Abstract
Modern rectal cancer management is dependent on preoperative staging, and radiological assessment is a crucial part of this process. Imaging must provide sufficient information to guide preoperative decision-making that is reliable and reproducible. Different methods have been used for local staging; however, magnetic resonance imaging (MRI) has shown to be the most reliable tool for this purpose. MRI offers prognostic information about the patients and guides the decision between neoadjuvant treatment and total mesorectal excision alone. Also, not only the initial staging but also restaging by MRI can provide significant information regarding tumor response that is essential when considering alternative approaches.
Keywords: rectal cancer, magnetic resonance imaging, local staging
Modern rectal cancer management is dependent on preoperative staging, and radiological assessment is a crucial part of this process. Imaging must provide sufficient information to guide preoperative decision-making that is reliable and reproducible. Imaging provides not only prognostic data but also detailed information on anatomical planes, thereby serving the surgeon in anticipating potential difficulties that may develop intraoperatively. The primary modality for local staging of rectal cancer is high-resolution magnetic resonance imaging (MRI). When performed in accordance with the recommended standards, MRI enables accurate staging of both early and advanced rectal cancer, accurate response assessment, the delineation of recurrent disease, and the planning of surgical treatment. 1 2 3 4
There is a paucity of literature with validated outcome data concerning the use of diffusion-weighted imaging (DWI) and positron emission tomography (PET)/computed tomography (CT). In the absence of any validated methods and outcome data, their use in initial assessment and restaging is limited to research protocols. Thus at the present moment, the use of CT and PET-CT is limited to the assessment of metastatic disease, and there is no role for such modalities in local staging and surgical orientation of rectal cancer.
MRI combined with CT is essential for the assessment of distant spread and recurrent disease, and currently PET-CT is sometimes used in the evaluation of patients with recurrent and metastatic disease. 2
Magnetic Resonance Imaging
Outcomes for rectal cancer have significantly improved over the last two decades with advances in surgical techniques, preoperative therapy, and imaging modalities. 5 MRI of the rectum has been shown to accurately predict the depth of extramural spread and involvement of the surgical resection margin 6 7 8 and as such, is recommended by United Kingdom and European guidelines for local staging and preoperative assessment of patients with rectal cancer, 9 although there is still international variability with continued use of endoscopic ultrasound and CT staging in some centers with suboptimal local staging. 10
When imaging the primary tumor, high-resolution MRI has been proven to give accurate prognostic information. 11 As such, the radiologist plays a critical role in guiding preoperative multidisciplinary team decisions to help achieve better outcomes for patients. 12
Technique
Patient Preparation
To obtain a high-quality study, good patient preparation is a necessity. Patients should be counseled that scans can take up to 40 minutes and if there is concern regarding a patient's ability to stay still during this time, the appropriate use of analgesia and/or sedation should be considered before the patient's arrival. Patients should also empty their bladder before the scan. No bowel preparation, filling of the rectum with contrast agents, or air insufflation is required. In addition, intravenous or intramuscular antispasmodic agents are not mandatory but can be helpful in improving image quality. Intravenous contrast enhancement with gadolinium is not recommended for the staging of rectal cancer and can result in loss of clarity of the pelvic planes and can cause the vessels to enhance, so rather than aiding diagnosis, the use of intravenous contrast can lead to overstaging of the tumor. 4 13
Coil Positioning
The development of the multichannel, multiarray pelvic phases array coil has revolutionized rectal MRI, enabling imaging of the entire mesorectum in relative comfort for the patient, without the difficulties associated with traversing strictures or obstructing tumors, or problems of near-field distortion that was seen with endorectal coils. 14 The position of the coil is paramount and is dependent on the position of the tumor within the rectum. A 1.5T system is used with phased array coils that maintain the high signal required but will obtain greater coverage than endorectal coils. 1
For low rectal tumors, the center of the coil should be positioned over the pubic symphysis, as higher placement results in the loss of detailed anatomy of the lower rectum with added noise, thereby limiting interpretation, and assessment of tumor stage. For rectosigmoid tumors, the coil should be centered more superiorly. 4 15 It is crucial that the coil is optimally centered to ensure adequate coverage of the rectum, mesorectum, and anal sphincter complex. In addition, to encompass the lymph node draining territory, which is 5 cm above the tumor; 16 the lower edge of the tumor must lie at least 10 cm below the symphysis pubis and the upper limit of the sacral promontory. 1
Sequences
High-resolution T2 images are the mainstay of rectal cancer imaging. The magnetic resonance protocol has been published 13 and validated by the MERCURY Study Group. 6
Localization images in the coronal and sagittal planes are initially required to plan further high-resolution images that will allow the detailed interpretation of the tumor stage. A narrow field of view (160 × 160 mm, 256 × 256 matrix) with a minimum of four signal averages ensures a 0.6 × 0.6 × 3 mm high-resolution image (1 mm 3 voxel size). The first series are T2-weighted sagittal, turbo spin-echo sequences from one pelvic sidewall to the other that enable identification of the tumor. A 3 mm slice thickness results in a higher resolution sagittal image, which allows assessment of the presacral space. The second series consists of wide-field-of-view axial sections of the whole pelvis. The third series consists of high-resolution images that are T2-weighted thin-section axial images through the rectal cancer and adjacent tissues. To avoid partial volume effects, which can cause a distorted image prone to overstaging, it is vital that these sequences are performed perpendicular to the long axis of the rectum and at the level of the tumor (3 mm slices). 1 To achieve this, multiple slabs may be required which may increase the time taken for the examination slightly, but will result in a diagnostic study. For patients with low rectal tumors, the fourth series consists of high-spatial resolution coronal imaging that will display the levator muscles, the sphincter complex, the intersphincteric plane, and the relationship to the rectal wall at an optimum which is essential for staging low rectal tumors. 13
Diffusion-weighted MRI is no longer recommended in the primary staging of rectal cancer as it does not help to delineate the relationship of the tumor to the pelvic anatomy and can detract from accurate local staging. 17
Normal Anatomy and Relevance to Staging
For the clinical management of rectal cancer, the rectum is divided into upper, mid, and lower thirds. The sagittal scans allow tumors to be depicted in relation to the important anatomical landmarks, namely, the anal verge, the peritoneal reflection, the seminal vesicles/uterus, the sacrum, and the coccyx.
The upper third of the rectum (10–15 cm from the anal verge) lies above the peritoneal reflection. Here, surgical dissection is more straightforward and as a result, these tumors tend to have a lower associated morbidity than the mid and lower rectal tumors. 18 However, it is still important to identify those tumors with poor prognostic features, such as mesorectal fascia invasion posteriorly, and therefore require preoperative therapy to render them resectable. If extension through the peritoneal reflection occurs and invasion of adjacent structures is apparent, for example, bladder, then a different operative approach will be required to limit the risk of intraoperative tumor spillage.
Mid rectal tumors (5–10 cm from the anal verge) and lower third tumors (< 5 cm from the anal verge) can be more challenging and the goal remains resection with a clear circumferential margin.
Peritoneal Reflection
This is seen as a low-signal intensity linear structure on sagittal images. It can be traced back from the surface of the bladder to the rectal wall anteriorly, where it attaches to the rectum and may be more easily identified in a male patient. The point of attachment produces a v -shaped configuration on axial section. The anterior covering of the rectum by peritoneal reflection widens in the cranial direction ( Figs. 1 and 2 ).
Fig. 1.
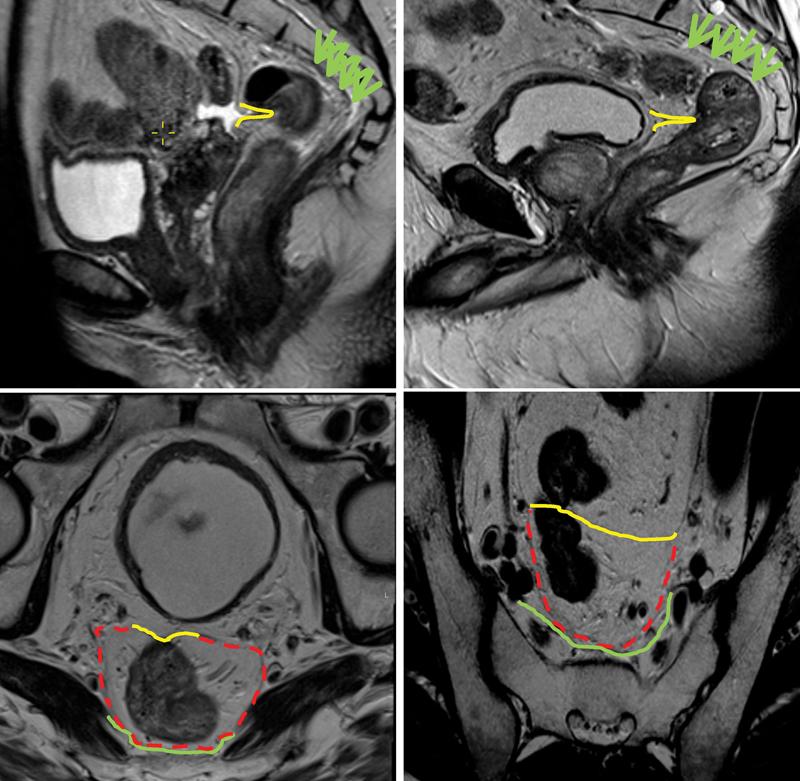
The yellow line demonstrates the level of the peritoneal reflection in a female patient (left sagittal image) and a male patient (right sagittal image). The green arrows and the green lines delineate the presacral fascia with red dashed lines indicating the mesorectal fascia.
Fig. 2.
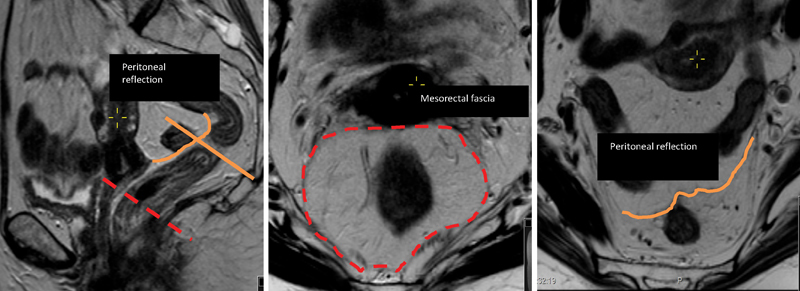
Peritoneal reflection: (left) sagittal T2 image and (right) upper T2-axial image; (center) mesorectal fascia. The red dashed line shows mesorectal fascia, and the orange line demarcates the peritoneal reflection.
