Abstract
In recent years, organ preservation has been considered a feasible alternative to total mesorectal excision for patients with locally advanced rectal cancer with a clinical complete response to neoadjuvant therapy. However, the degree of tumor response to neoadjuvant therapy is variable. A fraction of the patients who did not achieve a complete response had grossly visible tumors. These patients, with clearly incomplete clinical response, need a total mesorectal excision. In addition, some patients with a significant tumor response still have some abnormalities in the bowel wall, such as superficial ulceration or tissue nodularity, which, while not conclusive for the presence of a tumor, are indicative of the possibility of a residual tumor in the bowel wall or in mesorectal lymph nodes. The management of patients with a so-called near-complete clinical response to neoadjuvant therapy is controversial. In this article, we will review the clinical and radiological criteria that define a clinical response to neoadjuvant therapy, possible treatment strategies, and follow-up protocols. We will also discuss patient and tumor characteristics that in our opinion can be useful in selecting the most appropriate treatment alternative. Although organ preservation and quality of life are important, the primary goal of treatment for these patients should be local tumor control and long-term survival.
Keywords: rectal cancer, neoadjuvant chemoradiation, local excision, near-complete clinical response
Background
Rectal cancer treatment has evolved significantly in the past 15 years. The standard treatment for patients with locally advanced rectal cancer (LARC) includes fluoropyrimidine-based neoadjuvant chemoradiation therapy (CRT) followed by total mesorectal excision (TME) and postoperative systemic chemotherapy (CT), most often 4 months of modified FOLFOX6 (intravenous oxaliplatin, leucovorin and 5-FU; continuous infusion for 6 months) or CAPOX (intravenous oxaliplatin and oral capecitabine for a total of 6 months). The most recent version of the National Comprehensive Cancer Network (NCCN) guidelines also considers acceptable moving the adjuvant systemic CT to the neoadjuvant setting, before the start of CRT. 1 In this new treatment algorithm, the patient receives 4 months of CT, followed by fluoropyrimidine-based CRT and TME. This new approach is known as total neoadjuvant therapy, indicating that all local and systemic adjuvant therapy is delivered before surgery. The rationale for delivering traditional adjuvant CT in the neoadjuvant setting was to increase compliance and the efficacy of systemic CT in patients with potentially occult micrometastatic disease. What has been observed is that these total neoadjuvant protocols enhance tumor regression and might increase the proportion of patients who achieve a complete or near-complete clinical response (NCCR).
Rectal cancer response to neoadjuvant therapy is variable. In some patients, pathologists are unable to find cancer cells in the TME specimen. This select group of patients with a pathological complete response (pCR) have very low recurrence rates and excellent survival compared with those in which the tumor persists, 2 and some authors suggest alternative approaches to radical surgery, including local excision (LE) or active close surveillance protocols (“watch and wait,” “wait and see,” or “nonoperative management, NOM”), for these patients. 3 4 5 The challenge then is to differentiate between patients with a true pCR and those with tumor persistence, either in the rectal wall or mesorectal lymph nodes. A group of international experts agree on a set of morphological criteria that were best associated with a pCR: a white scar with telangiectasia and a normal digital rectal examination (DRE) 6 ( Table 1 ). Tumors meeting these criteria were considered to have achieved a complete clinical response (cCR). Unfortunately, more recent reports have found these criteria too restrictive. According to at least two retrospective case series, many patients with a cCR were found to have a pCR, but many patients not meeting those criteria were also found to have a pCR. 7 8 Although clinical assessment tends to underestimate tumor response, clinicians are concerned about the possibility of tumor persistence in or behind the scar in the rectal wall. 9 Computed tomography, endorectal ultrasound, and fluorine-18 fluorodeoxyglucose positron emission tomography provide an estimate of tumor regression but are not sensitive enough to identify pCR. 10 Conventional magnetic resonance imaging (MRI) morphological sequences (e.g., T2- and T1-weighted images) cannot differentiate residual tumor from surrounding fibrosis, but diffusion-weighted MRI sequences may improve the diagnostic performance of morphological MRI sequences for differentiating pCR from residual tumor. 11 The definition of response undoubtedly influences clinical outcomes: a strict definition reduces the proportion of eligible patients but increases the chance of success with NOM, whereas less strict criteria increase not only the number of eligible patients but also the risk of local tumor regrowth and distant metastasis. With all of this in mind, our institution has developed a new set of criteria categorizing response in a three-tiered system that is currently being tested in a prospective clinical trial ( Table 2 ). 12
Table 1. Complete clinical response: characterization of clinical and endoscopic findings by international consensus of experts 6 .
| Compatible | Noncompatible |
|---|---|
| White scar | Residual ulcer |
| Telangiectasia | Nodule at DRE (even in the presence of mucosal integrity) |
| Loss of pliability at insufflation | Stenosis/stricture |
Abbreviation: DRE, digital rectal examination.
Table 2. Three-tier system of response assessment developed for the OPRA trial.
| Complete response | Near-complete response | Incomplete response | |
|---|---|---|---|
| Endoscopy | • Flat, white scar • Telangiectasia • No ulcer • No nodularity |
• Irregular mucosa • Small mucosal nodules or minor mucosal abnormality • Superficial ulceration • Mild persisting erythema of the scar |
• Visible tumor |
| Digital rectal examination | • Normal | • Smooth induration or minor mucosal abnormalities | • Palpable tumor nodules |
| T2W-MRI | • Normal appearing bowel wall without any fibrosis in the tumor bed • Only dark T2 signal, no intermediate T2 signal • No visible lymph nodes or very few, small (<5 mm) nodes |
• Mostly dark T2 signal, some remaining intermediate signal and / or • Partial regression of lymph nodes |
• More intermediate than dark T2 signal, no T2 scar and / or • No regression of lymph nodes |
| DW-MRI | • No visible signal on B800–B1000 and / or • Uniform, linear signal in the wall above the tumor is acceptable |
• Significant regression of signal on B800–B1000 | • Insignificant regression of signal on B800–B1000 |
Abbreviations: DW-MRI, diffusion-weighted magnetic resonance imaging; OPRA, organ preservation in rectal adenocarcinoma; T2W-MRI, T2-weighted magnetic resonance imaging.
