Abstract
During the last 15 years, a significant evolution has emerged in the surgical treatment of rectal cancer and restoration of bowel continuity has been one of the main goals. For many years the treatment of distal rectal cancer would necessarily require an abdominoperineal resection and end colostomy. The surgical procedure of intersphincteric resection has been proposed to offer sphincter preservation in patients with low rectal cancer and has been legitimized if executed according to adequate oncologic criteria. This article will discuss the best indications, technical aspects, functional, and oncological outcomes of intersphicteric resection in the management of rectal cancer.
Keywords: rectal cancer, intersphincteric resection, radiochemotherapy
In patients with rectal cancer, the development of surgical technique with total mesorectum excision, as developed by Heald et al, led to the improvement of local control of the disease and patient survival. 1 During the last 15 years, a significant evolution has emerged in the surgical treatment of rectal cancer and restoration of bowel continuity has been one of the main goals. The surgical procedure of intersphincteric resection (ISR) has been proposed to offer sphincter preservation in patients with low rectal cancer and has been legitimized if executed according to adequate oncologic criteria. 2 The goal of ISR is to divide the rectum transanally and to remove partly or totally of the internal anal sphincter (IAS), to obtain adequate distal margin and restore bowel continuity. Further understanding of the safe distal resection margin coupled with advanced surgical procedures and tools has allowed an increased incidence of sphincter-saving procedures without compromising oncologic outcomes. 3 4 5
Anatomy of the Low Rectum and the Anal Canal
The Low Rectum and the Pelvic Floor
The low rectum is usually defined as the lower third of the rectum within 6 cm from the anal verge. 6 It can be considered as the area of the rectum below the origin of the levator ani muscle where the mesorectum fuses with the rectosacral fascia and tapers together at the anorectal junction. At this level, the rectum is supported by:
The rectosacral fascia which goes from the presacral fascia in front of S4 to the mesorectum above the pelvic floor,
The levator ani muscle which is formed by three main components: puborectalis, pubococcygeus, iliococcygeus, and the fourth variable component, the iliococcygeus or coccygeus muscle.
The puborectalis muscle forms a strong u -shaped sling of striated muscle that pulls the lower rectum anteriorly just above the anal canal and blends with the top of the external anal sphincter (EAS) forming the anorectal ring.
The Anal Canal and the Intersphincteric Space
The anal canal varies from 2 to 5 cm in length with a significant difference between genders. 7 The wall of both the low rectum and anal canal is composed of three main layers: the mucosa, the submucosa, and the muscularis. However, the muscularis layer is different between the low rectum and the anal canal ( Fig. 1 ). Indeed, the muscularis of the low rectum comprises:
Fig. 1.
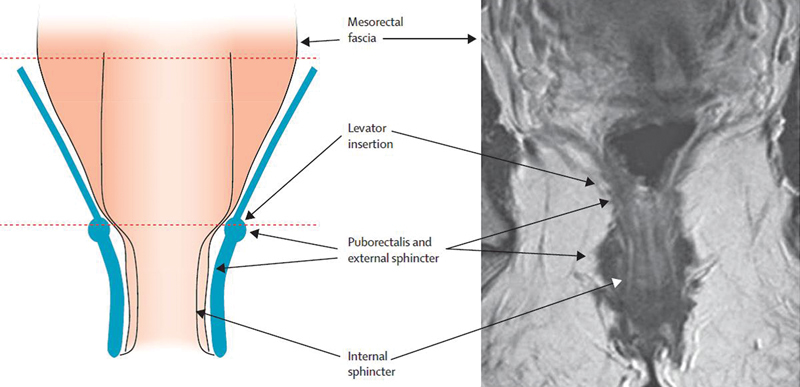
Anatomy of the low rectum. (Reprinted with permission from Shihab OC, Heald RJ, Rullier E, et al. Defining the surgical plane on MRI improves surgery for cancer of the low rectum. Lancet Oncol. 2009;10(12):1207–1211.)
An inner circular muscle which condenses to form the IAS of the anal canal;
An outer longitudinal muscle which fuses, at the top of the anal canal, with fibers from the puborectalis and fragment together into a small layer of connective tissue to form the intersphincteric space of the anal canal. This latter fibroelastic layer separates the IAS from the EAS which comprises striated muscle fibers that blend with the puborectalis component of the levator ani muscle to form a muscular anal ring.
The upper third of the anal canal is located between the anal ring and the dentate line which represents histologically the point at which the columnar epithelium of the rectum becomes the transitional epithelium. 8 Below the dentate line, in the lower two-third of the anal canal, the EAS extends further than IAS to the anal verge. This point is very important for the assessment of the lower edge of the tumor from the anal verge in an awake patient.
These anatomical characteristics of the low rectum and the anal canal emphasize the decrease of tissue surrounding low rectal cancer leading to a potentially greater risk of resection margin involvement compared with mid and high rectal cancer.
Surgical Margins of the Low Rectal Cancer
The Distal Resection Margin
Historically, 5 cm of distal bowel margin was required to remove the rectal cancer, and abdominoperineal resection (APR) was used for both mid and low rectal cancer. 9 Since the 1980s comparison between local recurrence and pathologic data suggested that a 2 cm distal bowel margin was adequate. 10 11 Therefore, only low rectal cancer defined by tumors < 5 cm from the anal verge or < 2 cm from the anal ring were treated by APR. More recently, since 2005 it is admitted that the distal resection margin can be shortened to 1 cm for most tumors. 12 13
Some authors suggest the feasibility to decrease the distal resection margin close to 5 mm after radiochemotherapy. 14 However, this can increase the risk of R1 resection due to positive distal margin, because the distal edge of the tumor may be difficult to identify after neoadjuvant treatment, especially in the case of good response. Our surgical strategy is therefore to keep the 1 cm distal rule and to decide the level of rectal transection before irradiation. Finally, we must keep in mind that by removing part or the whole of the internal sphincter, a safe distal resection margin can be achieved in all cases. 15 Using the technique of interphincteric resection modifies the concept of decision-making for sphincter conservative surgery, which finally does not depend on the distance between the tumor and the anal ring.
The Circumferential Resection Margin
In modern surgery for low rectal cancer, the concept of the distal rule must be, in significant part, replaced by the concept of the circumferential resection margin (CRM). Patients with positive CRM (≤ 1 mm) are associated with a high rate of local recurrence, as compared with those with negative CRM (> 1 mm) following rectal excision with or without neoadjuvant radiotherapy. 16 17 In the case of low rectal cancer, positive CRM means infiltration by the tumor into the skeletal muscles of the pelvic floor, that is, the EAS or the levator ani muscles. Pelvic magnetic resonance imaging (MRI) using external phased-array coils on high-magnetic-field (1.5 T) scanners with thin section (2.5 mm) are crucial for staging low tumors. 18 19 In our practice, in addition to classic series, high spatial resolution T2-weighted thin-section images in the coronal plane, parallel to the anal canal, are used for optimal visualization of the levator ani, the sphincter complex, the intersphincteric plane, and the relationship to the rectal wall. Invasion of the intersphincteric plane by the tumor at MRI is the absolute contraindication for intersphincteric resection.
