Infants with TSC can often be identified before neurologic symptoms present, allowing for improved surveillance and the possibility of disease-modifying treatment.
Abstract
OBJECTIVES:
Tuberous sclerosis complex (TSC) is a neurocutaneous genetic disorder with a high prevalence of epilepsy and neurodevelopmental disorders. TSC can be challenging to diagnose in infants because they often do not show many clinical signs early in life. In this study, we describe the timing and pattern of presenting and diagnostic features in a prospective longitudinal study of infants with TSC.
METHODS:
Two multicenter, prospective studies enrolled 130 infants with definite TSC by clinical or genetic criteria and followed them longitudinally up to 36 months of age. Periodic study visits included medical and seizure histories, physical and neurologic examinations, and developmental assessments. Ages at which major and minor features of TSC and seizures were first identified were analyzed.
RESULTS:
The most common initial presenting features of TSC were cardiac rhabdomyomas (59%) and hypomelanotic macules or other skin findings (39%), and 85% of infants presented with either or both. Ultimately, the most prevalent diagnostic TSC features were hypomelanotic macules (94%), tubers or other cortical dysplasias (94%), subependymal nodules (90%), and cardiac rhabdomyomas (82%). Thirty-five percent of infants presented prenatally, 41% presented at birth or within the first month of life, and 74% met criteria for TSC diagnosis at or within 30 days of presentation. Seizure onset occurred before or at initial presentation in only 15% of infants, but 73% developed epilepsy within the first year of life.
CONCLUSIONS:
Infants with TSC can often be identified early, before the onset of neurologic sequelae, enabling earlier diagnosis, surveillance, and possibly disease-modifying treatment.
What’s Known on This Subject:
Tuberous sclerosis complex (TSC) can be challenging to diagnose in infants because they often do not show many clinical signs early in life. Early diagnosis enables more effective disease surveillance, especially for epilepsy, which is highly prevalent in TSC.
What This Study Adds:
In this prospective longitudinal study, a majority of infants with TSC could be identified early by cardiac rhabdomyomas or hypomelanotic skin macules before epilepsy onset. Different presentation patterns may be related to risk of developing epilepsy.
The understanding and treatment of tuberous sclerosis complex (TSC) have advanced significantly in the last 2 decades.1 After the identification and sequencing of the genes responsible for TSC in the 1990s, the biochemical pathway at the root of the disorder was mapped, leading to effective treatments aimed at the underlying disease mechanism.2,3 However, much remains to be discovered. Why does TSC vary widely in presentation and severity from patient to patient, particularly in its neurodevelopmental effects? How early can we identify patients with TSC? Could targeted treatment safely and effectively modify the course and prognosis of the disease? The TSC Autism Center of Excellence Research Network is conducting prospective longitudinal observational studies in infants with TSC to answer these and other questions. In this article, we analyze the timing and pattern of clinical presenting and diagnostic features in infants with TSC to better understand how TSC presents in this unique population and how it can be diagnosed and treated earlier.
TSC is a neurocutaneous genetic disease with an incidence of ∼1 in 6000 live births.4 It presents with a wide range of manifestations caused by localized cellular overgrowth, leading to benign tumors (hamartomas) in multiple organs. In the brain, these include areas of abnormal cortical and subcortical cellular development (tubers), subependymal nodules (SENs), and subependymal giant cell astrocytomas (SEGAs). The skin, heart, eyes, kidneys, and lungs may also be affected in varying degrees.5 Dysfunction of hamartin and tuberin, the protein products of the TSC1 and TSC2 genes, results in upregulation of the mechanistic (formerly mammalian) target of the rapamycin (mTOR) pathway and produces dysregulated cellular growth.6,7 mTOR inhibitors are used to treat SEGAs, renal angiomyolipomas, facial angiofibromas, and pulmonary lymphangioleiomyomatosis.2 Most patients with TSC also develop neurologic and neuropsychiatric disorders: up to 90% develop epilepsy,8,9 and up to 50% develop autism.10 Studies have shown that mTOR inhibitors may have disease-modifying effects in TSC-associated neurologic and neuropsychiatric disorders.11 Treating infants with TSC and epilepsy earlier with antiepileptic medications or surgery may result in better neurologic outcomes, reinforcing the importance of early diagnosis.12,13
TSC can be diagnosed by the presence of clinical criteria and by genetic testing. Two major features or 1 major and 2 minor features are needed for a definite clinical diagnosis.14 The variability of presentation and later onset of certain clinical features can make diagnosing TSC in infants more challenging.15,16 Early identification of infants with TSC allows clinicians to provide appropriate, disease-related surveillance and anticipatory guidance to patients. Early identification is also critical for clinical researchers, who can study these infants from early in life to better understand the natural history of TSC and develop targeted treatments.
