Abstract
Transanal total mesorectal excision (taTME) has evolved over the past decade fueled by advances in minimally invasive surgery. The technique aims to overcome the constraints posed by a narrow rigid pelvis and poor TME visualization that are encountered during “top-down” rectal surgery. A more accurate pelvic dissection should subsequently result in safer oncological resections and better preservation of pelvic autonomic nerves. taTME is an advanced complex technique that requires dedicated training and experience in TME surgery. Initial results from small cohorts are promising and confirmation by randomized controlled trials is eagerly awaited.
Keywords: transanal, bottom-up, total mesorectal excision, rectal cancer
Colorectal cancer remains the third most common cancer worldwide, predominantly occurring in developed countries and accounting for more than 9% of all cancer incidences. 1 The management of rectal cancer has undergone significant changes over the last few decades with increased use of neoadjuvant chemoradiotherapy and the adoption of less invasive surgical approaches. The gold standard operative treatment of rectal cancer surgery still remains total mesorectal excision (TME), which has been shown to optimize locoregional clearance by reducing positive circumferential resection margins (CRMs). 2 3 Traditionally, TME was completed via an open anterior abdominal approach. However, advances in technology and surgical innovation have led to the introduction of minimally invasive techniques including laparoscopic TME, robotic TME, and, recently, transanal TME (taTME). In particular, the widespread interest in taTME has been fueled by the unique set of surgical challenges that rectal cancer surgery poses; in particular, in obese, male patients with a narrow pelvis and in any patient with a bulky mid to distal rectal cancer.
A variety of acronyms have been used for the transanal approach including taTME, transanal minimally invasive surgery (TAMIS) TME, and “bottom-up” TME. TaTME has in fact been inspired and evolved from a combination of other techniques, namely, transanal endoscopic microsurgery (TEM), 4 transanal transabdominal approach, 5 natural orifice transluminal endoscopic surgery, 6 7 and TAMIS. 8 Extensive preliminary work in animal laboratories 9 10 and on human cadavers 7 11 was performed to demonstrate feasibility of the concept and establish the critical steps of the operation. In 2010, the first human clinical case was reported by Sylla et al, 12 with a subsequent rapid rise in the adoption of taTME.
This article will explore the evolution and rationale for taTME ( why ), when and who should undertake this approach, and how the operation is performed.
Transanal Total Mesorectal Excision: Why?
Laparoscopic surgery has been associated with several advantages over open abdominal surgery including less postoperative pain, fewer wound infections, and shorter hospital stay. 13 However, two recent randomized controlled trials (RCTs), ACOSOG Z6051 14 and ALaCaRT, 15 could not demonstrate noninferiority of laparoscopic TME over open TME for histopathological outcomes and morbidity. The positive CRM rate for laparoscopic versus open TME surgery in these trials was 7 to 12.1 versus 3 to 7.7%, respectively. Previous RCTs for rectal cancer surgery 16 17 also found similar positive CRM rates of 16 and 10% overall; even reaching up to 22% for low rectal cancers in the open group of the COLOR II study.
Furthermore, high conversion rates from laparoscopic to open surgery have been reported in the COLOR II 17 and ACOSOG Z6051 14 trials, 16 and 11·3%, respectively. The introduction of the robot for TME surgery does not appear to significantly improve these results either. Recent findings from the RObotic versus LAparoscopic Resection for Rectal Cancer) trial 18 still demonstrate a conversion rate of 8.1% in the robotic arm (odds ratio [OR]: 0.61, confidence interval [CI]: 0.31–1.21, p = 0.158). Although conversion rates tended to be lower in the robotic arm compared with the laparoscopic group on subgroup analysis of certain risk groups including males (8.7 vs. 16.0%, OR: 0.46, CI: 0.21–0.99), obese (18.9 vs. 27.8%, OR: 0.58, CI: 0.21–1.60), and low tumors (7.2 vs. 13.3%, OR: 0.49, CI: 0.21–1.12), rates still reached as high as 18.9% for the obese and never lower than 7%.