Classification of Low Rectal Cancer and Standardization of Surgery
Neoadjuvant radiochemotherapy for low rectal cancer induces downsizing and downstaging that theoretically facilitates sphincter-saving surgery, at least in experienced hands. 20 21 22 23 24 25 26 Reproducibility of such experience, however, remains questionable, as suggested by four reviews which failed to demonstrate any actual decrease in rate of APR following preoperative radiotherapy. 27 28 29 30 Some reasons could explain this discrepancy. First, there is no set definition of low rectal cancer. Low rectal cancer is usually described as a tumor with a lower edge below 5 or 6 cm from anal verge, or less than 2 cm from the dentate line, while the length of the anal canal and the level of the dentate line can vary between patients. 7 Second, surgery for low rectal cancer is not standardized, as underlined by the high variation rate of APR for rectal cancer, from 8.5 to 53%, observed across English hospitals. 31 Third, surgeons and oncologist usually do not reclassify or restage rectal cancer after neoadjuvant therapy, limiting changes in surgery type. Therefore, we have proposed a new surgical classification for low rectal cancer using MRI in association with standardization of surgery according to tumor type. 32 The objective of the classification was to help surgeons in the decision for sphincter-saving surgery versus APR, and which type of sphincter-saving procedure. It also permits to restage the tumor after treatment and thus to change the type of surgery. We classified the tumors into four categories dedicated to four distinct surgical procedures ( Fig. 2 ):
Fig. 2.
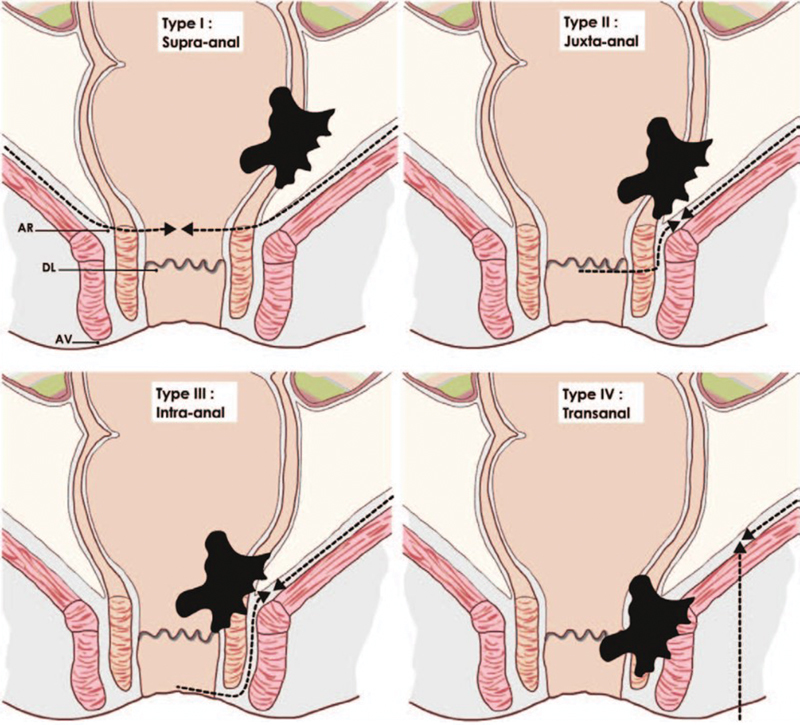
Classification of low rectal cancer. Type I are treated by conventional coloanal anastomosis, type II by partial intersphincteric resection (ISR), type III by total ISR, and type IV by APE. APE: Abdominoperineal excision, AR: anal ring, DL: dentate line; AV: anal verge. (Reprinted with permission from Rullier E, Denost Q, Vendrely V, Rullier A, Laurent C. Low rectal cancer: classification and standardization of surgery. Dis Colon Rectum. 2013;56(5):560–567.)
type I supra-anal (> 1 cm from the anal ring) had ultralow anterior resection;
type II juxta-anal (< 1 cm from the anal ring) had partial ISR;
type III intra-anal (IAS invasion) had total ISR;
type IV transanal (EAS or levator ani invasion) had APR.
Type IV was divided into three subgroups depending upon the level of invasion of the anal sphincter complex: IVa levator ani muscles, IVb EAS, IVc levator ani muscles, and EAS. Anatomical structures were considered as invaded when the radiological circumferential margin was ≤ 1 mm. 18 19 Infiltration of the intersphincteric plane was considered as invasion of the external sphincter.
Surgical Technique of Intersphincteric Resection
The anal canal is exposed with a self-retaining retractor (Lone Star Retractor; Lone Star Medical Products Inc., Houston, TX) and a gauze is introduced into the rectum to limit the risk of tumor spillage. A circular incision of the anal canal is performed 1 cm below the tumor ( Fig. 3 ). Both the mucosa and the muscular layer are incised to transect the IAS. Conventionally, the incision at the level of the dentate line or just below, removing one-third or half of the IAS, is a partial ISR. By contrast, incision 1 or 2 cm below the dentate line, removing two-third or the whole of the internal sphincter, is a subtotal or total ISR. Dissection is performed between the internal and the external sphincter by using scissors or cautery in a bloodless plane ( Fig. 4 ). It begins posteriorly then laterally, where the external sphincter is easier to identify, to finish anteriorly where the plane presents more adhesions. At the top of the anal canal, that is, the level of the anal ring, the rectum is closed by suture to avoid intraoperative tumor seeding, and the dissection is followed along the fibers of the levator ani muscle by using conventional anal retractors or a laparoscopic single port through the anus. The dissection is performed posteriorly and then anteriorly to finish laterally after visualization of the neurovascular bundle. Posteriorly, low rectal dissection is performed behind the sheath of the levator ani muscle, which is usually thickened due to irradiation ( Fig. 5 ). This sheath is then transected to join the mesorectal plane. Anteriorly the dissection is performed along the prostate or the vagina up to the seminal glands or the cervix, respectively, leaving the Denonvillier's fascia on the rectum. Laterally, the definition of the plane of dissection is enhanced by the previous posterior and anterior dissection, allowing a more accurate dissection with regards to the neurovascular bundle. Therefore, the lateral dissection establishes the connection between both posterior and anterior planes, pushing outside the nerve route. After performing the transanal dissection of the low and mid rectum and mesorectum up to the peritoneal reflection, a conventional five-port laparoscopic procedure is performed.