Methods
Study Protocols, Design, and Participant Demographics
Participants were enrolled across 5 geographically distributed sites: Boston Children’s Hospital; Cincinnati Children’s Hospital Medical Center; the University of Alabama at Birmingham; the University of California, Los Angeles; and the University of Texas Health Science Center at Houston. Two concurrent prospective longitudinal observational studies were conducted, and eligible participants could be enrolled in 1 or both studies: (1) Potential EEG Biomarkers and Antiepileptogenic Strategies for Epilepsy in TSC (P20NS080199) and (2) Early Biomarkers of Autism Spectrum Disorders in Infants with Tuberous Sclerosis Complex (U01NS082320, a TSC Autism Center of Excellence grant). The enrollment goal was 150 infants; infants were eligible if they met genetic or clinical diagnostic criteria for TSC on the basis of current recommendations for diagnostic evaluation.14 Data collected at study visits included medical and seizure histories, physical and neurologic examinations, and developmental assessments. Full inclusion and exclusion criteria, protocols, and study design are detailed in Table 1. The study protocols were approved by the internal review boards at each site with direction from the leading regulatory core at Cincinnati Children’s Hospital Medical Center. Informed consent was obtained from the parents or legal guardians of all participants. The study was conducted in accordance with Good Clinical Practice guidelines. Data from each study site were entered into a Web-based, distributed data management system meeting Health Insurance Portability and Accountability Act privacy regulations. Subject demographics are summarized in Table 2.
TABLE 1.
Study Protocol and Design
| TACERN study inclusion criteria | TSC-EBS study inclusion criteria |
| Meets genetic or clinical diagnostic criteria for TSC on the basis of current recommendations for diagnostic evaluation, including physical examination, neuroimaging, and echocardiogram14 | Meets genetic or clinical diagnostic criteria for TSC on the basis of current recommendations for diagnostic evaluation, including physical examination, neuroimaging, and echocardiogram14 |
| Age criteria: 3–12 mo at time of enrollment. For study purposes, 3 mo is defined as ≥9 wk, 1 d and 12 mo is defined as ≤13.5 mo. | Age criteria: <6 mo at time of enrollment. |
| — | Seizure free at the time of study enrollment |
| TACERN study exclusion criteria | TSC-EBS study exclusion criteria |
| Gestational age <36 wk at time of delivery | Gestational age <30 wk at time of delivery |
| Taking an mTOR inhibitor such as rapamycin, sirolimus, or everolimus (other than topical formulations) at the time of study enrollment | Taking vigabatrin or mTOR inhibitor before study enrollment |
| Has taken an investigational drug as part of another research study within 30 d before study enrollment | Participant’s neonatal course, time of delivery, or history includes CNS infection, hypoxic-ischemic encephalopathy, or intraventricular hemorrhage |
| SEGA requiring medical or surgical treatment at the time of study enrollment | History of seizures and/or infantile spasms |
| History of epilepsy surgery at the time of study enrollment | — |
| Contraindications to MRI scanning, such as metal implants and/or noncompatible medical devices or medical conditions | — |
| TACERN study visit ages | TSC-EBS study visit ages |
| 3, 6, 9, 12, 18, 24, and 36 mo | Before 3 wk, at 6 wk, and at 3, 4.5, 6, 9, 12, 18, and 24 mo |
| Information collected at baseline visit in both studies | |
| Demographics and medical history including seizures, medications, family history, physical and neurologic examinations, developmental assessment, and EEGs | |
| Information collected at follow-up visits in both studies | |
| Seizures types and frequency, interval medical history, medications, physical and neurologic examinations, developmental assessment, EEGs, and results of yearly clinical brain MRI | |
| TSC-EBS (P20NS080199 and NCT01767779) | |
| TACERN (U01NS082320 and NCT01780441) | |
CNS, central nervous system; TACERN, TSC Autism Center of Excellence Research Network; TSC-EBS, Potential EEG Biomarkers and Antiepileptogenic Strategies for Epilepsy in TSC; —, not applicable.
TABLE 2.
Participant Demographics
| Sex | ||
| Female | 62 | 48% |
| Male | 68 | 52% |
| Mean enrollment age, mo, SD | 5.2 | 3.3 |
| Race | ||
| White | 115 | 88% |
| Black or African American | 4 | 3% |
| Asian American | 10 | 8% |
| American Indian or Alaskan native | 5 | 4% |
| Native Hawaiian or other Pacific Islander | 1 | 1% |
| Other | 8 | 6% |
| Not reported | 7 | 5% |
| Reported >1 race | 17 | 13% |
| Ethnicity | ||
| Hispanic or Latino | 23 | 18% |
| Not Hispanic or Latino | 107 | 82% |
Data Collection and Analysis
Data from 130 participants were used in this interim analysis. The date of onset for each TSC feature was used to calculate the days between date of birth and onset or to determine if reported onset was prenatal. If a date of onset was unknown, the date of the study visit when the feature was first noted was used as a conservative replacement when needed for calculations. The date of diagnosis was defined as the earliest date when the subject met genetic or clinical criteria for a definite TSC diagnosis.14 For participants who had cardiac rhabdomyomas or other imaging findings reported at birth or as an initial presenting feature, study sites provided the reason for the imaging study and the date it was performed. For participants who did not have neuroimaging findings reported in the study database, sites provided anonymized reports from any MRI neuroimaging performed at the study site. These reports were reviewed for any findings of cortical tubers or dysplasia, SENs, or SEGAs. A SEGA was defined as a tumor at the caudothalamic groove larger than 10 mm in any direction or any SEN that had demonstrated growth over consecutive imaging studies.17 If an imaging report referred to findings seen in previous imaging for comparison, the date of the previous imaging was used as the date of onset for that feature; otherwise, the date of the first report with a finding was used as the date of onset. For participants who did not have ophthalmologic findings reported, sites provided dates and findings from documented clinical ophthalmologic examinations; ophthalmologic examination results were reported for 87 subjects. For participants who did not have full genetic testing results reported, sites provided deidentified copies of reports from clinical genetic testing, including parental testing, when available. Some subjects who had not had clinical genetic testing had testing done on a research basis; research results were included when available. Reported P values are for χ2 tests of independence except when noted.