On one end of the spectrum are patients who have clinical complete response and therefore may qualify for “watch and wait.” On the other end are those with incomplete response characterized by grossly visible tumor and in whom TME would be the treatment of choice. However, in the middle, there is a select group of patients for whom tumors present significant regression, but not enough to meet the criteria of a clinical complete response. These are the patients known to have an NCCR ( Figs. 1 and 2 ).
Fig. 1.
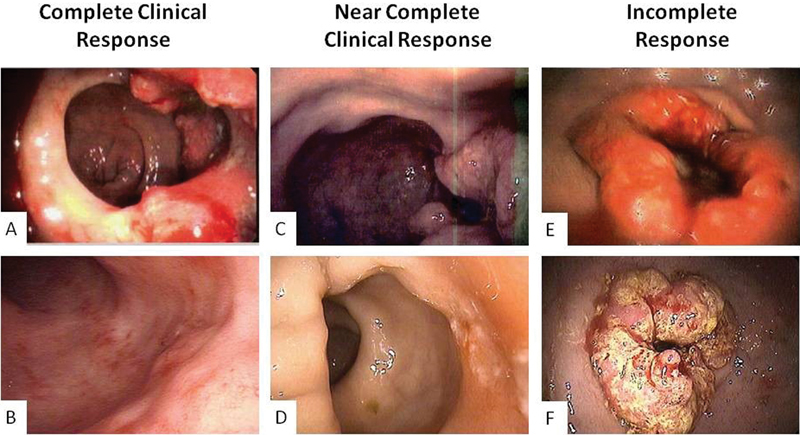
Endoscopic images at presentation and after neoadjuvant treatment completion. ( A, B ) Complete clinical response. ( C, D ) Near-complete response. ( E, F ) Incomplete response.
Fig. 2.
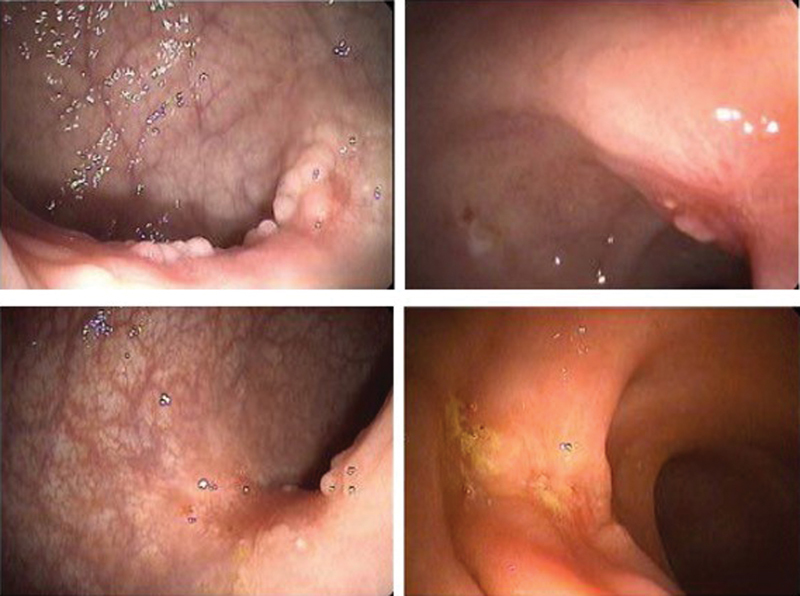
Endoscopic findings compatible with near-complete clinical response.
Management of Patients with an NCCR to Neoadjuvant Therapy
The compiled evidence from the retrospective series published so far suggests that almost 25% of patients with a clinical complete response entered in a watch-and-wait protocol develop tumors regrowth in the first 2 years after completion of neoadjuvant therapy. 13 It is expected that the rate of regrowth may be even higher for patients with an NCCR. For these patients, TME may be the most prudent approach for maximizing oncological outcomes. However, a significant proportion of these patients will have a pCR and therefore will not have any oncological benefit from an operation that will impact their quality of life (QOL) permanently. Alternatives to immediate TME in patients with a NCCR include enhancing the tumor response by intensifying neoadjuvant therapy, active surveillance, LE of the tumor site, contact radiotherapy, or a combination of all.
Increasing Neoadjuvant Chemotherapy
A potential way to increase tumor response is the addition of CT to CRT. This could be done before (induction CT) or after CRT (consolidation CT). Furthermore, neoadjuvant CT addresses the concern that extended periods of observation (in which the patient does not receive further treatment) may allow residual cancer cells to grow, locally or at distant sites.
Some groups have explored the use of induction CT with fluoropyrimidines and oxaliplatin with different results. pCR rates have been described between 11 and 36%, varying according to the regimen and time until surgery ( Table 3 ). Although clinical response has not been assessed in these trials, the pCR rate could be used as surrogate marker.