Studies have identified patient and tumor-related risk factors that predict intraoperative difficulty and potentially can lead to a poor oncological specimen. 19 20 Such factors include male gender, high body mass index (BMI), visceral obesity, a narrow pelvis, bulky tumors, and advanced T stage. These features present technical challenges during both laparoscopic and open surgeries, due to poor exposure of the mesorectal plane and difficulty introducing instruments down a narrow space with a fixed bony pelvis, which can subsequently lead to inaccurate dissection and uncertain margins.
The transanal approach to pelvic dissection was pioneered to overcome these inherent shortcomings of abdominal (“top-down”) dissection. This “bottom-up” approach offers a clearer visualization of the dissection plane even in a narrow pelvis and avoids excessive manipulation of the specimen to obtain exposure, thus allowing a more precise and trauma-free dissection. This in turn will facilitate better oncological resections as well as preserving the pelvic autonomic nerves with potentially improved bowel, urinary, and sexual function.
A recent systematic review including 36 studies on taTME, with a total of 510 patients has shown promising results. 21 The overall morbidity and mortality rates were comparable to those for laparoscopic TME surgery at 35 and 0.2%, respectively. The positive CRM rate was 5% and distal resection margin (DRM) rate only 0.3%, while a good TME specimen without major defects was obtained in 94%. Twelve conversions to open were reported. The full potential of taTME will hopefully be elucidated in RCTs and future larger scale national studies.
Transanal Total Mesorectal Excision: When?
This question can refer to both the patient (i.e., patient selection) and the surgeon (i.e., surgeon education and training; when is a surgeon ready to perform taTME?).
Patient Selection
The second international taTME conference held in Paris in July 2014 brought together surgeons with experience and expertise in taTME surgery. 22 They discussed the current status and development of the technique and formed consensus statements including guidance on patient selection and indications for surgery. The consensus reached was that taTME can be utilized for both benign and malignant conditions where accurate dissection of the distal and mid-rectum is required. Patient-related and tumor-related factors that can benefit from a taTME approach include: (1) male gender, (2) narrow and/or deep pelvis, (3) visceral obesity and/or a BMI > 30 kg/m 2 , (4) prostatic hypertrophy, (5) a tumor height of less than 12 cm from the anal verge, (6) tumor diameter > 4 cm, (7) distorted tissue planes due to neoadjuvant radiotherapy, and (8) impalpable, low primary tumor requiring accurate placement of the distal resection margin.
Benign conditions that may be preferably approached using taTME include inflammatory bowel disease requiring proctectomy, rectal strictures, complex fistulae, fecal incontinence, familial adenomatous polyposis, radiation proctitis, and completion proctectomy. Failure to progress during traditional abdominal surgery, whereby the only alternative would be an abdominoperineal excision, can also be an indication for conversion to the transanal approach. The expert group agreed that contraindications to taTME should include obstructing rectal tumors, T4 tumors, and patients requiring rectal surgery in an emergency setting.
Different opinions on patient selection for a taTME approach do however exist among practicing surgeons, as demonstrated in a recent systematic review of published taTME series and comparative studies. 23 From a total of 20 included articles, 8 studies only selected patients for taTME who had low rectal tumors of < 5 cm from the anal verge, while another 3 studies accepted a tumor height of up to 12 cm. T4 tumors were included in most studies, while one center only allocated anteriorly located tumors to the transanal approach and another three specified a pubococcygeal diameter of < 10 cm in their inclusion criteria. This variation is likely to be secondary to surgeon choice and experience, available local resources, as well as familiarity of the whole multidisciplinary team with taTME. The indications for a new technique also tend to be much broader at the start of its adoption and then later refined as the cases that benefit the most from the technique are identified.
Further studies are exploring alternative parameters that could guide patient selection for taTME, such as the use of computed tomography and magnetic resonance imaging pelvimetry measurements. 24 Therefore, with increasing evidence and experience, the selection criteria for taTME are likely to be updated in the future.