Latent Class Analysis and Relationship to Genetic Variant
TSC manifestations of 103 participants with available neuroimaging and genetic testing were analyzed by means of latent class (LC) mixture modeling to identify the presence of classes that share similar patterns of TSC manifestations. After identification of distinct classes on the basis of latent factors of TSC features and seizures, the classes were regressed on genetic testing results indicating a variant in TSC1, TSC2, or no mutation identified (NMI). See the Supplemental Information for details of the mathematical analysis used in LC modeling.
Results
Timing and Pattern of Presenting Features and TSC Diagnosis
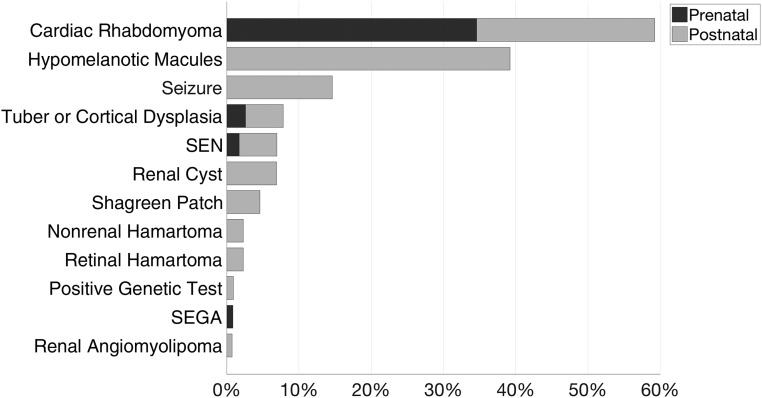
The most common initial presenting features were cardiac rhabdomyomas (59%) and hypomelanotic macules (39%), and 85% of patients presented with either or both. Cardiac rhabdomyomas were seen on prenatal imaging in 35% of patients, and 3% met diagnostic criteria for TSC prenatally with brain involvement also seen on imaging (Fig 1). Of 12 infants with rhabdomyomas recorded postnatally as the initial presenting feature, 4 had a heart murmur, 3 had another clinical indication for an echocardiogram (concern for aortic coarctation, abnormal heart sounds, and perinatal distress), and 2 had a positive family history. No reason for imaging was reported for the remaining 3 infants with rhabdomyomas and for 2 others who had other imaging findings reported as the initial presenting feature. However, all 5 had hypomelanotic macules reported as present within the first few months of life, so early skin findings may have prompted imaging but were reported by parents as occurring later.
FIGURE 1.
Prevalence of TSC features at initial presentation. Thirty-six infants (28%) had >1 initial presenting feature.
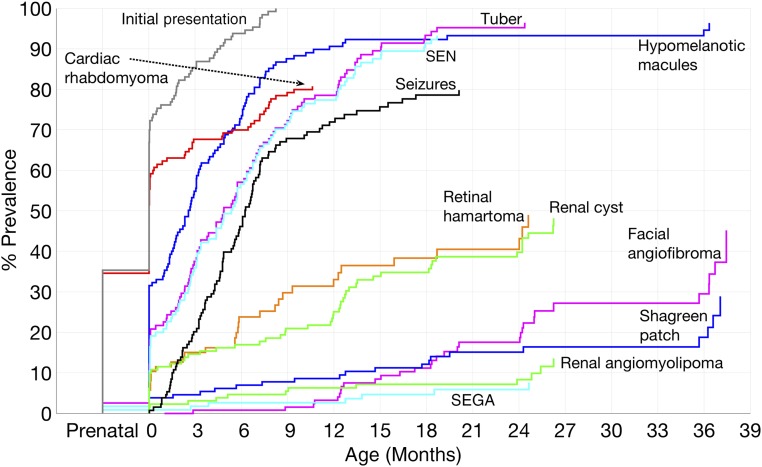
The mean postnatal age of initial feature onset was 48 days (SD 72 days). The median age, including prenatal presentations, was at birth. Thirty-five percent of infants presented prenatally, whereas 41% initially presented at birth or within the first month of life. The mean postnatal diagnosis age was 72 days (SD 85 days), and the median diagnosis age was 32 days. Fifty percent were diagnosed with TSC within the first month of life. Twenty-four percent met TSC diagnosis criteria at initial presentation; an additional 49% met diagnosis criteria within 1 month of initial presentation. Age of onset or recognition of the most prevalent major TSC features plus seizures and renal cysts is shown in Fig 2.