Fig. 3.
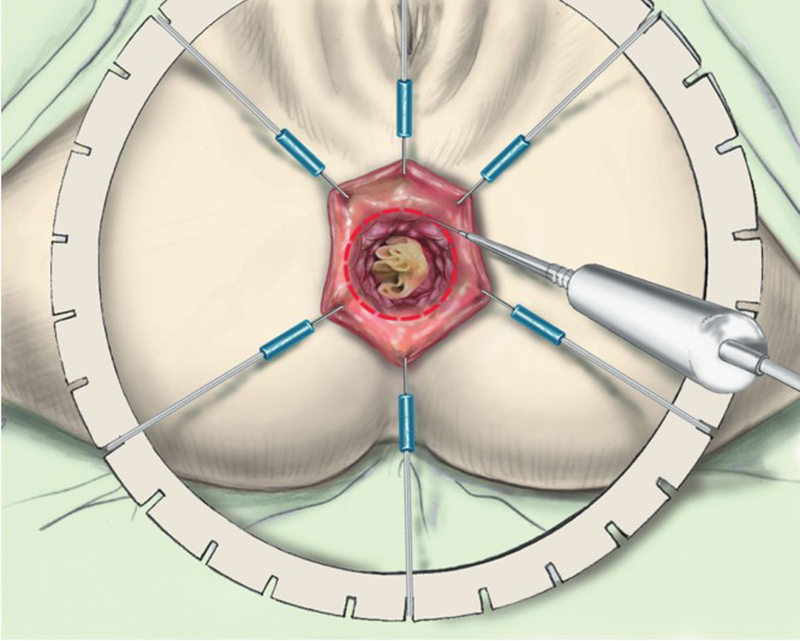
Exposition and circular incision of the anal canal. (Reprinted with permission from Laurent C, Rullier E. Intersphincteric rectal resection [in French]. J Chir (Paris) 2007;144(3):225–230.)
Fig. 4.
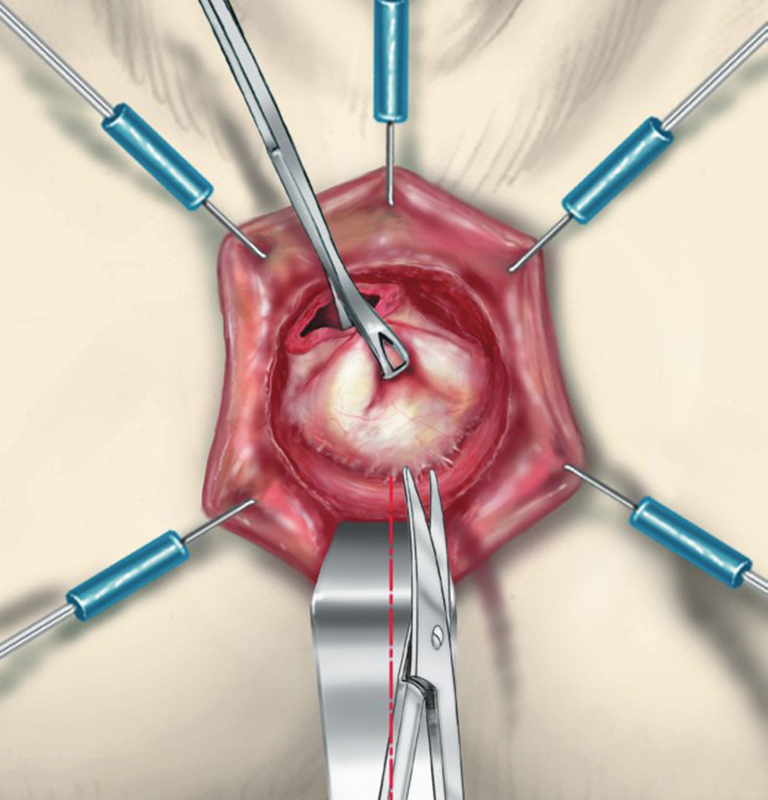
Dissection of the intersphincteric bloodless plane by scissors. (Reprinted with permission from Laurent C, Rullier E. Intersphincteric rectal resection [in French]. J Chir (Paris) 2007;144(3):225–230.)
Fig. 5.
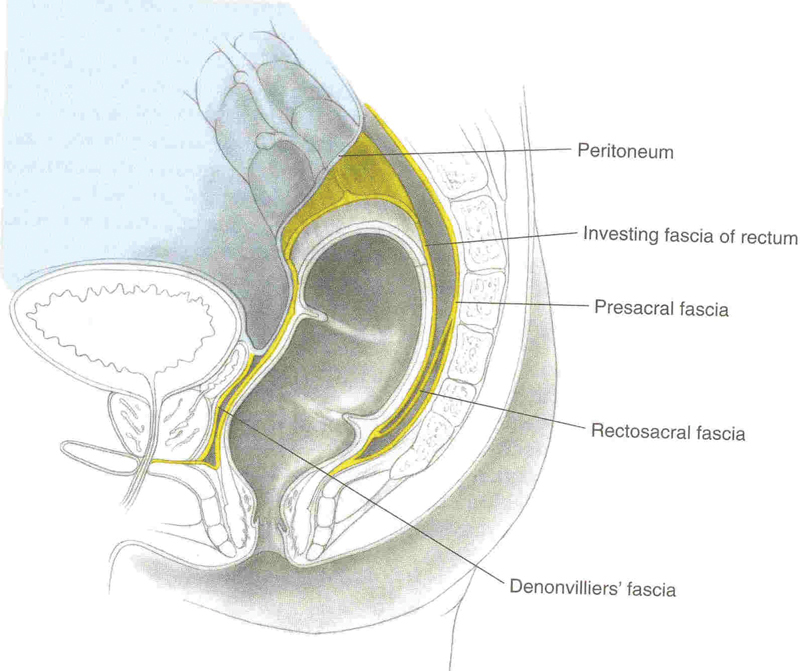
Posterior dissection. The plane between the internal and the external sphincters is initially behind the presacral facia and becomes in front to find the mesorectal plane.
During the laparoscopic procedure, a high ligation of the inferior mesenteric artery and a full mobilization of the left colon, including the splenic flexure, are systematically performed. This allows to achieve a tension-free coloanal anastomosis and to permit transanal extraction of the specimen as well. During this step, care is taken to open enough window into the mesentery to optimize left colon mobilization, but avoiding injury of the marginal colonic artery. The pelvic dissection is performed by conventional scissors with monopolar coagulation. The dissection of the mesorectum begins posteriorly to continue laterally first on the right then on the left and finishes anteriorly. After performing transanal total mesorectal excision (TME), this step is really short and safe.