Surgeon Education and Training
The introduction of any new surgical technique must occur in a safe and monitored manner to avoid unacceptable patient harm. TaTME is a complex minimally invasive technique that requires advanced surgical skills as well as the knowledge and experience of recognizing anatomical planes, structures from a very different viewpoint. Although the learning curve has yet to be established, there is a consensus among experienced taTME surgeons and outlined in national guidelines that a minimal pre-requisite experience with various aspects of rectal cancer surgery (i.e., laparoscopic TME surgery, TEM/TAMIS, and intersphincteric approaches to low rectal cancer) and training are necessary before any surgeon undertakes this procedure in patients. 22 25 An international taTME educational collaborative has recently been formed and aims to establish the essential elements for an optimum taTME training curriculum, provide guidance on the implementation and assessment of the training program while also creating an international network with shared communication that will drive the educational standard of taTME. 26 The structure of the proposed curriculum is similar to the robotic surgery training pathway and involves online learning modules, dry laboratory purse-string practice, cadaveric courses, and proctored live cases.
McLemore et al 27 described the training pathway followed by pioneers in the taTME technique and the process of implementation into clinical practice. The article highlights the importance of previous experience in transanal endoscopic and minimally invasive TME surgery prior to undertaking preclinical transanal training. A cadaveric course, not only for the surgeon, but for the entire operative team is a fundamental part of training before starting live cases. Proctoring of the initial clinical cases is also advised to offer guidance and further training, as well as preventing unwanted intraoperative adverse events at the beginning of a surgeon's learning curve. The final recommendation encourages participation in a clinical registry with publication of outcomes. The first international taTME registry 28 was launched in July 2014 and captures data from more than 29 different countries worldwide. Registry results on the first 720 registered patients are due to be published soon.
Transanal Total Mesorectal Excision: How?
TaTME is currently most commonly performed as a hybrid approach with an abdominal (robotic, laparoscopic, or open) and perineal phases. These phases can either occur simultaneously with separate abdominal and perineal teams or consecutively by one operative team, starting either transabdominally or transanally. Entire transanal mobilization of the complete left colon with high ligation of the mesentery and complete takedown of the splenic flexure has been reported; 29 however, the hybrid approach is more routinely performed in current practice. Although taTME has been applied to benign cases, the technique was primarily pioneered for the meticulous excision of low rectal tumors. The following section will therefore focus on the steps involved in the resection of a low rectal cancer.
Initial transanal dissection will vary depending on the location of the tumor and the operation to be performed, that is, TME, partial mesorectal excision, abdominoperineal excision, or intersphincteric resection. For tumors encroaching on the anorectal junction (< 1.5 cm), partial intersphincteric open dissection is performed prior to luminal occlusion with a purse string when the level of the pelvic floor is reached. 22 In more proximal tumors with > 1.5 cm distal clearance from the anorectal junction, a circumferential rectal purse string is placed ensuring a safe distal margin. In general, the procedure can be divided into the following five key steps ( Fig. 1 ): (1) distal purse-string placement, (2) full-thickness rectotomy, (3) TME dissection, (4) specimen extraction, and (5) anastomosis.
Fig. 1.
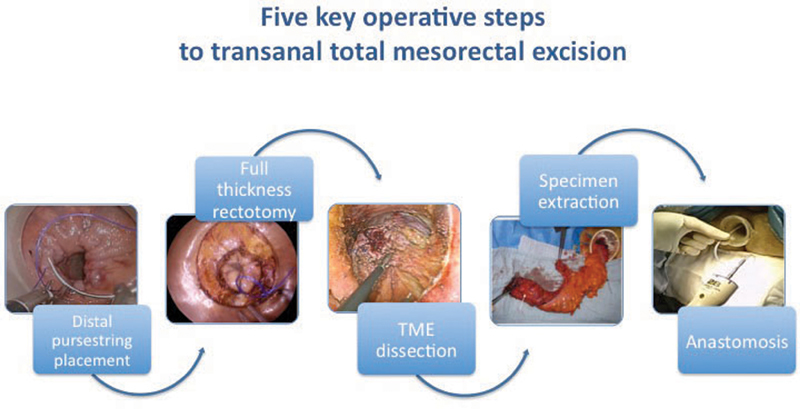
Five key operative steps to transanal total mesorectal excision.