FIGURE 2.
Age of onset or recognition of the most prevalent TSC features in infants. Hypomelanotic macules, tubers, SENs, and cardiac rhabdomyomas are often seen before the onset of seizures, whereas other manifestations are more commonly first seen later in life.
Prevalence of Major and Minor TSC Features and Contribution to Diagnosis
The most prevalent major TSC criteria were hypomelanotic macules (94%), tubers or other cortical dysplasias (94%), SENs (90%), and cardiac rhabdomyomas (82%). Every infant had at least 1 of these features, and 61% had all 4. Of 87 infants with documented ophthalmologic examinations or findings, 43% had retinal hamartomas, and 6% had retinal achromic patches. Renal cysts were the only relatively common minor criteria, occurring in 45% of patients (Table 3). All patients with only 1 major criterion had no minor criteria present and were diagnosed with definite TSC on the basis of genetic testing. Thus, minor criteria did not contribute to the diagnosis of TSC in any infant. No TSC diagnostic criteria differed significantly by sex (P > .05).
TABLE 3.
Prevalence of TSC Criteria
| % (n) | |
|---|---|
| Major criteria | |
| Hypomelanotic macules | 94 (122) |
| Tuber or cortical dysplasia | 94a (108) |
| SEN | 90a (104) |
| Cardiac rhabdomyoma | 82 (106) |
| Retinal hamartoma | 43b (37) |
| Angiofibroma or cephalic plaque | 25 (32) |
| Shagreen patch | 18 (24) |
| Renal angiomyolipoma | 11 (14) |
| SEGA | 6a (7) |
| Ungual fibroma | 1 (1) |
| Minor criteria | |
| Renal cysts | 45 (58) |
| Retinal achromic patch | 6b (5) |
| Confetti skin lesions | 5 (7) |
| Nonrenal hamartoma | 5 (7) |
| Dental pits | 2 (2) |
| Intraoral fibroma | 1 (1) |
Out of 115 participants with neuroimaging data available.
Out of 87 participants with documented ophthalmologic examinations.
TSC Features in Infants Presenting Prenatally
As expected, infants presenting prenatally had a higher prevalence of cardiac rhabdomyomas (100% prenatal, 71% postnatal; P < .001). Infants presenting prenatally also had a lower prevalence of hypomelanotic macules (87% prenatal, 98% postnatal; P = .02), confetti skin lesions (none prenatal, 8% postnatal; P = .05; Fisher’s exact test), and all seizure types (65% prenatal, 82% postnatal; P = .03), although the difference was not statistically significant for individual seizure types. Other TSC features did not differ in prevalence across prenatal and postnatal presentations (P > .05).
Neuroimaging Findings
Of 115 participants with neuroimaging results available, 94% had tubers or cortical dysplasias, 90% had SENs, and 89% had both. SEGAs occurred in 6% of participants. Neuroimaging findings were seen at presentation in 16% of participants; an additional 37% had neuroimaging findings at diagnosis. Neuroimaging findings contributed to a TSC diagnosis in 38% of infants. All neuroimaging was read as normal in 4%, whereas 3% had initial neuroimaging that was read as normal with subsequent neuroimaging showing a tuber or cortical dysplasia.
Genetic Testing Findings and Family History
Of 109 infants with reported results from genetic testing, 14% had a pathogenic TSC1 variant, 72% had a pathogenic TSC2 variant, 3% had a TSC2 sequence variant of uncertain significance, and 11% had NMI. A family history of TSC was reported in 15% of infants: 5% maternal, 7% paternal, and 5% in a sibling, with 2% having both an affected parent and a sibling. In 40 subjects for whom both parents also had genetic testing, 30% had an inherited variant, and 70% had a de novo variant. Two infants (2%) were diagnosed with definite TSC by genetic diagnostic criteria.14
We examined the relationship of each TSC feature and type of variant to the affected TSC gene. Seizures of any type occurred at a lower frequency in infants with TSC1 variants (20%) than those with TSC2 variants (87%) or NMI (67%) (P < .001). This was also the case for infantile spasms (TSC1 7%, TSC2 68%, and NMI 42%; P < .001) and focal seizures (TSC1 13%, TSC2 66%, and NMI 42%; P < .001) but not for other seizure types. There were no significant relationships between the affected gene and the presence of any other major or minor TSC criteria (P > .05). Nonsense variants were more common in TSC1 (53%) than TSC2 (24%) (P = .02), and missense variants and small and large deletions were only seen in TSC2. Table 4 summarizes the prevalence of each type of genetic variant by TSC gene, and the full listing of variants and types for each subject are listed in Supplemental Table 5.
