Abstract
OBJECTIVES:
Childhood obesity is rarely identified in hospitalized pediatric patients despite the high prevalence of obesity and potential for associated morbidity. The purpose of this study was to identify specific patient characteristics associated with the documentation of obesity and related weight management recommendations in the inpatient setting.
METHODS:
Retrospective chart review was conducted on all pediatric patients ages 2 to 18 years old and discharged between January 1, 2012, and December 31, 2014, to determine the following: (1) if obesity was noted in the clinical documentation of those with a BMI ≥95th percentile; and (2) if those with documented obesity had evidence of an obesity-specific management plan. Using χ2 and multivariable logistic regression, we determined patient characteristics associated with the documentation of obesity and presence of a management plan.
RESULTS:
Only 26% (214 of 809) of inpatients with obesity had documentation of weight status. The odds of obesity documentation were higher in patients with comorbid cholelithiasis, severe obesity, and older age. Of those with obesity documentation, 23% (49 of 214) had an obesity management plan. Comorbid sleep apnea and admission to a surgical service with a pediatric hospital medicine consult were significantly associated with the presence of an obesity management plan.
CONCLUSIONS:
Increased efforts are necessary to improve obesity diagnosis and management in younger children who have not yet developed comorbidities. Additionally, the role of pediatric hospitalists as consultants for surgical patients should be further explored as a tool for addressing obesity during inpatient hospitalization.
Childhood obesity (BMI ≥95th percentile) is a national epidemic that affects ∼17% of children in the United States.1 Pediatric obesity not only impacts acute and chronic diseases of childhood but also is a major risk factor for obesity and related diseases in adulthood.2 As with any other public health issue, the first step in curbing the epidemic is to identify and understand the problem. In the clinical setting, this translates to increasing the recognition and diagnosis of obesity.
In 2003, the American Academy of Pediatrics recommended annual obesity screenings beginning at the age of 2 years.3 Yet in a nationally representative sample of outpatient preventive visits from 2005 to 2007, only 1 in 5 pediatric patients with obesity had their weight status identified by a primary care provider. Younger age, white race, and living in the Midwest were all associated with higher risk of not receiving an obesity diagnosis despite having a BMI ≥95th percentile.4 In several other single-center outpatient studies, less severe obesity (lower BMI percentile) was also a risk factor for having no documentation of weight status despite having a BMI in the obese range.5,6 Documenting this diagnosis in primary care has been linked to increased rates of dietary counseling, screening, and treatment of comorbid conditions.7 In contrast, failure to notify parents of their child’s weight status is a strong predictor of parental weight misclassification,8 which can negatively affect a family’s interest in making healthy lifestyle changes.9
In 2011, the National Association of Children’s Hospitals and Related Institutions Healthy Hospital Environment Subcommittee recommended that the “identification of obesity and treatment (or referral for treatment) should occur in all inpatient and outpatient settings in children’s hospitals.” In part, this is because of a growing recognition that weight status can impact safety and clinical outcomes in the inpatient setting.10 Several studies have shown that compared with their healthy weight peers, children with obesity have higher rates of admission for cholelithiasis and asthma,11,12 more severe influenza infections,13 higher risk of hospital-acquired infections14 and venous thromboembolism,15 and higher rates of morbidity and mortality from severe traumatic injury,16 organ transplant, and critical illness.17 Several analyses of large administrative databases have revealed longer length of stay (LOS) and higher hospital charges associated with a discharge diagnosis of obesity.18,19 However, it is difficult to interpret these results, specifically because the documentation and coding of obesity occurs even less frequently in the hospital than in primary care. Rates of obesity documentation or diagnosis in the hospital range from 8% to 18% in single-center studies,20–23 with obesity more often identified in girls, children of color, and older children.20
To better understand the factors driving inpatient provider documentation of obesity, we conducted a retrospective chart review to identify patient characteristics associated with the documentation of weight status and related obesity management recommendations. We hypothesized that similar to findings in the primary care setting, age, ethnicity, and severity of obesity may impact weight status documentation and obesity management by inpatient providers.
Methods
Sample and Setting
We reviewed medical record data of all pediatric patients aged 2 to 18 years and discharged from a 74-bed children’s hospital within a hospital between January 1, 2012, and December 31, 2014. This hospital has ∼2600 pediatric discharges (excluding NICU discharges) per year. All pediatric floor patients are either admitted to a surgical service, with a general pediatric consult performed by a pediatric resident and pediatric hospital medicine (PHM) attending, or to a pediatric service under a PHM attending, a community pediatrician, or a pediatric subspecialty attending.