Pelvic Sidewall Nerves
The inferior hypogastric plexus is a densely fenestrated meshwork of nerves for genitourinary function. They lie just laterally to the mesorectal fascia in the sagittal plane, running downwards and forward from the sacral plexus. At the level of the seminal vesicles, the nerves lie very close to the mesorectal fascia ( Fig. 3 ).
Fig. 3.
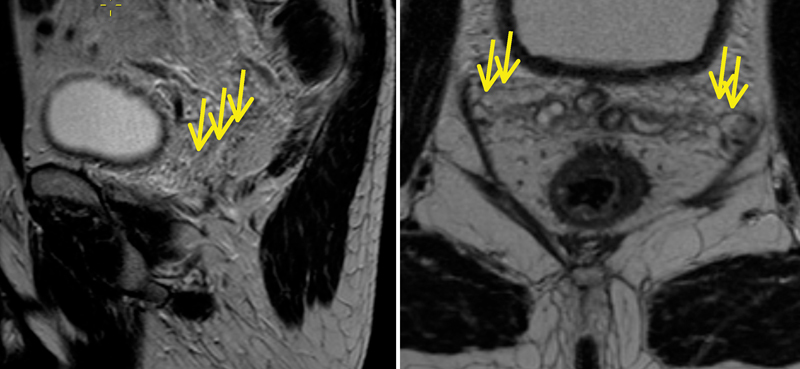
Hyperintense tubular/cystic structures along the seminal vesicles (yellow arrows on sagittal and axial images) show the level of the neurovascular bundles.
Mesorectal Fascia
This is seen as a low intensity signal layer that surrounds the mesorectum. Inferiorly this layer fuses with the endopelvic fascia that lies over the surface of the levator muscles and superiorly the fascia fuses with the peritoneal reflection anteriorly and the parietal fascia posteriorly ( Fig. 1 ).
Denonvilliers' Fascia
The mesorectal fascia fuses with the remnant of the urogenital septum at the level of the seminal vesicles. In the males, this is a dense band of connective tissue and separates the prostate and seminal vesicles from the rectum. During total mesorectal excision (TME), the anterior dissection occurs in the plane between Denonvilliers' fascia and the prostate ( Fig. 4 ).
Fig. 4.
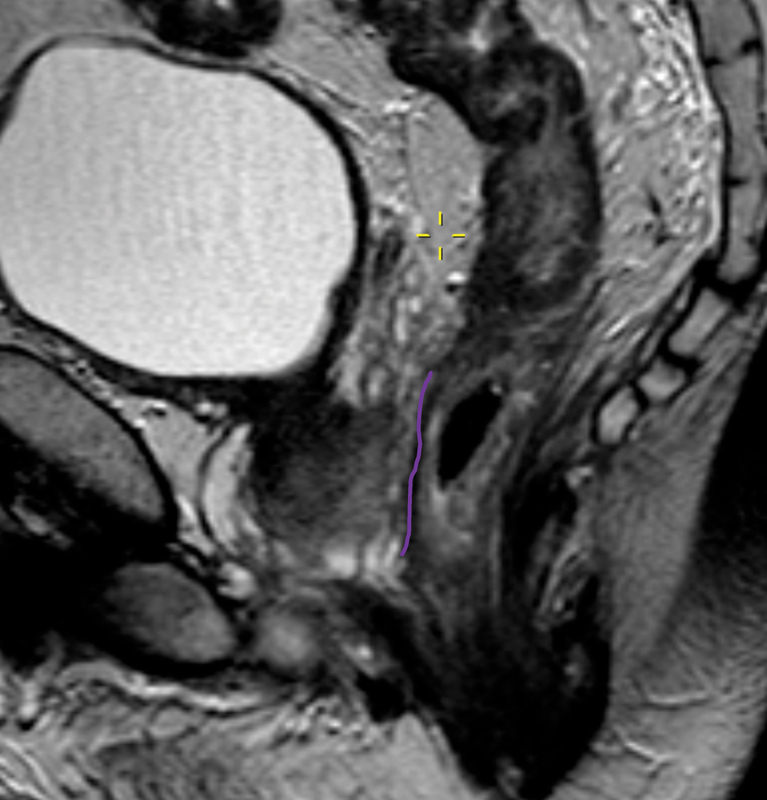
The purple line indicates Denonvilliers' fascia in a male patient.
Anal Canal
Anatomically, the anal canal is defined as the level at which the puborectalis sling sweeps around the rectum creating the anorectal junction. This results in angulation between the rectum and anal canal. The total length of the anal canal is variable; longer in males than females and on average represents the distal 4 cm of the gastrointestinal tract.
On MRI, the sphincter is depicted as an almost complete ring of muscle. The superior margin of the external anal sphincter is defined by the lower edge of the puborectalis sling. Inferiorly, the fibers curve inwards just below the internal sphincter. The internal anal sphincter is formed by a thickened segment of the circular muscle coat from distal rectum. Ridges of mucosal membrane that form anal columns characterize the upper third of the anal canal. Below this, the mucosa is smooth surfaced and the dentate line marks this point of transition ( Fig. 5 ).
Fig. 5.
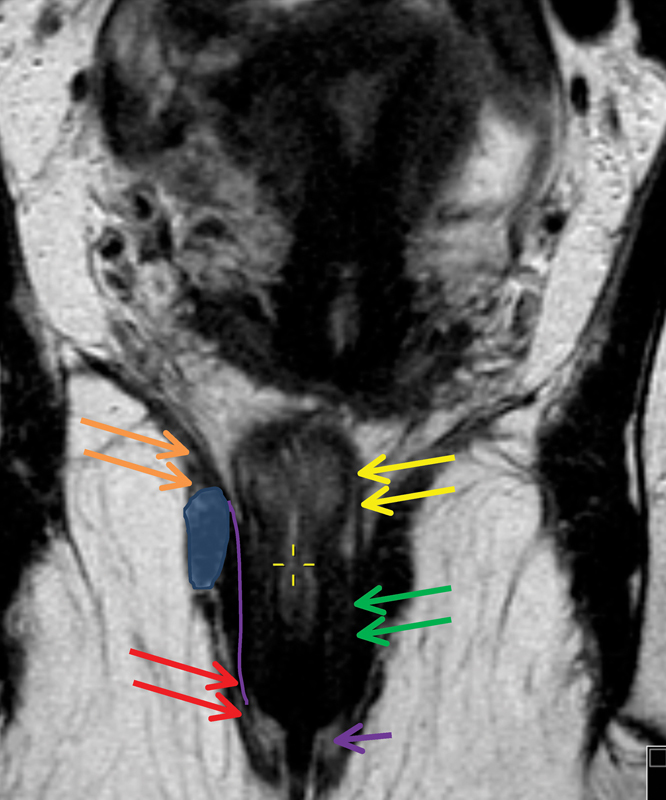
The coronal image shows the anatomical structures of the anal canal: Levators (orange) continue to the external sphincter (red arrow), and muscularis propria layer of the rectal wall (yellow arrows) continues as the internal sphincter (green arrow). The tiny hyperintense line between the internal and external sphincters indicates an intersphincteric plane (purple line) and the purple arrow indicates the interspnincteric groove which corresponds to the dentate line not visible on magnetic resonance imaging. The blue oval demonstrates the level of puborectalis sling.
Levator Muscle
The levator ani complex comprises the puborectalis, pubococcygeus, ileococcygeous, and coccygeous muscles. These collectively form a sheet that supports the pelvic floor. The levator muscle sheet is best seen on coronal images that depict the muscle sheet running obliquely toward the anorectal junction ( Fig. 5 ).
Presacral Fascia
The presacral fascia is a thickened parietal fascial covering that overlies the presacral veins and fat, and fuses with, and directly covers the muscles and vessels of the pelvic sidewall. It lies behind the mesorectal fascia. The lymph nodes of the pelvic sidewall accompany branches of the internal and external iliac vessels and are therefore in a separate compartment from the mesorectum. It is because of this that the pelvic sidewall nodes are not routinely visualized during surgery, unless the compartment is opened by dissecting through the presacral or parietal fascia. It is best assessed initially on the sagittal images with correlation to the high-resolution axials 19 ( Fig. 1 ).
MRI Anatomy Related to TME Surgery
Using distinct signal characteristics on T2-weighted images, MRI can identify the layers of muscle and mucosa. The mucosal layer is seen as a very fine layer of low-signal intensity overlying the much thicker and higher signal of the submucosa. Outside of this, the muscularis propria can be seen as a dual layer representing the inner circular and the outer longitudinal muscle layers sometimes separated by the intermediate signal intensity layer of the myenteric plexus. The outer layers have a characteristic irregular appearance due to vessels crossing the rectal wall. The perirectal fat is seen as a high signal with vessels seen as signal void areas encompassing the relatively low-signal intensity of the muscularis. Enveloping all of this is the mesorectal fascia, which is seen as a fine layer of low-signal intensity. To determine the T-stage of a tumor, careful assessments of the images is required with respect to signal characteristics and correct field alignment. The T-stage is defined by the extent of signal intensity within and beyond the submucosa of the rectal wall 1 20 ( Fig. 6 ).
Fig. 6.
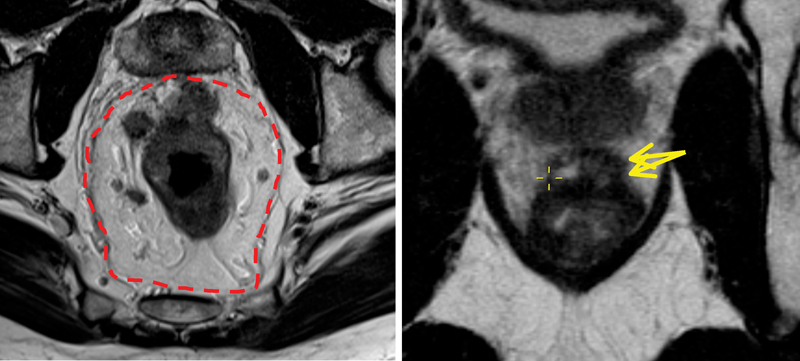
Axial pretreatment T2 magnetic resonance imaging (MRI) (left) shows a semiannular lesion which infiltrated the rectal wall at 9 to 3'o clock position with evidence of extramural spread at the site of infiltrating border. Tumor infiltrates the mesorectal fascia (distance to circumferential resection margin is < 1 mm) and abuts the prostate. (Right) Posttreatment magnetic resonance image, persistence intermediate signal intensity tumor (yellow arrow) at the site of the primary disease, suggesting mrTRG4. mrTRG, MRI tumor regression grade.