Table 3. Experiences with induction chemotherapy.
| Author (y) | pCR rate (%) | Induction regimen | RT dose (Gy) | Time CRT-surgery (wk) |
|---|---|---|---|---|
| Calvo et al (2006) 43 | 29 | FOLFOX4, two cycles | 45–50.4 | 4–6 |
| Chua et al (2010) 44 | 20 | CAPOX, three cycles (12 wk) | 54 | 6 |
| Schou et al (2012) 45 | 23 | CAPOX, two cycles | 54 | 6 |
| Maréchal et al (2012) 46 | 25 | FOLFOX6, two cycles | 45 | 6–8 |
| Dewdney et al (2012) 47 | 11 W/cetuximab 9 no cetuximab |
CAPOX, four cycles with and without cetuximab | 54 | 6–8 |
| Cercek et al (2014) 48 | 36 (cCR + pCR) | FOLFOX6, seven cycles | 50.4 | 6–8 |
Abbreviations: cCR, complete clinical response; CRT, chemoradiation therapy; Gy, gray; pCR, pathological complete response; RT, radiotherapy; wk, week.
The second proposed strategy is consolidation CT. Habr-Gama et al pioneered this approach by giving extra cycles of 5-fluorouracil (5-FU) during the resting period of CRT. 14 With this extended regimen, the cCR rate at initial evaluation was 68%, whereas sustained cCR rate was reported as 57% 1 year after completion of CRT. Of note, in this new regimen, patients were assessed at 9 weeks after CRT completion, 1 week later than within the conventional regimen, and the RT dose was 5,400 cGy instead of the conventional 5,040 cGy. These impressive numbers should be analyzed with caution since this is a retrospective series in a very specific population.
Recently, a multicenter prospective phase 2 trial studying the effect of adding FOLFOX to the treatment regimen during the resting period between CRT and surgery was published. The TIMING (Timing of Rectal Cancer Response to Chemoradiation) trial was a study consisting of four sequential groups of patients receiving increasing cycles of CT. 15 By adding mFOLFOX6 during the resting period, the mean time from the end of CRT to surgery was sequentially increased from 8.5 weeks in the first group to 19.3 weeks in the fourth group. The first group was considered the standard therapy group and had a pCR rate of 18%, whereas the fourth group (treated with six cycles of FOLFOX) reached a pCR rate of 38%, one of the highest reported rates for LARC ( Fig. 3 ). It should be noted that one patient died during CT and that 36% of patients in the fourth group had grade 3 or 4 adverse events to CT, although these numbers are consistent with trials examining outcomes of adjuvant CT. Although cCR rates are not mentioned in this study, it seems likely that with the significantly increased rate of pCR, there would be a similar increase in cCR with an additional 11 weeks to allow residual mucosal abnormalities to reside.
Fig. 3.
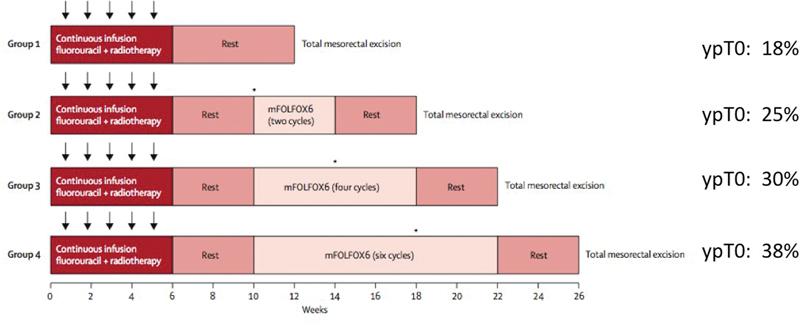
TIMING (Timing of Rectal Cancer Response to Chemoradiation) Trial. Complete response rates according to treatment received.
The question of using induction CT or consolidation CT is actually being addressed prospectively in the OPRA (Organ Preservation for Rectal Adenocarcinoma) trial, an ongoing trial comparing CRT followed by consolidation CT versus induction CT followed by CRT and the relative cCR rates. The study schema is shown in Fig. 4 . Briefly, patients in this trial are restaged by clinical examination, endoscopy, and MRI and then, according to response, are entered into a watch-and-wait protocol or undergo TME. At the time of the writing of this article, the trial is open to accrual at 20 centers across the United States. 12
Fig. 4.
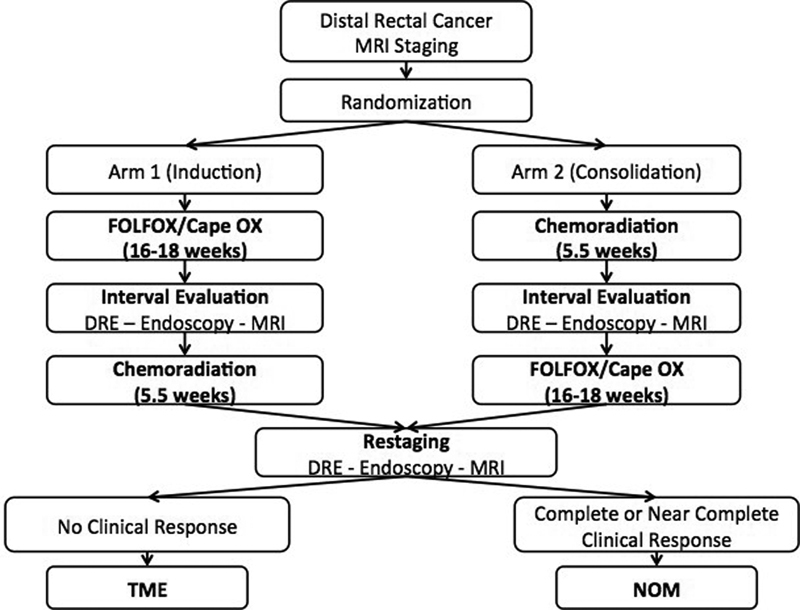
OPRA (Organ Preservation in Rectal Adenocarcinoma) trial design.
