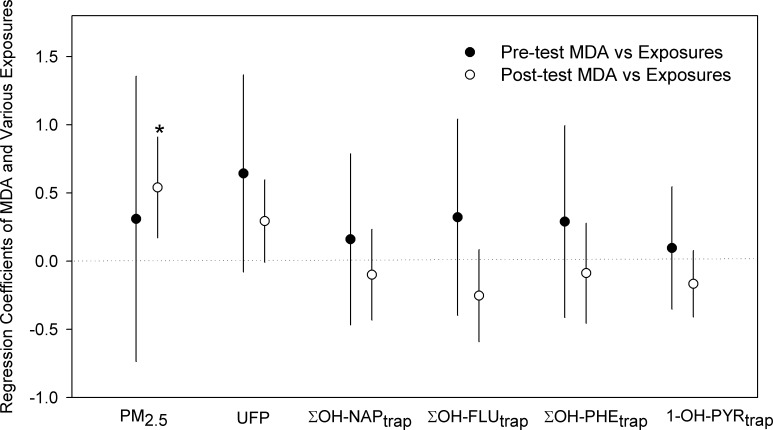
To examine the association between PM and PAH exposures and MDA, a mixed effect linear model was developed. Other factors that might affect the MDA levels, such as temperature, relative humidity, and weekday/weekend, were also included to the initial models, but the results showed that they were not significant, and didn’t affect the associations between exposures and the MDA. Thus the final models only include exposures and individual effects. Linear regression coefficients and their confidence intervals were summarized in Fig 1 to show the significance of the correlations at α = 0.05 level. The taxi driver post-test urinary MDA levels were observed to have significant positive correlations with their in-cabin PM2.5 exposure during the 6-hr driving test, and have marginally significant positive correlations with their in-cabin UFP levels (p = 0.07) (Fig 1).
Fig 1. Regression coefficients of pre- and post-test urinary MDA and various exposure indicators including PM2.5 and UFP levels inside taxi as well as PAH metabolites in urine.
* indicates significant correlations (p < 0.05), error bars indicate 95% confidence intervals.
Discussion
Taxi driver PM and PAH exposures
The PM2.5 and UFP levels measured inside taxis under NM were on the similar levels with those measured inside private vehicles [3, 23]. However, since taxi drivers, on average, drive for 12 hours on each working day, which composes of 50% of their daily activities, their total exposures to roadway PM2.5 and UFPs are about six times higher than other Southern California regular commuters, given that an average commuter in Southern California spends 7.2% of his/her day driving in traffic [38].
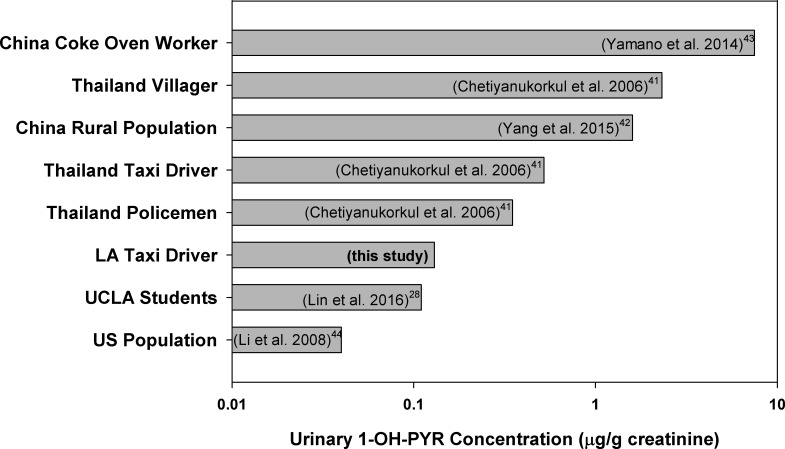
Since urinary 1-OH-PYR is a widely used biomarker for human total PAH exposure assessments [39, 40], urinary 1-OH-PYR concentration was used to compare the taxi drivers with other population. The results are illustrated in Fig 2. The median concentration of 1-OH-PYR of this taxi driver group were about 125% higher than the U.S. general population median from National Health and Nutrition Examination Survey (NHANES), 50% higher than a group of University of California Los Angeles (UCLA) students [28], but lower than taxi drivers or other occupational groups in Thailand and China [41–44].
Fig 2. Comparison of Los Angeles taxi driver urinary 1-PYR concentrations with literature data from U.S. population (NHANES), UCLA students, and some other occupational groups.
