ABSTRACT
Phosphatidic acid phosphatases are involved in the biosynthesis of phospholipids and triacylglycerol, and also act as transcriptional regulators. Studies to ascertain their role in lipid metabolism and membrane biogenesis are restricted to Opisthokonta and Archaeplastida. Here, we report the role of phosphatidate phosphatase (PAH) in Tetrahymena thermophila, belonging to the Alveolata clade. We identified two PAH homologs in Tetrahymena, TtPAH1 and TtPAH2. Loss of function of TtPAH1 results in reduced lipid droplet number and an increase in endoplasmic reticulum (ER) content. It also results in more ER sheet structure as compared to wild-type Tetrahymena. Surprisingly, we did not observe a visible defect in the nuclear morphology of the ΔTtpah1 mutant. TtPAH1 rescued all known defects in the yeast pah1Δ strain and is conserved functionally between Tetrahymena and yeast. The homologous gene derived from Trypanosoma also rescued the defects of the yeast pah1Δ strain. Our results indicate that PAH, previously known to be conserved among Opisthokonts, is also present in a set of distant lineages. Thus, a phosphatase cascade is evolutionarily conserved and is functionally interchangeable across eukaryotic lineages.
KEY WORDS: Phosphatidic acid hydrolase, Lipin, Tetrahymena thermophila, Lipid droplet, Nuclear membrane expansion, Endoplasmic reticulum
Summary: Tetrahymena possesses two PAH homologs, TtPAH1 and TtPAH2. TtPAH1 regulates lipid droplet biogenesis and ER morphology in Tetrahymena and functionally replaces yeast PAH1.
INTRODUCTION
Eukaryotic cell organelles are enclosed by a membrane composed of the lipid bilayer and proteins. Phospholipids constitute the major structural components of lipid bilayers and play a central role in membrane biogenesis, lipid metabolism and signaling (Van Meer et al., 2008). The lipid composition of the membrane is critical for maintaining the shape, size and number of organelles, and is established through synthesis, transport and modification of phospholipids (McMahon and Gallop, 2005). The regulation of lipid synthesis and storage is critical for maintaining lipid homeostasis since both excess and poor fat storage results in various lipid-associated disorders (Klingenspor et al., 1999; Reue et al., 2000; Péterfy et al., 2001). However, the molecular mechanisms that link lipid production to organelle morphology remain unclear.
Pah/lipin proteins are Mg2+-dependent phosphatidic acid phosphatases (3-sn-phosphatidate phosphohydrolase, EC 3.1.3.4) (Han et al., 2006). Members of the Pah/lipin protein family perform dephosphorylation of phosphatidic acid (PA) to generate diacylglycerol (DAG), the penultimate step in glycerolipid synthesis (Lin and Carman, 1989). DAG can be converted back to PA by DAG kinase. PA and DAG are the central precursors which control the levels of phospholipids, govern membrane structure and lipid storage. In yeast, phospholipid biosynthesis occurs by two pathways: the cytidine diphosphate diacylglycerol (CDP-DAG) pathway (de novo) and the Kennedy pathway (salvage) (Fig. 1A) (Carman and Zeimetz, 1996; Carman and Henry, 1999). DAG is converted to triacylglycerol (TAG) (Han et al., 2006), which forms the lipid droplet. The dual function of TAG, as a reservoir of cellular energy and precursor for membrane phospholipids, makes it a key player in lipid homeostasis. DAG derived from PA is used for the synthesis of membrane phospholipids phosphatidylethanolamine (PE) and phosphatidylcholine (PC) via the Kennedy pathway (Carman and Kersting, 2004). Through the CDP-DAG pathway, phosphatidic acid serves as the precursor for the synthesis of the phospholipids PE, PC and phosphatidylserine (PS). Apart from the synthesis of lipids, PA and DAG act as lipid second messengers in signaling events that trigger membrane expansion, secretion and endocytosis (Kearns et al., 1997; Nadra et al., 2008). In yeast, PA positively regulates phospholipid synthesis through sequestration of a transcription repressor Opi1, thereby activating the transcription of genes encoding lipid biosynthetic enzymes (White et al., 1991; Loewen et al., 2004).
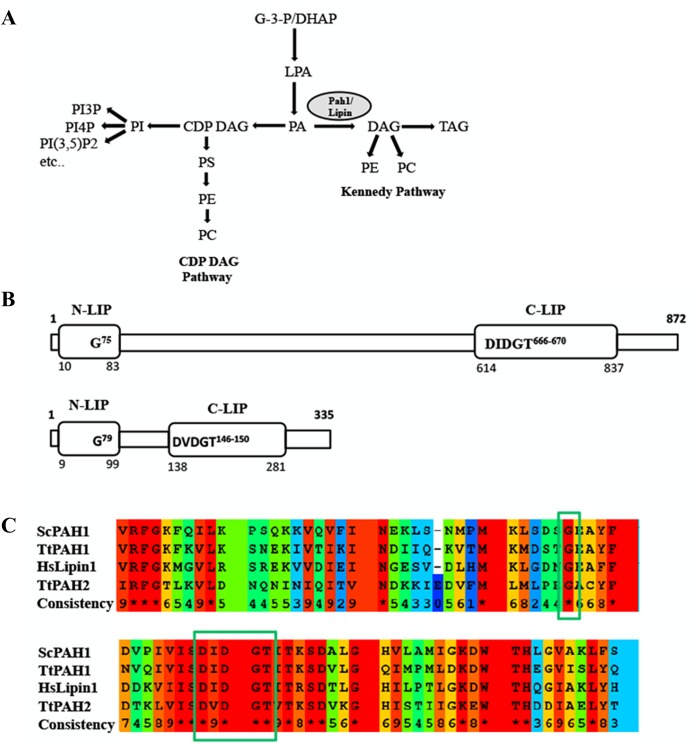
Fig. 1.
Domain organization, sequence analysis and function of PAH protein. (A) Schematic representation of the role of PAH in lipid biosynthesis. PA is a key precursor used for the synthesis of PE and PC through the CDP-DAG pathway. In the presence of choline and ethanolamine, these phospholipids are synthesized through the Kennedy pathway. In metazoans, the pathway that converts CDP-DAG to PC/PE (CDP-DAG pathway) does not exist, whereas both the pathways are present in yeast. G-3-P, glycerol-3-phosphate; LPA, lysophosphatidate; PI3P, phosphatidylinositol-3-phosphate; PI4P, phosphatidylinositol-4-phosphate; PI(3,5)P2, phosphatidylinositol-3, 5-biphosphate; PS, phosphatidylserine. (B) Domain organization of TtPAH1 and TtPAH2. Predicted N-LIP and C-LIP domains are indicated in the boxes. Also shown are the positions of a conserved glycine residue in N-LIP and the HAD with its conserved DXDXT/V motif in C-LIP. (C) Multiple sequence alignment showing partial sequences of N-LIP (top) and C-LIP (bottom) of PAH proteins from T. thermophila, S. cerevisiae and H. sapiens. Assigned colors of the particular residues are based on alignment consensus. Conserved glycine residue in N-LIP and catalytic motif (DXDGT/V) in C-LIP are indicated inside the box.
Lipins are relatively large proteins close to 100 kDa, and are primarily found in the cytosol. These proteins contain a carboxy-terminal region (C-LIP) with a haloacid dehalogenase (HAD)-like domain, possessing the DXDXT/V catalytic motif and an amino-terminal domain (N-LIP) of unknown function (Santos-Rosa et al., 2005). Lipin 1 was initially identified through positional cloning as the mutated gene in the fatty liver dystrophy (fld) mouse, which is characterized by abnormal development of adipose tissue that results in lipodystrophy and insulin resistance (Reue et al., 2000; Péterfy et al., 2001). In Saccharomyces cerevisiae, a single lipin orthologue, PAH1, is present, whereas mammals express three lipin paralogues, LIPIN1, LIPIN2 and LIPIN3, exhibiting distinct but overlapping expression patterns (Han et al., 2006; Donkor et al., 2007). The first lipin protein shown to function as a Mg2+-dependent phosphatidic acid phosphatase enzyme was S. cerevisiae Pah1 (Han et al., 2006). Deletion of PAH1 in yeast causes aberrant expansion of nuclear/endoplasmic reticulum (ER) membrane, increased phospholipid synthesis, decreased TAG level and lipid droplet number, and slow growth (Siniossoglou et al., 1998; Adeyo et al., 2011). In Caenorhabdtis elegans, downregulation of lipin affects the dynamics of the peripheral ER and nuclear envelope (Golden et al., 2009; Gorjánácz and Mattaj, 2009). Defects in mammalian lipins lead to various metabolic disorders including lipodystrophy and insulin resistance, rhabdomyolysis, peripheral neuropathy and inflammation (Reue et al., 2000; Müller-felber et al., 2010).
Besides serving enzymatic functions, lipins also act as transcriptional regulators (Finck et al., 2006; Zhang and Reue, 2017). Mammalian lipins regulate gene expression by modulating the activity of key transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ), PPAR co-activator 1α (PGC-1α) and sterol regulatory element binding protein (Phan et al., 2004; Peterson et al., 2011; Kim et al., 2013). Yeast Pah1 translocates to the nucleus where it interacts with the promoter of phospholipid synthesis genes (Santos-Rosa et al., 2005).
Phosphorylation and dephosphorylation at multiple sites regulate the activity and subcellular localization of PAH proteins. In yeast, Cdc28 phosphorylation of Pah1 is critical for cell cycle progression while phosphorylation by Pho85 plays other roles; in mammals, mTOR kinases phosphorylate lipins (Laplante and Sabatini, 2009; Peterson et al., 2011; Choi et al., 2012). Dephosphorylation of Pah1 by a nuclear/ER membrane complex consisting of a catalytic phosphatase subunit nuclear envelope morphology protein 1 (Nem1), and its regulatory subunit, sporulation-specific protein 7 (Spo7), activates its catalytic function and recruits it to the ER membrane, where it acts on its substrate PA (Santos-Rosa et al., 2005; Karanasios et al., 2010).