The electronic medical record (EMR) system used at the study site automatically calculates and plots anthropometric measurements on appropriate Centers for Disease Control and Prevention (CDC) growth curves, including BMI, but does not report percentiles or z scores. This automatic growth curve plotting function was present in the EMR during the entire study period. However, the use of the EMR for clinical documentation did not begin until October 2013. Before that time, all notes were written on paper or typed and printed, kept in a paper chart, and scanned into the EMR after discharge.
Incomplete records, which are defined as those without an admission history and physical examination and a progress note for each subsequent hospital day, were excluded. Additionally, patients with conditions that limit the accuracy of BMI in estimating adiposity, including nephrotic syndrome, heart failure, or liver failure, were excluded. Of these 3 diagnoses, only nephrotic syndrome was among the discharge diagnoses seen in our sample. Unique inpatient encounters were used as the unit of analysis rather than individual patients to allow for the evaluation of each hospitalization as an opportunity for obesity recognition. Human subjects approval was obtained from the hospital’s institutional review board.
Independent Variables
Predictor variables were electronically abstracted from medical records. These were chosen based on previous literature demonstrating an association with provider recognition, screening, or documentation of obesity (inpatient or outpatient) and included anthropometric, sociodemographic, clinical data.4,7,20,24
Height and weight were measured on admission and used to calculate BMI by using the formula BMI = kg/m2. This was converted to BMI percentile by using SAS software (SAS Institute, Inc, Cary, NC) for the CDC 2000 Growth Charts.25 Patient records were then categorized into 3 weight status groups based on age- and sex-specific norms.26 Patients with a BMI <95th percentile for age and sex were categorized as “not obese” and excluded from further analysis. The remaining patients were categorized as “obese” or “severely obese” (ie, >120% of the 95th percentile).27 CDC standards for identifying outliers in anthropometric data were applied to exclude subjects with biologically implausible BMI (adjusted z score of <−4 or >5).25,28
Sociodemographic predictors included age, race and/or ethnicity, primary language, and insurance type. Patients were categorized into 3 age groups (2–4, 5–11, and 12–18 years). Race and/or ethnicity was designated as white, Hispanic, African American, Asian American, or other to capture the 4 most common racial groups in the sample. Similarly, each patient’s primary language was designated as English, Spanish, or other. Insurance type was categorized as private or public.
Clinical predictors included the presence of obesity-related comorbidities, primary inpatient service, LOS, and presence of >1 admission during the study period. Among all possible discharge diagnoses, those known to be associated with obesity, including asthma, sleep apnea, cholelithiasis, deep venous thrombosis (DVT), diabetes mellitus, and pseudotumor cerebri, were coded as present or absent and referred to as obesity-related comorbidities. Primary service was defined as surgical if the primary attending was a general surgeon, pediatric surgeon, otolaryngologist, orthopedist, or urologist, and it was defined as nonsurgical if the primary attending was a hospitalist, community pediatrician, gastroenterologist, pulmonologist, hematologist and/or oncologist, neurologist, or pediatric intensivist. LOS was calculated by subtracting the admission date and time from the discharge date and time and dichotomized at the median.
Dependent Variables
Data for the 2 dependent variables were obtained by a manual review of the history and physical examination, progress notes, consult notes, and discharge summaries in each medical record. Documentation of obesity was defined by the presence of the words “obese,” “obesity,” or “severe obesity” in the history (history of present illness, review of systems, past medical and surgical history, or social history), physical examination, or assessment. Presence of an obesity management plan was defined as documentation of referral to a specialist, referral to a nutritionist, follow-up with a primary medical doctor, direct weight-related counseling by the inpatient team, or other recommendation made specifically to address weight status.
Chart review was conducted by the principal investigator and 2 trained pediatric residents using a standardized data abstraction protocol. All charts were reviewed independently by 2 reviewers (94.2% agreement). Discrepant findings between reviewers were resolved by consensus among all 3 reviewers.
Analysis
χ2 tests were used to explore the associations between each independent variable and the primary outcome. Variables with a P value <.1 in the bivariate analysis were then used in multivariable logistic regression. In the multivariable model, age, BMI z score, and LOS were entered as continuous variables and as categorical variables with similar results. Categorical variables were used in the final model, and findings were considered significant if P was <.05.
After completing initial analyses, we performed a subgroup analysis in those with obesity documentation to determine if there was a unique set of characteristics specifically associated with the documentation of an obesity management plan. We conducted χ2 tests for the same set of predictor variables with obesity management plan as the outcome. All variables with a P value of <.1 were included in a multivariable regression model. Again, age, BMI z score, and LOS were entered as continuous and categorical variables with similar results; categorical variables were kept in the final model. Findings from the multivariable regression model were considered significant if P was <.05. All analyses were conducted by using SPSS version 23 (IBM SPSS Statistics, IBM Corporation, Armonk, NY).