TABLE 4.
Genetic Variants
| TSC1, % (n) | TSC2, % (n) | TSC2 (VUS), % (n) | NMI, % (n) | Total, % (n) | |
|---|---|---|---|---|---|
| Nonsense | 7 (8) | 18 (20) | 0 (0) | — | 26 (28) |
| Any frameshift | 6 (6) | 20 (22) | 0 (0) | — | 26 (28) |
| Missense | 0 (0) | 13 (14) | 2 (2) | — | 15 (16) |
| Any splice | 1 (1) | 12 (13) | 1 (1) | — | 14 (15) |
| Large deletion | 0 (0) | 6 (7) | 0 (0) | — | 6 (7) |
| Small deletion | 0 (0) | 3 (3) | 0 (0) | — | 3 (3) |
| Total | 14 (15) | 72 (79) | 3 (3) | 11 (12) | 100 (109) |
| De novo | 13a (5) | 58a (23) | 0a (0) | — | 70a (28) |
| Inherited | 8a (3) | 20a (8) | 3a (1) | — | 30a (12) |
VUS, variant of unknown significance; —, not applicable.
Out of 40 subjects for whom both parents also had genetic testing.
Epilepsy Onset
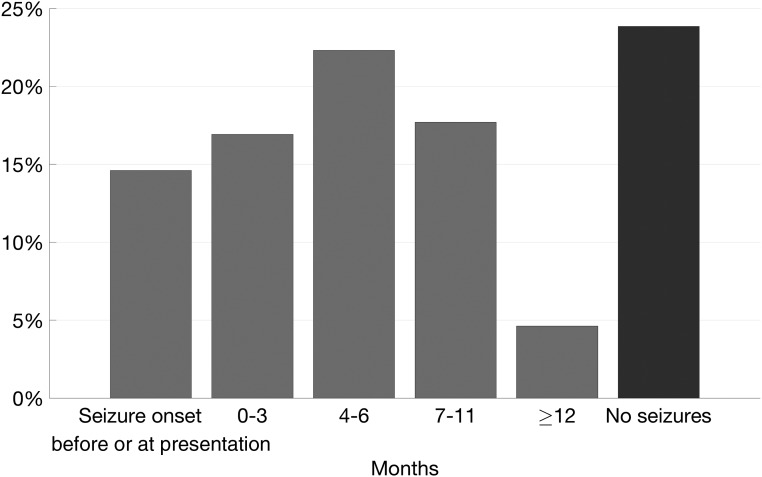
Seizures are not a diagnostic criterion for TSC, but epilepsy prevalence in TSC is as high as 90%.8,9 New-onset seizures may be the symptom that first brings patients with TSC to medical attention, prompting closer examination and studies that then lead to a TSC diagnosis. In this cohort, 15% of infants had a seizure onset date before or at the recorded onset dates of other TSC criteria, suggesting that seizure was an initial presenting symptom. Seizure onset was within 3 months after initial presentation in 17% of infants, within 6 months in 39%, and within 12 months in 57% (Fig 3).
FIGURE 3.
Time from initial presentation to first seizure.
To date, the overall prevalence of epilepsy in this cohort is 76%, with 57% having infantile spasms, 55% having focal seizures, and 12% having another seizure type. Thirty-two percent of infants had 1 seizure type, 41% had 2 seizure types, and 3% had 3 seizure types. Both infantile spasms and focal seizures were seen in 36% and were more likely to co-occur than to occur alone in an individual (P = .02).
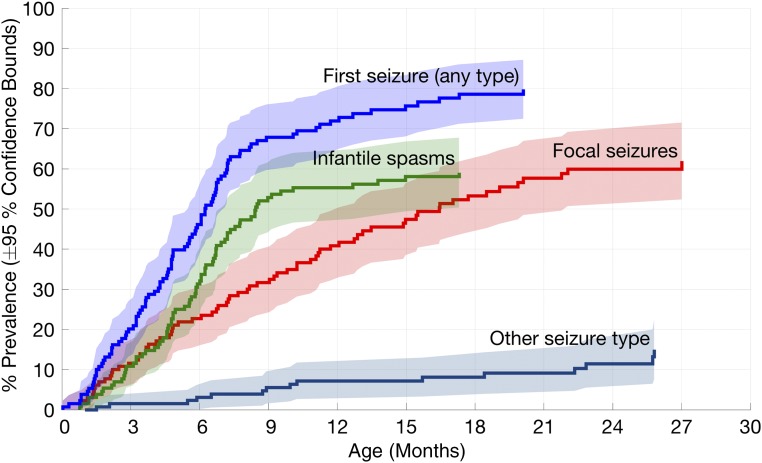
Seizure onset prevalence by age and seizure type (infantile spasms, focal seizures, or other) is shown in Fig 4. The rate of onset for a first seizure of any type was greatest in the first year of life but began to level off at ∼9 months. Infantile spasms had the highest rate of onset between 3 and 9 months, focal seizures had a relatively constant rate of onset up to 21 months, and other seizure types had an onset up to 26 months. Overall seizure prevalence was higher in girls (84%) than boys (69%) (P = .05), although the difference was not statistically significant for each individual seizure type.