MRI and Local Rectal Cancer Staging
Early Rectal Cancer Staging
Early rectal cancer can be defined as invasive disease confined to the submucosa and/or the muscle of the rectal wall, without lymph node metastasis. 21 It is currently understood that T1 sm1 lesions can be safely removed by local excision without further therapy, since the likelihood of local relapse and tumor recurrence is low in these patients. Patients with T1 sm2 or greater are offered TME surgery, which is considered a good option for patients who are fit for surgery and in whom sphincters can be conserved. However, for low rectal polyps and early-stage tumors within 10 mm of the puborectalis sling, surgery most often requires either an abdominoperineal excision of the rectum or an ultralow intersphincteric anastomosis. Both of these procedures result in a significant impact on quality of life due to impairment or loss of sphincter function. Instead, many patients opt for chemoradiotherapy (CRT) and surveillance following local excision or transanal endoscopic microsurgery (TEM) of an early-stage lesion. 22
Careful examination of the advancing edge of the tumor is needed to judge the safety of TEM preoperatively. Precise documentation of both the quadrant (clock position) and height of tumor is needed. Certain interfaces may limit the extent for TEM excision: the anterior rectal wall and the prostate, the tumor height and the relationship to levator muscles, distal TME plane, and the peritoneal reflection. Imaging assessment should therefore include a measurement of the closest distance to these structures if clearance beyond the invasive edge of the tumor is likely to encroach upon these. 23
Assessment of Polypoidal and Sessile Lesions
To evaluate polypoidal lesions preoperatively, it is important to first determine the site of the stalk and then assess the extension of tumor into the fibromuscular stalk and beyond ( Fig. 7 ).
Fig. 7.
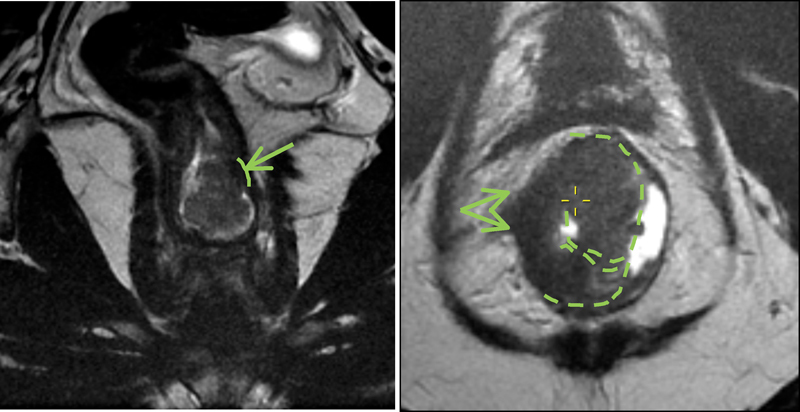
Left coronal image shows a polypoidal lesion within the lower/mid rectum. The fibromuscular stalk arises at the left lateral wall, and the maximum degree of invasion would be assessed at this very level (green arrow and line). (Right) The image indicates a low rectal advanced tumor is infiltrating rectal wall at 7 to 10 o'clock position with depression portion at 9 o'clock (green arrow) and rolled ages (dashed green lines). The intramural and extramural contiguous invasion should be assessed at this level and measured in millimeters.
Sessile or flat lesions are identified by a raised rolled edge laterally (noninvasive portion) and a depressed portion that forms the advancing edge of the tumor centrally ( Fig. 7 ).
Multiplanar assessment of the central depressed portion of the tumor and the degree of preservation identifiable in the submucosa and muscularis propria at the advancing edge is required preoperatively. If submucosa is evident on any single view at the base of the stalk, or at the central invasive base of a sessile tumor, it is diagnostic of a T1 lesion suitable for a local excision approach.
Assessment of T-stage tumor depth within and beyond the rectal wall is achieved by assessing the extent of the intermediate signal intensity and its extent of spread into the submucosa, muscularis, and beyond. The depth of extension into the submucosa should be assessed but measurement of the thickness of preserved submucosa is key; a thickness of > 1 mm in any plane increases the likelihood of detection of a sm1 or sm2 lesion which could be definitively treated by a local excision approach. The lack of any measurable high signal intensity layer on any plane imaged between the advancing edge of the tumor and the low-signal intensity of the muscularis suggests a high probability of a T1sm3 or early T2 tumor. The distinction between a T1sm3 and an early T2 is prognostically and clinically irrelevant since a local excision without removal of the full thickness of the underlying muscularis propria would result in a positive deep margin of < 1 mm. 23
Advanced Rectal Cancer Staging
Tumor Stage
The T-stage represents the depth of tumor invasion in relation to the bowel wall and is a key component of the staging classification. There are four T-stages: T1, submucosal invasion; T2, muscularis propria invasion; T3, beyond muscularis propria into the serosa, or into nonperitonealized pericolic or perirectal tissue; T4, perforation of visceral peritoneum (T4a) or direct invasion of peritoneum (T4b). 24
The degree of extension beyond the muscularis propria and into the mesorectum is of greater prognostic importance than T-stage. Tumors with invasion into the mesorectum > 5 mm are associated with a worse prognosis and higher recurrence rates. Thus, within the T3-stage there is a great deal of variability in terms of outcome. For this reason, the MRI (mr) and pathology (p) T3-stage should be subclassified to give a meaningful indication of prognosis. T3a minimal invasion: < 1 mm beyond the border of the muscularis propria are associated with survival outcomes that are identical to T1 and T2 tumors; T3b slight invasion: 1 to 5 mm beyond the border of the muscularis propria are also associated with very low rates of recurrence; however T3c moderate invasion: > 5 to 15 mm beyond the border of the muscularis propria; pT3d extensive invasion: > 15 mm beyond the border of the muscularis propria have significantly worse outcomes. This has been confirmed in numerous histopathologic studies. 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39
A prospective study comparing MRI and pathological assessment of T-stage showed a 94% agreement. 11 The multicenter MERCURY study directly compared extramural depth of invasion measured by MRI with histopathology in 295 patients and found that MRI was equivalent to histopathology assessment of depth to within 0.5 mm (mean difference: −0.046 mm; standard deviation: + 3.85 mm; 95%confidence interval [CI]: −0.49 to 0.4 mm). 7 This suggests that local staging on high-resolution MRI is not only accurate but also reproducible in a multicenter setting.
The widest range in survival is seen in patients with T3 tumors, which make up 80% of rectal tumors seen in clinical practice. 40 Survival rates are dependent on the depth of penetration into the mesorectum. pT3 tumors with > 5 mm of extramural invasion (≥pT3c) have a significantly worse 5-year disease-free survival (DFS) compared with tumors with an invasion depth of ≤ 5mm (≤pT3b); 54 versus 85% DFS, regardless of nodal status. 30 Conversely, those patients with tumors staged at ≤pT3a (< 1 mm invasion into the mesorectum) have a more favorable prognosis. Survival and local recurrence rates for T2 and early T3 tumors are identical; therefore, the decision regarding preoperative chemoradiotherapy should be based on the T3b/T3c cutoff. 1
A meta-analysis of 21 studies performed by Al-Sukhni et al investigated the accuracy of MRI in assessing T-stage. Specificity was found to be 75% and sensitivity 87%. 41 It should be noted, however, that many of the included studies used suboptimal imaging techniques without high-resolution T2-weighted images in the correct planes.
Circumferential Resection Margin
Of the preoperative prognostic features, circumferential resection margin (CRM) has emerged as one of the most powerful predictors of outcome. Regardless of local stage of the tumor, the presence of tumor within 1 mm of the surgical CRM predicts the development of local recurrence. 19
The surgical CRM is defined as the surgical cut surface of the connective tissues that encase the rectum. It is important to note that the areas of the rectum that have a peritoneal covering do not constitute the CRM following TME surgery. The CRM, therefore, equates to the mesorectal fascia, which forms the plane of dissection in rectal cancer surgery.
Visualization of the mesorectal fascia and accurate assessment of the CRM is key for preoperative staging and surgical planning. MRI has the inherent advantage of being able to consistently depict the mesorectal fascia. The CRM can be seen on MRI as the mesorectal fascia encompassing the entire mesorectum below the level of the peritoneal reflection. 42 When tumor extends to within 1 mm of this fascia, infiltrates, or extends beyond the fascia, it is predictive of subsequent margin involvement 11 ( Fig. 1 ).
Accurate measurement of the minimum distance from the tumor to the CRM requires high-resolution T2-weighted images perpendicular to the long axis of the tumor. In upper and mid rectal tumors, the CRM distance is measured from the closest point of the tumor to the mesorectal fascia. Additional high-resolution T2-weighted axial images perpendicular to the long axis of the anal canal, and coronal images parallel to the anal canal are needed for low tumors to assess the relationship of the tumor to the sphincters and levators. 43 In the lower rectum anteriorly, the mesorectal fascia is in contact with the prostate in men and the vagina in women, posteriorly, it forms the CRM. Below the origin of the levators, the mesorectal fascia is in contact with the levator sling, which then forms the CRM. This is continuous with the external sphincter inferiorly, which becomes the CRM for tumors extending into the anal canal.
It is important to assess CRM distance on the sagittal and coronal images as well as the correctly orientated high-resolution axial images to avoid errors, which can occur from partial voluming when the anteroposterior angulation of the rectum changes. 17
A single-center prospective study reported that MRI accurately identified involvement of the mesorectal fascia in 92% when compared with pathological assessment (kappa = 0.81). 11 These results were validated with the multicenter, prospective MERCURY study, which confirmed high-resolution MRI to be the most accurate imaging modality for identifying the CRM. 349 patients were predicted to have clear margins on preoperative MRI assessment and this was confirmed in 327 patients on histopathology (specificity 92%). 6 Around 53% of pathology samples were found to have an involved CRM when the tumor was ≤ 1mm from the potential CRM on MRI. If however, the tumor was > 1 mm from the CRM on MRI, CRM positivity was only 7%. 20
A meta-analysis to assess the accuracy of MRI in predicting CRM was performed by Xie et al consisting of 14 studies and 1,600 patients. When CRM involvement was defined as a tumor within 1 mm of the mesorectal fascia, MRI had a specificity and sensitivity of 76 and 88%, respectively. 44
CRM involvement is a poor prognostic factor in rectal cancer. 45 46 Long-term follow-up of the MERCURY study showed that 5-year overall survival was 62.2% in the CRM-negative group compared with 42.2% in the CRM-positive group and was the only significant prognostic risk factor for overall survival (OS), DFS, and local recurrence (LR) on multivariate analysis. 47 There is widespread agreement that all patients with an involved CRM on MRI should be offered neoadjuvant CRT. 48 49 50
Nodal Staging
Before the adoption of TME surgery, any nodal involvement predicted for pelvic recurrence. 51 Presently, if suboptimal TME surgery is performed, the risk of local recurrence is seen to significantly increase as pathological lymph nodes are left behind within the residual mesorectum. 52 However, when high-quality TME surgery is performed, the mesorectum and all draining lymph nodes contained within it are removed. In such cases, there is evidence to show that when the burden of nodal involvement is confined to the mesorectum (N1), without extracapsular breach or a positive CRM, there is no marked increase in the risk of pelvic recurrence, 53 although involvement of pelvic side wall nodes outside the mesorectum are more common in patients with involved mesorectal nodes. 54 Therefore, nodal staging is of questionable importance as a predictor of local recurrence in those patients undergoing radical surgery for primary tumors. 2 55 56
Accurate nodal staging remains one of the most challenging aspects of preoperative assessment for all imaging modalities, including MRI. Several criteria for lymph node staging have been proposed including lymph node size, morphology, and signal characteristics.