Results
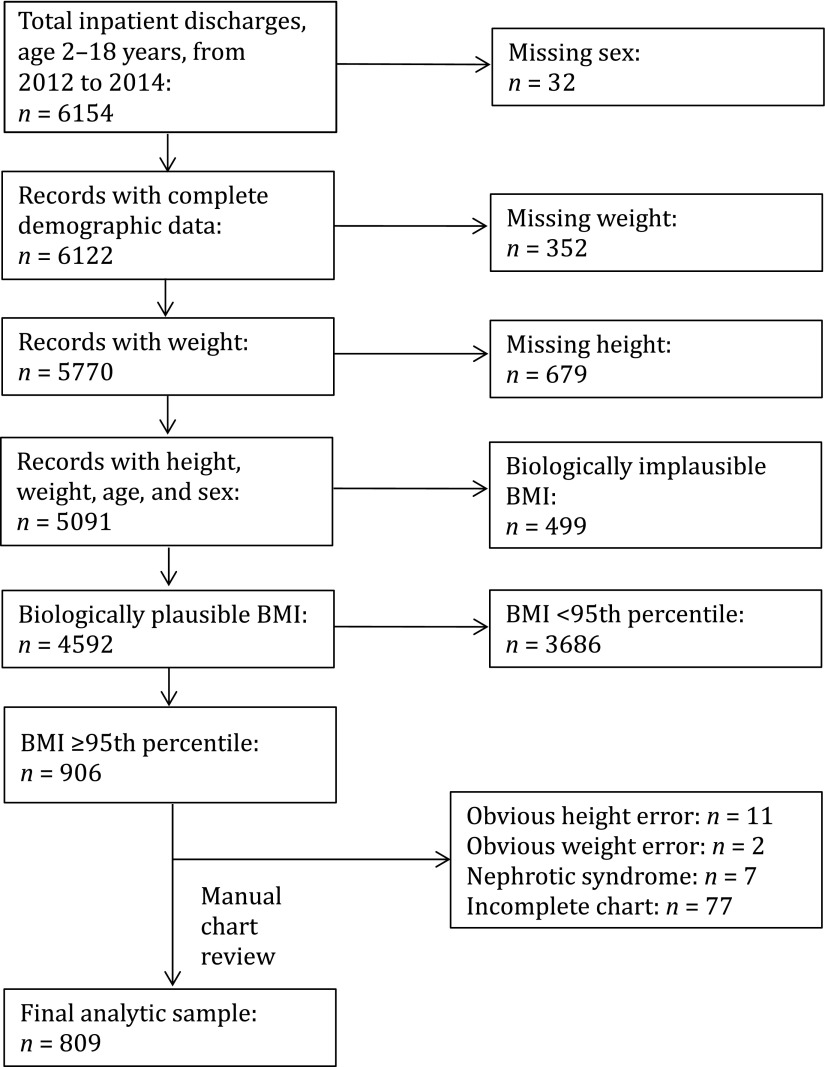
During the study period, there were 5091 discharges of patients aged 2 to 18 years whose records contained complete demographic, billing, height, and weight data. After testing for biologic plausibility, 499 (10%) were excluded, leaving 4592 records for weight status classification. All records of patients who were classified as obese were then manually reviewed, and exclusion criteria were applied. The final analytic sample contained 809 medical records of inpatients with a BMI ≥95th percentile (Fig 1).
FIGURE 1.
Formation of analytic sample.
Of the 809 participants with a BMI ≥95th percentile, 43% were female, 56% were children of color, 26% had a primary language other than English, and 70% were publicly insured (Table 1). More than one-third had at least 1 obesity-related comorbidity. The median age was 10.3 years (interquartile range 6.3–3.9), the median LOS was 52.4 hours (interquartile range 36.5–97.5), and mean BMI z score was 2.16 (SD 0.37). Seventy-two percent of the children were admitted to a primary medical pediatric service, with the remainder being admitted to a surgical service receiving a PHM consult per hospital policy.
TABLE 1.