Exposure mitigation
In this study, only 2-ring (NAP), 3-ring (FLU and PHE), and 4-ring (PYR) PAHs were measured by their urinary monohydroxylated metabolites. In all collected urine samples, on average, the two OH-NAPs (2.28 and 5.20 μg/g cr) were most abundant, followed by the three OH-FLUs (0.13–0.42 μg/g cr), the three OH-PHEs (0.06–0.15 μg/g cr), and the 1-OH-PYR (0.13 μg/g cr). The two di-cyclic aromatic hydrocarbon metabolites (∑OH-NAP), on average, contributed 83% to the ∑OH-PAH in mass. This is comparable with the 82% in the U.S. population from the NHANES results [44]. NAP, FLU, and PHE are mostly in gaseous phase in the ambient air, while PYR is on the borderline but mainly in gaseous under relatively high ambient temperature [45]. The 1-OH-PYR only composed of about 1% of the total analyzed OH-PAHs in mass in this study. The recorded sampling temperature during the field sampling time ranged from 13.7°C to 34.9°C with an average of 27.8°C. This relatively high ambient temperature made the ambient PYR more likely to be found in the gaseous phase [46].
Compared with in-cabin PM2.5 and UFP, the reduction of PAH exposure by WC+HECA was not significant, which is probably due to the gaseous nature of the measured PAHs. The HECA filter was less effective for gaseous PAHs than particles. Notably, PAHs were found to be bound to airborne particles of different sizes [47], and some PAH species in particle phase (more than 4 rings) are also likely to be at high levels on road and cause health risks [48]. Therefore, the results of this study only indicate that the effect of HECA filters was limited for gas phase PAHs, but might be still effective for particle-bound and higher molecular weight PAH reduction, which was not measured in this study.
PM/PAH exposures and health effects
The significant positive correlations between the in-cabin PM exposures and the post-test urinary MDA observed in this study support the previous findings that PM exposures induce systematic oxidative stress in human. The possible pathways linking PM exposures and urinary MDA generation include: (i) activation of leukocyte NADPH oxidase and myeloperoxidase [49]; (ii) interference with normal mitochondrial functions [50]; and (iii) depletion of antioxidant capacities [51]. Some chemical compositions of PM2.5 and UFPs can also contribute to the oxidative stress through other pathways. In addition, the decrease of post-test MDA level under WC+HECA suggests the potential health benefit of the tested mitigation strategy.
Previous studies have reported significant association between urinary OH-PAHs and MDA [15, 42]. But such association was not observed in this study. This is probably due to the differences in the study design. In this study, we measured the urinary MDA levels immediately after the exposures, reflecting the acute health effects, whereas most other studies on PAHs and oxidative stress association were based on long term observations. For example, one of the longitudinal studies found significant association between urinary OH-PAHs and MDA based on samples collected in several months [28]. Different from the PM, the PAHs’ capacity of inducing ROS depends on their relatively longer and more complex metabolizing processes. Given that the median half-lives of the analyzed PAHs in human body range from 2.5 to 6.1 hours, the insignificant association between OH-PAHtrap and MDA in our study is possibly due to the fact that the post-test samples collected right after the 6-hr driving cannot reflect the MDA generation induced by the 6-hr PAH exposures. Nevertheless the difference in our results between PAHs and PM suggest different mechanisms in inducing oxidative stress.
There are several limitations of this study. First, 17 taxis and drivers were relatively a small sample size. However, the study power was increased with repeated-measures study design, enabling each subject to serve as his/her own control. Second, no environmental PAH data were collected during the tests because of the instrument restrictions. Instead, urinary metabolites and a pharmacokinetic model were used to calculate the exposure surrogates, which may induce uncertainties. Specifically, only population mean elimination rates were available and used to calculate the OH-PAHtrap, and individual variations in PAH metabolism among the drivers were not taken into consideration. However, this interference from individual variation could be at least partially controlled by the repeated-measures design. The elimination rates in the literature from both dietary and inhalation intake were compared in Table C in S1 File. Although the calculated OH-PAHtrap values were different between dietary and inhalation routes, the association between the OH-PAHtrap and MDA remains insignificant. Finally, the concentration of MDA could also be influenced by factors other than the PM through inhalation, such as other inhalable pollutants and diet. However, the post-test MDA shows a similar trend with the in-cabin PM2.5 and UFP suggesting PM exposure may play an important role in affecting MDA levels.
To our knowledge, this is the first intervention study using repeated-measures design to examine the effectiveness of various mitigation strategies and the health effects of TRAPs on taxi drivers. HECA filters were found to reduce both PM2.5 and UFP levels significantly inside taxis. The positive association between the in-cabin PM levels and the drivers’ urinary MDA concentrations shows that oxidative stress is a possible mechanism for the adverse health effects associated with TRAP exposures.