Studies of phosphatidic acid phosphatase have focused on Opisthokonta (fungi, nematode, flies and mammals) (Santos-Rosa et al., 2005; Han et al., 2006; Donkor et al., 2007; Golden et al., 2009; Gorjánácz and Mattaj, 2009; Ugrankar et al., 2011) and Plantae (Nakamura et al., 2009) clades. These enzymes and the regulatory cascades in which they participate have not been reported in organisms including Amoebozoa, Alveolata and Excavata. Tetrahymena thermophila belongs to the Alveolata, a major evolutionary branch of eukaryotic protists, in which cells display functional complexity comparable to the cells of humans and other metazoans. In this study, we report the role of phosphatidic acid phosphatase (Pah) in regulating lipid homeostasis and membrane biogenesis in this ciliate. We also investigated the cellular functions of PAH/LIPIN homologs in Excavata to understand the evolutionary conservation of this cascade.
We found two homologs for PAH in the Tetrahymena Genome Database. The larger protein is TtPah1, and a smaller one is TtPah2. We investigated the role of TtPAH1 in regulating lipid homeostasis, maintaining nuclear morphology and ER organization. We characterized the effects of loss of function of TtPAH1 and also performed complementation studies in the pah1Δ yeast strain. Deletion of TtPAH1 in Tetrahymena led to a reduction in lipid droplet number, thus confirming its role in lipid homeostasis. However, unlike in yeast, TtPAH1 was not required to maintain nuclear morphology. Overall, we provide evidence for the evolutionary conservation of this Mg2+-dependent phosphatidic acid phosphatase in Alveolata and Excavata.
RESULTS
Tetrahymena harbors two PAH homologs
We identified two homologs of phosphatidic acid phosphatase (PAH/LIPIN) in the Tetrahymena Genome Database and designated them as TtPAH1 (TTHERM_00189270) and TtPAH2 (TTHERM_00215970). TtPah1 contains 872 amino acids and is comparable to Pah1 proteins in other organisms, whereas TtPah2 (335 amino acids) is smaller than other known lipins. Both TtPah1 and TtPah2 proteins possess two specific phosphatidic acid phosphatase (PAP) domains, N-LIP and C-LIP, suggesting that these are the Mg2+-dependent phosphatidate phosphatases (Fig. 1B). All Mg2+-dependent phosphatidic acid phosphatases contain an essential catalytic DXDXT/V motif in the HAD-like domain of the C-LIP region. This catalytic motif is present in the C-LIP domain of both TtPah1 (666 DIDGT 670) and TtPah2 (146 DVDGT 150) (Fig. 1B,C). While the amino acid sequence of TtPah1 has 24% identity with yeast Pah1 and 31% identity with human lipin, TtPah2 has 22% identity with yeast Pah1 and 34% with human lipin. Similar to other phosphatidic acid phosphatases, the amino acids are more conserved in the N-LIP (50% and 49% identity for TtPah1, 35% and 30% identity for TtPah2 with yeast Pah1 and human lipin1, respectively) and C-LIP regions (49% identity for TtPah1 and 44% identity for TtPah2 with both yeast Pah1 and human lipin1). A conserved G residue in N-LIP is critical for PAH function since its mutation in mammalian lipin1 causes lipodystrophy. We have also identified the conserved G residue in N-LIP of both TtPah1 (G75) and TtPah2 (G79) (Fig. 1B,C).
TtPAH1 localizes on ER and encodes functional phosphatidate phosphatase
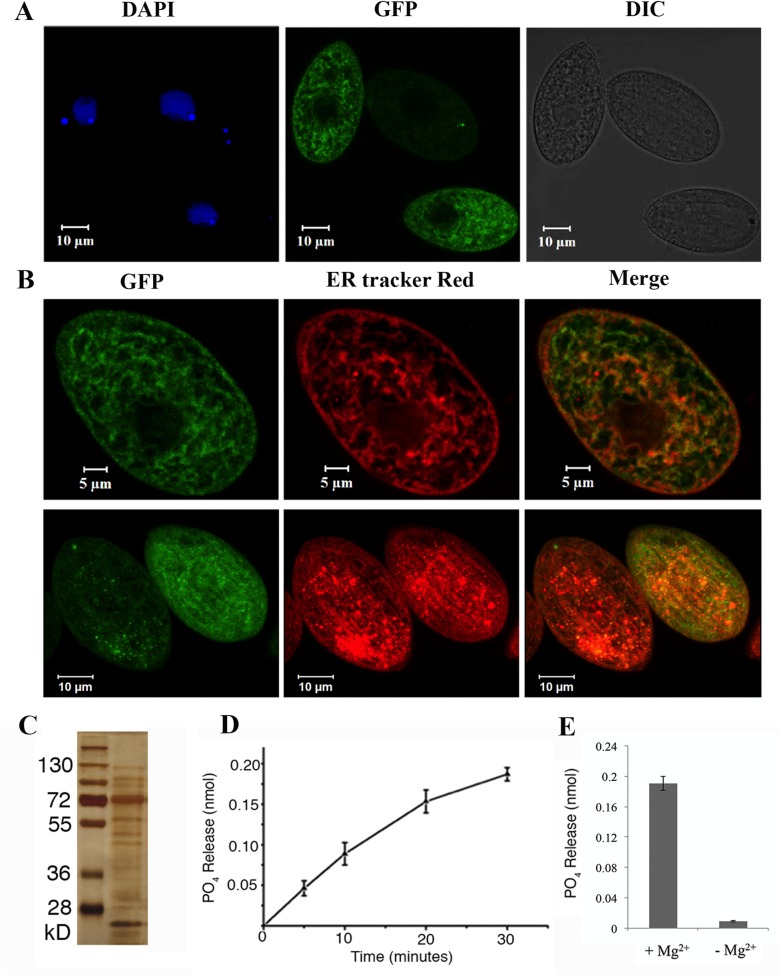
We focused our study on TtPAH1. To assess its localization, we overexpressed it bearing a green fluorescent protein (GFP) tag. Analysis of confocal images showed that TtPah1-GFP was distributed throughout the cell (Fig. 2A). To evaluate whether TtPah1 associates with ER membrane, Tetrahymena cells expressing TtPah1-GFP were labeled with ER-Tracker Red dye, and analyzed by confocal microscopy. The results revealed that TtPah1-GFP is localized to ER membrane in addition to the cytoplasm (Fig. 2B). To examine whether TtPAH1 encodes a functional phosphatidate phosphatase, we expressed a tandem affinity purification (TAP)-tagged fusion protein in Tetrahymena. We then purified the protein from lysates and measured phosphatidate phosphatase activity using a colorimetric assay. The purified protein migrated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at its expected size, near 100 kDa, but there were also more abundant smaller species, probably corresponding to proteolytic products (Fig. 2C). This purified protein dephosphorylated PA in a Mg2+-dependent manner (Fig. 2D,E). Taken together, these results confirm that TtPah1 is a functional PAH in Tetrahymena.
Fig. 2.
TtPAH1 localizes on ER and encodes functional phosphatidate phosphatase. (A) Localization of TtPAH1-GFP in Tetrahymena cells. Confocal image of fixed Tetrahymena cells expressing TtPAH1-GFP after DAPI staining; DAPI-stained nuclei (left), TtPAH1-GFP (middle) and DIC image of the fixed growing cell (right). (B) TtPAH1 associates with ER. Confocal images of fixed cells expressing TtPAH1-GFP after staining with ER-Tracker Red; TtPAH1-GFP (upper left), ER-Tracker Red (upper middle) and merge (upper right). Confocal stack of a different Tetrahymena cell expressing TtPAH1-GFP and stained with ER-Tracker Red is shown in the lower panels. (C) TtPAH1 purified as TAP-tag fusion in Tetrahymena. The silver-stained gel of purified TtPAH1 along with standard molecular weight marker is shown, with the molecular weights indicated on the left. In addition to the expected band of ∼100 kDa, many smaller species likely reflect partial proteolysis of the full-length protein. (D) TtPAH1 displays phosphatidate phosphatase activity. TtPAH1 protein (1 µM) purified from Tetrahymena was used to measure phosphatidic acid phosphatase activity, using a colorimetric assay. The average phosphate released (nmol) (n=3) was plotted against time. (E) The phosphatidate phosphatase assay performed either in the presence (+Mg2+) or absence (−Mg2+) of magnesium. The assay was carried out for 30 min before measuring the activity. TtPAH1 showed activity only in the presence of Mg2+, confirming it to be a PAP1 enzyme. The average phosphate released (nmol) (n=3) is shown.
TtPAH1 is dispensable for normal growth of Tetrahymena and loss of TtPAH1 does not affect expression of TtPAH2
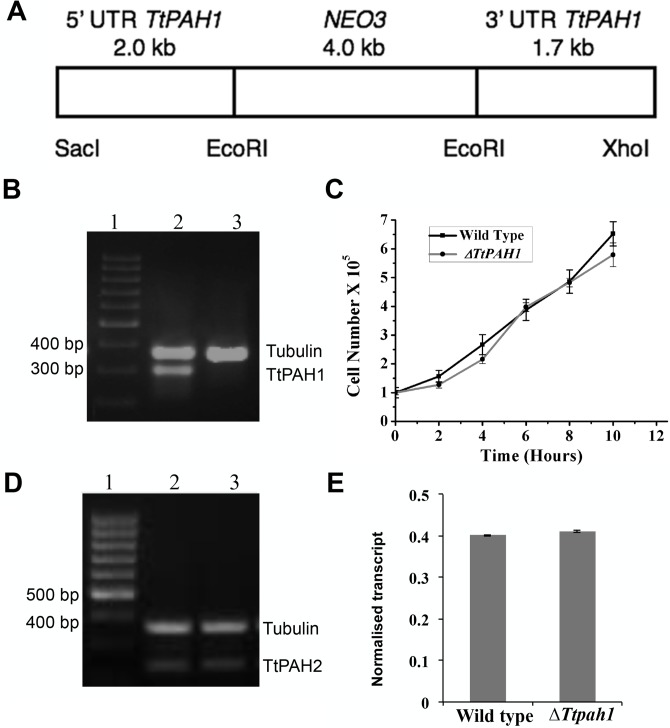
In many organisms such as Saccharomyces cerevisiae, C. elegans and Drosophila melanogaster, PAH is required for normal growth (Santos-Rosa et al., 2005; Golden et al., 2009; Ugrankar et al., 2011). To assess whether TtPAH1 is essential for normal growth of Tetrahymena, we generated the knockout strain by removing all 45 copies of TtPAH1 from the macronucleus of wild-type Tetrahymena by homologous recombination. The knockout strains thus generated (ΔTtpah1) were analyzed by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR), which confirmed the absence of TtPAH1 transcripts (Fig. 3A,B). The growth of ΔTtpah1 cells was not significantly different from that of wild-type cells (Fig. 3C). Moreover, there was no visible defect in the morphology of the knockout cells (data not shown). To rule out the possibility that the lack of growth defect in ΔTtpah1 is due to compensatory overexpression of TtPAH2 in these cells, we compared the expression of TtPAH2 in ΔTtpah1 with that in wild-type cells. The expression of TtPAH2 was not enhanced in ΔTtpah1 cells (Fig. 3D,E). Taken together, these results suggest that TtPAH1 is dispensable for normal growth of Tetrahymena.