Characteristics of Obese Subjects With and Without Obesity Documentation in the Inpatient Record
| Characteristic | All Obese (N = 809) | No Obesity Documentation (n = 595) | Obesity Documentation (n = 214) | P |
|---|---|---|---|---|
| Age, y, n (%) | <.01 | |||
| 2–4 | 141 (17.4) | 134 (22.5) | 7 (3.3) | |
| 5–11 | 370 (45.7) | 286 (48.1) | 84 (39.3) | |
| 12–18 | 298 (36.8) | 175 (29.4) | 123 (57.5) | |
| Severe obesity (BMI ≥120% of 95th percentile), n (%) | 262 (32.4) | 138 (23.2) | 124 (57.9) | <.01 |
| LOS ≥52 h, n (%) | 405 (50.1) | 282 (48.7) | 123 (57.9) | .01 |
| Female sex, n (%) | 347 (42.9) | 243 (40.8) | 104 (48.6) | .05 |
| Race, n (%) | .02 | |||
| White | 353 (43.6) | 253 (42.5) | 100 (46.7) | |
| Hispanic | 178 (22.0) | 123 (20.7) | 55 (25.7) | |
| African American | 76 (9) | 54 (9) | 22 (10) | |
| Asian American | 65 (8.0) | 58 (9.7) | 7 (3.3) | |
| Other | 137 (16.9) | 107 (18.0) | 30 (14.0) | |
| Primary language, n (%) | .11 | |||
| English | 597 (73.8) | 430 (72.3) | 167 (78.0) | |
| Spanish | 130 (16.1) | 97 (16.3) | 33 (15.4) | |
| Other | 82 (10.1) | 68 (11.4) | 14 (6.6) | |
| Medicaid, n (%) | 564 (69.7) | 430 (72.3) | 134 (62.6) | .01 |
| Comorbidity types present, n (%) | ||||
| Asthmaa | 218 (26.9) | 152 (25.5) | 66 (30.8) | .15 |
| Sleep apneaa | 42 (5.2) | 26 (4.4) | 16 (7.5) | .10 |
| Cholelithiasisa | 22 (2.7) | 5 (1) | 17 (7.9) | <.01 |
| Diabetes mellitusa | 16 (2.0) | 10 (1.7) | 6 (2.8) | .39 |
| Pseudotumor cerebria | 4 (0.4) | 2 (0.3) | 2 (0.9) | .29 |
| DVTa | 3 (0.4) | 0 (0) | 3 (1.4) | .02 |
| Surgical service with PHM consult | 223 (28) | 153 (25.7) | 70 (32.7) | .06 |
| >1 admission | 170 (21.0) | 113 (19.0) | 57 (26.6) | .02 |
Analyzed separately as dichotomous variables (and therefore not mutually exclusive).
Obesity documentation was present in 26.5% of the medical records studied. Patients with obesity documentation were more likely to be older and have severe obesity than those without obesity documentation. Patient ethnicity and cholelithiasis were also significantly associated with obesity documentation in bivariate analysis. Cholelithiasis was significantly associated with obesity documentation, whereas asthma, DVT, pseudotumor cerebri, sleep apnea, and diabetes mellitus were not. In the multivariable model, older age (adjusted odds ratio [aOR] 7.43; 95% confidence interval [CI] 1.45–7.54), severe obesity (aOR 3.92; 95% CI 2.74–5.62; P <.01), and comorbid cholelithiasis (aOR 6.20; 95% CI 2.08–18.5; P <.01) remained significant after adjusting for all other significant variables (Table 2).
TABLE 2.
Multivariable Analysis of Factors Associated With Obesity Documentation in the Inpatient Setting
| Characteristic | aOR (95% CI) | P |
|---|---|---|
| Age (reference, 2–4 y) | ||
| 5–11 | 3.31 (1.45–7.54) | <.01 |
| 12–18 | 7.43 (3.27–16.84) | <.01 |
| Severe obesity (BMI ≥120% of 95th percentile) | 3.92 (2.74–5.62) | <.01 |
| Cholelithiasis | 6.20 (2.08–18.50) | <.01 |
Sex, race and/or ethnicity, primary language, Medicaid, >1 admission, and LOS >52 hours were also included in the model but were not significant.
Of the 214 records with obesity documentation, 22.9% contained an obesity management plan. All 49 of these records also contained documentation of obesity in the history, physical examination, or assessment portion of the note, and 94% had management plans written by PHM attending physicians. This included 24 patients who were admitted to a surgical service, where the PHM attending performed a general pediatric consult. The most common type of management plan was direct counseling by the documenting clinician or primary team. In bivariate analysis (Table 3), admission to a surgical service with PHM consult, comorbid sleep apnea, and >1 inpatient encounter were significantly associated with obesity management plan documentation (P <.1). In the multivariable analysis, admission to a surgical service (aOR 2.78; 95% CI 1.39–5.54) and diagnosis of sleep apnea (aOR 3.98; 95% CI 1.31–12.10) remained significant predictors of plan documentation (Table 4).
TABLE 3.