In a meta-analysis of 21 studies, rectal MRI was found to have a sensitivity of 77% and a specificity of 71% for the identification of lymph node metastases. 41 However, many of these studies used size criteria for assessment of lymph node status.
Size has been shown to be a poor indicator of nodal status 55 and enlarged lymph nodes may be present for several reasons. Using size > 7 mm as the criteria for malignancy, MR was found to have a sensitivity of 71%, a specificity of 61%, a positive predictive value of 46%, and a negative predictive value of 81%, with an overall accuracy of node positive disease of 64%. 57 By increasing the margin to 10 mm, specificity is increased but sensitivity is reduced, and by lowering the margin to 5 mm, sensitivity is increased at the cost of specificity. Trying to use a size cutoff is invariably challenging due to the considerable overlap that exists between a benign and malignant lymph node. 55 58 By using morphological criteria, specificity is increased, although it should be noted that lymph nodes of < 3 mm cannot be accurately characterized on current MRI and 15% of these will be malignant. 55 59 It is therefore not recommended to attempt staging of nodes by using size criteria.
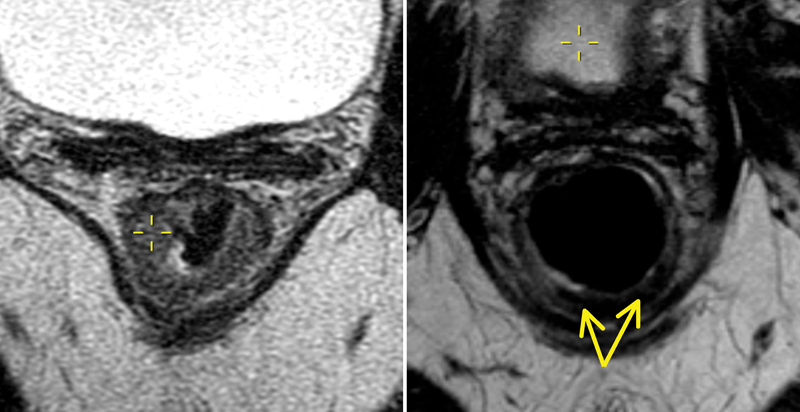
Currently, the most accurate method for nodal staging uses high-resolution MRI and morphological criteria. Normal, benign, or reactive lymph nodes are homogeneous with a preserved capsule, while malignant lymph nodes are irregular and heterogeneous and may demonstrate capsular breach 15 ( Fig. 8 ).
Fig. 8.
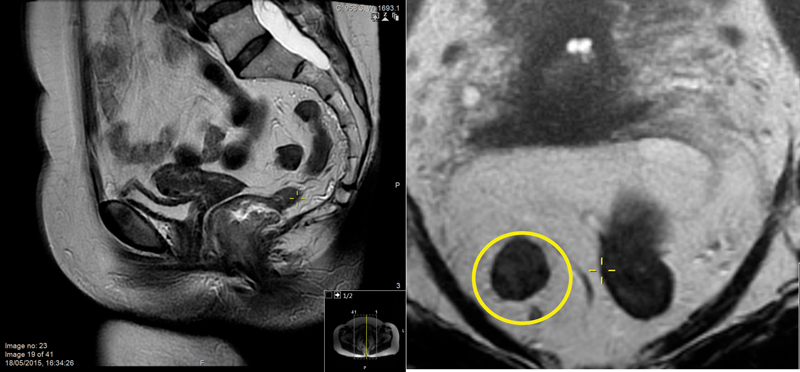
A mesorectal lymph node with a heterogeneous signal intensity seen at 9 o'clock position (yellow circle).
DWI has been shown to identify more mesorectal lymph nodes than T2-weighted turbo spin-echo imaging alone. Although, apparent diffusion coefficient (ADC) is increased in both malignant and reactive nodes and as such cannot be relied upon to usefully discriminate between the two. 60 61
Pelvic Sidewall Disease
The 16 cm field of view use of thin-section MRI enables the pelvic sidewall compartment to be assessed, as well as the mesorectal compartment. This is particularly valuable in identifying patients at risk of residual disease despite a successful TME operation due to pelvic sidewall nodal disease. These include common iliac nodes, internal iliac nodes, and external iliac lymph nodes. 62
Pelvic sidewall disease has been found to be a feature of aggressive tumors and is associated with poor survival. 63 In the United Kingdom and Europe, pelvic side wall nodal dissection is rarely performed due to the morbidity associated with the surgery, but the finding of pelvic sidewall disease should prompt the use of targeted radio- and/or chemotherapy. 19
Extramural Venous Invasion
Extramural venous invasion (EMVI) describes the presence of tumor beyond the outer limits of the muscularis propria in endothelium-lined vessels. 11 Histopathologically, this is seen as tumor involvement of a vascular structure with a smooth muscle wall that will contain elastin on elastin staining. This requires careful inspection and should be suspected if an isolated tumor deposit is seen close to an arterial structure, without an accompanying vein. As tumor is spread beyond the bowel wall, it is more commonly found in locally advanced tumors (T3 and T4), although can be seen in all stage tumors. 64
The reporting of pathological EMVI (pEMVI) remains highly variable, and detection rates range from 9 to 50% 64 65 66 67 with the worst results seen in preoperatively treated rectal cancers. High-resolution MRI produces high-quality anatomical images, and this three-dimensional view allows blood vessels to be tracked and traced; arguably MRI is better placed to identify EMVI. 1 EMVI should be scored as either present or absent on MR and once identified, it should be located as being within a small, medium, or large vessel 15 ( Fig. 9 ).
Fig. 9.
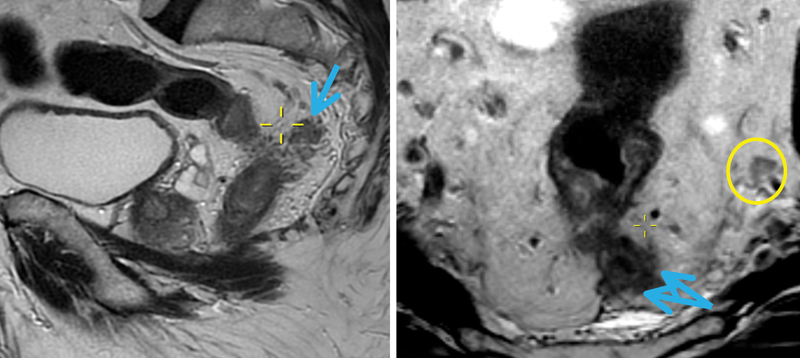
Extramural vascular invasion, affecting the large diameter superior rectal veins (blue arrows). The yellow circle encases the left internal iliac node with irregular borders.
Multiple studies have shown that venous invasion independently predicts for local and distant metastases and reduced survival 68 69 70 and in TME specimens, EMVI is an important predictor of both local and distant failure. 1 Both CT and MRI are capable of identifying EMVI, but in the context of local staging, MRI is clinically more relevant. Sensitivity and specificity have been reported to be between 62 and 100% and 88 and 89%, respectively. 6 70 71
The radiological characteristics of MRI-detected EMVI (mrEMVI) have been previously described. 64 Veins are recognizable on T2-weighted images as serpiginous or tortuous linear structures. Larger vessels appear black owing to signal void, and smaller vessels may be recognized because of tortuosity and branching. Small, unnamed vessels may radiate outward from the edge of the muscularis propria into the perirectal fat. Larger named vessels, such as the superior rectal vein and middle rectal vein, may be visualized in a consistent anatomic position, and a contralateral-paired vessel may also be present, helping with identification.
Assessment of MR images for features suggestive of EMVI must include the following four components: pattern of tumor margin (extension into small veins may produce a nodular border); location of tumor relative to major vessels; caliber of vessel (tumor causes vessel expansion and an increase in tumor signal in the lumen) and vessel border. Smaller venules may also be seen perforating the outer rectal wall and produce a low-to-intermediate signal intensity in tubular structures. EMVI into these smaller vessels can be recognized by their expansion and irregularity adjacent to the tumor. 64 72
An early prospective study of 98 patients undergoing TME surgery for biopsy-proven rectal cancer evaluated the accuracy of MRI in identifying EMVI. EMVI was considered present if a serpiginous extension of tumor signal within a vascular structure was seen on 3 mm slice MRI images. A total of 18 patients had large vessel EMVI visible to the naked eye on hematoxylin-eosin stain and 15 of these 18 cases were shown to have mrEMVI. 11 These were early studies and higher resolution images are now routinely available.