The specimen is extracted through the anus except in obese patient to avoid injury of the mesorectum, a colonic J-pouch and a diverting loop ileostomy are associated with the hand-sewn coloanal anastomosis.
Personal Experience of Intersphincteric Resection
Oncological Outcomes
Series of intersphincteric resection demonstrated the feasibility to achieve adequate distal resection margin for low rectal cancer. 33 34 35 The technique of transanal ISR enables us to obtain optimal distal margin in all cases, including the difficult narrow pelvis, obese patient, and those with the involvement of the IAS. Indeed, we showed in a previous report of 92 ISR in which the distal margin was safe in 98% of cases with a median size of 2 cm. 15
Possibilities to achieve a safe CRM was also reported, ranging from 4 to 8 mm for tumors lying less than 1 cm from the top of the anal sphincter. 15 33 35 In the last assessment of our experience regarding 303 ISR over the last 25 years (data not published), the median circumferential margin for such tumors was 5 mm (range: 0–18) without difference over time, whereas the distal resection margin decrease from 25 to 10 mm from the first period of time to the last 7 years of the study. The rate of R1 was 15.5%. We observed 4.8% of local recurrence, and half of the patients with local recurrence had a distant recurrence as well. These good oncological results observed after ISR for a tumor located very close or into the anal canal are due, in part, to the selection of the patients. Some proposed ISR mainly for T1 and T2 low rectal cancer, but in our experience, 44% of patients were ypT3–4. 34 36 In our opinion, by using neoadjuvant treatment, all grades, and stages of tumors may be considered for ISR, except invasion of the external sphincter.
Functional Outcomes
Beside this oncologic objective, the preservation of fecal continence is the second most important aim to reach an acceptable quality of life. ISR has been proposed as an alternative procedure to abdominoperineal excision (APE) to avoid a permanent colostomy. However, any patients experience low anterior resection syndrome with association of stool fragmentation, fecal urgency, and incontinence. 37 38 39 40 41 Such continence function disorders could have such a strong impact on patient's quality of life that colostomy might be a more satisfactory option for some of them. 42 We have previously reported from 101 patients that two-thirds of the patients with ISR had less than three stools a day, half had urgency, most of the patients had fragmentation and a quarter suffered from difficulties to evacuate. 43 Full continence was observed in 14% of the patients, incontinence to gas in 36%, minor fecal incontinence in 39%, major incontinence in 11% for whom a colostomy was required in half. Overall, half of the patients had a good bowel functional result in term of continence and 11% suffered from major incontinence. Patients with partial ISR had a better continence than those with total ISR as suggested by the Japanese experience. 44 Finally, only the height of the tumor, and subsequently those of the anastomosis, was associated with continence disorders. The long-term follow-up of the patients demonstrated an improvement of continence with time ( Fig. 6 ).
Fig. 6.
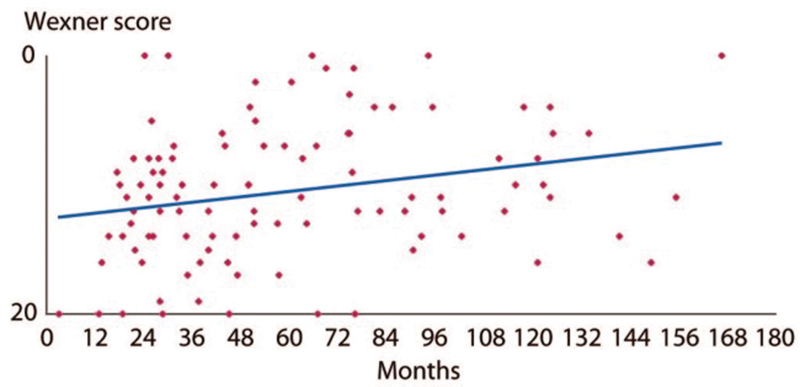
Evolution of Wexner's score after ISR. (Reprinted with permission from Denost Q, Laurent C, Capdepont M, Zerbib F, Rullier E. Risk factors for fecal incontinence after intersphincteric resection for rectal cancer. Dis Colon Rectum 2011;54(8):963–968.)
Moreover, beside the oncological and the bowel functional outcomes, the TME technique also influenced the postoperative urogenital function. The incidence of postoperative urinary and sexual dysfunctions after rectal excision for rectal cancer was 10 to 30% and 40 to 60%, respectively, after conventional rectal excision versus 0 to 12% and 10 to 35% after introduction of the TME. 45 46 47 48 49 50 51 52 53 54 The better functional outcome following TME is mainly due to the nerve-sparing technique which is part of the TME procedure. 55 Sexual and urinary functions dependent on dual autonomic (sympathetic and parasympathetic) innervation. Among 169 patients who underwent surgery for rectal cancer in our institution, we observed, through a prospective and longitudinal assessment of urogenital function, a temporary urinary dysfunction in men after radiotherapy. 56 By contrast, sexual disorders occurred in more than half of the study population 1 year after surgery, including loss of sexual activity in both males and females, and erectile and ejaculatory dysfunction in males. The male sexual function was still impaired at 12 months after surgery, and predictive factors for this dysfunction were related to tumor characteristics but not to the surgical technique, meaning that ISR procedure did not impact the urogenital function compared with partial or total TME and APE. Taking together the urinary and the male genital functions, 58% of patients had a normal urogenital function (no urinary or sexual dysfunction) before treatment, against 10% at 12 months after surgery ( p < 0.001) ( Fig. 7 ). The rate of sexual activity in females declined from 59% before treatment to 36% at 12 months after surgery ( p = 0.02). A temporary dyspareunia occurred three months after surgery and disappeared at 6 months. At 1 year after surgery, the quality of sexuality in active females did not differ from the baseline, that is, no difference of lubrications disorders, dyspareunia, sexual arousal, and sexual satisfaction was observed ( Fig. 8 ).
Fig. 7.
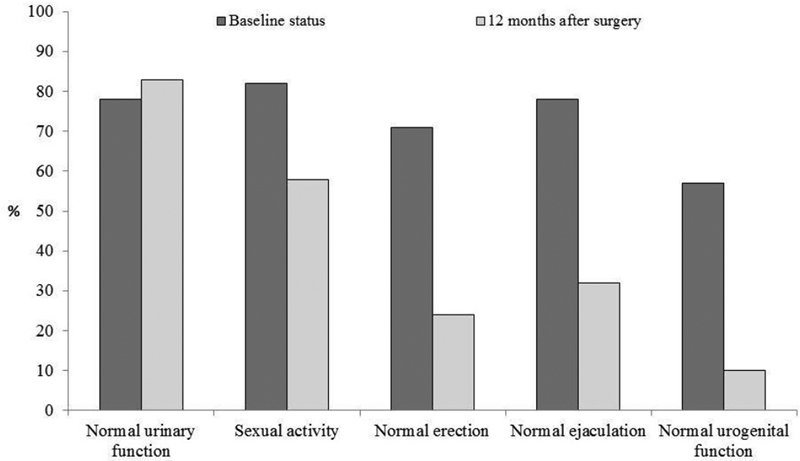
Male urogenital function after rectal surgery for cancer. (Reprinted with permission from Adam JP, Denost Q, Capdepont M, van Geluwe B, Rullier E. Prospective and longitudinal study of urogenital dysfunction after proctectomy for rectal cancer. Dis Colon Rectum 2016;59(9):822–830.)