Fig. 3.
TtPAH1 is dispensable for normal growth of vegetative Tetrahymena cells. (A) Schematic showing organization of the knockout construct used to disrupt TtPAH1 in the macronucleus. Gene disruption was performed by replacing the TtPAH1 ORF with NEO3 gene cassette, by homologous recombination. The NEO3 cassette confers resistance to paromomycin. (B) RT-PCR analysis of wild-type and ΔTtpah1 cells. Lane 1, standard molecular weight marker; lane 2, amplified products of cDNA from wild-type cells; lane 3, amplified products of cDNA from ΔTtpah1 cells. The top band just below the 400 bp marker corresponds to alpha-tubulin (387 bp), and the band near 300 bp represents TtPAH1. The absence of a 300 bp band corresponding to TtPAH1 confirms that knockout is complete. (C) Growth curve of Tetrahymena wild-type versus ΔTtpah1 cells. The cell numbers were counted every 2 h, and the number of cells/ml was plotted against time. Loss of TtPAH1 does not affect Tetrahymena growth significantly. (D) Semi-quantitative RT-PCR showing expression of TtPAH2 in wild-type and ΔTtpah1 cells. Lane 1, standard molecular weight marker; lane 2, amplified products of cDNA from wild-type cells; lane 3, amplified products of cDNA from ΔTtpah1 cells. The top band in lanes 2 and 3 corresponds to alpha-tubulin (387 bp), and the band near 238 bp represents TtPAH2. (E) The graph shows quantitation of TtPAH2 after normalization with the alpha-tubulin band. The expression of TtPAH2 is not enhanced by the loss of TtPAH1.
TtPAH1 is required to maintain lipid droplet number in Tetrahymena
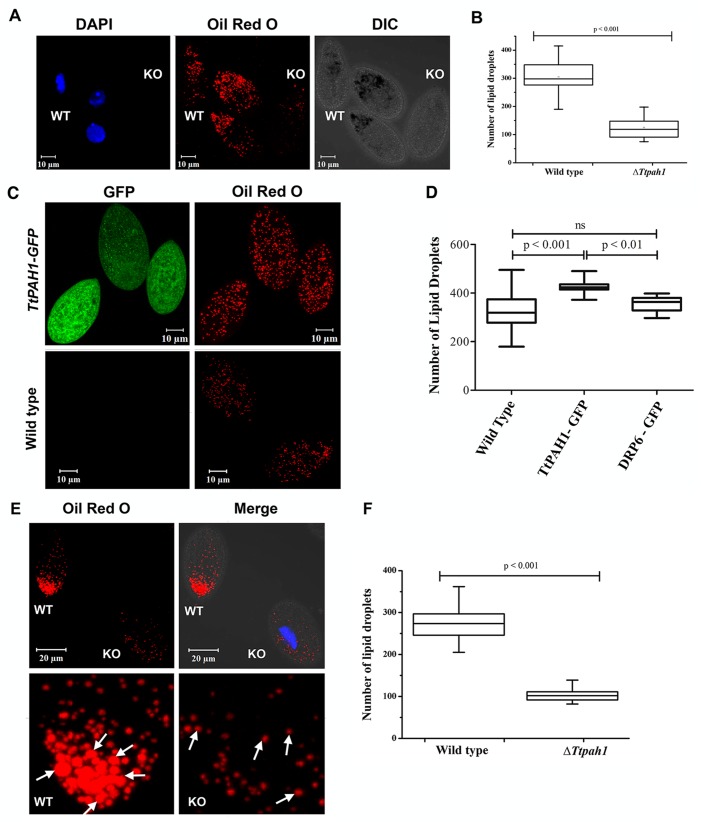
Lipid droplets are ubiquitous eukaryotic organelles mainly used for storing lipids (Murphy, 2001). They consist of a hydrophobic core of neutral lipids, such as triacylglycerol, sterols and sterol esters, surrounded by a phospholipid monolayer originating from the ER (Tauchi-Sato et al., 2002; Farese and Walther, 2009; Radulovic et al., 2013). Lipid droplet growth occurs either by localized synthesis of lipids or by fusion with other lipid droplets (Thiele and Spandl, 2008). Since Pah proteins are required for the synthesis of triacylglycerol, we compared lipid droplet numbers between ΔTtpah1 and wild-type cells. Lipid droplets were visualized by staining with Oil Red O, and the number of lipid droplets was counted after analyzing confocal images by LSM Image analyzer. The number of lipid droplets decreased significantly in ΔTtpah1 (Fig. 4A,B). Although there was no visible difference in the size of lipid droplets, quantitative analysis showed ∼60% reduction in lipid droplet numbers compared to wild type (Fig. 4B). To provide further evidence that TtPAH1 is involved in lipid droplet biogenesis, we overexpressed TtPAH1-GFP in wild-type Tetrahymena cells. Overexpression of TtPAH1 resulted in a ∼20% increase in lipid droplet number compared to wild type (Fig. 4C,D). To demonstrate the specificity of this effect, we similarly overexpressed DRP6-GFP (a dynamin-related protein in Tetrahymena) and observed that it did not affect the lipid droplet number (Fig. 4D). Hence, we conclude that TtPAH1 is required to maintain normal lipid droplet number in Tetrahymena. Decreased lipid droplet accumulation in ΔTtpah1 was not due to decreased nutrient uptake since we saw a similar reduction when the comparison between ΔTtpah1and wild-type was performed under starvation conditions (Fig. 4E,F). Under starvation conditions, we observed a ∼60% reduction in lipid droplet number in ΔTtpah1 cells. Moreover, the size of lipid droplet in ΔTtpah1 was smaller than in wild-type cells (Fig. 4E). Taken together, these results suggest that TtPAH1 influences the number and size of the lipid droplets in Tetrahymena.
Fig. 4.
TtPAH1 maintains lipid droplet number in Tetrahymena. (A) Confocal images of Tetrahymena cells showing lipid droplets stained with Oil Red O dye. Wild-type cells and knockout cells were imaged together simultaneously. The wild-type cells were stained with DAPI to distinguish them from knockout cells. (B) Box plot showing the distribution of lipid droplet numbers in wild-type (n=35) versus ΔTtpah1 (n=38) cells. (C) Confocal images of wild-type and TtPAH1-GFP-expressing cells showing lipid droplets after staining with Oil Red O dye. (D) Box plot showing lipid droplet numbers in wild-type cells, cells overexpressing TtPAH1-GFP (n=20) and cells overexpressing GFP-DRP6 (n=20). An increase in lipid droplet number is observed in cells expressing TtPAH1-GFP. (E) Confocal images of Tetrahymena cells showing lipid droplets stained with Oil Red O dye. Wild-type (WT) and knockout cells after starvation were imaged together simultaneously. Knockout cells were stained with DAPI to distinguish them from wild-type cells. Both the size and number of lipid droplets are reduced in ΔTtpah1 cells (KO). Lipid droplet size in wild-type cells appears to be larger than in the knockout cells as indicated by arrows. (F) Box plot showing lipid droplet numbers in wild-type (n=22) and ΔTtpah1 (n=22) cells under starved condition.
TtPAH1 is needed for maintaining tubular ER in Tetrahymena
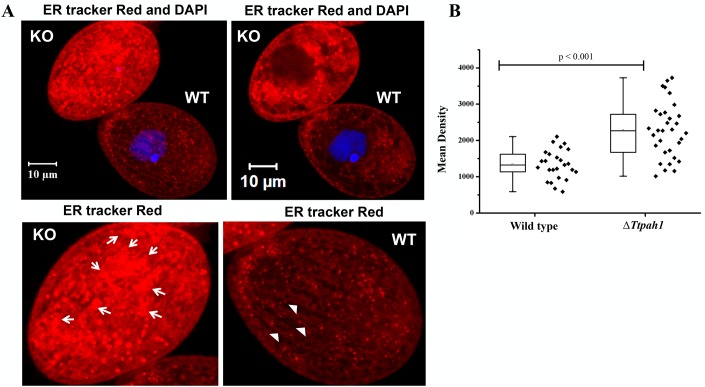
The ER is a complex network consisting of flat sheets and highly curved tubules, and their abundance varies with cell cycle stages. The ER serves as the primary site for de novo lipid biosynthesis. We hypothesized that PAH regulates ER morphology since phosphatidic acid, a major component of ER, is converted to DAG by PAH. To determine whether TtPAH1 is important in maintaining ER morphology, we stained both ΔTtpah1 and wild-type cells with ER-Tracker Red dye and analyzed morphology by confocal microscopy (Fig. 5A,B; Fig. S1). The ER content increased significantly in cells lacking TtPAH1, as measured by the mean density of ER-Tracker Red staining (Fig. 5C). Moreover, in wild-type cells, the ER appeared mainly as a network of fine tubules with occasional small patches, likely to represent ER sheets (Fig. 5A,B; Fig, S1). These patches seemed larger and more abundant in the absence of functional TtPAH1. This result suggests that TtPAH1 is required for creating and/or maintaining the ER structure.
Fig. 5.