Distal Purse-String Placement
A purse-string suture can be placed under direct vision or endoscopically through a transanal platform using a 2/0 or 0 monofilament suture. The purse string is placed > 1 cm distal to the tumor, thus ensuring a free distal resection margin. Small equal bites starting at 7 o'clock and continuing the purse string circumferentially without spirally up or down the lumen will occlude the lumen without leaving defects. It is essential to secure a tight seal with the purse string to avoid spillage of stool during the operation and excessive colonic insufflation. Generous washout using a tumoricidal solution helps prevent implantation of exfoliated tumor cells and bacterial contamination of the operative field. With the transanal platform in place a “pneumorectum” is created with an initial pressure of 8 to 10 mm Hg and standard laparoscopic instruments are used with either a 0-, 30-, or 45-degree high definition or 0-degree three-dimensional laparoscopy. Off-setting with different lengths the operating instruments to the scope, usually by using a longer scope, reduces clashing of instruments and unnecessary disruptions.
Full-Thickness Rectotomy
The insufflation pressure is increased to ∼10 to 15 mm Hg to allow for adequate rectal wall tension as well as facilitate the rectotomy. More modern insufflation systems, such as the AirSeal System (SurgiQuest Inc., Milford, CT) are preferred to conventional systems as they are able to evacuate smoke effectively and maintain a constant pressure without bellowing. 30
The mucosa is scored using monopolar diathermy at the extremities of the radial folds created by the purse string to outline the circle of dissection for the rectotomy. For right-handed surgeons, the rectotomy is more easily initiated in the posterior quadrant from a right to left direction. A circumferential full-thickness dissection through the muscular rectal wall and into the mesorectal plane must be ensured prior to proceeding with the TME dissection. Care must be taken to obtain a truly tangential rectotomy, as an intramural dissection can easily occur early in the learning curve. In very distal tumors, this can create a positive margin, or the rectal tube can be perforated if the intramural dissection is not recognized by the surgeon.
Total Mesorectal Excision Dissection
Dissection of the mesorectal “holy” plane should be initiated posteriorly at the 5 or 7 o'clock position and then joined in the middle. This initially avoids the fibrotic raphe found in the midline posteriorly, which makes identification of the TME plane at the start of dissection more difficult. Care should be taken to enter the avascular presacral plane between the parietal endopelvic fascia and mesorectal envelope, which is identified by the “angel hair” seen when enough traction is applied and insufflation gas enters the tissue planes. Dissecting too posteriorly significantly increases the risk of bleeding from presacral veins. Conversely, by not acknowledging the steep sacral angle and dissecting following a more horizontal line from the anus runs the risk of creating defects in the mesorectum, or even a close rectal dissection creating an incomplete TME specimen ( Fig. 2 ).
Fig. 2.