Smith et al performed a retrospective study of 142 patients and reported a sensitivity of 62% and specificity of 88% for the identification of EMVI on MR. 64 Sohn et al reported on mrEMVI in 447 patients and demonstrated a sensitivity and specificity of 28.2 and 94%, respectively. 73 Despite the low sensitivity, MRI is specific and is still more accurate in identifying EMVI than histology, especially after preoperative CRT. 74 The Royal College of Pathologists recommend that MRI be used to assess EMVI and then communicated to pathologists to try and improve detection rates of pEMVI. 75
mrEMVI is of prognostic significance. Smith et al demonstrated that 3-year recurrence free survival was 35% for mrEMVI-positive patients compared with 74.1% for mrEMVI-negative patients, which was similar to pathological EMVI positive and negative patients (34 and 74%, respectively). 64 In addition, it has been shown that a change in mrEMVI status from positive to negative post-CRT improves DFS: 3-year DFS 88%, 9% recurrence in those that convert from positive to negative versus 3-year DFS 46%, with 44% recurrence in those that remain positive ( p < 0.0001). 76 mrEMVI-positive patients with stage II disease had similar survival outcomes to those with stage III disease. 74 In a study by Sohn et al mrEMVI-positive patients were found to have an odds ratio of 3.02 for distant metastases, and that large vessel EMVI positive patients had an odds ratio of 5.27. 73 Smith et al have shown that mrEMVI-positive patients also had a fourfold increased risk of developing distant metastases (52 vs. 12%). 64
Adjacent Organ Involvement
Assessment of adjacent organ involvement is required for those patients undergoing exenterative surgery for locally advanced or recurrent rectal cancer. It allows preoperative surgical planning and time to involve the appropriate specialties. It also aids the selection of patients in whom an R0 resection is possible. The Beyond TME Collaborative 77 recommended the use of MRI as the primary imaging modality for imaging the pelvis in patients with cancers beyond the TME plane, although few studies have specifically addressed this issue. 17
Tumor Height: Low Rectal Cancers
Low rectal tumors are those that arise within 6 cm of the anal verge. A more objective description is that the low rectum begins at the level of origin of the levators. At this point, the natural tapering of the mesorectal envelope means that the distance between the outer muscularis propria and the mesorectal fascia decreases, which increases the likelihood of the tumor being nea, or breaching the distal TME plane. 15 In addition, surgery is often more challenging due to the limited access afforded in a narrow pelvis. These factors increase the risk of a positive CRM. 1 Studies have found a higher recurrence rate, 78 79 a higher mortality rate, 78 a greater risk of the permanent stoma 80 and higher rates of anastomotic dehiscence 81 in these patients. As a result, there is a conflict between the optimal oncological approach and how best to preserve bowel function.
With an increasing number of complex options, a validated and accurate radiological assessment is vital. Preoperative assessment must combine feasibility of sphincter preservation by assessing the distance from the distal edge of the tumor to the top of the puborectalis sling seen on MRI and assessment of the radial extent of the tumor in relation to the distal TME plane. To address these anatomical complexities, a specific staging system has been devised for low rectal tumors that have been validated in the MERCURY II: Low Rectal Cancer Study. 43 This identifies those tumors that encroach or breach the intersphincteric plane and allows preoperative planning for a more radical approach, such as an extralevator abdominoperineal excision (ELAPE). Fig. 10 demonstrates the consequences of inadequate preoperative staging, while Table 1 describes the four stages of the low rectal cancer (mLR) system. 1
Fig. 10.
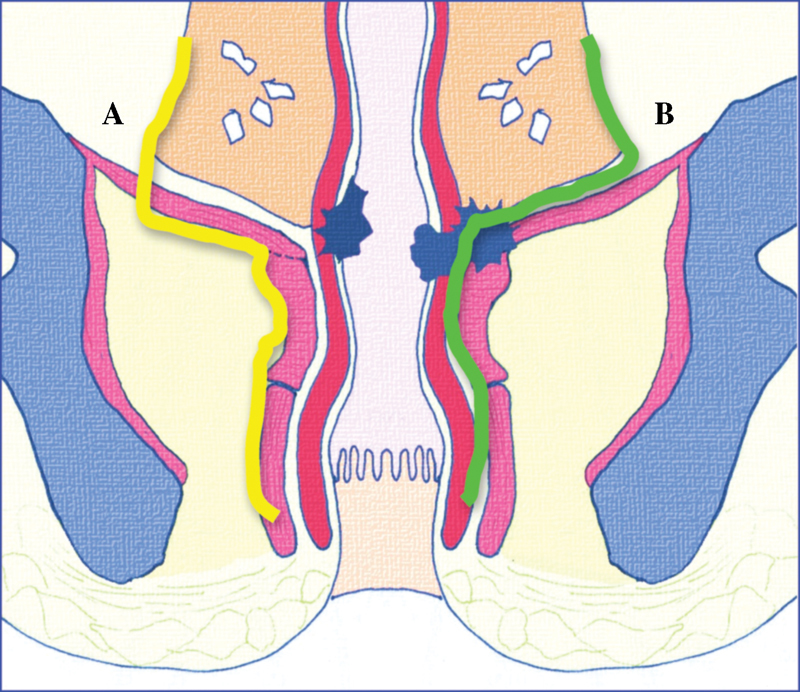
Pelvic floor and low rectum (oblique coronal view). The solid green line indicates intersphincteric plane; solid yellow line indicates plane for extralevator abdominoperineal excision (ELAPE). Early-stage tumor ( A ) has not invaded beyond the muscularis propria; overtreatment with ELAPE, yellow line. Late-stage tumor ( B ) has breached the muscularis propria/internal sphincter and has invaded beyond the intersphincteric space into the levator ani and puborectalis muscle; following the total mesorectal excision plane (green line) would lead to an involved circumferential resection margin.
Table 1. MRI low rectal cancer staging system 1 .
| MRI stage | Anatomical definition |
|---|---|
| mrLR1 | Tumor confined to the bowel wall and does not extend through the full thickness (intact outer muscle coat) |
| mrLR2 | Tumor replaces the muscle coat but does not extend into the intersphincteric plane |
| mrLR 3 | Tumor invades the intersphincteric space or lies within 1 mm of the levator muscle |
| mrLR 4 | Tumor invades the external anal sphincter and is within 1 mm and beyond levator muscle with or without invading the adjacent structures |
Abbreviation: MRI, magnetic resonance imaging.
Reassessment after Neoadjuvant Chemoradiotherapy
Given the huge variation in response to neoadjuvant CRT (with ∼20% who show no response, and up to 50% who may demonstrate a complete clinical response), accurate restaging of patients is vital to avoid under- and overtreatment. 82
T- and N-Stage
Memon et al performed a meta-analysis of 12 studies investigating the accuracy of MRI in the restaging of rectal cancer following neoadjuvant CRT in 2015. The average T-stage restaging accuracy was found to be 52% (95% CI: 44–59%) although this may in part be due to the wide variation in MRI technique seen within the studies. N-stage restaging accuracy was assessed in 14 articles and ranged from 60 to 88% with a mean accuracy of 72%. 83 Again, there was significant heterogeneity in the criteria used to identify malignant lymph nodes, with some studies using only the size, others using morphological criteria and those that remained, combined both.
Studies that have used high-resolution T2-weighted MRI rather than DWI or gadolinium-enhanced MRI have reported higher accuracy in the assessment of T-stage and N-stage after CRT. In addition, ymrT and ymrN responders were found to have a similar 3-year OS when compared with histopathological outcomes (80 and 79%, respectively). 84 ymrT-stage is of some value for assessing response to CRT, but it should be used in conjunction with other assessment measures such as post-CRT CRM involvement (ymrCRM), post-CRT EMVI (ymrEMVI), 74 and magnetic resonance tumor regression grade (mrTRG) ( Table 2 ). 85
Table 2. mrTRG score 85 .
| mrTRG grade | Radiological findings on MRI |
|---|---|
| 1 | Radiological complete response (linear/crescenteric 1–2 mm scar in mucosa or submucosa only) |
| 2 | Good response (dense fibrosis; no obvious residual tumor, signifying minimal residual disease or no tumor) |
| 3 | Moderate response (>50% fibrosis or mucin, and visible intermediate signal) |
| 4 | Slight response (little areas of fibrosis or mucin but mostly tumor) |
| 5 | No response (intermediate signal intensity, same appearances as original tumor/tumor regrowth) |
Abbreviations: MRI, magnetic resonance imaging; mrTRG, MRI tumor regression grade.
ymrCRM
The MERCURY Study Group prospectively investigated the accuracy of high-resolution MRI for detecting involvement of the surgical CRM after CRT in a subset of 97 patients. 6 Histopathological CRM (pCRM) involvement occurred in 1% (1/97) MRI-predicted CRM (mrCRM)–negative patients. When the posttreatment MRI predicted a positive CRM (ymrCRM), a histologically clear CRM (ypCRM) occurred in 55% (21/38) of patients. However, there is some evidence to suggest that pathology may actually understage. Hole et al have shown that reviewing pathology with the assistance of the posttreatment MRI significantly increases the likelihood of identifying pCRM involvement. 86 In addition, an involved mrCRM is itself, an independent predictor of local recurrence. 5-year OS and DFS was 62.2 and 67.2% in patients with a negative mrCRM compared with 42.2 and 47.3% in patients with an involved mrCRM, respectively. mrCRM was the only preoperative staging parameter that remained significant for OS, DFS, and LR on multivariate analysis. 3 50
ymrEMVI
In a study by Yu et al where 96% of patients had >mrT3c disease, 78% (219/281) patients had evidence of mrEMVI on their baseline MRI. These patients were significantly less likely to respond to neoadjuvant CRT than mrEMVI-negative patients (odds ratio: 2.5). However, when CRT did succeed in changing ymrEMVI status from positive to negative (seen in 81/281); this was associated with good overall outcomes. 84 The MARVEL study (NCT01995942) is a European multicenter observational study that is currently recruiting patients to validate an mrEMVI regression grade, 76 and it may be that in the future, such patients may benefit from treatment intensification or alternative preoperative therapy.
Post-CRT Tumor Response Assessment
Prediction and evaluation of response to neoadjuvant chemoradiotherapy are important. Differentiating between the good and poor responders allows treatment to be tailored to individual patients. Poor responders may benefit from a change in treatment; radiotherapy dose escalation, chemotherapy regime change, early surgery, extended plane surgery, or best supportive care, while prediction of complete pathological response may allow organ preservation. It has been suggested that complete pathological response is viewed as a lost opportunity to avoid surgery, as these patients have by definition have undergone resection of a disease-free organ with questionable oncological benefit. 17
Modalities such as endoanal ultrasound (EUS), CT, PET, and MRI have all been investigated for their ability to detect a posttreatment response.