TtPAH1 is needed for maintaining tubular ER. (A) The top panel shows wild-type (WT) and ΔTtpah1 (KO) cells imaged simultaneously in the same field after staining with ER-Tracker Red. The left panel represents the confocal stack; the right panel is a single mid plane confocal slice. Wild-type cells were stained with DAPI to distinguish them from ΔTtpah1 cells. The enlarged images of ΔTtpah1 (bottom left) and wild-type (bottom right) cells are shown, indicating ER sheet (arrows) and ER tubule (arrowheads) structures. To rule out the effect of DAPI staining on ER morphology, we also stained ΔTtpah1 cells with DAPI and imaged them simultaneously with wild-type cells and found similar results. (B) Box plot showing the mean density of ER-Tracker Red staining. The mean intensity of ΔTtpah1 (n=32) is significantly higher than that of wild type (n=25).
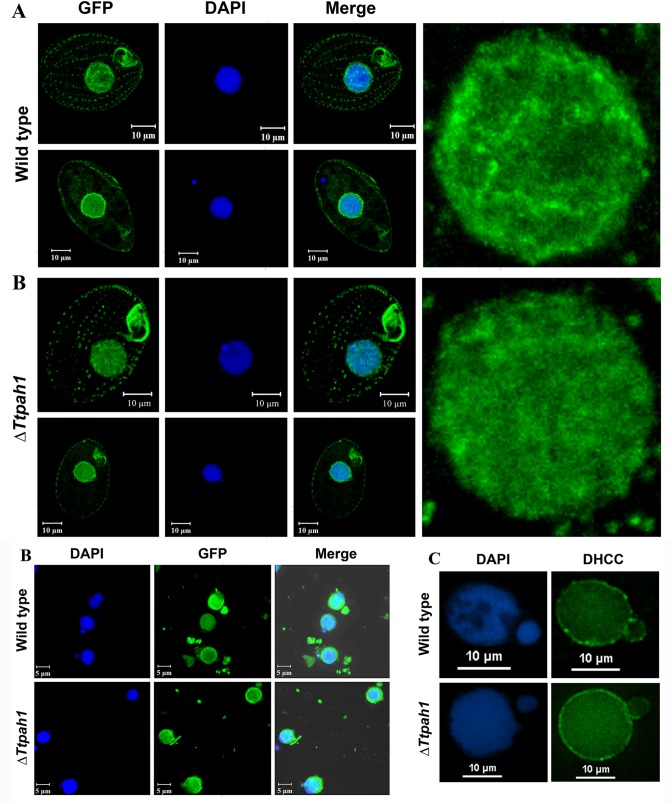
Loss of TtPAH1 does not manifest visible nuclear envelope defect in Tetrahymena
Tetrahymena harbors one polyploid, phenotypically active macronucleus (MAC) and a diploid transcriptionally silent germline micronucleus (MIC). To determine whether TtPAH1 function is necessary to maintain normal nuclear envelope (NE) morphology, we analyzed the NE by expressing and visualizing NUP3-GFP (a nuclear pore component marker specifically localizing to macronucleus) in ΔTtpah1 cells and wild-type cells. This comparison did not reveal any visible defect in size or shape of the NE in ΔTtpah1 cells (Fig. 6A). Like in wild-type, the 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI)-stained DNA appeared round, compact and nonfragmented (Fig. 6A). Consistent with this, isolated DAPI-stained nuclei from wild-type and mutant cells expressing NUP3-GFP seemed identical (Fig. 6B). To further confirm that deletion of TtPAH1 did not affect nuclear morphology, we stained isolated nuclei (both MAC and MIC) with a lipophilic dye (3,3′-dihexyloxacarbocyanine iodide, DHCC) to visualize nuclear membrane. As with Nup3-GFP, we did not observe any visible defect in nuclear membranes of MAC (Fig. 6C). We also did not observe any detectable change in MIC structure (Fig. 6C). These results suggest that TtPAH1 is not essential for maintaining normal nuclear morphology in Tetrahymena. Our results are in contrast to findings in S. cerevisiae, where cells lacking PAH1 showed abnormal expansion of nuclear envelope that appeared as a nuclear membrane projections lacking DNA. Our results, taken together with our analysis of the ER, suggest that defects in ER morphology in Tetrahymena do not necessarily affect nuclear morphology, unlike the coupling in other organisms.
Fig. 6.
Loss of TtPAH1 does not manifest visible nuclear envelope defect in Tetrahymena. (A) Confocal images of wild-type and ΔTtpah1 cells expressing NUP3-GFP after DAPI staining. In both wild-type and ΔTtpah1, the upper panel is the Z-stack and the lower panel is a single slice. The enlarged nucleus from the Z-stack is shown on the right side. (B) Confocal images of DAPI-stained nuclei isolated from wild-type (upper panel) and ΔTtpah1 (lower panel) cells expressing NUP3-GFP. (C) Fluorescence images of Tetrahymena nuclei of wild type (upper panel) and ΔTtpah1 (lower panel) after staining with DHCC and DAPI. The images are deconvoluted using NIS Advanced Research software.
TtPAH1 restores different phenotypes of pah1Δ yeast cells
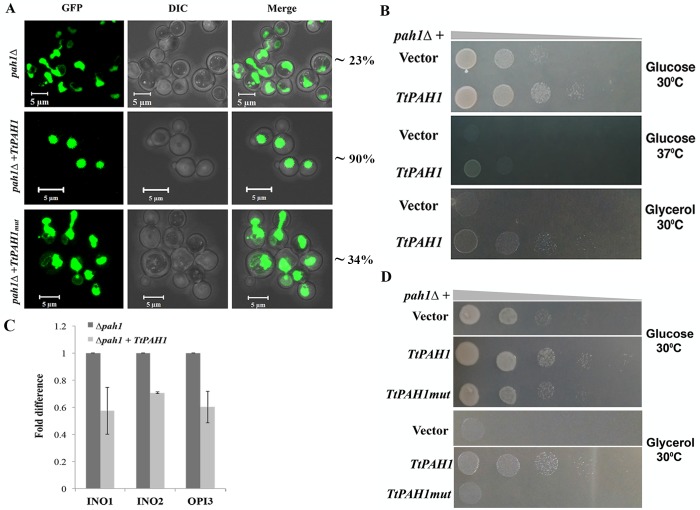
Though TtPAH1 is not required for regulating nuclear expansion and nuclear shape in Tetrahymena, we asked whether the ciliate protein could rescue the nuclear defects in S. cerevisiae pah1Δ, which might be expected if the homologous proteins retain the same enzymatic activity. To assess nuclear morphology in budding yeast, we expressed nucleoplasmic protein PUS as a GFP-fusion and visualized pah1Δ cells expressing TtPAH1.
In pah1Δ, the nuclei in nondividing cells often appeared as two lobes interconnected by a long nuclear membrane extension (Fig. 7A) (Santos-Rosa et al., 2005). In contrast, pah1Δ expressing TtPAH1 showed nearly normal nuclear morphology (Fig. 7A). This result suggests that TtPAH1 can substitute for one or more functions of the yeast homolog.
Fig. 7.
TtPAH1 rescues the nuclear structure defect, slow growth phenotype and respiratory deficiency of pah1Δ yeast strain, and the catalytic motif is essential for its function. (A) Confocal images of pah1Δ yeast cells transformed with either empty vector (top), TtPAH1 (middle) or TtPAHmut (bottom) along with PUS1-GFP (an intranuclear reporter). TtPAH1 but not TtPAHmut restores aberrant nuclei of pah1Δ yeast to wild-type spherical shape. Three different transformants per strain were analyzed and the number of cells counted for each transformant was 200-250 (n= 600-750). The percentage of cells containing round nucleus is indicated on the right. (B) The growth of pah1Δ yeast cells transformed with either TtPAH1 or empty vector grown on SD media containing either glucose or glycerol (lacking leucine and uracil) at either 30°C or 37°C as indicated. The experiment was repeated three times. (C) Quantitative RT-PCR analysis of INO1, INO2 and OPI3 mRNAs in pah1Δ yeast cells transformed with either empty vector or TtPAH1. Amplification of each sample was performed in triplicate and normalized to a control gene SEC63 in three independent experiments. (D) The growth of pah1Δ yeast cells transformed with either with empty vector, TtPAH1 or TtPAHmut grown on SD media lacking leucine and uracil and containing either glucose or glycerol as indicated.
pah1Δ also exhibits slow growth at 30°C, temperature-sensitive growth at 37°C (Han et al., 2006) and respiratory deficiency (i.e. growth defect) on nonfermentable carbon sources (Han et al., 2007). Along with rescue of the nuclear morphology defect, expression of TtPAH1 restored growth both at 30°C and 37°C (Fig. 7B). To evaluate the role of TtPAH1 in rescuing respiratory deficiency, we grew cells on plates containing glycerol as nonfermentable carbon source. The pah1Δ expressing TtPAH1 grew faster than control pah1Δ cells (Fig. 7B).
Nuclear expansion in yeast is linked to the induction of phospholipid biosynthetic genes (Santos-Rosa et al., 2005). Deletion of PAH1 induces the expression of inositol-3-phosphate synthase (INO1), the transcription factor INO2 and phosphatidyl-N-methylethanolamine N-methyltransferase (OPI3), which are involved in the induction of phospholipid biosynthetic genes, leading to overly developed ER and aberrant expansion of nuclear membrane (Santos-Rosa et al., 2005). To test whether TtPAH1 inhibits abnormal nuclear expansion in pah1Δ yeast by inhibiting the phospholipid biosynthesis genes, we have analyzed the mRNA levels of INO1, OPI3 and INO2 by quantitative real-time PCR using Sec 63 (a resident ER membrane protein unaffected by PAH1 deletion) as a control (Santos-Rosa et al., 2005). TtPAH1 repressed expression of all three genes tested, suggesting that TtPAH1 could replace yeast PAH1 in regulating expression of phospholipid biosynthesis genes (Fig. 7C). Taken together, these results suggest that TtPAH1 retains all the known functions of yeast PAH1, and hence is functionally conserved between yeast and Tetrahymena.
A conserved DXDXT/V motif at C-LIP is essential for the catalytic activity of Pah1/lipin in yeast and mammals (Finck et al., 2006; Han et al., 2007). We identified a similar motif (666 DIDGT 670) in the predicted C-LIP of TtPah1 and evaluated if the motif is important for the function of TtPAH1 by mutating two aspartate residues (D666,668E) (TtPAH1mut). Since TtPAH1 functionally replaces yeast PAH1, we attempted to complement pah1Δ yeast cells with TtPAH1mut, and evaluated nuclear morphology, and growth in different temperatures and media. The mutant protein did not rescue aberrant nuclear morphology, slow growth at 30°C and the respiratory defect to the wild-type level (Fig. 7A,D). These results suggest that the catalytic activity of TtPah1 is important for its function.