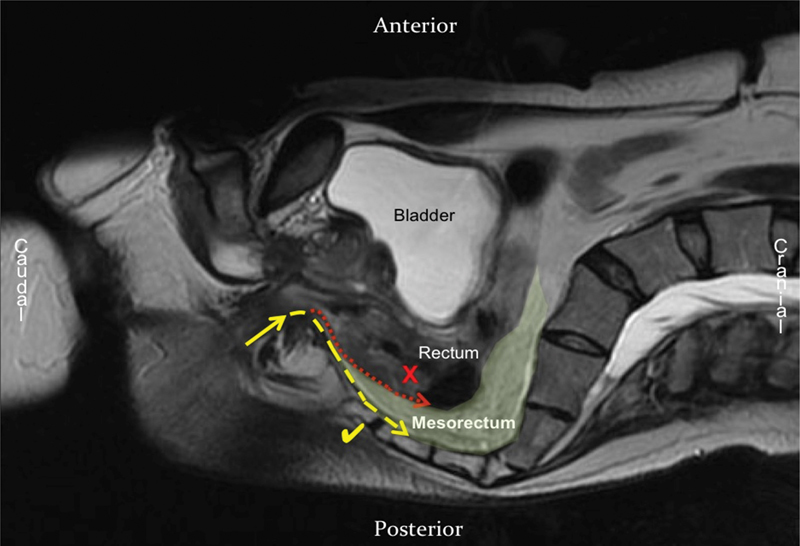
Sagittal T2-weighted MRI through the pelvis showing the correct plane of dissection (dashed line) that follows an acute angle backward along the sacral curvature. The dotted line shows an incorrect plan for TME surgery that would result in the mesorectum not being excised. MRI, magnetic resonance imaging; TME, total mesorectal excision.
Anterior dissection should be attempted next with identification of the lower border of the prostate. A plane either side of Denonvilliers' fascia can be selected depending on the position of the tumor. The membranous urethra in males is vulnerable to injury if dissection is too wide anteriorly with three cases reported in a recent systematic review. 21 Cylindrical or “sleeve-like” dissection should progress cephalad leaving the lateral pillars last, when the dissection plane becomes clearer thanks to its identification anteriorly and posteriorly. This “posterior–anterior–lateral” sequence helps prevent one of the commonest intraoperative dissecting errors; dissecting too widely on the pelvic sidewall and thus increasing the risk of injury to the lateral neurovascular bundles and pelvic sidewall vessels. This mistake is easily made as medial traction on the specimen pulls the sidewall structures inward and leads the surgeon into the wrong (too lateral) plane. Connection between the abdominal and perineal teams should only occur once the anterior and posterior dissections are almost complete, as early connection will lead to a less stable pneumoperitoneum and obscuring the bottom teams' view by fluid draining into the pelvis. The two teams will however be able to work together providing traction and counter-traction guiding each other with better views for dissection.
Specimen Extraction
Once TME dissection is completed and the rectal tube is fully mobilized, the specimen can be extracted either transanally or transabdominally. Typically, abdominal extraction occurs through a Pfannenstiel, midline, or umbilical incision, or at the site of a previous or future stoma. Extraction must be performed cautiously without exerting too much tension on the specimen to avoid tearing and perforation of the rectal tube with subsequent seeding of tumor cells and stool contamination. Therefore, in our opinion, the specimen should preferably be extracted through an auxiliary abdominal incision.
Anastomosis
Following extraction of the specimen, an open distal rectal stump will continue. The anastomosis can either be performed using a hand-sewn coloanal or stapled colorectal/coloanal technique. 31 Three stapling approaches have been described so far using either an EEA Hemorrhoid Stapler (AutoSuture; Covidien, Dublin, Ireland), 32 a standard diameter circular stapler either in combination with a guiding 10Fr redivac drain 33 or a pull through method. 31 The hand-sewn anastomosis appears to be more suitable for very low coloanal anastomoses, when there is insufficient stump length to place a purse string and stapler. Stapled anastomosis requires a double purse string: one on the open distal rectal stump and the second on the proximal colon or small bowel. Each technique offers unique advantages and disadvantages and may be more suited for anastomoses at different heights from the anal verge and customized to the patient characteristics, as outlined in Table 1 . However, the key principles for the formation of an optimal anastomosis are healthy, well-vascularized ends with a tension-free anastomosis.