Bivariate Analysis of Clinical and Sociodemographic Factors Associated With Obesity Management Plan
| Characteristic | No Plan (n = 165) | Plan (n = 49) | P |
|---|---|---|---|
| Age, y, n (%) | .43 | ||
| 2–4 | 4 (2) | 3 (6) | |
| 5–11 | 66 (40) | 18 (37) | |
| 12–18 | 95 (58) | 28 (57) | |
| Severe obesity (BMI ≥120% of 95th percentile), n (%) | 90 (55) | 27 (55) | >.99 |
| LOS >52 h, n (%) | 97 (58.8) | 26 (53.1) | .51 |
| Female sex, n (%) | 80 (48.5) | 24 (49.0) | >.99 |
| Race, n (%) | .80 | ||
| White | 79 (48) | 21 (43) | |
| Hispanic | 42 (26) | 13 (27) | |
| African American | 18 (11) | 4 (8) | |
| Asian American | 6 (4) | 1 (2) | |
| Other | 20 (12) | 10 (20) | |
| Primary language, n (%) | .118 | ||
| English | 134 (81.2) | 33 (67.3) | |
| Spanish | 22 (13.3) | 11 (22.4) | |
| Other | 9 (5.5) | 5 (10.2) | |
| Medicaid, n (%) | 99 (60.0) | 35 (71.4) | .18 |
| Comorbidity types present, n (%) | |||
| Asthmaa | 51 (30.9) | 15 (30.6) | >.99 |
| Sleep apneaa | 9 (5.5) | 7 (14.3) | .06 |
| Cholelithiasisa | 12 (7.3) | 5 (10.2) | .55 |
| Diabetes mellitusa | 5 (3.0) | 1 (2.0) | >.99 |
| Pseudotumor cerebria | 1 (0.6) | 1 (2.0) | .41 |
| DVTa | 3 (1.8) | 0 (0) | >.99 |
| Surgical service with PHM consult, n (%) | 45 (27.3) | 25 (51.0) | <.01 |
| >1 admission, n (%) | 49 (29.7) | 8 (16.3) | .07 |
Analyzed separately as dichotomous variables (and therefore not mutually exclusive).
TABLE 4.
Multivariable Analysis of Factors Associated With Obesity Management Plan
| Characteristic | aOR (95% CI) | P |
|---|---|---|
| Comorbid sleep apnea | 3.98 (1.31–12.10) | .02 |
| Surgical service with PHM consult | 2.78 (1.39–5.54) | <.01 |
More than 1 admission was also included in the model, but results were not significant. OR, odds ratio.
Discussion
To our knowledge, this is the largest sample of inpatient medical records to be studied for provider documentation of obesity and predictors of such documentation. We found that only 1 of every 4 obese pediatric inpatients had any documentation of obesity, and only a small fraction of those received counseling, recommendations, or referrals for weight management. The low rates of obesity documentation seen in our data corroborate previous findings that the majority of obese patients likely do not have appropriate recognition of their weight status in the hospital. However, it is important to note that the rate of documentation seen in our sample is more than twice as high as that seen in earlier inpatient studies and higher than some previously published outpatient reports.20,21,29–31 This is likely due to differences in methodology and sample characteristics across different institutions, but it may also reflect a shifting understanding of obesity’s relevance across the continuum of care over time.
Our findings suggest that provider documentation of obesity is affected by specific patient characteristics. Ethnicity was not a significant predictor of obesity documentation in our study, although it has been in others. This may be due to the specific ethnic and racial makeup of our hospital population, which has a much smaller percentage of non-Hispanic African American patients than other similar studies.7,24 Additionally, our choice to categorize patients into 5 distinct racial and/or ethnic subgroups may have limited our analytic power compared with other studies that have used race as a dichotomous variable.4,20
We did find that severe obesity and older age were significantly associated with obesity recognition. As others have reported in the outpatient setting, recognition, documentation, and diagnosis of obesity occurs less frequently in younger children regardless of BMI z score.7 Our study replicated this finding in the inpatient setting; children <5 years old represented only 3% of patients with obesity documentation despite being 17% of the overall sample with obesity. One 2014 study in which researchers surveyed primary care pediatricians found that <60% of respondents thought obesity should be discussed at well-child visits before the age of 5 years.32 It may be that inpatient providers have similar beliefs. Alternatively, our results may indicate that the problem is not merely one of discomfort with discussion but of failure to recognize a patient’s weight status on history and physical examination at that early age. Similar to age, a lower BMI z score is also associated with a lower likelihood of obesity recognition, both in our study and in others.5,6,29 These findings are of particular concern given that younger, less obese patients may be more likely to respond to obesity interventions.33
Our study of individual comorbidities associated with obesity documentation and the development of an obesity management plan revealed that comorbid cholelithiasis and sleep apnea influenced inpatient provider behaviors in regard to obesity assessment in distinct ways. Comorbid cholelithiasis was an independent predictor of obesity documentation. whereas comorbid sleep apnea was associated with the presence of a management plan. Cholelithiasis is well known to be related to obesity, and this association is often taught in medical school training. As such, its relation to obesity may be more apparent for physicians than that of other diagnoses, leading them to document obesity as a diagnosis for patients with cholelithiasis. Sleep apnea, although also a well-known consequence of obesity, results from a direct functional and mechanical impact of excess weight on one’s breathing in contrast to the complex metabolic pathways that lead to cholelithiasis. Thus, the mechanism of obesity’s contribution to sleep apnea may allow providers an opportunity to address weight status that is simple to explain and understand. This likely makes it easier for providers to overcome concerns about the appropriateness of the inpatient setting or a perceived lack of family receptiveness, which are 2 of the most common reasons cited by inpatient providers for not addressing obesity.34