Fig. 8.
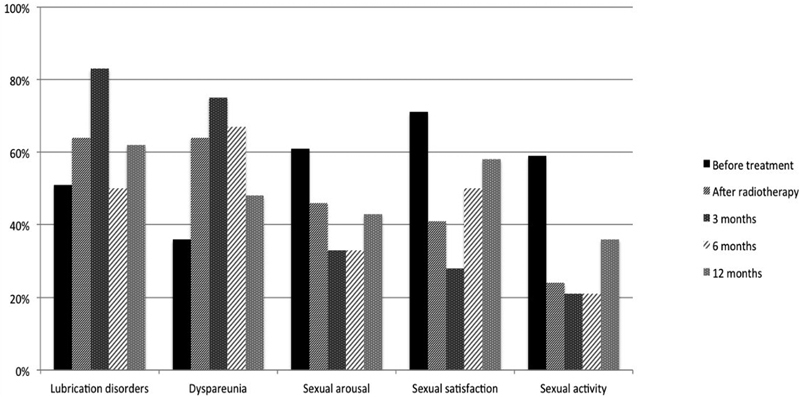
Female sexual function after rectal surgery for cancer. (Reprinted with permission from Adam JP, Denost Q, Capdepont M, van Geluwe B, Rullier E. Prospective and longitudinal study of urogenital dysfunction after proctectomy for rectal cancer. Dis Colon Rectum 2016;59(9):822–830.)
However, we have recently reported data from a prospective and randomized study, conducted at our institution, to compare the long-term functional results between transanal and laparoscopic distal rectal dissection in laparoscopic sphincter-saving resection for low rectal cancer with a median follow-up of 38 months. 57 Our data showed a similar bowel and urinary functions between the two groups. By contrast, we observe a marked impaired sexual function after laparoscopic dissection including sexual activity, erectile, and ejaculatory function in men. In multivariate analysis, among patients with preoperative sexual activity, the transanal approach of the low rectum was the only independent factor of healthy sexual activity.
All these information, regarding both bowel and urogenital functions, must be given to the patient before taking the decision to treat. The surgeon should be able to offer the best approach to their patients to give the best chance in terms of oncological and functional outcomes.
Risk of Definitive Stoma Formation
The TME trial reported that 19% of “temporary” stomas were not closed during follow-up after TME. 58 Age was reported to be a significant risk factor associated with a decreased likelihood of stoma reversal. 58 Broadly speaking, the nonstoma closure was the main reason of definitive stoma in the majority of studies and was due to early death, age, postoperative complication or adjuvant chemotherapy, and patient's tiredness. 50 51 52 53 54 55 56 57 58 59 60 61 Among our series of 297 patients who underwent TME and sphincter preservation with a temporary defunctioning stoma for low rectal cancer until 2010, 180 (61%) had an ISR. 62 The rate of definitive stoma formation has reached 20% after a median follow-up of 69 months. Actuarial rates of definitive stoma were 11% at 1 year and increased by 1%/y to reach 22% at 10 years. Interestingly, ISR did not expose to a higher risk of definitive stoma formation compared with low anterior resection with low colorectal anastomosis or coloanal anastomosis without ISR.
Perspectives
How to Decrease the Risk of R1?
The rectum, pelvic floor, and anal canal have been described as “a tube within a funnel.” The rectum and the internal sphincter form the tube, whereas the levator ani and the external sphincter (both striated muscles) form the funnel. The levator ani muscles are covered by a parietal fascia or pelvic sheath. The plane of dissection may differ according to the surgical approach. The plane is usually mesorectal, that is, anterior to the pelvic sheath during an abdominal, low rectal dissection, whereas it can be posterior to the sheath in case of transanal low rectal dissection ( Fig. 9 ). We demonstrated a significantly decreased rate of positive CRM from 18 to 4% after transanal dissection compared with abdominal dissection of the low rectum. 63 Our results suggest the transanal approach more consistent than the conventional abdominal dissection to achieve free CRM in the case of low rectal cancer.
Fig. 9.
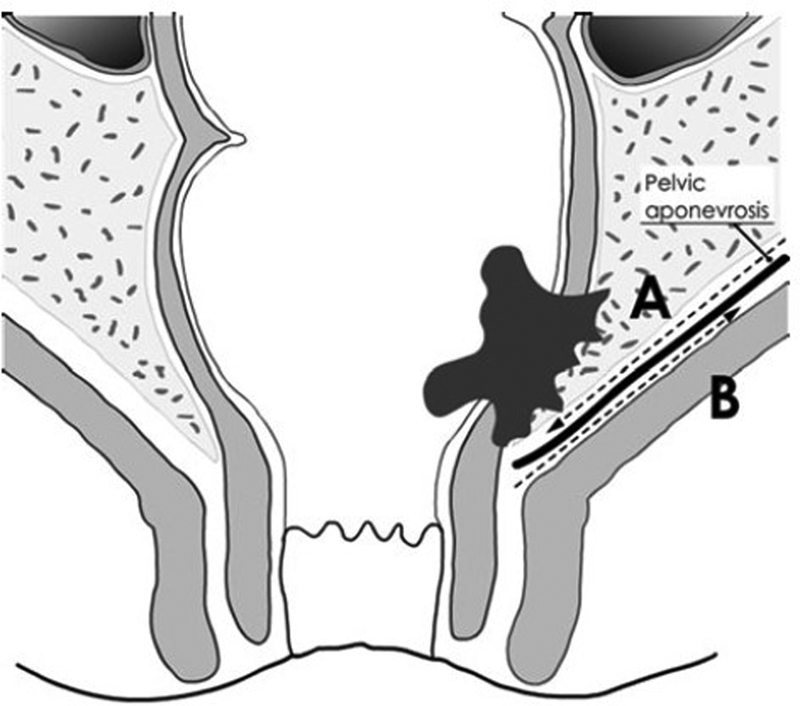
Plane of dissection of the low rectum accordingly to the abdominal (plane A) or transanal approach (plane B). (Reprinted with permission from Denost Q, Adam JP, Rullier A, Buscail E, Laurent C, Rullier E. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg 2014;260(6):993–999.)