Supporting information
(DOCX)
(TIF)
Acknowledgments
This study was supported by the National Institute of Occupational Safety and Health (NIOSH, 1R21OH0010196-01A1), and targeted research training program of the Southern California NIOSH Education and Research Center (SCERC), with Grant Agreement Number T42 OH008412 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of CDC. The authors want to thank California Department of Public Health (CDPH) for analyzing the urine samples for PAH metabolites. We thank the taxi drivers who contributed to this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Institute of Occupational Safety and Health (NIOSH, 1R21OH0010196-01A1 to YZ), and targeted research training program of the Southern California NIOSH Education and Research Center (SCERC), with grant agreement number T42 OH008412 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of CDC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cohen G, Levy I, Yuval, JD Kark, N Levin, DM Broday, et al. Long-term exposure to traffic-related air pollution and cancer among survivors of myocardial infarction: A 20-year follow-up study. European Journal of Preventive Cardiology. 2017;24(1):92–102. doi: 10.1177/2047487316669415 [DOI] [PubMed] [Google Scholar]
- 2.Zeng Q, Ni Y, Jiang GH, Li GX, Pan XC. The short term burden of ambient particulate matters on non-accidental mortality and years of life lost: A ten-year multi-district study in Tianjin, China. Environmental Pollution. 2017;220:713–9. doi: 10.1016/j.envpol.2016.10.036 [DOI] [PubMed] [Google Scholar]
- 3.Zhu YF, Fung DC, Kennedy N, Hinds WC, Eiguren-Fernandez A. Measurements of ultrafine particles and other vehicular pollutants inside a mobile exposure system on Los Angeles freeways. Journal of the Air & Waste Management Association. 2008;58(3):424–34. [DOI] [PubMed] [Google Scholar]
- 4.Tartakovsky L, Baibikov V, Czerwinski J, Gutman M, Kasper M, Popescu D, et al. In-vehicle particle air pollution and its mitigation. Atmospheric Environment. 2013;64:320–8. [Google Scholar]
- 5.Wu J, Tjoa T, Li L, Jaimes G, Delfino RJ. Modeling personal particle-bound polycyclic aromatic hydrocarbon (pb-pah) exposure in human subjects in Southern California. Environmental health: a global access science source. 2012;11:47. Epub 2012/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu YF, Eiguren-Fernandez A, Hinds WC, Miguel AH. In-cabin commuter exposure to ultrafine particles on Los Angeles freeways. Environmental Science & Technology. 2007;41(7):2138–45. [DOI] [PubMed] [Google Scholar]
- 7.Su JG, Apte JS, Lipsitt J, Garcia-Gonzales DA, Beckerman BS, de Nazelle A, et al. Populations potentially exposed to traffic-related air pollution in seven world cities. Environment International. 2015;78:82–9. doi: 10.1016/j.envint.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 8.United Nations. Prospects of world urbanization. 1989 Contract No.: 57.
- 9.Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology. 2005;16(1):41–8. [DOI] [PubMed] [Google Scholar]
- 10.Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, et al. Long-Term Exposure to Traffic Emissions and Fine Particulate Matter and Lung Function Decline in the Framingham Heart Study. American Journal of Respiratory and Critical Care Medicine. 2015;191(6):656–64. doi: 10.1164/rccm.201410-1875OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karottki DG, Spilak M, Frederiksen M, Andersen ZJ, Madsen AM, Ketzel M, et al. Indoor and Outdoor Exposure to Ultrafine, Fine and Microbiologically Derived Particulate Matter Related to Cardiovascular and Respiratory Effects in a Panel of Elderly Urban Citizens. International Journal of Environmental Research and Public Health. 2015;12(2):1667–86. doi: 10.3390/ijerph120201667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araujo JA. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Quality Atmosphere and Health. 2011;4(1):79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li RJ, Kou XJ, Xie LZ, Cheng FQ, Geng H. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environmental Science and Pollution Research. 2015;22(24):20167–76. doi: 10.1007/s11356-015-5222-z [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Staimer N, Gillen DL, Tjoa T, Schauer JJ, Shafer MM, et al. Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort. Environmental Research. 2016;150:306–19. doi: 10.1016/j.envres.2016.