Table 1. Comparison of hand-sewn and stapling techniques for coloanal and colorectal anastomoses post Transanal Total Mesorectal Excision, including suggested cutoff distances of tumor from anorectal junction to determine the anastomotic technique.
| Anastomotic technique | Tumor distance from anorectal junction (cm) | Advantages | Disadvantages |
|---|---|---|---|
| Hand-sewn coloanal | Coloanal | ▪ Suitable for coloanal and low colorectal anastomoses ▪ Suture placement and depth of suture controlled by surgeon under direct vision ▪ Avoids the difficult step of placing a rectal purse string |
▪ Difficult anastomosis if a long rectal stump due to: − Inadequate visual exposure − Too far to reach with “open” instruments ▪ Potentially worse functional outcomes compared with colorectal anastomoses |
| Stapled—EEA Hemorrhoid Stapler 33 mm | > 4 or wide colon/pelvis | ▪ Long central rod allows passage through the anal canal and attachment to the spindle prior to purse string closure ▪ Good for long rectal stumps |
▪ Large 33 mm stapler diameter posing a risk to adjacent structures, such as anal sphincters and vagina ▪ Needs sufficient rectal stump length to form the rectal purse string |
| Abdominal Double purse string stapled—28 or 31 mm CEEA stapler |
3–4 | ▪ Smaller stapler diameter posing less risk to adjacent structures ▪ Precise placement of the anvil through the center of the purse string under direct vision ▪ Abdominal conventional anvil-stapling device attachment |
▪ Needs sufficient rectal stump length to form the rectal purse string ▪ May be difficult to connect the anvil to the spindle laparoscopically in an obese narrow pelvis with poor visualization |
| Transanal Double purse string stapled—28 or 31 mm CEEA stapler |
2–3 | ▪ Smaller stapler diameter posing less risk to adjacent structures ▪ Precise placement of the anvil through the center of the purse string under direct vision ▪ Transanal stapling technique for low anastomoses |
▪ Can be used only for low anastomoses. Good transanal exposure is essential and therefore not suitable for heights above 4 cm. For higher anastomoses, the two other techniques are preferred |
Source : Reproduced and modified with permission from Penna et al. 31
Ongoing modifications and advances to taTME are being developed, including robotic taTME 34 35 36 and stereotactic navigation. 37 38 These approaches have the potential to further improve the precision of pelvic dissection, thus ensuring better oncological resections and preserving functional outcomes. Future studies in this field are eagerly awaited.
In conclusion, an incredible evolution in rectal surgery has occurred over the last few decades with increasing focus and interest on the transanal approach. Initial experience and reports on taTME appear very promising with acceptable morbidity rates and excellent TME specimen quality and clear margins. The upcoming COLOR III trial, 39 comparing laparoscopic versus transanal TME for rectal cancer, will hopefully provide more conclusive evidence of the true risks and benefits of this novel technique.
Footnotes
Disclosure None.
References
- 1.World Cancer Research Fund and American Institute for Cancer Research Food.Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective Washington, DC: American Institute for Cancer Research; 2007 [Google Scholar]
- 2.Heald R J. A new approach to rectal cancer. Br J Hosp Med. 1979;22(03):277–281. [PubMed] [Google Scholar]
- 3.Heald R J, Moran B J, Ryall R D, Sexton R, MacFarlane J K. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133(08):894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 4.Buess G, Theiss R, Hutterer F et al. Transanal endoscopic surgery of the rectum - testing a new method in animal experiments [in German] Leber Magen Darm. 1983;13(02):73–77. [PubMed] [Google Scholar]