Another association revealed by our data is that admission to a surgical service was an independent predictor of an obesity-specific management plan. Although our intention in studying primary service was to determine differences in provider behavior that may be associated with clinical specialty, a manual chart review revealed that this difference was actually a reflection of having a PHM consult, which is a requirement in this children’s hospital. Notably, the PHM attending who performs the consults is the same attending who concurrently oversees the care of the majority of nonsurgical patients. Perhaps the act of performing a general pediatric consult provided an additional opportunity for hospitalists to consider their patients’ health more broadly, including the relevance and consequences of obesity. There is growing literature examining the benefits of comanagement on acute, hospital-based outcomes in surgical patients, resource use, LOS, patient safety, and nursing satisfaction.35 No researchers have specifically looked at the degree to which PHM involvement may improve screening for obesity, weight-related comorbidities, or other medical conditions. This is an area worth further study as the field of PHM continues to expand and define its role.
This study had several limitations. It was performed at a single academic institution, which limits its generalizability. As with any retrospective study, we cannot deduce causality, but the temporal relationship between relatively fixed or chronic patient characteristics and provider documentation during a single hospitalization can be presumed. The transition to electronic notes during our study period may have impacted provider documentation behavior in a manner that is difficult to isolate and quantify. However, automatic growth curve plotting (likely the most useful EMR feature for recognizing obesity) remained the same throughout the study period.
Excluding cases for biologic implausibility did introduce the risk of excluding true outliers. This was weighed against the risk of drawing erroneous conclusions from analyzing inaccurate data, which we attempted to balance by using programming developed by the CDC for this purpose.25,28 The retrospective design also limited the level of detail available in our data regarding illness acuity, transfers between clinical services, and provider characteristics, all of which may have acted as confounders or alternative predictors. Indeed, there are important provider and system-level factors that also contribute to the under-recognition of weight status in hospitalized patients. As noted in a recent survey of hospitalists across the country, providers have limited time and numerous other, competing priorities and are frequently concerned that patients may not be receptive to a weight-related discussion.34 However, there is evidence to suggest that the majority of families want to hear from a doctor about their children’s weight if it is abnormal and are receptive to obesity screening both on the inpatient floor36,37 and in the emergency department.38
Our choice to strictly define obesity documentation as the use of the word “obese” or “obesity” may have missed cases in which “overweight” was used erroneously, leading to an under-representation of provider recognition of obesity in our results. Furthermore, focusing on documentation as the outcome may not truly reflect the discussion of weight status with the patient because these 2 behaviors are frequently discordant.39 As such, our data may under- or over-represent the true rate of obesity recognition in our sample. Finally, the data on obesity-related comorbidities were limited by the relatively low prevalence of those diagnoses, and it may be that although we found significant associations in 2 of the 6 diagnoses examined, researchers in a larger study could find significant associations between obesity recognition and other comorbidities.
Conclusions
These results add to a growing body of evidence that inpatient providers often do not accurately document weight status in obese pediatric patients, although our rates were higher than previously reported. Additionally, these results suggest that this missed opportunity is more pronounced in younger patients with less severe obesity and no associated comorbidities. Because these characteristics are known to be associated with greater responsiveness to intervention,33 efforts to improve obesity diagnosis and management in younger children who have not yet developed comorbidities should be increased, and interventions to curb the obesity epidemic should take the disparities of obesity recognition into account. Additionally, the role of PHM attending physicians as general pediatric consultants should be further explored as a tool for addressing obesity during inpatient hospitalization. It is apparent in our sample that when acting as a consultant, PHM attending physicians address weight status in surgical patients more frequently than in their own patients. Barriers to doing so among medical patients should be investigated further to inform the development of systems and policies that support accurate documentation and diagnosis of obesity for all hospitalized children.
Acknowledgments
We acknowledge the tireless work of Hannah Bodenstein, Lauren Castner, and Hanna Siddiqui in data extraction and chart review.