How to Change Surgical Procedure: From APE to ISR?
Data from the literature failed to demonstrate that neoadjuvant radiotherapy changes type of surgery in rectal cancer. 27 28 29 30 After demonstrating the interest of our surgical classification for low rectal cancer in association with standardization of surgery according to tumor type, we have assessed the reclassification of the rectal tumor after radiochemotherapy by using a restaging MRI (data not published). 32 In 50 patients with low rectal cancers and neoadjuvant radiochemotherapy, we observed tumor downsizing which induced an increased radiological distal margin and free circumferential margin from the anal sphincter complex (levator ani muscles, internal and external sphincter), as compared with the initial MRI. The objective of MRI in this reassessment was not T-restaging because imaging is not accurate in this setting after irradiation, as shown by the MERRION study group. 64 It was downsizing, circumferential margin, and free margin from the anal sphincter. Indeed, MRI permits to diagnose a 1 mm margin which is the key to the surgical decision. 18 19 MERCURY study group also suggest the importance of restaging the primary tumor with a willingness to change the initial plan selectively. 65 Using our classification for restaging after chemoradiotherapy might help the surgeon to change surgical procedure from APE to ISR.
How to Treat Bad Functional Outcome?
The first intention treatment of fecal incontinence after ISR aims to improve colonic emptying by using both a low fiber diet and bulking agents and/or glycerol-based enemas (130 mL; Normacol, Norgine Pharma, Paris, France). The dose of bulking agents is determined for each patient according to the daily number and consistency of stools. In the case of failure, treatment by loperamide might be proposed in association with sphincter re-education by Biofeedback consisted weekly exercises of anal contraction assisted by a specialized nurse. Sacral nerve stimulation is suggested after the failure of medical treatment in association with anal re-education. 66 Finally, in the case of failure of all these procedures, we propose cecostomy by the colonoscopic approach to achieve a full colonic emptying by using an anterograde enema. Patients report a high level of satisfaction with this procedure. In the end, 5% of our patients need a definitive stoma formation due to major and refractory fecal incontinence. 62
In the case of stool fragmentation, we usually propose in first intention a medical treatment by using mucillage with graded dose increase from one to three doses per day in 3 weeks.
Conclusion
Although definition and impact of fecal incontinence after ISR may vary depending on the surgeon and the patient, it appears clearly that the main limit of ISR is functional rather the oncologic outcome. It is, therefore, necessary to explain to the patient before surgery that ISR includes a significant risk and symptoms of low anterior resection syndrome, mainly the risk of fecal incontinence. Improvement of functional results including both bowel, urinary and sexual functions after rectal excision for cancer should be the main goal in the future treatment of low rectal treatment and ISR.
References
- 1.Heald R J, Moran B J, Ryall R D, Sexton R, MacFarlane J K. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133(08):894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 2.Schiessel R, Karner-Hanusch J, Herbst F, Teleky B, Wunderlich M. Intersphincteric resection for low rectal tumours. Br J Surg. 1994;81(09):1376–1378. doi: 10.1002/bjs.1800810944. [DOI] [PubMed] [Google Scholar]
- 3.Lavery I C, Lopez-Kostner F, Fazio V W, Fernandez-Martin M, Milsom J W, Church J M.Chances of cure are not compromised with sphincter-saving procedures for cancer of the lower third of the rectum Surgery 199712204779–784., discussion 784–785 [DOI] [PubMed] [Google Scholar]
- 4.Rullier E, Zerbib F, Laurent C et al. Intersphincteric resection with excision of internal anal sphincter for conservative treatment of very low rectal cancer. Dis Colon Rectum. 1999;42(09):1168–1175. doi: 10.1007/BF02238569. [DOI] [PubMed] [Google Scholar]
- 5.Gamagami R A, Liagre A, Chiotasso P, Istvan G, Lazorthes F. Coloanal anastomosis for distal third rectal cancer: prospective study of oncologic results. Dis Colon Rectum. 1999;42(10):1272–1275. doi: 10.1007/BF02234212. [DOI] [PubMed] [Google Scholar]
- 6.Salerno G, Sinnatamby C, Branagan G, Daniels I R, Heald R J, Moran B J. Defining the rectum: surgically, radiologically and anatomically. Colorectal Dis. 2006;8 03:5–9. doi: 10.1111/j.1463-1318.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 7.Nivatvongs S, Stern H S, Fryd D S. The length of the anal canal. Dis Colon Rectum. 1981;24(08):600–601. doi: 10.1007/BF02605754. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen O O. Anorectal function. Dis Colon Rectum. 1994;37(04):386–403. doi: 10.1007/BF02053604. [DOI] [PubMed] [Google Scholar]
- 9.Goligher J C, Dukes C E, Bussey H JR. Local recurrences after sphincter saving excisions for carcinoma of the rectum and rectosigmoid. Br J Surg. 1951;39(155):199–211. doi: 10.1002/bjs.18003915504. [DOI] [PubMed] [Google Scholar]
- 10.Pollett W G, Nicholls R J. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg. 1983;198(02):159–163. doi: 10.1097/00000658-198308000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams N S, Dixon M F, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients' survival. Br J Surg. 1983;70(03):150–154. doi: 10.1002/bjs.1800700305. [DOI] [PubMed] [Google Scholar]
- 12.Shirouzu K, Isomoto H, Morodomi T, Kakegawa T. Carcinomatous lymphatic permeation. Prognostic significance in patients with rectal carcinoma--a long term prospective study. Cancer. 1995;75(01):4–10. doi: 10.1002/1097-0142(19950101)75:1<4::aid-cncr2820750103>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H, Mochizuki H, Hashiguchi Y et al. Preoperative parameters expanding the indication of sphincter preserving surgery in patients with advanced low rectal cancer. Ann Surg. 2004;239(01):34–42. doi: 10.1097/01.sla.0000103070.13030.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pahlman L, Bujko K, Rutkowski A, Michalski W. Altering the therapeutic paradigm towards a distal bowel margin of < 1 cm in patients with low-lying rectal cancer: a systematic review and commentary. Colorectal Dis. 2013;15(04):e166–e174. doi: 10.1111/codi.12120. [DOI] [PubMed] [Google Scholar]