FIGURE 4.
Seizure onset prevalence in TSC by age and seizure type. Infantile spasms had the highest rate of onset between 3 and 9 months, whereas focal seizures had a more constant rate of onset up to 21 months, and other seizure types had a rate of up to 26 months.
Of the 108 individuals with tubers or cortical dysplasias seen on neuroimaging, 80% developed seizures. Only 1 of the 7 individuals without tubers or cortical dysplasias seen on neuroimaging developed seizures, a significant difference from subjects with tubers (P < .001; Fisher’s exact test). This individual’s MRI was done at 6 months of age, when sensitivity to detecting tubers may be lower because of the stage of white-matter myelination.
LC Analysis
An LC model using 3 classes best fit the data by using both information criteria and a test of log-likelihood functions (Supplemental Table 6). All included TSC major features and seizures were significant indicators of LC membership. Renal cysts were the only minor feature prevalent enough to be included in analysis, but they were not a significant class indicator. With the affected gene treated as a grouping covariate, only TSC1 was significantly different among groups (Supplemental Table 7).
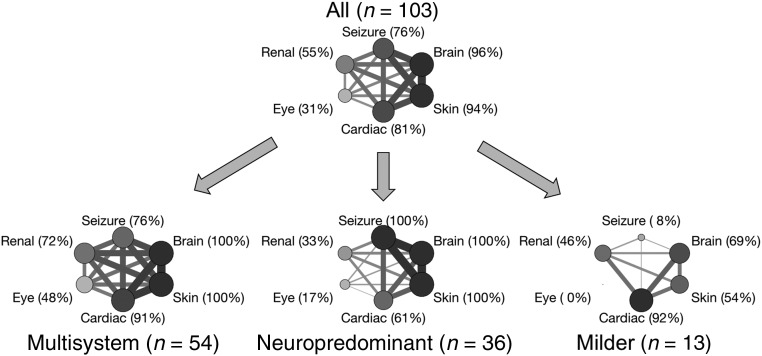
To compare patterns of organ system manifestations and co-occurrence across classes, we divided TSC manifestations into 5 organ systems (skin, brain, cardiac, renal, and eye), with seizures considered separately from structural brain manifestations of tubers and SENs, similar to the Medical Inventory of TSC Organ System Codes used by Kingswood et al.18 The subjects in the largest class (n = 54) had a high proportion of multisystem involvement, with a mean of 4.9 organ systems involved (median 5, SD 0.8) and the highest co-occurrence rates being among the brain, skin, and cardiac systems. The multisystem class included TSC1, TSC2, and NMI variants. The second-largest class (n = 36) had a neuropredominant presentation, with all subjects having structural brain manifestations and seizures; the highest co-occurrence rates were between the brain, skin, and seizures. The neuropredominant class had a mean of 4.1 organ systems involved (median 4, SD 0.7) and included only TSC2 variants and a single NMI subject. The smallest class (n = 13) had an overall milder presentation, with a mean of 2.7 organ systems involved (median 2, SD 0.8), a high prevalence of cardiac rhabdomyomas, and only 1 subject with seizures. The proportion of infants who had involvement of each organ system and the proportion who had involvement of each pair of organ systems are depicted graphically for all subjects and for each class separately in Fig 5.
FIGURE 5.
Frequency of organ system involvement in all subjects and in LCs. Vertex size is proportional to the prevalence of organ system involvement in a class. Edge width is proportional to relative co-occurrence of adjoining organ systems. Note the different patterns and prevalences of organ system involvement and co-occurrence in each class.
Discussion
A key finding of this prospective longitudinal multicenter study is that a few specific, nonneurologic TSC findings appear early in infancy. Every infant in this cohort had either hypomelanotic macules, cardiac rhabdomyomas, or both. An analysis of developmental outcomes in this cohort found that earlier seizure onset and higher seizure frequencies were associated with worse developmental outcomes.19 Early diagnosis of infants with TSC opens a window of opportunity to prevent or mitigate the often severe and disabling later neurologic manifestations of the disease, including epilepsy, developmental delays, or other TSC-associated neuropsychiatric disorders. However, this window may be as small as a few months (Fig 3). Early findings of TSC in infants are often subtle and asymptomatic and may be missed if a child is not completely evaluated, leading to delayed diagnosis.16
Epilepsy incidence in this cohort was 49% by 6 months, 73% by 12 months, and 80% by 24 months. Boys, infants who presented prenatally, and individuals with TSC1 gene variants had a lower epilepsy prevalence. Neuroimaging without tubers or cortical dysplasias had a high negative predictive value for the development of epilepsy by age 36 months. However, neuroimaging in TSC may initially appear normal, with tubers or cortical dysplasias only becoming apparent later with progressive myelination, as occurred in 3 individuals.
