Abstract
Microbial infections lead to neurological damages either by direct infection in the nervous tissues or by uncontrolled immune responses (immunopathology). For example, in Zika virus infection, microcephaly can be caused by the former, i.e., direct viral infection in the brain, while Guillain-Barré syndrome (GBS) seems to be antibody-mediated immunopathology. Although a variety of factors affect immunopathology, two essential systems maintaining whole-body homeostasis had long been neglected: 1) the lymphatic system and 2) microbiota. Only recently, the role of the lymphatic system in immunopathology is beginning to be clarified. During infection, increased lymphatic flow limits edema and prevent tissue dendritic cell retention, while lymphostasis can lead to chronic inflammation. The role of gut microbiota, particularly bacterial community, in immunopathology has also been clarified recently; “bad bacteria” are proposed to exacerbate any immunopathology. For example, Helicobacter pylori is associated with not only gastritis but also extra-intestinal diseases, including neuromyelitis optica (NMO) and Alzheimer’s disease. However, H. pylori and another bad bacterium Clostridium perfringens type A have been proposed to be protective against multiple sclerosis (MS). The above discrepancy on the roles of microbiota can be attributed to several conflicting factors, such as oversimplification, methodology, and taxonomy, which are summarized as “10 pitfalls of microbiota studies.”
Keywords: Micobiome, CNS demyelinating diseases, Experimental autoimmune encephalomyelitis (EAE), Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease (TMEV-IDD), virome
Graphical abstract
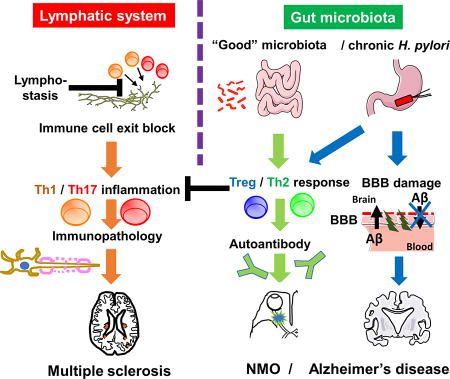
During infection, increased lymphatic flow limits edema and prevent tissue dendritic cell retention, while lymphostasis can lead to chronic inflammation. Helicobacter pylori is associated with not only gastritis but also extra-intestinal diseases, including neuromyelitis optica (NMO) and Alzheimer’s disease, while H. pylori and another bad bacterium Clostridium perfringens type A have been proposed to be protective against multiple sclerosis (MS). The above discrepancy on the roles of microbiota can be attributed to several conflicting factors, such as oversimplification, methodology, and taxonomy, which are summarized as “10 pitfalls of microbiota studies.”
Infections with microbes, such as viruses and bacteria, result in damages of the peripheral nervous system (PNS) or the central nervous system (CNS) either by microbial replication in the nervous tissues (microbial pathology) or by uncontrolled immune responses (immunopathology). For example, as reviewed by Dr. Kutsuna (1), Zika virus infection has been associated with two neurological complications: microcephaly and Guillain-Barré syndrome (GBS). Microcephaly can be caused by the direct microbial infection (viral replication) in the brain (viral pathology), while GBS is antibody-mediated immunopathology (Note. Zika virus induced-GBS may be heterogenous; a group of patients had a “parainfectous” onset, not a postinfectous onset typically seen in GBS) (2).
Two systems affect microbial pathology and immunopathology
Although a variety of factors can affect immunopathology triggered by microbial infections, two essential systems maintaining whole-body homeostasis had long been neglected in the field: the lymphatic system and microbiota. Although the lymphatic system and microbiota have been described in most medical textbooks of anatomy, immunology, and microbiology, their roles in immunopathology associated with microbial infections had not been investigated until recently. Although involvement of the lymphatics and microbiota in microbial immunopathology is intuitively plausible, the reason why they have long been neglected by pathologists may be due to their invisibility in routine hematoxylin and eosin-stained sections. While most mucosal microbes are washed out during the tissue processing, lymphatics is either indistinguishable with blood vessels or invisible in traditional histology staining; lymphatic specific immunohistochemistry and/or gene-targeting become available only recently.
Although regional lymph nodes have been used to study immune responses in health and diseases, lymphatic vessels have not been investigated by most immunologists. In medical education and clinical settings, only some disease conditions (e.g., filariasis, chylothorax, lymph node metastasis in cancer, and lymph node swelling in infections) remind us of the presence of the lymphatic vessels. This is partly due to the fact that lymphadenectomy does not necessarily result in expected adverse effects, such as lymphedema and local infection. In many immunology experiments, even in the situation where some immunologists misunderstand that lymph nodes are connected with blood vessels, the misconception affects neither experimental results nor their interpretation in most cases.