- 15.Rullier E, Laurent C, Bretagnol F, Rullier A, Vendrely V, Zerbib F. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg. 2005;241(03):465–469. doi: 10.1097/01.sla.0000154551.06768.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quirke P, Durdey P, Dixon M F, Williams N S.Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision Lancet 19862(8514):996–999. [DOI] [PubMed] [Google Scholar]
- 17.Birbeck K F, Macklin C P, Tiffin N J et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235(04):449–457. doi: 10.1097/00000658-200204000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor F G, Quirke P, Heald R J et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011;98(06):872–879. doi: 10.1002/bjs.7458. [DOI] [PubMed] [Google Scholar]
- 19.Taylor F G, Quirke P, Heald R J et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32(01):34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 20.Marks G, Mohiuddin M, Eitan A, Masoni L, Rakinic J. High-dose preoperative radiation and radical sphincter-preserving surgery for rectal cancer. Arch Surg. 1991;126(12):1534–1540. doi: 10.1001/archsurg.1991.01410360108018. [DOI] [PubMed] [Google Scholar]
- 21.Wagman R, Minsky B D, Cohen A M, Guillem J G, Paty P P. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Phys. 1998;42(01):51–57. doi: 10.1016/s0360-3016(98)00180-1. [DOI] [PubMed] [Google Scholar]
- 22.Janjan N A, Khoo V S, Abbruzzese J et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44(05):1027–1038. doi: 10.1016/s0360-3016(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 23.Rullier E, Goffre B, Bonnel C, Zerbib F, Caudry M, Saric J. Preoperative radiochemotherapy and sphincter-saving resection for T3 carcinomas of the lower third of the rectum. Ann Surg. 2001;234(05):633–640. doi: 10.1097/00000658-200111000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouanet P, Saint-Aubert B, Lemanski Cet al. Restorative and nonrestorative surgery for low rectal cancer after high-dose radiation: long-term oncologic and functional results Dis Colon Rectum 20024503305–313., discussion 313–315 [DOI] [PubMed] [Google Scholar]
- 25.Gerard J P, Chapet O, Nemoz C et al. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: the lyon R96-02 randomized trial. J Clin Oncol. 2004;22(12):2404–2409. doi: 10.1200/JCO.2004.08.170. [DOI] [PubMed] [Google Scholar]
- 26.Weiser M R, Quah H M, Shia J et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249(02):236–242. doi: 10.1097/SLA.0b013e318195e17c. [DOI] [PubMed] [Google Scholar]
- 27.Bujko K, Kepka L, Michalski W, Nowacki M P. Does rectal cancer shrinkage induced by preoperative radio(chemo)therapy increase the likelihood of anterior resection? A systematic review of randomised trials. Radiother Oncol. 2006;80(01):4–12. doi: 10.1016/j.radonc.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Leibold T, Guillem J G. The role of neoadjuvant therapy in sphincter-saving surgery for mid and distal rectal cancer. Cancer Invest. 2010;28(03):259–267. doi: 10.3109/07357900802112719. [DOI] [PubMed] [Google Scholar]
- 29.Baker B, Salameh H, Al-Salman M, Daoud F. How does preoperative radiotherapy affect the rate of sphincter-sparing surgery in rectal cancer? Surg Oncol. 2012;21(03):e103–e109. doi: 10.1016/j.suronc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Gerard J P, Rostom Y, Gal J et al. Can we increase the chance of sphincter saving surgery in rectal cancer with neoadjuvant treatments: lessons from a systematic review of recent randomized trials. Crit Rev Oncol Hematol. 2012;81(01):21–28. doi: 10.1016/j.critrevonc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Morris E, Quirke P, Thomas J D, Fairley L, Cottier B, Forman D. Unacceptable variation in abdominoperineal excision rates for rectal cancer: time to intervene? Gut. 2008;57(12):1690–1697. doi: 10.1136/gut.2007.137877. [DOI] [PubMed] [Google Scholar]
- 32.Rullier E, Denost Q, Vendrely V, Rullier A, Laurent C. Low rectal cancer: classification and standardization of surgery. Dis Colon Rectum. 2013;56(05):560–567. doi: 10.1097/DCR.0b013e31827c4a8c. [DOI] [PubMed] [Google Scholar]
- 33.Saito N, Ono M, Sugito M et al. Early results of intersphincteric resection for patients with very low rectal cancer: an active approach to avoid a permanent colostomy. Dis Colon Rectum. 2004;47(04):459–466. doi: 10.1007/s10350-003-0088-4. [DOI] [PubMed] [Google Scholar]
- 34.Tiret E, Poupardin B, McNamara D, Dehni N, Parc R. Ultralow anterior resection with intersphincteric dissection--what is the limit of safe sphincter preservation? Colorectal Dis. 2003;5(05):454–457. doi: 10.1046/j.1463-1318.2003.00508.x. [DOI] [PubMed] [Google Scholar]
- 35.Vorobiev G I, Odaryuk T S, Tsarkov P V, Talalakin A I, Rybakov E G. Resection of the rectum and total excision of the internal anal sphincter with smooth muscle plasty and colonic pouch for treatment of ultralow rectal carcinoma. Br J Surg. 2004;91(11):1506–1512. doi: 10.1002/bjs.4330. [DOI] [PubMed] [Google Scholar]
- 36.Köhler A, Athanasiadis S, Ommer A, Psarakis E. Long-term results of low anterior resection with intersphincteric anastomosis in carcinoma of the lower one-third of the rectum: analysis of 31 patients. Dis Colon Rectum. 2000;43(06):843–850. doi: 10.1007/BF02238025. [DOI] [PubMed] [Google Scholar]
- 37.Chamlou R, Parc Y, Simon Tet al. Long-term results of intersphincteric resection for low rectal cancer Ann Surg 200724606916–921., discussion 921–922 [DOI] [PubMed] [Google Scholar]
- 38.Bittorf B, Stadelmaier U, Göhl J, Hohenberger W, Matzel K E. Functional outcome after intersphincteric resection of the rectum with coloanal anastomosis in low rectal cancer. Eur J Surg Oncol. 2004;30(03):260–265. doi: 10.1016/j.ejso.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Gamagami R, Istvan G, Cabarrot P, Liagre A, Chiotasso P, Lazorthes F. Fecal continence following partial resection of the anal canal in distal rectal cancer: long-term results after coloanal anastomoses. Surgery. 2000;127(03):291–295. doi: 10.1067/msy.2000.103487. [DOI] [PubMed] [Google Scholar]