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae S, Pan XC, Kim SY, Park K, Kim YH, Kim H, et al. Exposures to Particulate Matter and Polycyclic Aromatic Hydrocarbons and Oxidative Stress in Schoolchildren. Environmental Health Perspectives. 2010;118(4):579–83. doi: 10.1289/ehp.0901077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo CX, Xia YY, Niu PY, Jiang LZ, Duan JC, Yu Y, et al. Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-kappa B signaling. International Journal of Nanomedicine. 2015;10:1463–77. doi: 10.2147/IJN.S76114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le NA. Lipoprotein-Associated Oxidative Stress: A New Twist to the Postprandial Hypothesis. International Journal of Molecular Sciences. 2015;16(1):401–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition Metabolism and Cardiovascular Diseases. 2005;15(4):316–28. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen M, Autrup H, Moller P, Hertel O, Jensen SS, Vinzents P, et al. Linking exposure to environmental pollutants with biological effects. Mutation Research-Reviews in Mutation Research. 2003;544(2–3):255–71. [DOI] [PubMed] [Google Scholar]
- 20.Delfino RJ, Staimer N, Vaziri ND. Air pollution and circulating biomarkers of oxidative stress. Air Quality Atmosphere and Health. 2011;4(1):37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saffari A, Daher N, Shafer MM, Schauer JJ, Sioutas C. Seasonal and Spatial Variation of Trace Elements and Metals in Quasi-ultrafine (PM0.25) Particles in the Los Angeles Metropolitan Area and Characterization of Their Sources. Environmental Pollution. 2013;181:14–23. doi: 10.1016/j.envpol.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 22.Fruin S, Westerdahl D, Sax T, Sioutas C, Fine PM. Measurements and predictors of on-road ultrafine particle concentrations and associated pollutants in Los Angeles. Atmospheric Environment. 2008;42(2):207–19. [Google Scholar]
- 23.Hudda N, Kostenidou E, Sioutas C, Delfino RJ, Fruin SA. Vehicle and Driving Characteristics That Influence In-Cabin Particle Number Concentrations. Environmental Science & Technology. 2011;45(20):8691–7. [DOI] [PubMed] [Google Scholar]
- 24.Lee ES, Zhu Y. Application of a High-Efficiency Cabin Air Filter for Simultaneous Mitigation of Ultrafine Particle and Carbon Dioxide Exposures Inside Passenger Vehicles. Environmental Science & Technology. 2014;48(4):2328–35. [DOI] [PubMed] [Google Scholar]
- 25.Blasi G, and Leavitt J. Driving Poor: Taxi Drivers and the Regulation of the Taxi Industry in Los Angeles . University of California Los Angeles, 2006. [Google Scholar]
- 26.Shu S, Yu N, Wang Y, Zhu Y. Measuring and modeling air exchange rates inside taxi cabs in Los Angeles, California. Atmospheric Environment. 2015;122:628–35. [Google Scholar]
- 27.Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, et al. Assessing human exposure to polycyclic aromatic hydrocarbons by urinary biomonitoring. Abstracts of Papers of the American Chemical Society. 2006;232:606-. [Google Scholar]
- 28.Lin Y, Qiu XH, Yu N, Yang QY, Araujo JA, Zhu YF. Urinary Metabolites of Polycyclic Aromatic Hydrocarbons and the Association with Lipid Peroxidation: A Biomarker-Based Study between Los Angeles and Beijing. Environmental Science & Technology. 2016;50(7):3738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanstaden JF. Determination of Creatinine in Urine and Serum by Flow-injection analysis using the Jaffe Reaction. Fresenius Zeitschrift Fur Analytische Chemie. Fresenius Zeitschrift Fur Analytische Chemie. 1983;315(2):141–4. [Google Scholar]
- 30.Garde AH, Hansen AM, Kristiansen J. Evaluation, including effects of storage and repeated freezing and thawing, of a method for measurement of urinary creatinine. Scandinavian Journal of Clinical & Laboratory Investigation. 2003;63(7–8):521–4. [DOI] [PubMed] [Google Scholar]
- 31.Yanosky JD, Williams PL, MacIntosh DL. A comparison of two direct-reading aerosol monitors with the federal reference method for PM2.5 in indoor air. Atmospheric Environment. 2002;36(1):107–13. [Google Scholar]
- 32.Zhang QF, Zhu YF. Measurements of ultrafine particles and other vehicular pollutants inside school buses in South Texas. Atmospheric Environment. 2010;44(2):253–61. [Google Scholar]
- 33.Kearney E. Internal Quality Control. In: Wheeler MJ, editor. Hormone Assays in Biological Fluids, 2nd Edition 2013. p. 277–89. [Google Scholar]