Different seizure types demonstrated different onset timing: most infantile spasms started between 3 and 9 months, whereas focal seizures had consistent onset of up to 21 months (Fig 4). This may reflect a relationship between infantile spasm onset and specific neurodevelopmental processes.20–22 Wu et al23 reported on 28 infants from this cohort enrolled in the Potential EEG Biomarkers and Antiepileptogenic Strategies for Epilepsy in TSC study before epilepsy onset, finding that of the 19 infants who developed epilepsy, 14 (74%) had EEG abnormalities seen before the onset of clinical seizures. Early, prospective use of EEGs may enable risk stratification in studies of epilepsy prevention in infants with TSC. The antiepileptic medication vigabatrin is particularly effective in treating infantile spasms in TSC2,24 and has mTOR-inhibiting effects.25 Vigabatrin is currently in clinical trials to determine its efficacy at preventing epilepsy in patients with TSC (Preventing Epilepsy Using Vigabatrin In Infants in the United States [NCT02849457] and Long-term, Prospective Study Evaluating Clinical and Molecular Biomarkers of Epileptogenesis in a Genetic Model of Epilepsy - Tuberous Sclerosis Complex in the European Union [NCT02098759]). To be most effective, treatment should be started at the earliest possible opportunity in patients at the highest risk of developing epilepsy.12,13,26,27
mTOR inhibitors have been successfully used to treat multiple TSC manifestations and have shown some efficacy as adjunctive treatment of refractory epilepsy.28–31 Current management guidelines determined at an international consensus meeting do not recommend using mTOR-inhibitor therapy in infants who are newly diagnosed with TSC.2 Small subgroup analyses have shown that mTOR inhibitors are generally well tolerated in young children,32,33 but concerns remain that the use of these drugs in young children may adversely affect early development or cause other sequelae, especially if long-term treatment is needed.34,35 Future studies are needed to determine the safety and efficacy of mTOR inhibitors in this age group.
Neuroimaging findings of tubers, cortical dysplasias, or SENs were highly prevalent in this cohort but were often identified after initial presentation. The high prevalence of cardiac rhabdomyomas in this cohort when compared with other large population-based studies9,18 is likely due to the regression of these tumors in older individuals and is comparable to the youngest patients in previous studies in which researchers examined TSC manifestations in pediatric populations.36,37 Minor TSC features other than renal cysts were infrequently found, and minor features did not contribute to the diagnosis of any infant.
LC analysis identified 3 classes: the largest, with multisystem organ involvement; the next largest, with a neuropredominant presentation and no TSC1 variants; and the smallest, with a milder presentation and fewer organ systems affected (Fig 5). This suggests that there may be identifiable subtypes of TSC associated with specific patterns of organ system involvement and co-occurrence. Clinicians should be aware that early involvement of multiple organ systems may indicate a patient is at higher risk of seizures. Previous genotype-phenotype studies found an overall more severe phenotype in patients with TSC2 variants but with a high degree of variability across individuals.38 Additional studies are needed to investigate whether groupings of TSC manifestations can be mechanistically related to specific genetic variants and their effects on TSC protein function and if they predict clinical or developmental outcomes.
One limitation of this study is that genetic testing was limited to TSC1 and TSC2 sequence and deletion or duplication testing. Twenty-one patients did not have genetic testing results available, and in many cases, 1 or both parents had not had testing for variants found in their child, possibly because of a lack of parental availability or family inability to obtain or pay for testing. We attempted to obtain all available clinical genetic testing results for infants and parents and were able to perform research genetic testing on a number of infants who did not have clinical testing. The 30% inherited and 70% de novo rate in infants with both parents tested is consistent with previously reported rates in TSC.38–42 Future studies of more detailed genotype-phenotype relationships in infants with TSC are needed, and data from this study will contribute to those studies.
To date, this is the largest prospective study of infants with TSC. Data were collected in a standardized, rigorous manner, and study sites were queried for clarifications and to provide missing data. Other limitations generally reflected the study design. The onset dates of some features were based on parental report and thus were susceptible to recall bias, inaccurate reporting, or were unknown. However, in most cases, onset dates were based on physical examination findings or testing reports. When dates of onset were unknown, conservative replacements were used, making it more likely that the true age was earlier than the estimated age of feature onset or TSC diagnosis. Because study enrollment was restricted to infants, individuals with milder or mosaic forms of TSC (who typically present and are diagnosed later in life) may not have been included. Infants from urban areas closer to the 5 TSC center study sites were probably overrepresented in this cohort and more likely to come to medical attention early. Thus, these infants probably represent a best-case scenario in early TSC diagnosis, demonstrating the opportunity for prompt recognition of signs of TSC with rapid follow-up testing, leading to early diagnosis, surveillance, and treatment.