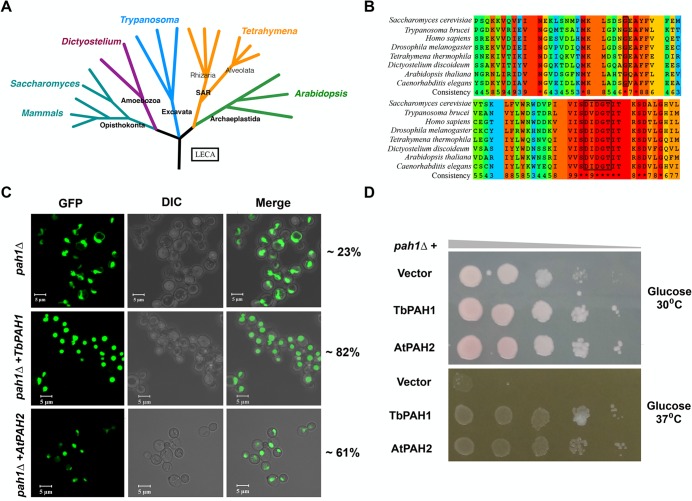
Phosphatidate phosphatase is conserved across eukaryotic lineages
Prior studies on the role of PAH proteins in the regulation of lipid homeostasis and membrane biogenesis have focused mainly on the Opisthokont and Archaeplastid clades. The cellular function of PAH is not yet known in organisms belonging to clades distantly related to Opisthokont, such as the Excavata. Fig. 8A shows an evolutionary tree with representative organisms for each clade. The sequence analysis of PAH homologs from organisms belonging to different clades suggest that it is conserved across eukaryotic lineages (Fig. 8B). In this study, we established the role of PAH1 in regulating lipid homeostasis and membrane biogenesis in Tetrahymena, an Alveolate. By complementation of pah1Δ yeast cells with Trypanosoma PAH1 (TbPAH1), we further show that conservation appears to extend to another group, the Excavates. TbPAH1 rescued the growth, respiratory and nuclear defects of pah1Δ yeast cells (Fig. 8C,D).
Fig. 8.
Phosphatidate phosphatase is conserved across eukaryotic lineages. (A) Eukaryotic evolutionary tree. Five clades with representative organisms from each clade are shown. (B) Multiple sequence alignments showing parts of N-LIP (top) and C-LIP (bottom) of PAH proteins from various organisms. Assigned colors of the specific residues are based on alignment consensus. The boxes indicate conserved Glycine at the N-LIP and conserved catalytic motif (DXDXT) at the C-LIP. (C) Confocal images of pah1Δ yeast cells complemented either with Trypanosoma PAH (TbPAH1) or with Arabidopsis PAH (AtPAH2). Both TbPAH1 and AtPAH2 rescued the nuclear defect of pah1Δ yeast cells. The nucleus is visualized by expression of PUS-GFP. Two different transformants per strain were analyzed and the number of cells counted for each one was 200 (n=400). The percentage of cells containing a round nucleus is indicated on the right. (D) Rescue of growth defect of pah1Δ yeast cells by TbPAH1 and AtPAH2 on SD media containing glucose but lacking leucine and uracil at 30°C or 37°C as indicated. Two different transformants per strain were spotted and analyzed.
The Arabidopsis PAH homolog AtPAH2 has previously been shown to possess some functions of S. cerevisiae PAH1, based on its ability to rescue the slow growth phenotype of pah1Δ yeast. However, it was not reported whether the plant homolog also rescues the nuclear envelope defect (Nakamura et al., 2009; Mietkiewska et al., 2011). We used AtPAH2 to complement the pah1Δ yeast strain. In addition to rescuing the growth phenotype, AtPAH2 mitigated the aberrant nuclear morphology of pah1Δ yeast cells, confirming conservation between Opisthokonta and Archaeplastida (Fig. 8C,D). Taken together, these results along with results from earlier reports suggest that the PAH phosphatase cascade is functionally conserved across eukaryotic lineages, indicating that it originated before the lineages diverged very early in eukaryotic evolution.
DISCUSSION
In this study, we have identified two homologs of LIPIN/PAH in T. thermophila. We report that TtPAH1 is a phosphatidic acid phosphatase involved in the regulation of lipid droplet biogenesis and ER morphology in Tetrahymena. Regulation of lipid homeostasis and membrane biogenesis is fundamental to all eukaryotes, and the presence of a regulation cascade comprising Pah1 and its phosphatase complex Nem1-Spo7 has been shown in yeast (Siniossoglou et al., 1998; Péterfy et al., 2001; Han et al., 2006; Golden et al., 2009; Nakamura et al., 2009). Similar cascades are also reported in plants (Nakamura et al., 2009; Mietkiewska et al., 2011), mammals, (Kim et al., 2007), worms (Golden et al., 2009) and flies (Ugrankar et al., 2011). All studies are restricted to Opisthokonta and Archaeplastida clades. The presence of such a cascade is not reported in the distantly related lower eukaryotic clades such as Alveolata and Excavata. In the present study, we observed that PAH from the clades belonging to Excavata and Alveolata functionally replaces yeast PAH1. We, therefore, conclude that this phosphatidic acid phosphatase cascade regulating membrane biogenesis and lipid homeostasis is conserved across the eukaryotic evolutionary tree.
Fungi (S. cerevisiae), nematodes (C. elegans) and insects (D. melanogaster) express one PAH homolog (Han et al., 2006; Golden et al., 2009; Ugrankar et al., 2011), whereas mammals express three and plants (Arabidopsis thaliana) express two homologs (Donkor et al., 2007; Nakamura et al., 2009). The presence of two PAH homologs in a lower eukaryote, such as Tetrahymena, is unusual since multiple homologs are mainly found in higher organisms. Previous studies have shown that deletion of PAH leads to growth and development defects in yeast (Han et al., 2006, 2007; Adeyo et al., 2011), D. melanogaster (Ugrankar et al., 2011) and C. elegans (Golden et al., 2009). In contrast, loss of PAH1 in Tetrahymena did not result in growth defect. The normal growth and development of ΔTtpah1 mutant cells may be attributed to the presence of another homolog, TtPAH2.
TtPah1 displays cytoplasmic as well as membrane localization consistent with previously characterized mammalian lipin and yeast Pah1 (Péterfy et al., 2001; Han et al., 2006). Dephosphorylation of Pah1 regulates its subcellular localization and promotes its translocation from the cytoplasm into ER, where it converts PA to DAG (Karanasios et al., 2010). PA phosphatase regulates lipid droplet number by generating its precursor TAG from the substrate PA (Adeyo et al., 2011). The role of PAH/ LIPIN in lipid droplet biogenesis or lipid storage has been established in yeast (Adeyo et al., 2011), Drosophila (Ugrankar et al., 2011) and C. elegans (Golden et al., 2009). By generating the deletion of TtPAH1 in Tetrahymena, we demonstrated its role in lipid droplet biogenesis. Overexpression of TtPAH1 in wild-type cells leads to an increase in lipid droplet number, further corroborating its role in lipid droplet biogenesis.
The role of PAH proteins in maintaining ER structure is well established in yeast and C. elegans (Siniossoglou et al., 1998; Campbell et al., 2006; Golden, Liu and Cohen-Fix, 2009). The loss of PAH produces a morphological change in many organelles in Drosophila, but perturbation of ER morphology was not reported (Ugrankar et al., 2011). Interestingly, deletion of macronuclear copies of TtPAH1 in Tetrahymena alters ER morphology, resulting in an increased proportion of sheet to tubule structure. One possibility for the altered ER morphology could be the change in phospholipid flux arising from the loss of PAH1, leading to change in the phospholipid composition of ER. We observed increased intensity of ER-Tracker Red dye in ΔTtpah1 cells, indicating higher levels of sulphonyl urea receptor (SUR) of ATP-sensitive K+ channel in these cells. The expansion of the ER by deletion of PAH1 is in general associated with increased expression of membrane proteins that include ER resident proteins in other organisms such as Schizosacchatamyces pombe, C. elegans and Yarrowia lipolytica (Tange, 2002; Golden et al., 2009; Guerfal et al., 2013). Therefore, we speculate that the increased ER membrane synthesis in ΔTtpah1 cells concomitantly increases the production of ER-associated proteins and might include ER resident proteins such as SUR. However, this remains to be tested in Tetrahymena.
Loss of PAH in mammals and C. elegans results in a defect in nuclear envelope breakdown (NEBD) without any nuclear expansion (Golden et al., 2009; Gorjánácz and Mattaj, 2009). The regulation of nuclear expansion by PAH is restricted to yeast, which could be explained by the presence of the CDP-DAG pathway in yeast and its absence in mammals and C. elegans (Bahmanyar et al., 2014; Bahmanyar, 2015). The accumulation of PA due to loss of PAH1 leads to the excess synthesis of phospholipids PE and PC via the CDP-DAG pathway, resulting in massive nuclear expansion in yeast (Santos-Rosa et al., 2005; Han et al., 2006; Bahmanyar et al., 2014). It is interesting to note that although Tetrahymena possesses the CDP-DAG pathway for phospholipid synthesis, nuclear expansion was not visible in ΔTtpah1. Although we have used only NUP-GFP as a marker to detect nuclear expansion, it might be useful to test with other nuclear markers as well. However, we believe that NUP3-GFP is also a reliable marker since nuclear membrane flares seen in yeast contain assembled nuclear pore structures (Siniossoglou et al., 1998). Further, by staining the nuclear membrane with a lipophilic dye that should stain any membranous structure, we failed to detect any visible flares in both micronucleus and macronucleus of ΔTtpah1. These results suggest that unlike yeast, in which expansion of the nuclear membrane is very prominent, there is no extensive expansion of the nuclear membrane in Tetrahymena upon deletion of TtPAH1. Nuclear volume in Tetrahymena is variable presumably due to differential ploidy level in the MAC (Raikov 1976; Gorovsky 1980; Bodenbender et al., 1992). Therefore, one could speculate a different mechanism that allows plasticity in nuclear expansion to accommodate different nuclear volumes.