Lymphatic system in immunopathology
Dr. Al-Kofahi and colleagues from Dr. J. Steven Alexander’s group excellently reviewed anatomy and functions of the lymphatic system, particularly in the gastrointestinal tract, the heart, and the CNS (3). During acute microbial infection, lymphatic flow is increased in the infected tissue, limiting edema as well as providing more soluble microbial antigens and antigen-laden dendritic cells into the regional lymph nodes for antigen-presentation. The authors have proposed that dysfunction of the lymphatics leads to persistence of immune cells and mediators in the tissue (Figure 1). This lead to chronic inflammation and tissue damage by immunopathology, while the lymphostasis may confine pathogens locally, limiting systemic spread of the microbes. This theory is based on experimental findings by Dr. Alexander’s group and others; for example, Al-Kofahi et al. (4) have demonstrated that dysfunction of lymphatic vessels can contribute to prolonged inflammation in inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis, as well as myocarditis using a mouse model induced with Theiler’s murine encephalomyelitis virus (TMEV). Furthermore, Dr. Alexander’s group previously showed that downregulation of a set of serum lymphatic markers, such as prospero homeobox 1 (Prox-1) and angiopoietin-2 (Ang2), may be characteristic to secondary progressive (SP)-MS; here, lymphatic-specific molecules can be biomarkers to distinguish between SP-MS and relapsing-remitting (RR)-MS (5).
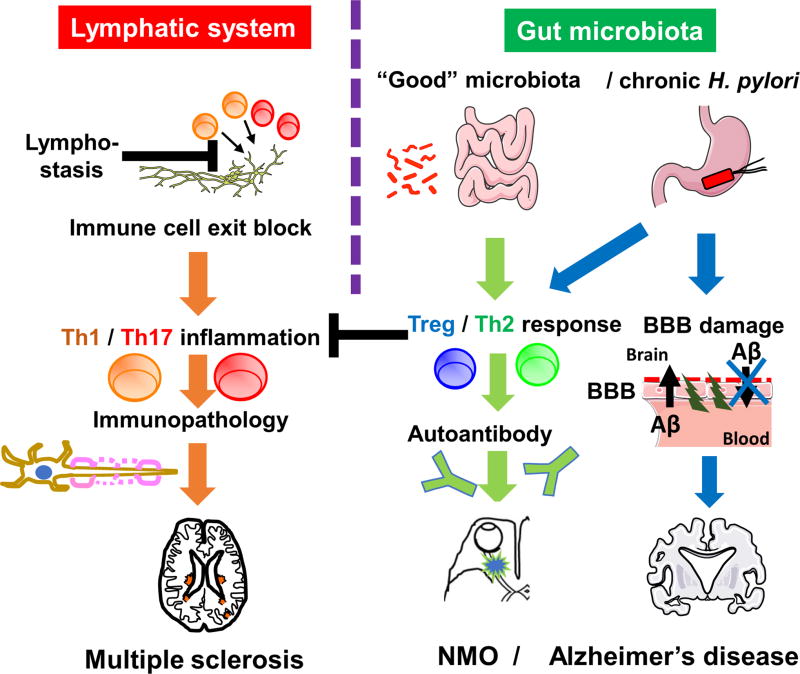
Figure 1. Lymphatic system and gut microbiota in neuroinflammatory diseases.
(Left) Lymphatic vessels drain immune cells and edema fluid from inflamed tissues into regional lymph nodes. Dysfunction of lymphatics blocks the exit of inflammatory cells from the tissue, leading to chronic inflammation and/or immunopathology, such as multiple sclerosis (MS), in the brain (where the presence of the lymphatic system has been proposed recently). (Right) “Good bacteria” in the gut microbiota as well as chronic H. pylori infection change the T helper (Th) cell subset balance toward regulatory T (Treg) / Th2 responses. Tregs and Th2 cells can suppress pro-inflammatory Th1/Th17 inflammation, preventing immune-mediated tissue damage, for example, gastritis in the stomach and MS in the brain. On the other hand, increased Th2 cytokines may enhance autoantibody production, exacerbating antibody-mediated disease, including neuromyelitis optica (NMO). Since antibody against H. pylori has no role in bacterial clearance, the suppression of anti-bacterial Th1/Th17 immunity leads to H. pylori persistence, which has been associated with blood-brain barrier (BBB) dysfunction. BBB dysfunction can not only increase the accumulation of amyloid-β (Aβ) from the periphery but also decrease the clearance of Aβ from the brain, contributing to progression of Alzheimer’s disease (AD).