Despite the popularity of EUS as a staging tool for rectal cancer, it is hampered post-CRT. It has been well documented, that sonographically, it is impossible to distinguish tumor necrosis, fibrosis, and inflammation from residual tumor postradiotherapy. There is a tendency therefore for EUS to overstage patients. 87 88 Pastor et al used EUS to assess tumor response after neoadjuvant CRT in 235 patients. A total of 20% were misclassified as clinical complete response (cCR), and overall, overstaging was seen in 37%. They concluded that this was unacceptably high. 89 A study by Rau et al in 84 patients had similar results, and the authors drew parallel conclusions. 90
A review article by Schaefer et al. summarized results for six studies that examined the use of CT in the detection of the first recurrence of rectal cancer and found that the sensitivity ranged from 1 to 41%. 91 A study of 23 patients that looked specifically at the ability of CT to assess response to treatment found that CT had a sensitivity of 54% and a specificity of 80%. 92 Despite the use of CT in the surveillance of colorectal cancer, it's inability to distinguish tumor from fibrosis posttreatment, reduces its value in assessing for a cCR. 93
PET scans are highly sensitive and specific and as such, are recommended for use in the workup of metastatic colorectal cancer. However, the cost and limited availability of PET remain disadvantages. A study by Chennupati looked at 35 patients and found no correlation between standardized uptake value and metabolic tumor volume when comparing good and poor responders. 94 In a prospective study by Guillem et al, 121 patients with rectal cancer underwent PET before and after CRT. Results were compared with pathology specimens. PET was found to detect a pathological complete response (pCR) in 54% and a non-pCR in 66%. 95 The authors concluded that there was only limited value in using PET to assess response to treatment.
At present, MRI is the staging modality of choice for rectal cancer posttreatment. 96 97 Increasing focus has been placed on the ability of MRI to predict complete pathological response following re-imaging after treatment. Wu et al. performed a meta-analysis of 14 studies in 2013 to investigate this. Five of the earlier studies used morphological change on T2-weighted imaging alone to predict response and were found to have a relatively low sensitivity (64%) with a high specificity (88%) for predicting pathological response. Nine of the later studies included DWI with or without ADC map cutoff values, together with T2-weighted imaging, and had a significantly higher sensitivity of 92% although a slightly lower specificity of 75% for predicting pathological response. 98
Yeo et al. investigated tumor volume reduction rate (TVRR) on rectal MRI following neoadjuvant CRT as a means to predict pathological tumor regression, complete pathological response, DFS, and OS in 430 patients. TVRR was significantly associated with tumor regression, complete response, DFS, and OS. 99 100 However, despite promising results, there are no published precise/accurate cutoff values to predict a favorable response. 2 Modified mrRECIST is based on objective measurements of the change in craniocaudal length of measurable disease and has been proposed as a tool for tumor response assessment. Guidelines state that a 30% reduction should be considered a favorable response. 101 However, there is no established threshold for defining response in a luminal organ such as the rectum.
mrTRG is based on the principles of histopathology tumor regression grading, and most resemble the Mandard pathological TRG score (pTRG). 102 The ability to differentiate between fibrosis and residual disease on MRI has improved with advances in technology 1 and the techniques used to score these patients are reproducible. 3 When examining the images, radiologists should initially decide whether the residual mass is predominantly tumor or fibrosis. After that, determining the exact TRG depends on the relative degree of fibrosis and residual tumor 15 ( Figs. 6 , 11 12 13 ).
Fig. 11.
Upper row (pre-CRT): a midrectal tumor infiltrating rectal wall at 5 to 8 o'clock position and discontinuous vascular spread (extramural venous invasion [EMVI]) at 5 o'clock (blue arrow) that abuts the mesorectal fascia. Lower row (post-CRT): a tiny scar at the site of treated tumor (barely visible and a completely fibrotic vascular deposit in the mesorectum at the level of EMVI (blue arrow), suggesting mrTRG1. mrTRG, MRI tumor regression grade.
Fig. 12.
Pretreatment (left) semiannular mass infiltrating rectal wall at 4 to 7 o'clock at which level tumor borders the interspincteric plane. Posttreatment image shows a good response to treatment with dense fibrotic scar (yellow arrow) at the site of the treated disease (mrTRG2). mrTRG, MRI tumor regression grade.
Fig. 13.
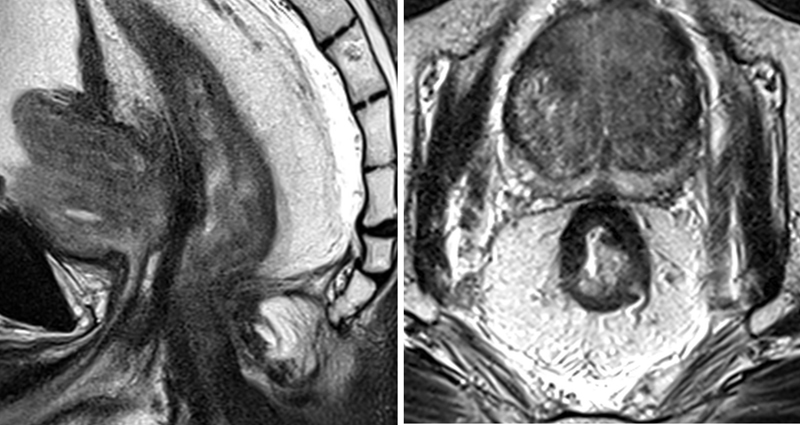
Midrectal tumor invading the anterior quadrant of the rectal wall (left). Posttreatment MRI shows predominating dense fibrosis with some minimal intermediate signal (right), suggesting mrTRG3. MRI, magnetic resonance imaging; mrTRG, MRI tumor regression grade.
Patel et al demonstrated a significant difference in DFS and OS between mrTRG1–3 (good response) and mrTRG4–5 (poor response) ( p < 0.001); the 5-year DFS was 72 and 27%, respectively. 85 The EXPERT-C trial data reported similar findings; a significant difference in DFS and OS was reported: mrTRG1–2 (good response), mrTRG3 (intermediate response), and mrTRG4–5 (poor response) had a 3-year DFS of 82, 72, and 61%, respectively. A recent study examined interobserver reliability between 35 radiologists and 1 central reviewer for mrTRG. Kappa agreement had a median value of 0.57 (95% CI: 0.37–0.77) indicating an overall good agreement. In 65.9 and 90% of cases, the radiologists were able to correctly highlight good and poor responders, respectively. The authors concluded that with minimal training, good agreement and differentiation between good and intermediate/poor responders could be achieved. 103
The mrTRG appears to be a valuable way of differentiating between the “responder” and “nonresponder.” It has been evaluated in several prospective trials and is emerging as a promising biomarker for evaluation of response, which may be used to stratify rectal cancer patients with regard to future management. Patients that show a “good” response to CRT (mrTRG1&2) appear to behave similarly to those who achieve a complete pathological response. 3 This cohort may benefit from the option of a deferral of surgery approach within a controlled trial. Equally, recognition of non-responders (mrTRG4&5) may prompt the use of further therapy and/or a novel pharmacotherapy in the hope of changing a patients' mrTRG status to a prognostically more favorable group. 1 104 We would, therefore, currently recommend using mrTRG on high-resolution T2-weighted images postneoadjuvant CRT to assess and predict response. Stratifying patients according to their mrTRG status will be conclusively tested in the prospective randomized clinical TRIGGER trial (UKCRN 20576). Patients will be randomized between a standard of care that does not re-evaluate tumor stage after CRT versus a mrTRG-based approach that offers deferral of surgery to favorable mrTRG patients or additional pharmacotherapy to non-responders to try and further downstage the tumor.
Posttreatment Assessment of the Distal TME Plane (ymrLR)
Low rectal cancers are associated with a favorable response to CRT. When the inferior tumor border is ≤ 5cm from the anal verge, greater downstaging is seen compared with that seen in upper and mid rectal tumors. This may be because of reduced organ mobility and a greater likelihood of the rectum receiving the prescribed dose of radiotherapy. 84 The mrLR staging system is currently being prospectively evaluated in the MERCURY II low rectal cancer trial. It is intended to assess the extent of tumor downstaging and assess the feasibility of a CRM clear intersphincteric resection. Although, whether surgeons will change their operative plane following downstaging of the tumor remains to be seen. 1
Five-Year View
MRI is central to the anatomical assessment of rectal cancer and looks set to remain the imaging modality of choice for the local assessment of rectal cancer. Improvements in MRI field strength and homogeneity, multichannel transmitting and receiving coils, and MRI sequences will lead to continuing improvement in anatomical imaging.
Despite a good deal of interest in physiological tumor assessment with multiparametric MRI, a multiplicity of parameters has been identified in small studies that have not been validated in external studies. The supporting published evidence for the use of MRI has been based on high-resolution narrow field view. There is a danger that publications using poor-quality MRI may mislead the clinician and limit the clinical application of MRI. Instead, further education and training in the accurate interpretation of correctly acquired high-resolution T2-weighted MRI images are what is likely to deliver improved patient care.
In the coming years, the incorporation of accurate MRI-based tumor assessment into therapeutic clinical trials will allow the development of evidence-based treatment strategies and individualized care with each stage of the disease. This will enable patient-tailored treatment based on prognostic features at presentation, response to neoadjuvant therapy and recurrent disease during follow-up with the hope of minimizing morbidity without compromising oncological outcomes.
Acknowledgment
Thank you to Mr Nick Battersby for providing image 10.