- 5.Marks J H, Frenkel J L, D'Andrea A P, Greenleaf C E.Maximizing rectal cancer results: TEM and TATA techniques to expand sphincter preservation Surg Oncol Clin N Am 20112003501–520., viii–ix [DOI] [PubMed] [Google Scholar]
- 6.Rattner D, Kalloo A; ASGE/SAGES Working Group.ASGE/SAGES Working Group on natural orifice translumenal endoscopic surgery. October 2005 Surg Endosc 20062002329–333. [DOI] [PubMed] [Google Scholar]
- 7.Whiteford M H, Denk P M, Swanström L L. Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc. 2007;21(10):1870–1874. doi: 10.1007/s00464-007-9552-x. [DOI] [PubMed] [Google Scholar]
- 8.Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24(09):2200–2205. doi: 10.1007/s00464-010-0927-z. [DOI] [PubMed] [Google Scholar]
- 9.Sylla P, Willingham F F, Sohn D K, Gee D, Brugge W R, Rattner D W. NOTES rectosigmoid resection using transanal endoscopic microsurgery (TEM) with transgastric endoscopic assistance: a pilot study in swine. J Gastrointest Surg. 2008;12(10):1717–1723. doi: 10.1007/s11605-008-0637-1. [DOI] [PubMed] [Google Scholar]
- 10.Trunzo J A, Delaney C P. Natural orifice proctectomy using a transanal endoscopic microsurgical technique in a porcine model. Surg Innov. 2010;17(01):48–52. doi: 10.1177/1553350609359516. [DOI] [PubMed] [Google Scholar]
- 11.Telem D A, Han K S, Kim M C et al. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc. 2013;27(01):74–80. doi: 10.1007/s00464-012-2409-y. [DOI] [PubMed] [Google Scholar]
- 12.Sylla P, Rattner D W, Delgado S, Lacy A M. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24(05):1205–1210. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 13.Breukink S, Pierie J, Wiggers T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2006;(04):CD005200. doi: 10.1002/14651858.CD005200.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Fleshman J, Branda M, Sargent D J et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson A R, Solomon M J, Lumley J W et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363. doi: 10.1001/jama.2015.12009. [DOI] [PubMed] [Google Scholar]
- 16.Jayne D G, Guillou P J, Thorpe H et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25(21):3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 17.van der Pas M H, Haglind E, Cuesta M A et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(03):210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 18.RObotic Versus LAparoscopic Resection for Rectal Cancer (ROLARR) trial. (ClinicalTrials.gov ID: NCT01736072). Available at:https://www.fascrs.org/video/robotic-vs-laparoscopic-resection-rectal-cancer-rolarr-trial. Accessed January 4,2016
- 19.Targarona E M, Balague C, Pernas J C et al. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg. 2008;247(04):642–649. doi: 10.1097/SLA.0b013e3181612c6a. [DOI] [PubMed] [Google Scholar]
- 20.Oh S J, Shin J Y. Risk factors of circumferential resection margin involvement in the patients with extraperitoneal rectal cancer. J Korean Surg Soc. 2012;82(03):165–171. doi: 10.4174/jkss.2012.82.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simillis C, Hompes R, Penna M, Rasheed S, Tekkis P P. A systematic review of transanal total mesorectal excision: is this the future of rectal cancer surgery? Colorectal Dis. 2016;18(01):19–36. doi: 10.1111/codi.13151. [DOI] [PubMed] [Google Scholar]
- 22.Motson R W, Whiteford M H, Hompes R, Albert M, Miles W F; Expert Group.Current status of trans-anal total mesorectal excision (TaTME) following the Second International Consensus Conference Colorectal Dis 2016180113–18. [DOI] [PubMed] [Google Scholar]