Footnotes
Dr Katzow conceptualized and designed the study and data collection instruments, coordinated and supervised data collection, performed the final data analysis, and drafted the initial manuscript; Dr Homel conducted the initial analyses, reviewed and revised the manuscript, and approved the manuscript as initially submitted (because of his untimely death on April 24, 2017, he was unable review the final revision of the manuscript); Dr Rhee critically reviewed the manuscript; and Drs Katzow and Rhee approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funding for this project provided in part to Dr Katzow by an NIH training grant (HRSA NRSA T32: HP22238) and NIH Clinical and Translational Science Award grants at NYU (UL1TR001445, KL2TR001446, and TL1R001447). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Must A, Phillips SM, Naumova EN. Occurrence and timing of childhood overweight and mortality: findings from the Third Harvard Growth Study. J Pediatr. 2012;160(5):743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs NF, Jacobson MS; American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112(2):424–430 [DOI] [PubMed] [Google Scholar]
- 4.Patel AI, Madsen KA, Maselli JH, Cabana MD, Stafford RS, Hersh AL. Underdiagnosis of pediatric obesity during outpatient preventive care visits. Acad Pediatr. 2010;10(6):405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow SE, Bobra SR, Elliott MB, Brownson RC, Haire-Joshu D. Recognition of childhood overweight during health supervision visits: does BMI help pediatricians? Obesity (Silver Spring). 2007;15(1):225–232 [DOI] [PubMed] [Google Scholar]
- 6.O’Brien SH, Holubkov R, Reis EC. Identification, evaluation, and management of obesity in an academic primary care center. Pediatrics. 2004;114(2). Available at: www.pediatrics.org/cgi/content/full/114/2/e154 [DOI] [PubMed] [Google Scholar]
- 7.Dilley KJ, Martin LA, Sullivan C, Seshadri R, Binns HJ; Pediatric Practice Research Group. Identification of overweight status is associated with higher rates of screening for comorbidities of overweight in pediatric primary care practice. Pediatrics. 2007;119(1). Available at: www.pediatrics.org/cgi/content/full/119/1/e148 [DOI] [PubMed] [Google Scholar]
- 8.Hernandez RG, Cheng TL, Serwint JR. Parents’ healthy weight perceptions and preferences regarding obesity counseling in preschoolers: pediatricians matter. Clin Pediatr (Phila). 2010;49(8):790–798 [DOI] [PubMed] [Google Scholar]
- 9.Rhee KE, De Lago CW, Arscott-Mills T, Mehta SD, Davis RK. Factors associated with parental readiness to make changes for overweight children. Pediatrics. 2005;116(1). Available at: www.pediatrics.org/cgi/content/full/116/1/e94 [DOI] [PubMed] [Google Scholar]
- 10.Halvorson EE, Irby MB, Skelton JA. Pediatric obesity and safety in inpatient settings: a systematic literature review. Clin Pediatr (Phila). 2014;53(10):975–987 [DOI] [PubMed] [Google Scholar]
- 11.Fradin K, Racine AD, Belamarich PF. Obesity and symptomatic cholelithiasis in childhood: epidemiologic and case-control evidence for a strong relation. J Pediatr Gastroenterol Nutr. 2014;58(1):102–106 [DOI] [PubMed] [Google Scholar]
- 12.Carroll CL, Stoltz P, Raykov N, Smith SR, Zucker AR. Childhood overweight increases hospital admission rates for asthma. Pediatrics. 2007;120(4):734–740 [DOI] [PubMed] [Google Scholar]
- 13.Chen W-H, Lu C-Y, Shao P-L, et al. Risk factors of severe novel influenza A (H1N1) infections in hospitalized children. J Formos Med Assoc. 2012;111(8):421–426 [DOI] [PubMed] [Google Scholar]
- 14.Bechard LJ, Duggan C, Touger-Decker R, et al. Nutritional status based on body mass index is associated with morbidity and mortality in mechanically ventilated critically ill children in the PICU. Crit Care Med. 2016;44(8):1530–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vu LT, Nobuhara KK, Lee H, Farmer DL. Determination of risk factors for deep venous thrombosis in hospitalized children. J Pediatr Surg. 2008;43(6):1095–1099 [DOI] [PubMed] [Google Scholar]
- 16.Backstrom IC, MacLennan PA, Sawyer JR, Creek AT, Rue LW, III, Gilbert SR. Pediatric obesity and traumatic lower-extremity long-bone fracture outcomes. J Trauma Acute Care Surg. 2012;73(4):966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bechard LJ, Rothpletz-Puglia P, Touger-Decker R, Duggan C, Mehta NM. Influence of obesity on clinical outcomes in hospitalized children: a systematic review. JAMA Pediatr. 2013;167(5):476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trasande L, Liu Y, Fryer G, Weitzman M. Effects of childhood obesity on hospital care and costs, 1999-2005. Health Aff (Millwood). 2009;28(4):w751–w760 [DOI] [PubMed] [Google Scholar]