The NE is connected with the ER, and changes in ER structure lead to defects in the NE. For example, while overexpression of reticulons and DP1 inhibits nuclear envelope formation and nuclear expansion, loss of their functions enhances nuclear envelope assembly (Anderson and Hetzer, 2008). In yeast, loss of PAH1 leads to an overdeveloped ER membrane, which in turn results in nuclear expansion (Siniossoglou et al., 1998; Tange, 2002). As discussed above, loss of PAH1 in Tetrahymena demonstrates a change in ER content and structure. Although the loss of TtPAH1 increases the ER sheet structure (Fig. 5A), it does not manifest visible defect in the nuclear envelope. It appears that in Tetrahymena, unlike in other organisms, ER content and structure are functionally isolated from mechanisms underlying nuclear expansion. However, further studies are required to clearly understand the regulation of nuclear expansion and its relation to ER in Tetrahymena.
Mutation of the catalytic motif in TtPah1 leads to loss of function, suggesting that the catalytic activity is necessary for its function. The role of PAH, other than catalytic function is identified in other organisms. For example, PAH acts as transcriptional co-activator in mammals and as a transcription factor in yeast (Santos-Rosa et al., 2005; Finck et al., 2006; Kim et al., 2013). However, further studies are required to show if Tetrahymena PAH1 has a direct role in transcription.
PAH homolog is functionally conserved (interchangeable) between Opisthokonta and Plantae (Nakamura et al., 2009; Mietkiewska et al., 2011). We have now extended functional conservation of PAH to lower eukaryotic lineages such as Alveolata and Excavata. Though there is no report of the presence of such a cascade in Amoebozoa, the PAH homolog is present in the genome sequence of Dictyostelium (an Amoebozoan). Therefore, it can be concluded that cascade comprising PAH for regulation of lipid homeostasis and membrane biogenesis was present in common ancestors before the divergence of lineages, and this cascade remained functionally conserved without allowing change or modification in these functions, since lipid homeostasis and membrane biogenesis regulation is important for the normal growth of all eukaryotes. PAH, in addition to lipid homeostasis and membrane biogenesis, has an additional role such as NEBD (in C. elegans) (Golden et al., 2009; Bahmanyar et al., 2014) and nuclear expansion (in yeast) (Santos-Rosa et al., 2005), suggesting that the lineage-specific role of PAH is adopted after divergence from the common ancestor. However, PAH homologs from all the lineages discussed here rescue abnormal nuclear expansion. Therefore, it can be concluded that though all known functions of PAH were present before lineage divergence, different lineages have adopted these functions to regulate various cellular processes.
Overall, our results along with results from previous studies as discussed above clearly demonstrate a common regulatory cascade across eukaryotic lineages and may have appeared before the divergence of lineages. Our results also show that unlike other known PAH homologs, TtPAH1 does not regulate nuclear morphology.
MATERIALS AND METHODS
Strains and culture conditions
Wild-type CU428.1 and B2086 strains of T. thermophila were grown at 30°C in SPP medium (2% proteose peptone, 0.2% dextrose, 0.1% yeast extract, 0.003% ferric EDTA). For conjugation, cells of different mating types were grown to log phase, washed and starved in DMC (0.17 mM sodium citrate, 0.1 mM NaH2PO4, 0.1 mM Na2HPO4, 0.65 mM CaCl2 and 0.1 mM MgCl2) for 16-24 h at 30°C (Orias et al., 2000). For long-term storage, wild-type or knockout cells were starved and frozen in liquid nitrogen in 4% DMSO (Bruns et al., 2000).
Construction and expression of TtPAH1-GFP, TtPAH1-TAP and NUP3-GFP
To generate the TtPAH1-GFP construct, full-length TtPAH1 was amplified from genomic DNA using specific primers (Table S1). The amplified product was cloned into an entry vector using a pENTR/D-TOPO kit (Invitrogen). This was further cloned into the destination vector pIGF (Tetrahymena-specific rDNA-based vector, a gift from Doug Chalker, Washington University, USA) using LR clonase. For expressing TtPAH1 as TAP-tagged protein, full-length TtPAH1 was PCR amplified using specific primers with a XhoI restriction site in the forward primer and an Apa1 restriction site in the reverse primer (Table S1), and the amplified product was cloned into Tetrahymena-specific vector pVGF (from Meng-Chao Yao, University of Washington, USA) using XhoI and ApaI restriction sites.
TtPAH1-TAP and TtPAH1-GFP were transformed into wild-type Tetrahymena cells using 20 μg of the plasmid by electroporation (Gaertig et al., 1994). Transformants were selected with 100 μg/ml paromomycin sulfate and induced with 1 μg/ml cadmium chloride for 4-5 h to stimulate transcription of the transgene from the MTT1 promoter. The NUP3-GFP in NCVB vector (from Aaron Turkewitz, University of Chicago, USA) was linearized and introduced biolistically into vegetative Tetrahymena by particle bombardment, and the transformants were selected using 60 μg/ml blasticidin in the presence of 1 μg/ml cadmium chloride (Rahaman et al., 2008).
Disruption of TtPAH1
5′UTR and 3′UTR of TtPAH1 were PCR amplified and cloned into the pCRII vector (Invitrogen). To amplify 5′UTR, SacI and EcoRI restriction sites were incorporated in the forward and reverse primer, respectively (Table S1). For amplification of 3′UTR, EcoRI and XhoI restriction sites were included in the forward and reverse primer, respectively (Table S1). Finally, the NEO3 cassette was introduced between 5′UTR and 3′UTR using EcoRI restriction sites. The resulting knockout construct was linearized by digesting with SacI and XhoI restriction enzymes and introduced biolistically into vegetative Tetrahymena by particle bombardment as previously described (Gaertig et al., 1994; Cassidy-Hanley, 2003). The complete replacement of endogenous TtPAH1 was achieved by growing the transformants in the presence of increasing concentrations of paromomycin sulfate (≤1.2 mg/ml) with 1 µg/ml cadmium chloride.
Semi-quantitative RT-PCR
Total RNA was isolated from ΔTtpah1 cells and wild-type cells using a RNeasy Mini Kit (Qiagen). A QuantiTect Reverse Transcription Kit (Qiagen) was used to synthesize cDNA. PCR reactions were performed with 100 ng cDNA using alpha-tubulin (ATU1)- and TtPAH1-specific primers (Table S1) in the same reaction for 25-40 cycles.
Purification of TtPah1-TAP
For purification of TtPah1-TAP, Tetrahymena cells harboring TtPAH1-pVGF were grown to a density of 3×105 cells/ml. The culture was induced with 1 µg/ml cadmium chloride for 5 h at 30°C, and cells from 300 ml cultures were collected by centrifugation. The cell pellet was resuspended in 10 ml lysis buffer [20 mM Tris-HCl (pH 8.00), 100 mM NaCl, 0.5% NP-40, 10% glycerol] supplemented with a mixture of protease inhibitors (pepstatin, E-64, aprotinin and protease inhibitor cocktail). The lysate was clarified by ultracentrifugation (Optima L100K, 70Ti rotor, Beckman Coulter, Brea, CA, United States) for 1 h at 250,000 g. To minimize proteolysis, all subsequent steps were carried out at 4°C unless mentioned otherwise. Rabbit-IgG agarose slurry (Sigma-Aldrich) pre-equilibrated with wash buffer was added to the clarified lysate and was kept for binding for 2 h. Resin was collected by centrifugation (1 min at 3000 g) and washed with 50 bed volumes of wash buffer [20 mM Tris-HCL (pH 8.00), 2 mM MgCl2, 0.2 mM EGTA, 0.1% Tween 20, 10% glycerol, 1 mM DTT, 0.1 mM PMSF]. Resin was incubated with 2 µl TeV protease in 200 µl cleavage buffer [10 mM Tris-HCl (pH 8.00), 0.1 M NaCl, 0.1% Tween 20, 0.5 mM EDTA, 1 mM DTT] for 1.5 h at room temperature, followed by further incubation at 4°C overnight. The eluate after proteolytic cleavage was adjusted to 3 mM CaCl2 and mixed with three volumes of calmodulin binding buffer [10 mM Tris-HCl (pH 8.00), 100 mM NaCl, 1 mM Mg acetate, 1 mM imidazole, 2 mM CaCl2, 0.1% Tween 20, 10 mM βME]. This was incubated with 100 µl calmodulin resin (GE Healthcare, Buckinghamshire, UK) at 4°C for 1 h. The resin was recovered by centrifugation and washed with calmodulin binding buffer. Protein was eluted with calmodulin elution buffer [10 mM Tris-HCl (pH 8.00), 100 mM NaCl, and 1 mM Mg acetate, 1 mM imidazole, 10 mM EGTA, 0.1% Tween 20, 10 mM 2-mercaptoethanol] (Witkin and Collins, 2004). Eluted fractions were loaded on 10% SDS polyacrylamide gel, and the protein was detected by silver staining.
Growth analysis
TtPAH1 knockout cells and wild-type cells were grown in triplicate. When the cell number reached 1×105/ml, cells were counted using a hemocytometer at 2 h intervals after fixation with formalin. The averaged cell density was plotted against time.
Isolation of nuclei
Tetrahymena cells (50 ml, 5×105 cells/ml) were centrifuged (5 min at 1100 g) at 4°C and cell pellets were washed with pre-chilled Solution A (sucrose 0.1 M, gum arabic 4% v/v, MgCl2 0.0015 M, Spermidine Hydrochloride 0.01% v/v) and resuspended in pre-chilled Solution B (sucrose 0.1 M, gum arabic 4% v/v, MgCl2 0.0015 M, Spermidine Hydrochloride 0.01% v/v, octanol 24 mM). The suspension was shaken vigorously for 5 min followed by centrifugation (Allen, 2000). The nuclear pellet was resuspended in Buffer A and imaged by fluorescence microscope after staining with DAPI.