Gut microbiota in immunopathology and neuropathology
In biomedical education, the gut microbiota had also been taught in association with only limited subjects, such as production of vitamin K and Clostridium difficile-induced pseudomembranous colitis. Recently, however, the gut microbiota has been investigated in a variety of health and disease conditions, where there seems to be a myth that “good bacteria” are beneficial for everything from aging, obesity, and infections, to brain diseases, while “bad bacteria” are bad for anything. As reviewed by Dr. Park et al. (6), this is likely not the case in MS and two “bad bacteria” Clostridium perfringens type A and Helicobacter pylori. While the former causes food poisoning and gas gangrene and the latter are associated with gastritis, gastric cancer and idiopathic thrombocytopenic purpura (ITP), the presence of both bacteria are lower in MS patients than controls. On the other hand, H. pylori is associated with exacerbation of neuromyelitis optica (NMO) and Alzheimer’s disease (AD).
The above contrasting roles of H. pylori can be explained when comparing and contrasting 1) anti-microbial immune responses versus immunopathology and 2) cellular immunity / pro-inflammatory T helper (Th) 1 and Th17 cells versus humoral immunity / anti-inflammatory Th2 and regulatory T cells (Tregs). For eradication of H. pylori, cellular immunity plays a key role. However, when hosts failed to eradicate H. pylori, to prevent uncontrolled pro-inflammatory responses that can cause gastritis, the immune response is skewed to anti-inflammatory, which protects immunopathology in the expense of bacterial persistence. This may be the case in asymptomatic carriers of H. pylori. Here, suppression of Th1/Th17 is protective against MS, while enhanced humoral responses play no role to eradicate H. pylori but may lead to antibody-mediated autoimmune diseases, such as ITP and NMO. Suppression of pro-inflammatory T cells in chronic H. pylori infection, may explain a lack of T cell infiltration in the brain lesions of AD, despite activation of resident innate cells, including microglia, while H. pylori infection can also contribute to dysfunction of the blood-brain barrier (BBB) observed in AD.
“10 pitafalls of microbiota studies”
Oversimplification and/or overestimation of the roles of the gut bacterial community (bacteriome) in microbiota studies can be explained by “10 pitfalls of microbiome studies” proposed by Dr. Park et al. (6): 1) the presence of fungal (mycobiome) and viral communities (virome); 2) microbial taxonomy/classification; 3) fecal bacteria ratio underrepresentation; 4) “dysbiosis” as the outcome; 5) discrepancy with primary immunodeficiency diseases (PID); 6) age, gender, and country; 7) good bacteria is not always good; 8) antibiotics affect systemic microbiota and immunity, 9) fecal microbiome transplantation (FMT) methodology and safety, and 10) tailor-made therapy. The proposal is useful to plan and evaluate microbiota studies, clinically and experimentally.
Acknowledgments
This work was supported by grants from the National Institute of General Medical Sciences COBRE Grant (P30-GM110703), the Japan Society for the Promotion of Science (JSPS, Grants-in-Aid for Scientific Research-KAKENHI, 16H07356) and Novartis Pharma Research Grants.
Footnotes
Conflict of interests
The author declares no Conflict of Interests for this article.
References
- 1.Kutsuna S. Zika virus infection: an overview with a summary of Japanese cases. Clin Exp Neuroimmunol. 2017;8(3) in press. [Google Scholar]
- 2.Tsunoda I, Omura S, Sato F, Kusunoki S, Fujita M, Park A-M, Hasanovic F, Yanagihara R, Nagata S. Neuropathogenesis of Zika virus infection : Potential roles of antibody-mediated pathology. Acta medica Kindai University. 2016;41(2):37–52. Epub 2016/01/01. [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Kofahi M, Yun JW, Minagar A, Alexander JS. Anatomy and roles of lymphatics in inflammatory diseases. Clin Exp Neuroimmunol. 2017;8(3) doi: 10.1111/cen3.12400. Epub 2017/07/20 http://onlinelibrary.wiley.com/wol1/doi/10.1111/cen3.12400/full. [DOI] [Google Scholar]
- 4.Al-Kofahi M, Becker F, Gavins FN, Woolard MD, Tsunoda I, Wang Y, et al. IL-1beta reduces tonic contraction of mesenteric lymphatic muscle cells, with the involvement of cycloxygenase-2 and prostaglandin E2. British journal of pharmacology. 2015;172(16):4038–51. doi: 10.1111/bph.13194. Epub 2015/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaitanya GV, Omura S, Sato F, Martinez NE, Minagar A, Ramanathan M, et al. Inflammation induces neuro-lymphatic protein expression in multiple sclerosis brain neurovasculature. J Neuroinflammation. 2013;10:125. doi: 10.1186/1742-2094-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park A-M, Omura S, Fujita M, Sato F, Tsunoda I. Helicobacter pylori and gut microbiota in multiple sclerosis versus Alzheimer’s disease: 10 pitfalls of microbiome studies. Clin Exp Neuroimmunol. 2017;8(3) doi: 10.1111/cen3.12401. Epub 2017/07/23 http://onlinelibrary.wiley.com/doi/10.1111/cen3.12401/full. [DOI] [PMC free article] [PubMed] [Google Scholar]