- 19.Wier LM, Encinosa W. Obesity in Children: Hospitalizations From 2000 to 2009: Statistical Brief #138. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012:1–9 [PubMed] [Google Scholar]
- 20.Woo JG, Zeller MH, Wilson K, Inge T. Obesity identified by discharge ICD-9 codes underestimates the true prevalence of obesity in hospitalized children. J Pediatr. 2009;154(3):327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sleeper EJ, Ariza AJ, Binns HJ. Do hospitalized pediatric patients have weight and blood pressure concerns identified? J Pediatr. 2009;154(2):213–217 [DOI] [PubMed] [Google Scholar]
- 22.Azhdam DB, Reyhan I, Grant-Guimaraes J, Feinstein R. Prevalence and documentation of overweight and obesity in hospitalized children and adolescents. Hosp Pediatr. 2014;4(6):377–381 [DOI] [PubMed] [Google Scholar]
- 23.King MA, Nkoy FL, Maloney CG, Mihalopoulos NL. Physicians and physician trainees rarely identify or address overweight/obesity in hospitalized children. J Pediatr. 2015;167(4):816.e1–820.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorsey KB, Wells C, Krumholz HM, Concato J. Diagnosis, evaluation, and treatment of childhood obesity in pediatric practice [published correction appears in Arch Pediatr Adolesc Med. 2008;162(5):417. Arch Pediatr Adolesc Med. 2005;159(7):632–638 [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. A SAS program for the 2000 CDC growth charts (ages 0 to <20 years). 2016. Available at: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Accessed February 5, 2015
- 26.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27 [PubMed] [Google Scholar]
- 27.Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl Health Stat Rep. 2013;(63):1–3 [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Modified z-scores in the CDC growth charts. 2016. Available at: https://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/biv-cutoffs.pdf. Accessed February 5, 2017
- 29.Borgmeyer A, Ercole PM, Niesen A, Strunk RC. Lack of recognition, diagnosis, and treatment of overweight/obesity in children hospitalized for asthma. Hosp Pediatr. 2016;6(11):667–676 [DOI] [PubMed] [Google Scholar]
- 30.O’Connor J, Youde LS, Allen JR, Baur LA. Obesity and under-nutrition in a tertiary paediatric hospital. J Paediatr Child Health. 2004;40(5–6):299–304 [DOI] [PubMed] [Google Scholar]
- 31.O’Connor J, Youde LS, Allen JR, Hanson RM, Baur LA. Outcomes of a nutrition audit in a tertiary paediatric hospital: implications for service improvement. J Paediatr Child Health. 2004;40(5–6):295–298 [DOI] [PubMed] [Google Scholar]
- 32.Chelvakumar G, Levin L, Polfuss M, Hovis S, Donohoue P, Kotowski A. Perception and documentation of weight management practices in pediatric primary care. WMJ. 2014;113(4):149–153; quiz 154 [PubMed] [Google Scholar]
- 33.Reinehr T, Kleber M, Lass N, Toschke AM. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. Am J Clin Nutr. 2010;91(5):1165–1171 [DOI] [PubMed] [Google Scholar]
- 34.Lee DS, Gross E, Rinke ML. Physician perspectives on obesity screening in hospitalized children. Clin Pediatr (Phila). 2016;55(10):983–985 [DOI] [PubMed] [Google Scholar]
- 35.Rappaport DI, Rosenberg RE, Shaughnessy EE, et al. Pediatric hospitalist comanagement of surgical patients: structural, quality, and financial considerations. J Hosp Med. 2014;9(11):737–742 [DOI] [PubMed] [Google Scholar]
- 36.Bradford K, Kihlstrom M, Pointer I, Skinner AC, Slivka P, Perrin EM. Parental attitudes toward obesity and overweight screening and communication for hospitalized children. Hosp Pediatr. 2012;2(3):126–132 [DOI] [PubMed] [Google Scholar]
- 37.McLean K, Wake M, McCallum Z. Overweight in medical paediatric inpatients: detection and parent expectations. J Paediatr Child Health. 2007;43(4):256–261 [DOI] [PubMed] [Google Scholar]
- 38.Vaughn LM, Nabors L, Pelley TJ, Hampton RR, Jacquez F, Mahabee-Gittens EM. Obesity screening in the pediatric emergency department. Pediatr Emerg Care. 2012;28(6):548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turer CB, Barlow SE, Montaño S, Flores G. Discrepancies in communication versus documentation of weight-management benchmarks: analysis of recorded visits with Latino children and associated health-record documentation. Glob Pediatr Health. 2017;4(244):2333794X16685190 [DOI] [PMC free article] [PubMed] [Google Scholar]