- 34.Westgard JO, Westgard SA. Quality control review: implementing a scientifically based quality control system. Annals of Clinical Biochemistry. 2016;53(1):32–50. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed T. Pharmacokinetics of Drugs Following IV Bolus, IV Infusion, and Oral Administration . Basic Pharmacokinetic Concepts and Some Clinical Applications 2015. [Google Scholar]
- 36.Jongeneelen FJ. Biological Monitoring of Environmental Exposure to Polycyclic Aromatic Hydrocarbons—1-Hydroxypyrene in Urine of People. Toxicology Letters. 1994;72(1–3):205–11. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, et al. Excretion Profiles and Half-Lives of Ten Urinary Polycyclic Aromatic Hydrocarbon Metabolites after Dietary Exposure. Chemical Research in Toxicology. 2012;25(7):1452–61. doi: 10.1021/tx300108e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Jiang C, Houston D, Baker D, Delfino R. Automated time activity classification based on global positioning system (GPS) tracking data. Environmental health: a global access science source. 2011;10:101. Epub 2011/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jongeneelen FJ, Anzion RBM, Scheepers PTJ, Bos RP, Henderson PT, Nijenhuis EH, et al. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Annals of Occupational Hygiene. 1988;32(1):35–43. [DOI] [PubMed] [Google Scholar]
- 40.Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Annals of Occupational Hygiene. 2001;45(1):3–13. [PubMed] [Google Scholar]
- 41.Chetiyanukornkul T, Toriba A, Kameda T, Tang N, Hayakawa K. Simultaneous determination of urinary hydroxylated metabolites of naphthalene, fluorene, phenanthrene, fluoranthene and pyrene as multiple biomarkers of exposure to polycyclic aromatic hydrocarbons. Analytical and Bioanalytical Chemistry. 2006;386(3):712–8. doi: 10.1007/s00216-006-0628-6 [DOI] [PubMed] [Google Scholar]
- 42.Yang QY, Qiu XH, Li R, Ma J, Li KQ, Li G. Polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress for a rural population from the North China Plain. Environmental Science and Pollution Research. 2015;22(3):1760–9. doi: 10.1007/s11356-014-3284-y [DOI] [PubMed] [Google Scholar]
- 43.Yamano Y, Hara K, Ichiba M, Hanaoka T, Pan GW, Nakadate T. Urinary 1-hydroxypyrene as a comprehensive carcinogenic biomarker of exposure to polycyclic aromatic hydrocarbons: a cross-sectional study of coke oven workers in China. International Archives of Occupational and Environmental Health. 2014;87(7):705–13. doi: 10.1007/s00420-013-0913-6 [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environmental Research. 2008;107(3):320–31. doi: 10.1016/j.envres.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 45.Xie MJ, Hannigan MP, Barsanti KC. Gas/particle partitioning of n-alkanes, PAHs and oxygenated PAHs in urban Denver. Atmospheric Environment. 2014;95:355–62. [Google Scholar]
- 46.Lin Y, Qiu X, Ma Y, Ma J, Zheng M, Shao M. Concentrations and spatial distribution of polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (NPAHs) in the atmosphere of North China, and the transformation from PAHs to NPAHs. Environmental Pollution. 2015;196:164–70. doi: 10.1016/j.envpol.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 47.Allen JO, Dookeran KM, Smith KA, Sarofim AF, Taghizadeh K, Lafleur AL. Measurement of polycyclic aromatic hydrocarbons associated with size-segregated atmospheric aerosols in Massachusetts. Environmental Science & Technology. 1996;30(3):1023–31. [Google Scholar]
- 48.Lin Y, Ma Y, Qiu X, Li R, Fang Y, Wang J, et al. Sources, transformation, and health implications of PAHs and their nitrated, hydroxylated, and oxygenated derivatives in PM2.5 in Beijing. Journal of Geophysical Research-Atmospheres. 2015;120(14):7219–28. [Google Scholar]
- 49.Magnani ND, Marchini T, Tasat DR, Alvarez S, Evelson PA. Lung oxidative metabolism after exposure to ambient particles. Biochemical and Biophysical Research Communications. 2011;412(4):667–72. doi: 10.1016/j.bbrc.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 50.Hou LF, Zhu ZZ, Zhang XA, Nordio F, Bonzini M, Schwartz J, et al. Airborne particulate matter and mitochondrial damage: a cross-sectional study. Environmental Health. 2010;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu XL, Meng ZQ. Effects of airborne fine particulate matter on antioxidant capacity and lipid peroxidation in multiple organs of rats. Inhalation Toxicology. 2005;17(9):467–73. doi: 10.1080/08958370590964467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.