- 40.Benoist S, Panis Y, Boleslawski E, Hautefeuille P, Valleur P. Functional outcome after coloanal versus low colorectal anastomosis for rectal carcinoma. J Am Coll Surg. 1997;185(02):114–119. doi: 10.1016/s1072-7515(97)00016-1. [DOI] [PubMed] [Google Scholar]
- 41.Bretagnol F, Troubat H, Laurent C, Zerbib F, Saric J, Rullier E. Long-term functional results after sphincter-saving resection for rectal cancer. Gastroenterol Clin Biol. 2004;28(02):155–159. doi: 10.1016/s0399-8320(04)94870-1. [DOI] [PubMed] [Google Scholar]
- 42.Grumann M M, Noack E M, Hoffmann I A, Schlag P M. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233(02):149–156. doi: 10.1097/00000658-200102000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denost Q, Laurent C, Capdepont M, Zerbib F, Rullier E. Risk factors for fecal incontinence after intersphincteric resection for rectal cancer. Dis Colon Rectum. 2011;54(08):963–968. doi: 10.1097/DCR.0b013e31821d3677. [DOI] [PubMed] [Google Scholar]
- 44.Saito N, Moriya Y, Shirouzu Ket al. Intersphincteric resection in patients with very low rectal cancer: a review of the Japanese experience Dis Colon Rectum 200649(10, Suppl):S13–S22. [DOI] [PubMed] [Google Scholar]
- 45.Fazio V W, Fletcher J, Montague D. Prospective study of the effect of resection of the rectum on male sexual function. World J Surg. 1980;4(02):149–152. doi: 10.1007/BF02393562. [DOI] [PubMed] [Google Scholar]
- 46.Santangelo M L, Romano G, Sassaroli C. Sexual function after resection for rectal cancer. Am J Surg. 1987;154(05):502–504. doi: 10.1016/0002-9610(87)90264-9. [DOI] [PubMed] [Google Scholar]
- 47.Kinn A C, Ohman U. Bladder and sexual function after surgery for rectal cancer. Dis Colon Rectum. 1986;29(01):43–48. doi: 10.1007/BF02555287. [DOI] [PubMed] [Google Scholar]
- 48.Chang P L, Fan H A. Urodynamic studies before and/or after abdominoperineal resection of the rectum for carcinoma. J Urol. 1983;130(05):948–951. doi: 10.1016/s0022-5347(17)51589-x. [DOI] [PubMed] [Google Scholar]
- 49.Pocard M, Zinzindohoue F, Haab F, Caplin S, Parc R, Tiret E. A prospective study of sexual and urinary function before and after total mesorectal excision with autonomic nerve preservation for rectal cancer. Surgery. 2002;131(04):368–372. doi: 10.1067/msy.2002.122371. [DOI] [PubMed] [Google Scholar]
- 50.Kim N K, Aahn T W, Park J K et al. Assessment of sexual and voiding function after total mesorectal excision with pelvic autonomic nerve preservation in males with rectal cancer. Dis Colon Rectum. 2002;45(09):1178–1185. doi: 10.1007/s10350-004-6388-5. [DOI] [PubMed] [Google Scholar]
- 51.Nesbakken A, Nygaard K, Bull-Njaa T, Carlsen E, Eri L M. Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg. 2000;87(02):206–210. doi: 10.1046/j.1365-2168.2000.01357.x. [DOI] [PubMed] [Google Scholar]
- 52.Maas C P, Moriya Y, Steup W H, Kiebert G M, Kranenbarg W M, van de Velde C J. Radical and nerve-preserving surgery for rectal cancer in The Netherlands: a prospective study on morbidity and functional outcome. Br J Surg. 1998;85(01):92–97. doi: 10.1046/j.1365-2168.1998.00530.x. [DOI] [PubMed] [Google Scholar]
- 53.Masui H, Ike H, Yamaguchi S, Oki S, Shimada H. Male sexual function after autonomic nerve-preserving operation for rectal cancer. Dis Colon Rectum. 1996;39(10):1140–1145. doi: 10.1007/BF02081416. [DOI] [PubMed] [Google Scholar]
- 54.Havenga K, Enker W E, McDermott K, Cohen A M, Minsky B D, Guillem J. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg. 1996;182(06):495–502. [PubMed] [Google Scholar]
- 55.Enker W E.Potency, cure, and local control in the operative treatment of rectal cancer Arch Surg 1992127121396–1401., discussion 1402 [DOI] [PubMed] [Google Scholar]
- 56.Adam J P, Denost Q, Capdepont M, van Geluwe B, Rullier E. Prospective and longitudinal study of urogenital dysfunction after protectomy for rectal cancer. Dis Colon Rectum. 2016;59(09):822–830. doi: 10.1097/DCR.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 57.Pontallier A, Denost Q, Van Geluwe B, Adam J P, Celerier B, Rullier E. Potential sexual function improvement by using transanal mesorectal approach for laparoscopic low rectal cancer excision. Surg Endosc. 2016;30(11):4924–4933. doi: 10.1007/s00464-016-4833-x. [DOI] [PubMed] [Google Scholar]
- 58.den Dulk M, Smit M, Peeters K C et al. A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol. 2007;8(04):297–303. doi: 10.1016/S1470-2045(07)70047-5. [DOI] [PubMed] [Google Scholar]
- 59.Lindgren R, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum. 2011;54(01):41–47. doi: 10.1007/DCR.0b013e3181fd2948. [DOI] [PubMed] [Google Scholar]
- 60.David G G, Slavin J P, Willmott S, Corless D J, Khan A U, Selvasekar C R. Loop ileostomy following anterior resection: is it really temporary? Colorectal Dis. 2010;12(05):428–432. doi: 10.1111/j.1463-1318.2009.01815.x. [DOI] [PubMed] [Google Scholar]
- 61.Gessler B, Haglind E, Angenete E. Loop ileostomies in colorectal cancer patients--morbidity and risk factors for nonreversal. J Surg Res. 2012;178(02):708–714. doi: 10.1016/j.jss.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Celerier B, Denost Q, Van Geluwe B, Pontallier A, Rullier E. The risk of definitive stoma formation at 10 years after low and ultralow anterior resection for rectal cancer. Colorectal Dis. 2016;18(01):59–66. doi: 10.1111/codi.13124. [DOI] [PubMed] [Google Scholar]
- 63.Denost Q, Adam J P, Rullier A, Buscail E, Laurent C, Rullier E. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg. 2014;260(06):993–999. doi: 10.1097/SLA.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 64.Hanly A M, Ryan E M, Rogers A C, McNamara D A, Madoff R D, Winter D C; MERRION Study Group.Multicenter Evaluation of Rectal cancer ReImaging pOst Neoadjuvant (MERRION) therapy Ann Surg 201425904723–727. [DOI] [PubMed] [Google Scholar]
- 65.Battersby N J, How P, Moran B et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: The MERCURY II study. Ann Surg. 2016;263(04):751–760. doi: 10.1097/SLA.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 66.Thomas G P, Bradshaw E, Vaizey C J. A review of sacral nerve stimulation for faecal incontinence following rectal surgery and radiotherapy. Colorectal Dis. 2015;17(11):939–942. doi: 10.1111/codi.13069. [DOI] [PubMed] [Google Scholar]