Active Surveillance
Ideally, before stating that a patient has an NCCR, physicians have to make sure the patient has achieved a maximal response. Unfortunately, there is no definitive way to identify when maximal response has occurred. The current evidence suggests that tumor response to CRT is a time-dependent phenomenon and complete regression might take months. Traditionally, surgeons have recommended surgery approximately 6 to 8 weeks after completion of neoadjuvant therapy. This time interval was considered sufficiently long enough to allow patients to recover from the radiation while not long enough to allow the tissue fibrosis associated with radiotherapy. The Lyon R90–01 trial was the first to show that increasing the interval after CRT from 2 weeks to 6 to 8 weeks increased the rates of PCR or microscopic residual disease from 10.3 to 26%. 16 Multiple retrospective case series and analysis of large databases have confirmed that an interval between completion of radiation and surgery longer than 12 weeks has been associated with higher pCR rates, without an apparent increase in surgical morbidity.
In a recent systematic review, Foster et al concluded that extended intervals between chemoradiotherapy and surgery appear to lead to higher rates of pCR and tumor downstaging but need further study in robust randomized trials to show long-term oncological benefits. 17 More recently, a meta-analysis by Petrelli et al, which overlapped with many of the papers cited by Foster, reported an increase in pCR rates from 13.7 to 19.5% when comparing an interval of 6 to 8 weeks to an interval of longer than 8 weeks. The authors calculated that waiting for more than 6 to 8 weeks conferred a relative risk of 1.42 for having a pCR compared with the standard, but also concluded that further study was needed in randomized trials. 18 Probst et al performed a retrospective review of more than 17,000 rectal cancer patients from the National Cancer Database and found that an interval of >8 weeks from the end of CRT was significantly associated with higher odds of pCR. Reported rates of pCR were relatively low in this study, although this potentially reflects “real-life” treatment outside of clinical trials, including patients who do not complete a full course of radiation. Rates of pCR in this study seemed to level off at 12 to 13 weeks from the end of CRT, potentially suggesting the maximum interval one should monitor for a complete response. Indeed, when comparing patients who had surgery at 6 to 8 weeks from CRT completion versus those who were operated at 10 to 11 weeks, the odds of having a pCR was 27% greater in the latter group. 19
In the prospective setting, the previously mentioned TIMING phase II trial confirmed the safety of TME after intervals as long as 20 weeks compared with shorter intervals. Nevertheless, it should be taken into account that during the waiting period, patients also received additional cycles of CT, and therefore the high rates of pCR could also be influenced by that and not only by the longer time to surgery. 15
There is a theoretical concern that delaying surgery out to 12 weeks from the end of CRT will lead to a significant delay until adjuvant therapy is started. This may have a negative effect on oncological outcomes, with higher rates of distant metastases that could have been prevented or treated with timely adjuvant CT. There are currently a few active trials (Stockholm III, 20 STARRCAT (Surgical Timing After Radiotherapy for Rectal Cancer Analysis of Technique), 21 GRECCAR-6 (Groupe de Recherche Chirurgicale sur le Cancer du Rectum-French Research Group of Rectal Cancer Surgery) 22 ) addressing the issue of interval to surgery, but it will still be years until final results are reported on long-term overall survival and disease-free survival (DFS).
Biopsy of Residual Mucosal Abnormality
In the setting of residual mucosal abnormalities after CRT, it may be tempting to perform an endoscopic biopsy for tissue diagnosis of residual tumor, but this in fact does not seem to be a useful diagnostic tool. Perez et al performed a retrospective review of patients with significant tumor downsizing after CRT who were considered to have an incomplete response. Thirty-nine patients underwent post-CRT endoscopic forceps biopsies (some had multiple biopsies), resulting in 53 total biopsies. Twenty-five of the patients were found to have positive biopsies, whereas 14 were negative. Of the 14 patients with negative biopsies, only three were actually found to have no residual tumor at resection. This gives a negative predictive value of 21%, which decreases even further to 11% when considering all biopsies that were taken. 23
Multiple authors have examined the significance of these residual mucosal abnormalities and the distribution of residual cancer cells at resection. Duldulao et al examined the distribution of cancer cells in specimens from 94 patients who had undergone CRT followed by surgery in ypT2–4 tumors. The authors found that residual cancer cells were preferentially located at or near the original invasive front of the tumor. Thirteen percent of patients had residuals cells in the mucosa, whereas 98% of patients had cells in the muscularis propria. 9 Smith et al had similar findings when examining the spread of residual cancer cells after CRT. The authors found that residual tumor cells could spread as far as 9 mm beyond the lateral border of residual mucosal abnormalities, while the deepest spread was consistently found directly below the mucosal abnormalities. 24 These two studies help to clarify why a superficial endoscopic biopsy may not adequately diagnose residual tumor cells, as they are frequently found well beyond the borders of a residual ulcer or significantly deeper than an endoscopic biopsy can reach. A full thickness biopsy would need to be performed to accurately diagnose residual disease.
What Is the Role of Local Excision for NCCR?
Since a superficial biopsy is of little diagnostic use, many surgeons have examined the use of LE with diagnostic and potentially therapeutic intent. LE can be performed as a traditional transanal excision, transanal endoscopic microsurgery (TEM), or transanal minimally invasive surgery (TAMIS). These methods will all be referred to as LE, except where specified for simplicity.