References
- 1.Battersby N J, Moran B, Yu S, Tekkis P, Brown G. MR imaging for rectal cancer: the role in staging the primary and response to neoadjuvant therapy. Expert Rev Gastroenterol Hepatol. 2014;8(06):703–719. doi: 10.1586/17474124.2014.906898. [DOI] [PubMed] [Google Scholar]
- 2.Balyasnikova S, Brown G. Optimal imaging strategies for rectal cancer staging and ongoing management. Curr Treat Options Oncol. 2016;17(06):32. doi: 10.1007/s11864-016-0403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel U B, Taylor F, Blomqvist L et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29(28):3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 4.Taylor F G, Swift R I, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191(06):1827–1835. doi: 10.2214/AJR.08.1004. [DOI] [PubMed] [Google Scholar]
- 5.Glynne-Jones R, Harrison M, Hughes R. Challenges in the neoadjuvant treatment of rectal cancer: balancing the risk of recurrence and quality of life. Cancer Radiother. 2013;17(07):675–685. doi: 10.1016/j.canrad.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 6.MERCURY Study Group.Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study BMJ 2006333(7572):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MERCURY Study Group.Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study Radiology 200724301132–139. [DOI] [PubMed] [Google Scholar]
- 8.Salerno G, Daniels I R, Moran B J, Wotherspoon A, Brown G. Clarifying margins in the multidisciplinary management of rectal cancer: the MERCURY experience. Clin Radiol. 2006;61(11):916–923. doi: 10.1016/j.crad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen L B, Wille-Jørgensen P. National and international guidelines for rectal cancer. Colorectal Dis. 2014;16(11):854–865. doi: 10.1111/codi.12678. [DOI] [PubMed] [Google Scholar]
- 10.Brown G. Staging rectal cancer: endoscopic ultrasound and pelvic MRI. Cancer Imaging. 2008;8 Spec No A:S43–S45. doi: 10.1102/1470-7330.2008.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown G, Radcliffe A G, Newcombe R G, Dallimore N S, Bourne M W, Williams G T. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90(03):355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 12.Burton S, Brown G, Daniels I R, Norman A R, Mason B, Cunningham D; Royal Marsden Hospital, Colorectal Cancer Network.MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer 20069403351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown G, Daniels I R, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol. 2005;78(927):245–251. doi: 10.1259/bjr/33540239. [DOI] [PubMed] [Google Scholar]
- 14.Stoker J, Rociu E, Zwamborn A W, Schouten W R, Laméris J S. Endoluminal MR imaging of the rectum and anus: technique, applications, and pitfalls. Radiographics. 1999;19(02):383–398. doi: 10.1148/radiographics.19.2.g99mr01383. [DOI] [PubMed] [Google Scholar]
- 15.Wale A, Brown G. A practical review of the performance and interpretation of staging magnetic resonance imaging for rectal cancer. Top Magn Reson Imaging. 2014;23(04):213–223. doi: 10.1097/RMR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 16.Koh D M, Brown G, Temple L et al. Distribution of mesorectal lymph nodes in rectal cancer: in vivo MR imaging compared with histopathological examination. Initial observations. Eur Radiol. 2005;15(08):1650–1657. doi: 10.1007/s00330-005-2751-8. [DOI] [PubMed] [Google Scholar]
- 17.Hunter C, Brown G. Pre-operative staging of rectal cancer: a review of imaging techniques. Expert Rev Gastroenterol Hepatol. 2016;10(09):1011–1025. doi: 10.1080/17474124.2016.1179577. [DOI] [PubMed] [Google Scholar]
- 18.Bhangu A, Rasheed S, Brown G, Tait D, Cunningham D, Tekkis P. Does rectal cancer height influence the oncological outcome? Colorectal Dis. 2014;16(10):801–808. doi: 10.1111/codi.12703. [DOI] [PubMed] [Google Scholar]
- 19.Brown G.Thin section MRI in multidisciplinary pre-operative decision making for patients with rectal cancer Br J Radiol 200578(Spec No 2):S117–S127. [DOI] [PubMed] [Google Scholar]
- 20.Taylor F G, Quirke P, Heald R J et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253(04):711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 21.Brown G.Mri IN STaging REctal polyp planes (2015)Available at:http://minstrelstudy.co.uk/downloads/trial-protocol.pdf. Accessed July 2016
- 22.Borstlap W A, Tanis P J, Koedam T W et al. A multi-centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer. 2016;16(01):513. doi: 10.1186/s12885-016-2557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz M P, Zalcberg J R, Glynne-Jones R et al. Second St. Gallen European Organisation for Research and Treatment of Cancer Gastrointestinal Cancer Conference: consensus recommendations on controversial issues in the primary treatment of rectal cancer. Eur J Cancer. 2016;63:11–24. doi: 10.1016/j.ejca.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Fleming I D, Cooper J S, Henson D E . Chicago, IL: American Joint Committee on Cancer; 1997. AJCC Cancer Staging Manual. 5th ed. [Google Scholar]
- 25.Compton C C, Greene F L. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54(06):295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 26.Cawthorn S J, Parums D V, Gibbs N Met al. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer [see comments] Lancet 1990335(8697):1055–1059. [DOI] [PubMed] [Google Scholar]
- 27.Dukes C E, Bussey H J. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958;12(03):309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jass J R, Love S B. Prognostic value of direct spread in Dukes' C cases of rectal cancer. Dis Colon Rectum. 1989;32(06):477–480. doi: 10.1007/BF02554501. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd N A.Mesorectal spread and involvement of lateral resection margin in rectal cancer [letter; comment] Lancet 1990335(8702):1402–1403. [DOI] [PubMed] [Google Scholar]
- 30.Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16(05):298–304. doi: 10.1007/s003840100309. [DOI] [PubMed] [Google Scholar]
- 31.Harrison J C, Dean P J, el-Zeky F, Vander Zwaag R. From Dukes through Jass: pathological prognostic indicators in rectal cancer. Hum Pathol. 1994;25(05):498–505. doi: 10.1016/0046-8177(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 32.Brandt W S, Yong S, Abood G, Micetich K, Walther A, Shoup M.The depth of post-treatment perirectal tissue invasion is a predictor of outcome in patients with clinical T3N1M0 rectal cancer treated with neoadjuvant chemoradiation followed by surgical resection Am J Surg 201420703357–360., discussion 360 [DOI] [PubMed] [Google Scholar]
- 33.Cho S H, Kim S H, Bae J H et al. Prognostic stratification by extramural depth of tumor invasion of primary rectal cancer based on the Radiological Society of North America proposal. AJR Am J Roentgenol. 2014;202(06):1238–1244. doi: 10.2214/AJR.13.11311. [DOI] [PubMed] [Google Scholar]
- 34.Cianchi F, Messerini L, Comin C E et al. Pathologic determinants of survival after resection of T3N0 (Stage IIA) colorectal cancer: proposal for a new prognostic model. Dis Colon Rectum. 2007;50(09):1332–1341. doi: 10.1007/s10350-007-0222-9. [DOI] [PubMed] [Google Scholar]
- 35.Merkel S, Weber K, Schellerer V et al. Prognostic subdivision of ypT3 rectal tumours according to extension beyond the muscularis propria. Br J Surg. 2014;101(05):566–572. doi: 10.1002/bjs.9419. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi M, Ueno H, Hashiguchi Y, Mochizuki H, Talbot I C. Extent of mesorectal tumor invasion as a prognostic factor after curative surgery for T3 rectal cancer patients. Ann Surg. 2006;243(04):492–498. doi: 10.1097/01.sla.0000205627.05769.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollheimer M J, Kornprat P, Pollheimer V S et al. Clinical significance of pT sub-classification in surgical pathology of colorectal cancer. Int J Colorectal Dis. 2010;25(02):187–196. doi: 10.1007/s00384-009-0801-4. [DOI] [PubMed] [Google Scholar]
- 38.Shin R, Jeong S Y, Yoo H Y et al. Depth of mesorectal extension has prognostic significance in patients with T3 rectal cancer. Dis Colon Rectum. 2012;55(12):1220–1228. doi: 10.1097/DCR.0b013e31826fea6a. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida K, Yoshimatsu K, Otani T, Yokomizo H, Ogawa K.The depth of tumor invasion beyond the outer border of the muscularis propria as a prognostic factor for T3 rectal/rectosigmoid cancer Anticancer Res 200828(3B, 3b):1773–1778. [PubMed] [Google Scholar]
- 40.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47(01):20–31. doi: 10.1080/02841860701697720. [DOI] [PubMed] [Google Scholar]
- 41.Al-Sukhni E, Milot L, Fruitman M et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19(07):2212–2223. doi: 10.1245/s10434-011-2210-5. [DOI] [PubMed] [Google Scholar]
- 42.Bissett I P, Fernando C C, Hough D M et al. Identification of the fascia propria by magnetic resonance imaging and its relevance to preoperative assessment of rectal cancer. Dis Colon Rectum. 2001;44(02):259–265. doi: 10.1007/BF02234302. [DOI] [PubMed] [Google Scholar]
- 43.Battersby N J, How P, Moran B et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: The MERCURY II study. Ann Surg. 2016;263(04):751–760. doi: 10.1097/SLA.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 44.Xie H, Zhou X, Zhuo Z, Che S, Xie L, Fu W. Effectiveness of MRI for the assessment of mesorectal fascia involvement in patients with rectal cancer: a systematic review and meta-analysis. Dig Surg. 2014;31(02):123–134. doi: 10.1159/000363075. [DOI] [PubMed] [Google Scholar]
- 45.Wibe A, Rendedal P R, Svensson E et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89(03):327–334. doi: 10.1046/j.0007-1323.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- 46.Adam I J, Mohamdee M O, Martin I Get al. Role of circumferential margin involvement in the local recurrence of rectal cancer Lancet 1994344(8924):707–711. [DOI] [PubMed] [Google Scholar]
- 47.Taylor F G, Quirke P, Heald R J et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32(01):34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 48.Gaertner W B, Kwaan M R, Madoff R D, Melton G B. Rectal cancer: An evidence-based update for primary care providers. World J Gastroenterol. 2015;21(25):7659–7671. doi: 10.3748/wjg.v21.i25.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiser M R, Fichera A, Schrag D, Boughey J C, You Y N. Progress in the PROSPECT trial: precision treatment for rectal cancer? Bull Am Coll Surg. 2015;100(04):51–52. [PubMed] [Google Scholar]
- 50.Taylor F G, Quirke P, Heald R J et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011;98(06):872–879. doi: 10.1002/bjs.7458. [DOI] [PubMed] [Google Scholar]
- 51.Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist L E, Wilking N; Swedish Rectal Cancer Trial.Improved survival with preoperative radiotherapy in resectable rectal cancer N Engl J Med 199733614980–987. [DOI] [PubMed] [Google Scholar]
- 52.Bondeven P, Hagemann-Madsen R H, Laurberg S, Pedersen B G. Extent and completeness of mesorectal excision evaluated by postoperative magnetic resonance imaging. Br J Surg. 2013;100(10):1357–1367. doi: 10.1002/bjs.9225. [DOI] [PubMed] [Google Scholar]