Staining and microscopy
For staining lipid droplets, Tetrahymena cells were pelleted down by centrifugation (1100 g for 2 min) at room temperature, washed with DMC and fixed with 4% paraformaldehyde. Fixed cells were washed with 10 mM HEPES and resuspended in the freshly prepared Oil Red O solution. Cells were tapped briefly and incubated in the dark in a nutating mixer at room temperature for 10 min. Stained cells were washed three times with 10 mM HEPES and resuspended in 10 mM HEPES before imaging in a confocal microscope (Binns et al., 2006).
For ER staining, Tetrahymena cells were grown to a density of 3-4×105 cells/ml, and 0.5 µM ER-Tracker Red dye (Invitrogen) was added to the culture and incubated for 60 min before fixing with 4% paraformaldehyde (50 mM HEPES, pH 7.5). To rule out any effect of differential pressure (during placing coverslips) on ER morphology in different samples, we imaged both wild-type cells and knockout cells simultaneously.
For Oil Red O staining images were taken at 543 nm excitation/619 nm emissions and for ER-Tracker Red images were taken at 587 nm excitation/615 nm emissions. Then, 3-5 µl of cells were mounted on glass slides, covered with cover glasses, sealed with nail polish and imaged with a LSM780 confocal microscope (Zeiss, Oberkochen, Germany).
For staining Tetrahymena nucleus, it was incubated with 5 μg/ml DHCC and 0.5 μg/ml DAPI for 10 min in dark, washed three times with Solution A and resuspended in the same solution before imaging in an Eclipse Ti fluorescence microscope (Nikon, Tokyo, Japan).
To quantitate ER content, the stacked images of ER-Tracker Red-stained cells were analyzed by ImageJ (https://imagej.nih.gov/ij/) after sum intensity projection. The mean intensity values were plotted for both wild-type (n=34) and ΔTtpah1 (n=32) cells using box plot.
Gene synthesis
The coding region of TtPAH1 was commercially synthesized (Eurofins, Louisville, KY, USA) after codon optimization and obtained in the pUC57 vector. This commercially synthesized gene was used for expression in bacteria and complementation assays in yeast.
Yeast culture conditions
Yeast cells were grown either in yeast extract peptone dextrose (YPD) medium or synthetic complete dextrose (SD) media containing 2% glucose with appropriate amino acids (Sherman, 2002). For growth analysis, yeast cells were grown in SD medium lacking leucine and uracil to early logarithmic phase, serially diluted (10-fold) and 5 µl of each dilution was spotted onto the solid SD medium lacking leucine and uracil and incubated at either 30°C or 37°C for 2-4 days. To check respiratory deficiency, glycerol (2%) in place of dextrose was used as the carbon source.
Site-directed mutagenesis
Point mutations (D666,668E) at the corresponding sites of the TtPAH1 coding region in YCplac111-PAH1 fusion construct were introduced using a Quik Change Site-Directed Mutagenesis protocol (Stratagene), and the mutations were confirmed by DNA sequencing.
Yeast complementation assay
The full-length coding sequence of TtPAH1 (T. thermophila PAH1), AtPAH2 (A. thaliana PAH2) and TbPAH1 (Trypanosoma brucei PAH1) were amplified using specific primers and cloned into YCplac111 (LEU) using SalI/ BamHI restriction sites. To assess nuclear membrane morphology and growth rescue, pah1Δ yeast cells (RS453 smp2Δ: ade2his3leu2trp1ura3 smp2::TRP1 were transformed with either TtPAH1 or TtPAH1mut or AtPAH2 or TbPAH1 along with PUS-GFP by standard lithium acetate protocol (Gietz and Woods, 2001). Transformants were screened on solid SD medium lacking uracil and leucine. The transformants were grown in the same media at 30°C to early log phase and analyzed by confocal microscopy. The results from three independent experiments were used for analysis of nuclear morphology.
Analysis of gene expression
Gene expression was analyzed by RT-PCR by isolating total RNA from cells grown in SD media containing adenine and histidine. The isolated RNA was used to synthesize single-stranded cDNA using Superscript II reverse transcriptase (Invitrogen). For quantitative analysis, RT-PCR was performed using the SYBR Green qPCR (Roche) in a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) following the manufacturer's instructions. All primer sequences used are listed in Table S1. The relative expression level was calculated using the comparative Ct method after normalizing to SEC 63 as a control gene.
Phosphatase assay
Phosphatidic acid phosphatase activity was measured by following the release of water-soluble Pi from chloroform-soluble PA. The standard reaction contained 50 mM Tris-HCl buffer (pH 7.5), 1 mM MgCl2, 10 mM Triton X-100, 10 mM 2-mercaptoethanol and 1 mM phosphatidic acid in a total volume of 100 μl. Reactions were initiated by the addition of recombinant proteins and carried out in triplicate at 30°C for 20 min. The reaction was terminated by adding 500 µl of 0.1 M HCl in methanol and 1 ml chloroform. To that mixture, 1 ml of water was added for phase separation, and one volume of upper phase was mixed with two volumes of Biomol Green to develop color. The absorbance was measured at 620 nm, and the amount of phosphate produced was quantified using a standard curve (Han and Carman, 2010).
Sequence analysis
Sequences of Tetrahymena PAH homologs (TTHERM_00189270 and TTHERM_00215970) were retrieved from the Tetrahymena Genome Database and domains were predicted with Interpro protein sequence analysis and classification tool (EMBL-EBI). Multiple sequence alignment was performed with PRALINE. Percent identity matrix was calculated using Clustal2.1. The sequences of PAH used in this study were S000004775 for S. cerevisiae, NM_001203528.1 for A. thaliana (AtPAH2), XM_841075 for Trypanosoma brucei, FBgn0263593 for D. melanogaster, BC030537.1 for Homo sapiens and DDB_G0271730 for Dictyostelium discoideum.
Supplementary Material
Acknowledgements
We thank Prof. Aaron Turkewitz (University of Chicago) for critical evaluation and useful comments on the manuscript; Symeon Siniossoglou (University of Cambridge) for providing pah1Δ yeast strain and PUS-GFP plasmid; and Laurie K. Read (SUNY at Buffalo) for providing Trypanosoma brucei genomic DNA.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.R.; Methodology: A.N.P., A.R.; Validation: A.N.P., A.R.; Formal analysis: A.N.P., A.R.; Investigation: A.N.P., S.S., A.R.; Resources: A.N.P., A.R.; Writing - original draft: A.N.P., S.S., A.R.; Writing - review & editing: A.N.P., S.S., A.R.; Visualization: A.N.P., S.S., A.R.; Supervision: A.R.; Project administration: A.R.; Funding acquisition: A.R.
Funding
This work was supported by the Department of Biotechnology, Ministry of Science and Technology (BT/PR14643/BRB/10/862/2010 to A.R.) and the Council of Scientific and Industrial Research (CSIR Fellowship to A.N.P.).
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.028233.supplemental
References
- Adeyo O., Horn P. J., Lee S. K., Binns D. D., Chandrahas A., Chapman K. D. and Goodman J. M. (2011). The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J. Cell Biol. 192, 1043-1055. 10.1083/jcb.201010111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. L. (2000). Isolation of micronuclear and macronuclear DNA. Methods Cell Biol. 62, 241-252. 10.1016/S0091-679X(08)61534-4 [DOI] [PubMed] [Google Scholar]
- Anderson D. J. and Hetzer M. W. (2008). Reshaping of the endoplasmic reticulum limits the rate for nuclear envelope formation. J. Cell Biol. 182, 911-924. 10.1083/jcb.200805140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmanyar S. (2015). Spatial regulation of phospholipid synthesis within the nuclear envelope domain of the endoplasmic reticulum. Nucleus 6, 102-106. 10.1080/19491034.2015.1010942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmanyar S., Biggs R., Schuh A. L., Desai A., Müller-Reichert T., Audhya A., Dixon J. E. and Oegema K. (2014). Spatial control of phospholipid flux restricts endoplasmic reticulum sheet formation to allow nuclear envelope breakdown. Genes Dev. 28, 121-126. 10.1101/gad.230599.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns D., Januszewski T., Chen Y., Hill J., Markin V. S., Zhao Y., Gilpin C., Chapman K. D., Anderson R. G. W. and Goodman J. M. (2006). An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 173, 719-731. 10.1083/jcb.200511125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenbender J., Prohaska A., Jauker F. and Hipke H., C. G. (1992). DNA elimination and its relation to quantities in the macronucleus of Tetrahymena. Dev. Genet. 13, 103-110. 10.1002/dvg.1020130203 [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Smith H. R. and Cassidy-Hanley D. (2000). Long-Term Storage. Methods Cell Biol. 62, 213-218. 10.1016/S0091-679X(08)61531-9 [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Lorenz A., Witkin K.L., Hays T., Loidl J. and Cohen-Fix O. (2006). Yeast nuclear envelope subdomains with distinct abilities to resist membrane expansion. Mol. Biol. Cell 17, 1768-1778. 10.1091/mbc.E05-09-0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman G. M. and Henry S. A. (1999). Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog. Lipid Res. 38, 361-399. 10.1016/S0163-7827(99)00010-7 [DOI] [PubMed] [Google Scholar]
- Carman G. M. and Kersting M. C. (2004). Phospholipid synthesis in yeast: regulation by phosphorylation. Biochem. Cell Biol. 82, 62-70. 10.1139/o03-064 [DOI] [PubMed] [Google Scholar]
- Carman G. M. and Zeimetz G. M. (1996). Regulation of phospholipid biosynthesis in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 271, 13293-13296. 10.1074/jbc.271.23.13293 [DOI] [PubMed] [Google Scholar]
- Bruns P. J. and Cassidy-Hanley D. (2003). Biolistic transformation of macro- and micronuclei. Methods Cell Biol. 62, 501-512. 10.1016/S0091-679X(08)61553-8 [DOI] [PubMed] [Google Scholar]
- Choi H.-S., Su W.-M., Han G.-S., Plote D., Xu Z. and Carman G. M. (2012). Pho85p-Pho80p phosphorylation of yeast pah1p phosphatidate phosphatase regulates its activity, location, abundance, and function in lipid metabolism. J. Biol. Chem. 287, 11290-11301. 10.1074/jbc.M112.346023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkor J., Sariahmetoglu M., Brindley D. N. and Reue K. (2007). Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 282, 3450-3457. 10.1074/jbc.M610745200 [DOI] [PubMed] [Google Scholar]