LE first gained popularity as a means of treating early stage rectal tumors, such as cT1–2 N0 tumors. However, several series demonstrated that the rates of local recurrence (LR) after LE for T1 and T2 tumors were higher than originally expected; they were specifically higher that the expected rate of nodal metastasis for T1 (8–10%) and T2 tumors (20–23%). 25 It should also be mentioned that LRs after LE for early rectal cancer are often extensive, with most patients requiring an extended pelvic resection for salvage. Postsalvage 5-year survival in these highly selected patients is just over 50%, which is rather low considering the early stage of the initial disease. 26 As a result, LE alone with curative intent is only recommended for T1 tumors without high-risk histological features (poor differentiation, perineural or lymphovascular invasion [LVI], positive margins) or alternatively as a palliative procedure for older patients who cannot tolerate a more radical resection.
The role of LE in LARC after CRT has also been questioned. The rate of lymph node metastasis is relatively low for ypT0 tumors (3.3–9%) but steadily increases in correlation with the depth of tumor penetration into the rectal wall/mesorectum: 11 to 17% for ypT1 and 21 to 29% for ypT2. 27 28 These numbers are troubling, as LE would not remove those mesorectal lymph nodes. But concern is not limited to lymph node status. Tumor shrinkage does not occur centripetally, and tumor cells tend to remain at the invasive front of the original tumor. Considering that for cT3 the invasive front is located in the mesorectum where landmarks can be difficult to identify after radiation, many question the indication of LE after CRT for cT3 tumors. 9
The American College of Surgeons Oncology Group (ACOSOG) Z6041 trial explored the use of LE in cT2N0 patients after CRT with an oxaliplatin/capecitabine regimen. Only 3 of 77 patients who received CRT + LE had a LR with 3 years of FU, with an estimated 3-year DFS of 88.2% (95% confidence interval: 81.3–95.8). 29 Although patients were operated irrespective of tumor response, these excellent oncological outcomes might favor the position of limiting LE to originally cT2 tumors. Moreover, a prospective randomized trial comparing TEM versus laparoscopic TME for cT2N0 patients after CRT showed similar LR rates (8 vs. 6%), with no difference in 5-year cancer-related survival (89 vs. 94%; p = 0.7), seeming to confirm the results of ACOSOG Z6041. Although these results have not been replicated and it is a small single-center series, it is the only prospective randomized trial comparing both approaches and suggests that for these select patients, LE seems to be equivalent to radical surgery. 30
On the other hand, it has been stated that baseline staging does not seem to matter when deciding to pursue a rectal preserving strategy in NCCR. A recent report from Perez et al with 46 patients undergoing TEM for NCCR after CRT showed that LR rates were not statistically different when comparing initially cT2N0 versus cStage II/III: 6.7% versus 22.6%, p = 0.18. 31 However, the interpretation of these results should be questioned as this was a retrospective case series without adequate power calculation, and, while not statistically different, these differences are probably clinically relevant. In addition, the median follow-up was relatively short (22 months). The mean time to LR after TME following CRT in large prospective randomized controlled trials is 3 years, with up to one-third of the cases occurring after 5 years of follow-up. It is expected that with longer follow-up, the recurrence rates in this series will continue to increase.
Furthermore, in another report from the same group, with an expanded series of 53 patients with NCCR after CRT for LARC, the 2-year LR rate after TEM was 22% (12/53 patients); nine of the patients were LR exclusively, and eight were deemed resectable by preoperative imaging and were operated with salvage intention. 32 Of note, a disappointing positive circumferential margin was obtained in most of the cases (7/8). The authors acknowledge that TME after LE might not be the best approach and make a call for completion TME immediately after TEM in the presence of bad pathological features (ypT2–3, LVI, perineural invasion, or poor differentiation). Had that approach been followed, all eight patients who had an LR exclusively would have received completion proctectomy.
A full-thickness LE after CRT, particularly when the surgeon intents to incorporate a portion of the mesorectum in the surgical specimen, may preclude a subsequent sphincter-saving procedure for patients found to have a positive margin or other unfavorable histological features requiring a TME. As the LE, particularly for anteriorly located tumor, often reaches the mesorectal fascia, it makes a future TME technically challenging, not infrequently leading to rectal perforation. In this context, sphincter preservation is sometimes impossible, even in patients who were initial candidates for a low coloanal anastomosis. In fact, in the aforementioned study, 7 of 8 patients requiring a TME after LE required an abdominoperineal resection. 32
Several series have reported the results of a treatment protocol for rectal cancer patients treated according to a protocol consisting on CRT followed by LE, followed by immediate TME for patients found to have high-risk features (i.e., positive margins, LVI, perineural invasion, and poor differentiation) in the LE specimen. An Italian multicenter phase II study, including 63 patients with cT2/T3 tumor with a significant response to CRT and found to have ypT2, positive margins, tumor regression grade ≥ 3, or LVI+ in the LE specimen, showed a 3-year OS of 91.5%, 3-year DFS of 91%, and a local-recurrence DFS of 96.9%. 33 Another report from Poland including 89 patients treated with either short-course RT or CRT reported a 7% LR rate among good responders (ypT0–T1, without adverse features). On the other hand, patients with adverse pathology features who refused TME had a 44% LR rate. Of note, the LR rate in patients treated only with LE according to clinical staging was 5.6% for cT1, 5.3% for cT2, and 28.6% for cT3, reinforcing the importance of initial clinical staging when selecting patients for this approach. 34 Finally, the CARTS (CApecitabine, Radiotherapy and Tem Surgery) study from the Netherlands included 47 patients (cT1–3N0) treated with CRT followed by TEM at 8 weeks from treatment completion. Radical surgery was advocated in ypT2 tumors, whereas ypT1–0 tumors were observed. With this approach, only four LR were recorded, 3 of them in ypT2 patients who refused surgery. 35 Although TME is safe, it has to be considered that not all patients will undergo TME after LE because of being unfit or refusal, putting them at a higher risk for LR; in fact, only 8/17 (47%) patients completed TME in the CARTS study and 8/26 (31%) in the Polish series.