- 23.Wolthuis A M, Bislenghi G, de Buck van Overstraeten A, D'Hoore A. Transanal total mesorectal excision: towards standardization of technique. World J Gastroenterol. 2015;21(44):12686–12695. doi: 10.3748/wjg.v21.i44.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferko A, Malý O, Örhalmi J, Dolejš J. CT/MRI pelvimetry as a useful tool when selecting patients with rectal cancer for transanal total mesorectal excision. Surg Endosc. 2016;30(03):1164–1171. doi: 10.1007/s00464-015-4324-5. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence.Transanal total mesorectal excision of the rectum. Interventional Procedure GuidanceMarch 2015. Available at:nice.org.uk/guidance/ipg514
- 26.Penna M, Hompes R, Mackenzie H, Carter F, Francis N K. First international training and assessment consensus workshop on transanal total mesorectal excision (taTME) Tech Coloproctol. 2016;20(06):343–352. doi: 10.1007/s10151-016-1454-2. [DOI] [PubMed] [Google Scholar]
- 27.McLemore E C, Harnsberger C R, Broderick R C. Transanal total mesorectal excision (taTME) for rectal cancer: a training pathway. Surg Endosc. 2016;30(09):4130–4135. doi: 10.1007/s00464-015-4680-1. [DOI] [PubMed] [Google Scholar]
- 28.Hompes R, Arnold S, Warusavitarne J. Towards the safe introduction of transanal total mesorectal excision: the role of a clinical registry. Colorectal Dis. 2014;16(07):498–501. doi: 10.1111/codi.12661. [DOI] [PubMed] [Google Scholar]
- 29.Leroy J, Barry B D, Melani A, Mutter D, Marescaux J.No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery JAMA Surg 201314803226–230., discussion 231 [DOI] [PubMed] [Google Scholar]
- 30.Nicholson G, Knol J, Houben B, Cunningham C, Ashraf S, Hompes R. Optimal dissection for transanal total mesorectal excision using modified CO2 insufflation and smoke extraction. Colorectal Dis. 2015;17(11):O265–O267. doi: 10.1111/codi.13074. [DOI] [PubMed] [Google Scholar]
- 31.Penna M, Knol J J, Tuynman J B, Tekkis P P, Mortensen N J, Hompes R. Four anastomotic techniques following transanal total mesorectal excision (TaTME) Tech Coloproctol. 2016;20(03):185–191. doi: 10.1007/s10151-015-1414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knol J J, D'Hondt M, Souverijns G, Heald B, Vangertruyden G. Transanal endoscopic total mesorectal excision: technical aspects of approaching the mesorectal plane from below--a preliminary report. Tech Coloproctol. 2015;19(04):221–229. doi: 10.1007/s10151-015-1275-8. [DOI] [PubMed] [Google Scholar]
- 33.Bracey E, Knol J, Buchs N et al. Technique for a stapled anastomosis following transanal total mesorectal excision for rectal cancer. Colorectal Dis. 2015;17(10):O208–O212. doi: 10.1111/codi.13075. [DOI] [PubMed] [Google Scholar]
- 34.Atallah S, Nassif G, Polavarapu H et al. Robotic-assisted transanal surgery for total mesorectal excision (RATS-TME): a description of a novel surgical approach with video demonstration. Tech Coloproctol. 2013;17(04):441–447. doi: 10.1007/s10151-013-1039-2. [DOI] [PubMed] [Google Scholar]
- 35.Verheijen P M, Consten E C, Broeders I A. Robotic transanal total mesorectal excision for rectal cancer: experience with a first case. Int J Med Robot. 2014;10(04):423–426. doi: 10.1002/rcs.1594. [DOI] [PubMed] [Google Scholar]
- 36.Gómez Ruiz M, Parra I M, Palazuelos C M et al. Robotic-assisted laparoscopic transanal total mesorectal excision for rectal cancer: a prospective pilot study. Dis Colon Rectum. 2015;58(01):145–153. doi: 10.1097/DCR.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 37.Atallah S, Nassif G, Larach S. Stereotactic navigation for TAMIS-TME: opening the gateway to frameless, image-guided abdominal and pelvic surgery. Surg Endosc. 2015;29(01):207–211. doi: 10.1007/s00464-014-3655-y. [DOI] [PubMed] [Google Scholar]
- 38.Buchs N C, Hompes R. Stereotactic navigation and augmented reality for transanal total mesorectal excision? Colorectal Dis. 2015;17(09):825–827. doi: 10.1111/codi.13058. [DOI] [PubMed] [Google Scholar]
- 39.Deijen C L, Velthuis S, Tsai A et al. COLOR III: a multicentre randomized clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30(08):3210–3215. doi: 10.1007/s00464-015-4615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