- Farese R. V. and Walther T. C. (2009). Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139, 855-860. 10.1016/j.cell.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck B. N., Gropler M. C., Chen Z., Leone T. C., Croce M. A., Harris T. E. Jr, J. C. L. and Kelly D. P. (2006). Lipin 1 is an inducible amplifier of the hepatic PGC-1 a / PPAR a regulatory pathway. Cell Metab. 4, 199-210. 10.1016/j.cmet.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Gaertig J., Gu L., Hai B. and Gorovsky M. A. (1994). High frequency vector-mediated transformation and gene replacement in tetrahymena. Nucleic Acids Res. 22, 5391-5398. 10.1093/nar/22.24.5391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D. and Woods R. A. (2001). Genetic transformation of yeast. BioTechniques 30, 816-831. [DOI] [PubMed] [Google Scholar]
- Golden A., Liu J. and Cohen-Fix O. (2009). Inactivation of the C. elegans lipin homolog leads to ER disorganization and to defects in the breakdown and reassembly of the nuclear envelope. J. Cell Sci. 122, 1970-1978. 10.1242/jcs.044743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorjánácz M. and Mattaj I. W. (2009). Lipin is required for efficient breakdown of the nuclear envelope in Caenorhabditis elegans. J. Cell Sci. 122, 1963-1969. 10.1242/jcs.044750 [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A. (1980). Genome organization and reorganization in Tetrahymena. Annu. Rev. Genet. 14, 203-239. 10.1146/annurev.ge.14.120180.001223 [DOI] [PubMed] [Google Scholar]
- Guerfal M., Claes K., Knittelfelder O., Rycke R. De Kohlwein S. D. and Callewaert N. (2013). Enhanced membrane protein expression by engineering increased intracellular membrane production. Microb. Cell Fact. 12, 122 10.1186/1475-2859-12-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G.-S. and Carman G.M. (2010). Characterization of the Human LPIN1 -encoded Phosphatidate Phosphatase Isoforms. J. Biol. Chem. 285, 14628-14638. 10.1074/jbc.M110.117747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G. S., Wu W. I. and Carman G. M. (2006). The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281, 9210-9218. 10.1074/jbc.M600425200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G.-S., Siniossoglou S. and Carman G. M. (2007). The cellular functions of the yeast lipin homolog Pah1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem. 282, 37026-37035. 10.1074/jbc.M705777200 [DOI] [PubMed] [Google Scholar]
- Karanasios E., Han G., Xu Z., Carman G. M. and Siniossoglou S. (2010). A phosphorylation-regulated amphipathic helix controls the membrane translocation and function of the yeast phosphatidate phosphatase. Proc. Natl. Acad. Sci. USA 107, 17539-17544. 10.1073/pnas.1007974107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns B. G., McGee T. P., Mayinger P., Gedvilaite A., Phillips S. E., Kagiwada S. and Bankaitis V. A. (1997). Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature 387, 101-105. 10.1038/387101a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Gentry M. S., Harris T. E., Wiley S. E., Lawrence J. C. and Dixon J. E. (2007). A conserved phosphatase cascade that regulates nuclear membrane biogenesis. Proc. Natl. Acad. Sci. USA 104, 6596-6601. 10.1073/pnas.0702099104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. E., Bae E., Jeong D. Y., Kim M.-J., Jin W.-J., Park S.-W., Han G.-S., Carman G. M., Koh E. and Kim K.-S. (2013). Lipin1 regulates PPARgamma transcriptional activity. Biochem. J. 453, 49-60. 10.1042/BJ20121598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenspor M., Xu P., Cohen R. D., Welch C. and Reue K. (1999). Altered gene expression pattern in the fatty liver dystrophy mouse reveals impaired insulin-mediated cytoskeleton dynamics. J. Biol. Chem. 274, 23078-23084. 10.1074/jbc.274.33.23078 [DOI] [PubMed] [Google Scholar]
- Laplante M. and Sabatini D. M. (2009). An emerging role of mTOR in lipid biosynthesis minireview. Curr. Biol. 19, R1046-R1052. 10.1016/j.cub.2009.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. and Carman G. M. (1989). Purification and characterization of phosphatidate phosphatase from Saccharomyces cerevisiae. J. Biol. Chem. 264, 8641-8645. [PubMed] [Google Scholar]
- Loewen C. J. R., Gaspar M. L., Jesch S. A. and Delon C. (2004). Phospholipid metabolism regulated by a transcription factor sensing phosphatidic acid. Science 304, 1644-1647. 10.1126/science.1096083 [DOI] [PubMed] [Google Scholar]
- McMahon H. T. and Gallop J. L. (2005). Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590-596. 10.1038/nature04396 [DOI] [PubMed] [Google Scholar]
- Mietkiewska E., Siloto R. M. P., Dewald J., Shah S., Brindley D. N. and Weselake R. J. (2011). Lipins from plants are phosphatidate phosphatases that restore lipid synthesis in a pah1 D mutant strain of Saccharomyces cerevisiae. FEBS J. 278, 764-775. 10.1111/j.1742-4658.2010.07995.x [DOI] [PubMed] [Google Scholar]
- Müller-felber W., Venkateswaran R., Ogier H., Desguerre I. and Altuzarra C. (2010). LPIN1 gene mutations: a major cause of severe rhabdomyolysis in early childhood. Hum.Mutat. 31, 1564-1573. 10.1002/humu.21282 [DOI] [PubMed] [Google Scholar]
- Murphy D. J. (2001). The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 40, 325-438. 10.1016/S0163-7827(01)00013-3 [DOI] [PubMed] [Google Scholar]
- Nadra K., Charles A.-S. d. P., Médard J. J., Hendriks W. T., Han G. S., Grès S., Carman G. M., Saulnier-Blache J. S., Verheijen M. H. G. and Chrast R. (2008). Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 22, 1647-1661. 10.1101/gad.1638008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Koizumi R., Shui G., Shimojima M., Wenk M. R. and Ito T. (2009). Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically. Proc. Natl. Acad. Sci. USA 106, 20978-20983. 10.1073/pnas.0907173106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Hamilton E. P. and Orias J. D. (2000). Tetrahymena as a laboratory organism: useful strains, cell culture, and cell line maintenance. Methods Cell Biol. 62, 189-211. 10.1016/S0091-679X(08)61530-7 [DOI] [PubMed] [Google Scholar]
- Péterfy M., Phan J., Xu P. and Reue K. (2001). Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nature Genet. 27, 121-124. 10.1038/83685 [DOI] [PubMed] [Google Scholar]
- Peterson T. R., Sengupta S. S., Harris T. E., Carmack A. E., Kang S. A., Balderas E., Guertin D. A., Madden K. L., Carpenter A. E., Finck B. N. et al. (2011). MTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408-420. 10.1016/j.cell.2011.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan J., Péterfy M. and Reue K. (2004). Lipin expression preceding peroxisome proliferator-activated receptor- γ is critical for adipogenesis in vivo and in vitro. J. Biol. Chem. 279, 29558-29564. 10.1074/jbc.M403506200 [DOI] [PubMed] [Google Scholar]
- Radulovic M., Knittelfelder O., Cristobal-Sarramian A., Kolb D., Wolinski H. and Kohlwein S. D. (2013). The emergence of lipid droplets in yeast: current status and experimental approaches. Curr. Genet. 59, 231-242. 10.1007/s00294-013-0407-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman A., Elde N. C. and Turkewitz A. P. (2008). A dynamin-related protein required for nuclear remodeling in tetrahymena. Curr. Biol. 18, 1227-1233. 10.1016/j.cub.2008.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikov I. B. (1976). Evolution of macronuclear organization. Annu. Rev. Genet. 10, 413-440. 10.1146/annurev.ge.10.120176.002213 [DOI] [PubMed] [Google Scholar]
- Reue K., Xu P., Wang X. and Slavin B. G. (2000). Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J. Lipid Res. 41, 1067-1076. [PubMed] [Google Scholar]
- Santos-Rosa H., Leung J., Grimsey N., Peak-Chew S. and Siniossoglou S. (2005). The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24, 1931-1941. 10.1038/sj.emboj.7600672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. (2002). Getting started with yeast contents. Methods Enzymol. 350, 3-41. 10.1016/S0076-6879(02)50954-X [DOI] [PubMed] [Google Scholar]
- Siniossoglou S., Santos-rosa H., Rappsilber J., Mann M. and Hurt E. (1998). A novel complex of membrane proteins required for formation of a spherical nucleus. EMBO J. 17, 6449-6464. 10.1093/emboj/17.22.6449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tange Y. (2002). An evolutionarily conserved fission yeast protein, Ned1, implicated in normal nuclear morphology and chromosome stability, interacts with Dis3, Pim1/RCC1 and an essential nucleoporin. J. Cell Sci. 115, 4375-4385. 10.1242/jcs.00135 [DOI] [PubMed] [Google Scholar]
- Tauchi-Sato K., Ozeki S., Houjou T., Taguchi R. and Fujimoto T. (2002). The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 277, 44507-44512. 10.1074/jbc.M207712200 [DOI] [PubMed] [Google Scholar]
- Thiele C. and Spandl J. (2008). Cell biology of lipid droplets. Curr. Opin. Cell Biol. 20, 378-385. 10.1016/j.ceb.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Ugrankar R., Liu Y., Provaznik J., Schmitt S. and Lehmann M. (2011). Lipin is a central regulator of adipose tissue development and function in drosophila melanogaster. Mol.Cell Biol. 31, 1646-1656. 10.1128/MCB.01335-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meer G., Voelker D. R. and Feigenson G. W. (2008). Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112-124. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. J., Hirschs J. P. and Henry A. (1991). The OPIl gene of Saccharomyces cerevisiae, a negative regulator of phospholipid biosynthesis, encodes a protein containing polyglutamine tracts and a Leucine Zipper. J. Biol. Chem. 266, 863-872. [PubMed] [Google Scholar]
- Witkin K. L. and Collins K. (2004). Holoenzyme proteins required for the physiological assembly and activity of telomerase. Genes Dev. 18, 1107-1118. 10.1101/gad.1201704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P. and Reue K. (2017). Lipin proteins and glycerolipid metabolism : Roles at the ER membrane and beyond. Biochim. Biophys. Acta 1859, 1583-1595. 10.1016/j.bbamem.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.