It is evident that there is a need for high-quality prospective data regarding how to manage these patients. Our institution is leading a multicenter prospective phase II trial in which patients with LARC are randomized to either induction CT followed by CRT or CRT followed by consolidation CT. Tumor response is assessed at 12 weeks after completion of the neoadjuvant treatment and classified according to strict criteria as one of three categories: cCR, no response, and NCCR ( Table 2 ). Nonresponders are treated with TME, whereas patients with cCR and NCCR are closely followed in an observational protocol without immediate surgery (NOM). Once completed and after adequate follow-up, information regarding the oncological outcomes of patients who develop NCCR will be available and hopefully will help clarify some aspects of the management of these patients. 12
Although LE alone is relatively well tolerated and is associated with less morbidity than TME, morbidity for LE after CRT is significant. In some series, up to 43% of patients required hospital readmission, most due to severe postoperative pain and wound dehiscence rates of 70% have been described. 36 Complications are sometimes severe enough to require a diverting stoma in some cases. 37 It is logical to think that the bigger the defect, the higher the chance of having a wound complication. It has also been suggested that the traditionally accepted 1-cm lateral margin for LE might not be enough after CRT. In a retrospective analysis of a series of 30 surgical specimens of NCCR patients treated by TEM, tumor regression in a fragmented pattern was present in 37% of the specimens and lateral intramucosal spread (LIS) in 53% of the cases. 38 The authors found that the mean distance between clusters of residual cells was 3.6 ± 2 mm and the maximum LIS was 7.2 mm; after combining both occurrences, the authors concluded that when fragmented regression and LIS are present, 1.5 cm would be a safer lateral margin than the traditional 1 cm. If these recommendations are followed, one could expect even higher rates of wound dehiscence and subsequent morbidity.
Finally, anorectal function and QOL are also affected by LE after CRT. In a recent report comparing QOL, anorectal manometry results and clinical incontinence status (evaluated with the Cleveland Clinic Incontinence Index [CCII]) between NCCR patients treated by TEM and cCR patients in a NOM protocol, the authors found that squeezing and resting pressures, CCII, and QOL were significantly worse in the TEM group. 39
Brachytherapy and Contact Radiation
Over the years, investigators have evaluated the role of brachytherapy to deliver higher doses of radiation as a boost to improve outcomes. Currently, there are three types of brachytherapy used in rectal cancer: contact X-ray brachytherapy (Papillon), high dose rate intraluminal rectal brachytherapy, and interstitial rectal brachytherapy implants. Of the three methods, only contact brachytherapy has been used in the setting of a NCCR.
Contact X-Ray Brachytherapy (Papillon)
Lamarque in Montpellier, France, was the first to use the Philips RT 50 machine to treat rectal adenocarcinoma. Papillon was responsible for popularizing this approach in the conservative treatment of rectal cancer in Lyon between 1950 and 1990. He was able to treat more than 300 patients with CXRT (contact radiotherapy), and this approach is named after him. 40
Regarding technique, for intrarectal irradiation, the tube is used with a rigid applicator or rectoscope, which provides a focal distance of 4 cm. The radiation output is very high, close to 20 Gy/minute.
Originally described for early rectal cancers, this technique has also been described as an adjuvant treatment in the case of T3–T2 tumors that present a good regression after CRT (final tumor size < 2 cm) in morbid patients with contraindications for radical surgery. Reports in the setting of a NCCR are anecdotal, and further investigation is guaranteed to define the role of this approach in this setting. 40 41 42
Our Current Approach to LARC and NCCR
At our institution, patients with LARC treated with curative intent receive total neoadjuvant therapy, most commonly induction CT (4 months of FOLFOX–CAPOX) followed by CRT (5,040 cGy of radiation and concurrent infusional 5-FU or capecitabine). Patients are often reassessed by DRE and flexible sigmoidoscopy for tumor response between CT and CRT. Tumor response is finally assessed by DRE, flexible sigmoidoscopy, rectal MRI, and CT CAP (chest, abdomen, and pelvis) approximately 8 weeks after completing CRT. At this point, cCR patients are offered an NOM with close follow-up. On the other end of the spectrum of tumor response are patients with tumor progression or no regression; these are offered TME. The decision is more complex in patients with an NCCR. While our preference is to proceed to a TME, we are often agreeable to wait another 8 weeks to reassess response again with DRE and flexible sigmoidoscopy in patients reluctant to have the rectum removed. While further response with this additional waiting is not guarantee, we have not seen dramatic growth during this relatively short interval. Patients who have not achieved a cCR during these additional 8 weeks should have a TME. Endoscopic biopsies are occasionally used to confirm the presence of cancer, but may give the surgeon a false sense of security when negative as they often miss cancer cells deep in the bowel wall. In general, we do not recommend LE for patients with NCCR who originally had a clinically stage II or III tumor, except when TME is contraindicated.
In cases when patients are seen for additional treatment after receiving CRT, we often recommend consolidation CT (4 months of FOLFOX–CAPOX) with similar assessment of response approximately 8 weeks after the last cycle of consolidation CT. Criteria for subsequent treatment are similar to patients receiving induction CT and CRT.