- 53.Hermanek P, Merkel S, Fietkau R, Rödel C, Hohenberger W. Regional lymph node metastasis and locoregional recurrence of rectal carcinoma in the era of TME [corrected] surgery. Implications for treatment decisions. Int J Colorectal Dis. 2010;25(03):359–368. doi: 10.1007/s00384-009-0864-2. [DOI] [PubMed] [Google Scholar]
- 54.Yano H, Moran B J, Watanabe T, Sugihara K.Lateral pelvic lymph-node dissection: still an option for cure Lancet Oncol 20101102114, author reply 114–115 [DOI] [PubMed] [Google Scholar]
- 55.Brown G, Richards C J, Bourne M W et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227(02):371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 56.Kim J H, Beets G L, Kim M J, Kessels A G, Beets-Tan R G. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52(01):78–83. doi: 10.1016/j.ejrad.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Arii K, Takifuji K, Yokoyama S et al. Preoperative evaluation of pelvic lateral lymph node of patients with lower rectal cancer: comparison study of MR imaging and CT in 53 patients. Langenbecks Arch Surg. 2006;391(05):449–454. doi: 10.1007/s00423-006-0066-0. [DOI] [PubMed] [Google Scholar]
- 58.Dworak O. Morphology of lymph nodes in the resected rectum of patients with rectal carcinoma. Pathol Res Pract. 1991;187(08):1020–1024. doi: 10.1016/S0344-0338(11)81075-7. [DOI] [PubMed] [Google Scholar]
- 59.Park J S, Jang Y J, Choi G S et al. Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: node-for-node matched histopathology validation of MRI features. Dis Colon Rectum. 2014;57(01):32–38. doi: 10.1097/DCR.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 60.Heijnen L A, Lambregts D M, Mondal D et al. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol. 2013;23(12):3354–3360. doi: 10.1007/s00330-013-2952-5. [DOI] [PubMed] [Google Scholar]
- 61.Lambregts D M, Maas M, Riedl R G et al. Value of ADC measurements for nodal staging after chemoradiation in locally advanced rectal cancer-a per lesion validation study. Eur Radiol. 2011;21(02):265–273. doi: 10.1007/s00330-010-1937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim D J, Chung J J, Yu J S, Cho E S, Kim J H. Evaluation of lateral pelvic nodes in patients with advanced rectal cancer. AJR Am J Roentgenol. 2014;202(06):1245–1255. doi: 10.2214/AJR.13.11228. [DOI] [PubMed] [Google Scholar]
- 63.Hida J, Yasutomi M, Fujimoto K, Maruyama T, Okuno K, Shindo K. Does lateral lymph node dissection improve survival in rectal carcinoma? Examination of node metastases by the clearing method. J Am Coll Surg. 1997;184(05):475–480. [PubMed] [Google Scholar]
- 64.Smith N J, Shihab O, Arnaout A, Swift R I, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191(05):1517–1522. doi: 10.2214/AJR.08.1298. [DOI] [PubMed] [Google Scholar]
- 65.Talbot I C, Ritchie S, Leighton M H, Hughes A O, Bussey H J, Morson B C. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67(06):439–442. doi: 10.1002/bjs.1800670619. [DOI] [PubMed] [Google Scholar]
- 66.Freedman L S, Macaskill P, Smith A N.Multivariate analysis of prognostic factors for operable rectal cancer Lancet 19842(8405):733–736. [DOI] [PubMed] [Google Scholar]
- 67.Chand M, Siddiqui M R, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol. 2016;22(04):1721–1726. doi: 10.3748/wjg.v22.i4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Günther K, Dworak O, Remke S et al. Prediction of distant metastases after curative surgery for rectal cancer. J Surg Res. 2002;103(01):68–78. doi: 10.1006/jsre.2001.6312. [DOI] [PubMed] [Google Scholar]
- 69.Rich T, Gunderson L L, Lew R, Galdibini J J, Cohen A M, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52(07):1317–1329. doi: 10.1002/1097-0142(19831001)52:7<1317::aid-cncr2820520731>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 70.Smith N J, Barbachano Y, Norman A R, Swift R I, Abulafi A M, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95(02):229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- 71.Koh D M, Smith N J, Swift R I, Brown G. The relationship between MR demonstration of extramural venous invasion and nodal disease in rectal cancer. Clin Med Oncol. 2008;2:267–273. doi: 10.4137/cmo.s370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown G, Kirkham A, Williams G T et al. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol. 2004;182(02):431–439. doi: 10.2214/ajr.182.2.1820431. [DOI] [PubMed] [Google Scholar]
- 73.Sohn B, Lim J S, Kim H et al. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. 2015;25(05):1347–1355. doi: 10.1007/s00330-014-3527-9. [DOI] [PubMed] [Google Scholar]
- 74.Chand M, Evans J, Swift R I et al. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg. 2015;261(03):473–479. doi: 10.1097/SLA.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 75.Balyasnikova S, Brown G. Imaging advances in colorectal cancer. Curr Colorectal Cancer Rep. 2016;12:162–169. doi: 10.1007/s11888-016-0321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chand M, Swift R I, Tekkis P P, Chau I, Brown G. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2014;110(01):19–25. doi: 10.1038/bjc.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beyond TME Collaborative.Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes Br J Surg 2013100081009–1014. [DOI] [PubMed] [Google Scholar]
- 78.Nagtegaal I D, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(02):303–312. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 79.Jörgren F, Johansson R, Damber L, Lindmark G. Oncological outcome after incidental perforation in radical rectal cancer surgery. Int J Colorectal Dis. 2010;25(06):731–740. doi: 10.1007/s00384-010-0930-9. [DOI] [PubMed] [Google Scholar]
- 80.Birbeck K F, Macklin C P, Tiffin N J et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235(04):449–457. doi: 10.1097/00000658-200204000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moran B J. Predicting the risk and diminishing the consequences of anastomotic leakage after anterior resection for rectal cancer. Acta Chir Iugosl. 2010;57(03):47–50. doi: 10.2298/aci1003047m. [DOI] [PubMed] [Google Scholar]
- 82.Habr-Gama A, Sabbaga J, Gama-Rodrigues J et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56(10):1109–1117. doi: 10.1097/DCR.0b013e3182a25c4e. [DOI] [PubMed] [Google Scholar]
- 83.Memon S, Lynch A C, Bressel M, Wise A G, Heriot A G. Systematic review and meta-analysis of the accuracy of MRI and endorectal ultrasound in the restaging and response assessment of rectal cancer following neoadjuvant therapy. Colorectal Dis. 2015;17(09):748–761. doi: 10.1111/codi.12976. [DOI] [PubMed] [Google Scholar]
- 84.Yu S K, Tait D, Chau I, Brown G. MRI predictive factors for tumor response in rectal cancer following neoadjuvant chemoradiation therapy--implications for induction chemotherapy? Int J Radiat Oncol Biol Phys. 2013;87(03):505–511. doi: 10.1016/j.ijrobp.2013.06.2052. [DOI] [PubMed] [Google Scholar]
- 85.Patel U B, Brown G, Rutten H et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012;19(09):2842–2852. doi: 10.1245/s10434-012-2309-3. [DOI] [PubMed] [Google Scholar]
- 86.Hole K H, Larsen S G, Grøholt K K, Giercksky K E, Ree A H. Magnetic resonance-guided histopathology for improved accuracy of tumor response evaluation of neoadjuvant treatment in organ-infiltrating rectal cancer. Radiother Oncol. 2013;107(02):178–183. doi: 10.1016/j.radonc.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 87.Alcaide J, Funez R, Rueda A et al. The role and prognostic value of apoptosis in colorectal carcinoma. BMC Clin Pathol. 2013;13(01):24. doi: 10.1186/1472-6890-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanagunas A, Lin D E, Stryker S J. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99(01):109–112. doi: 10.1046/j.1572-0241.2003.04019.x. [DOI] [PubMed] [Google Scholar]
- 89.Pastor C, Subtil J C, Sola J et al. Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum. 2011;54(09):1141–1146. doi: 10.1097/DCR.0b013e31821c4a60. [DOI] [PubMed] [Google Scholar]
- 90.Rau B, Hünerbein M, Barth C et al. Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc. 1999;13(10):980–984. doi: 10.1007/s004649901151. [DOI] [PubMed] [Google Scholar]
- 91.Schaefer O, Langer M. Detection of recurrent rectal cancer with CT, MRI and PET/CT. Eur Radiol. 2007;17(08):2044–2054. doi: 10.1007/s00330-007-0613-2. [DOI] [PubMed] [Google Scholar]
- 92.Denecke T, Rau B, Hoffmann K T et al. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005;15(08):1658–1666. doi: 10.1007/s00330-005-2658-4. [DOI] [PubMed] [Google Scholar]
- 93.Walker A S, Zwintscher N P, Johnson E K et al. Future directions for monitoring treatment response in colorectal cancer. J Cancer. 2014;5(01):44–57. doi: 10.7150/jca.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chennupati S K, Quon A, Kamaya A et al. Positron emission tomography for predicting pathologic response after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Am J Clin Oncol. 2012;35(04):334–339. doi: 10.1097/COC.0b013e3182118d12. [DOI] [PubMed] [Google Scholar]
- 95.Guillem J G, Ruby J A, Leibold T et al. Neither FDG-PET nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: a prospective study. Ann Surg. 2013;258(02):289–295. doi: 10.1097/SLA.0b013e318277b625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Neill B D, Brown G, Heald R J, Cunningham D, Tait D M. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 2007;8(07):625–633. doi: 10.1016/S1470-2045(07)70202-4. [DOI] [PubMed] [Google Scholar]
- 97.Dewhurst C E, Mortele K J. Magnetic resonance imaging of rectal cancer. Radiol Clin North Am. 2013;51(01):121–131. doi: 10.1016/j.rcl.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 98.Wu L M, Zhu J, Hu J et al. Is there a benefit in using magnetic resonance imaging in the prediction of preoperative neoadjuvant therapy response in locally advanced rectal cancer? Int J Colorectal Dis. 2013;28(09):1225–1238. doi: 10.1007/s00384-013-1676-y. [DOI] [PubMed] [Google Scholar]
- 99.Yeo S G, Kim D Y, Kim T H et al. Tumor volume reduction rate measured by magnetic resonance volumetry correlated with pathologic tumor response of preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;78(01):164–171. doi: 10.1016/j.ijrobp.2009.07.1682. [DOI] [PubMed] [Google Scholar]
- 100.Yeo S G, Kim D Y, Park J W et al. Tumor volume reduction rate after preoperative chemoradiotherapy as a prognostic factor in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82(02):e193–e199. doi: 10.1016/j.ijrobp.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 101.Therasse P, Arbuck S G, Eisenhauer E A et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(03):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 102.Shihab O C, Taylor F, Salerno G et al. MRI predictive factors for long-term outcomes of low rectal tumours. Ann Surg Oncol. 2011;18(12):3278–3284. doi: 10.1245/s10434-011-1776-2. [DOI] [PubMed] [Google Scholar]
- 103.Siddiqui M R, Gormly K L, Bhoday J et al. Interobserver agreement of radiologists assessing the response of rectal cancers to preoperative chemoradiation using the MRI tumour regression grading (mrTRG) Clin Radiol. 2016;71(09):854–862. doi: 10.1016/j.crad.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 104.Battersby N J, Balyasnikova S, Brown G. Guiding post-treatment decisions in rectal cancer: mrTRG is a practical place to start. Oncology (Williston Park) 2014;28(08):677–680. [PubMed] [Google Scholar]