Summary
The first step when facing an NCCR after CRT is to optimize tumor response. Strategies include longer waiting periods for response assessment and the use of induction or consolidation CT.
Unfortunately, we are still unable to identify the ideal candidates with an NCCR after CRT that could be treated with LE as definitive therapy, and TME remains as the safest option for these patients. Baseline staging might have implications when deciding who is a candidate for LE.
LE after CRT is associated with significant morbidity and worse functional outcomes compared with cCR managed with NOM.
Patients with any other clinical finding that is not a cCR after maximal neoadjuvant therapy should be treated with TME.
References
- 1.NCCN Clinical Practice Guidelines in Oncology - Rectal Cancer. Version 3.2015. Available atwww.NCCN.org. Accessed September 2015
- 2.Maas M, Nelemans P J, Valentini V et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(09):835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 3.Habr-Gama A, Perez R O, Nadalin Wet al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results Ann Surg 200424004711–717., discussion 717–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas M, Beets-Tan R G, Lambregts D M et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 5.Smith J D, Ruby J A, Goodman K A et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256(06):965–972. doi: 10.1097/SLA.0b013e3182759f1c. [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A, Perez R O, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692–1698. doi: 10.1007/DCR.0b013e3181f42b89. [DOI] [PubMed] [Google Scholar]
- 7.Smith F M, Chang K H, Sheahan K, Hyland J, O'Connell P R, Winter D C. The surgical significance of residual mucosal abnormalities in rectal cancer following neoadjuvant chemoradiotherapy. Br J Surg. 2012;99(07):993–1001. doi: 10.1002/bjs.8700. [DOI] [PubMed] [Google Scholar]
- 8.Smith F M, Wiland H, Mace A, Pai R K, Kalady M F. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2014;57(03):311–315. doi: 10.1097/DCR.0b013e3182a84eba. [DOI] [PubMed] [Google Scholar]
- 9.Duldulao M P, Lee W, Streja L et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum. 2013;56(02):142–149. doi: 10.1097/DCR.0b013e31827541e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samdani T, Garcia-Aguilar J. Imaging in rectal cancer: magnetic resonance imaging versus endorectal ultrasonography. Surg Oncol Clin N Am. 2014;23(01):59–77. doi: 10.1016/j.soc.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Lambregts D M, Lahaye M J, Heijnen L A et al. MRI and diffusion-weighted MRI to diagnose a local tumour regrowth during long-term follow-up of rectal cancer patients treated with organ preservation after chemoradiotherapy. Eur Radiol. 2016;26(07):2118–2125. doi: 10.1007/s00330-015-4062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith J J, Chow O S, Gollub M J et al. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. doi: 10.1186/s12885-015-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynne-Jones R, Hughes R. Complete response after chemoradiotherapy in rectal cancer (watch-and-wait): have we cracked the code? Clin Oncol (R Coll Radiol) 2016;28(02):152–160. doi: 10.1016/j.clon.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Habr-Gama A, Sabbaga J, Gama-Rodrigues J et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56(10):1109–1117. doi: 10.1097/DCR.0b013e3182a25c4e. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Aguilar J, Chow O S, Smith D D et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(08):957–966. doi: 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francois Y, Nemoz C J, Baulieux J et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(08):2396–2402. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 17.Foster J D, Jones E L, Falk S, Cooper E J, Francis N K. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56(07):921–930. doi: 10.1097/DCR.0b013e31828aedcb. [DOI] [PubMed] [Google Scholar]
- 18.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg. 2016;263(03):458–464. doi: 10.1097/SLA.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 19.Probst C P, Becerra A Z, Aquina C T et al. Extended intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation. J Am Coll Surg. 2015;221(02):430–440. doi: 10.1016/j.jamcollsurg.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettersson D, Cedermark B, Holm T et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg. 2010;97(04):580–587. doi: 10.1002/bjs.6914. [DOI] [PubMed] [Google Scholar]
- 21.STARRCAT Trial: Surgical timing after radiotherapy for rectal cancer. Available athttp://www.controlled-trials.com/ISRCTN88843062[cited May 5, 2016]
- 22.Lefevre J H, Rousseau A, Svrcek M, Parc Y, Simon T, Tiret E; French Research Group of Rectal Cancer Surgery (GRECCAR).A multicentric randomized controlled trial on the impact of lengthening the interval between neoadjuvant radiochemotherapy and surgery on complete pathological response in rectal cancer (GRECCAR-6 trial): rationale and design BMC Cancer 201313417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez R O, Habr-Gama A, Pereira G V et al. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Colorectal Dis. 2012;14(06):714–720. doi: 10.1111/j.1463-1318.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith F M, Wiland H, Mace A, Pai R K, Kalady M F. Depth and lateral spread of microscopic residual rectal cancer after neoadjuvant chemoradiation: implications for treatment decisions. Colorectal Dis. 2014;16(08):610–615. doi: 10.1111/codi.12608. [DOI] [PubMed] [Google Scholar]
- 25.Ricciardi R, Madoff R D, Rothenberger D A, Baxter N N. Population-based analyses of lymph node metastases in colorectal cancer. Clin Gastroenterol Hepatol. 2006;4(12):1522–1527. doi: 10.1016/j.cgh.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Weiser M R, Landmann R G, Wong W D et al. Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum. 2005;48(06):1169–1175. doi: 10.1007/s10350-004-0930-3. [DOI] [PubMed] [Google Scholar]
- 27.Park I J, You Y N, Skibber J M et al. Comparative analysis of lymph node metastases in patients with ypT0-2 rectal cancers after neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2013;56(02):135–141. doi: 10.1097/DCR.0b013e318278ff8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mignanelli E D, de Campos-Lobato L F, Stocchi L, Lavery I C, Dietz D W. Downstaging after chemoradiotherapy for locally advanced rectal cancer: is there more (tumor) than meets the eye? Dis Colon Rectum. 2010;53(03):251–256. doi: 10.1007/DCR.0b013e3181bcd3cc. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Aguilar J, Renfro L A, Chow O S et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16(15):1537–1546. doi: 10.1016/S1470-2045(15)00215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lezoche E, Baldarelli M, Lezoche G, Paganini A M, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99(09):1211–1218. doi: 10.1002/bjs.8821. [DOI] [PubMed] [Google Scholar]
- 31.Perez R O, Habr-Gama A, São Julião G P et al. Transanal local excision for distal rectal cancer and incomplete response to neoadjuvant chemoradiation - does baseline staging matter? Dis Colon Rectum. 2014;57(11):1253–1259. doi: 10.1097/DCR.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 32.Perez R O, Habr-Gama A, São Julião G P et al. Transanal endoscopic microsurgery (TEM) following neoadjuvant chemoradiation for rectal cancer: outcomes of salvage resection for local recurrence. Ann Surg Oncol. 2016;23(04):1143–1148. doi: 10.1245/s10434-015-4977-2. [DOI] [PubMed] [Google Scholar]
- 33.Pucciarelli S, De Paoli A, Guerrieri M et al. Local excision after preoperative chemoradiotherapy for rectal cancer: results of a multicenter phase II clinical trial. Dis Colon Rectum. 2013;56(12):1349–1356. doi: 10.1097/DCR.0b013e3182a2303e. [DOI] [PubMed] [Google Scholar]
- 34.Bujko K, Richter P, Smith F M et al. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders: a prospective multicentre study. Radiother Oncol. 2013;106(02):198–205. doi: 10.1016/j.radonc.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Verseveld M, de Graaf E J, Verhoef C et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study) Br J Surg. 2015;102(07):853–860. doi: 10.1002/bjs.9809. [DOI] [PubMed] [Google Scholar]
- 36.Perez R O, Habr-Gama A, São Julião G P, Proscurshim I, Scanavini Neto A, Gama-Rodrigues J. Transanal endoscopic microsurgery for residual rectal cancer after neoadjuvant chemoradiation therapy is associated with significant immediate pain and hospital readmission rates. Dis Colon Rectum. 2011;54(05):545–551. doi: 10.1007/DCR.0b013e3182083b84. [DOI] [PubMed] [Google Scholar]
- 37.Marks J H, Valsdottir E B, DeNittis A et al. Transanal endoscopic microsurgery for the treatment of rectal cancer: comparison of wound complication rates with and without neoadjuvant radiation therapy. Surg Endosc. 2009;23(05):1081–1087. doi: 10.1007/s00464-009-0326-5. [DOI] [PubMed] [Google Scholar]
- 38.Perez R O, Habr-Gama A, Smith F M et al. Fragmented pattern of tumor regression and lateral intramural spread may influence margin appropriateness after TEM for rectal cancer following neoadjuvant CRT. J Surg Oncol. 2014;109(08):853–858. doi: 10.1002/jso.23571. [DOI] [PubMed] [Google Scholar]
- 39.Habr-Gama A, Lynn P B, Jorge J M et al. Impact of organ-preserving strategies on anorectal function in patients with distal rectal cancer following neoadjuvant chemoradiation. Dis Colon Rectum. 2016;59(04):264–269. doi: 10.1097/DCR.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 40.Gérard J P, Ortholan C, Benezery K et al. Contact X-ray therapy for rectal cancer: experience in Centre Antoine-Lacassagne, Nice, 2002-2006. Int J Radiat Oncol Biol Phys. 2008;72(03):665–670. doi: 10.1016/j.ijrobp.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Sun Myint A, Grieve R J, McDonald A C et al. Combined modality treatment of early rectal cancer: the UK experience. Clin Oncol (R Coll Radiol) 2007;19(09):674–681. doi: 10.1016/j.clon.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Hershman M J, Myint A S, Makin C A. Multi-modality approach in curative local treatment of early rectal carcinomas. Colorectal Dis. 2003;5(05):445–450. doi: 10.1046/j.1463-1318.2003.00502.x. [DOI] [PubMed] [Google Scholar]
- 43.Calvo F A, Serrano F J, Diaz-González J A et al. Improved incidence of pT0 downstaged surgical specimens in locally advanced rectal cancer (LARC) treated with induction oxaliplatin plus 5-fluorouracil and preoperative chemoradiation. Ann Oncol. 2006;17(07):1103–1110. doi: 10.1093/annonc/mdl085. [DOI] [PubMed] [Google Scholar]
- 44.Chua Y J, Barbachano Y, Cunningham D et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(03):241–248. doi: 10.1016/S1470-2045(09)70381-X. [DOI] [PubMed] [Google Scholar]
- 45.Schou J V, Larsen F O, Rasch L et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23(10):2627–2633. doi: 10.1093/annonc/mds056. [DOI] [PubMed] [Google Scholar]
- 46.Maréchal R, Vos B, Polus M et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23(06):1525–1530. doi: 10.1093/annonc/mdr473. [DOI] [PubMed] [Google Scholar]
- 47.Dewdney A, Cunningham D, Tabernero J et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30(14):1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 48.Cercek A, Goodman K A, Hajj C et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12(04):513–519. doi: 10.6004/jnccn.2014.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]