Conclusions
This prospective study of infants with TSC recruited from 5 major medical centers across the United States demonstrates that we are capable of making an early diagnosis of TSC in many infants. Although many features of TSC do not appear until later in life, most infants with TSC can be identified with an echocardiogram for cardiac rhabdomyomas and a skin examination for hypomelanotic macules; both are noninvasive tests that do not require sedation. Early TSC diagnosis in infants opens a window of opportunity to treat before the onset of epilepsy or other neurodevelopmental disorders and allows for close surveillance for sequelae of TSC. Studies are underway to treat infants with TSC with the intention of preventing epilepsy, and future studies of disease-modifying therapies in TSC will also require early diagnosis. Because of the many neurodevelopmental comorbidities seen in TSC, findings gleaned from early diagnosis, surveillance, and treatment of children with TSC may also be applicable to other neurodevelopmental disorders.43
Acknowledgments
We are indebted to the families and patients in TSC clinics across the United States who contributed their time and effort to this study. We also thank the Tuberous Sclerosis Alliance for its continued support of TSC research.
The members of the TSC Autism Center of Excellence Research Network include the following: principal investigators were Sahin, M1; Krueger, D2; Bebin, M3; Wu, J4; and Northrup, H5. Co-investigators were Warfield, S1; Peters, J1; Scherrer, B1; and Goyal, M3. Project managers were Filip-Dhima, R1; Dies, K1; and Bruns, S2. The neuropsychological assessment team included Hanson, E1; Walsh, A1; Bing, N2; Kent, B2; Bucher, L3; O’Kelley, S3; William, M4; Pearson, D5; Mansour, R5; and Murray, D6. Study coordinators were Valle, M1; Gerhardt, R1; Carmody, E1; Griffith, M2; Krefting, J3; Martinez, A4; and Salazar, E5. From the Data Coordinating Center was Cutter, G7. From the Tuberous Sclerosis Alliance were Roberds, S8 and Nakagawa, JA8. National Institutes of Health project scientists were Mamounas, L9 and Kau, A10. Others included Scherrer, B1 and Leuchter, A4.
1Boston Children’s Hospital, Boston, Massachusetts; 2Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; 3University of Alabama at Birmingham, Birmingham, Alabama; 4University of California, Los Angeles, Los Angeles, California; 5University of Texas Health Science Center at Houston, Houston, Texas; 6Autism Speaks, Boston, Massachusetts; 7University of Alabama at Birmingham, Birmingham, Alabama; 8Tuberous Sclerosis Alliance, Silver Spring, Maryland; 9National Institute of Neurological Disorders and Stroke, Bethesda, Maryland; and 10Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Maryland.
Glossary
- LC
latent class
- mTOR
mechanistic target of rapamycin
- NMI
no mutation identified
- SEGA
subependymal giant cell astrocytoma
- SEN
subependymal nodule
- TSC
tuberous sclerosis complex
Footnotes
Dr Davis conceptualized the study, performed data analysis, drafted the initial manuscript, and revised the manuscript; Ms Filip-Dhima performed data collection and analysis, participated in manuscript preparation, and critically reviewed and revised the manuscript; Dr Sideridis performed statistical analyses, participated in manuscript preparation, and critically reviewed and revised the manuscript; Dr Peters contributed to the study design and data interpretation and critically reviewed and revised the manuscript; Dr Au reviewed and interpreted genetic data and critically reviewed and revised the manuscript; Drs Bebin, Krueger, Northrup, and Wu are co-principal investigators of the multicenter prospective study that supplied the data for this study, submitted and reviewed data, and critically reviewed and revised the manuscript; Dr Sahin conceptualized the study, is co-principal investigator of the multicenter prospective study that supplied the data for this study, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifiers NCT01780441 and NCT01767779).
FINANCIAL DISCLOSURE: Dr Bebin receives National Institutes of Health funding (U01NS082320, P20NS080199, and U01NS092595); Dr Wu receives research funding from the US Department of Defense and the Congressionally Directed Medical Research Program and the National Institutes of Health (P20NS080199, U01NS082320, R01NS082649, and U01NS092595); Dr Krueger has received research funding from the National Institutes of Health (U01NS082320 and U01NS092595); and Dr Sahin has received research funding from the National Institutes of Health (U01NS082320, U01NS092595, and U54HD090255) and the US Department of Defense (W81XWH-15-1-0189). Other than the items listed under Potential Conflicts of Interest, the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award numbers U01NS082320 and P20NS080199, and by the Tuberous Sclerosis Alliance. Dr Davis is supported by the Neurology Resident Research Education Program of the National Institute of Neurological Disorders and Stroke (R25NS070682), the Boston Children’s Hospital Office of Faculty Development, and the Tuberous Sclerosis Alliance. Dr Wu is supported by the University of California, Los Angeles Clinical and Translational Research Center National Institutes of Health grant UL1TR0000124. Drs Au, Bebin, Wu, Krueger, and Sahin are supported by the Developmental Synaptopathies Consortium (U54NS092090), which is a part of the National Center for Advancing Translational Sciences Rare Diseases Clinical Research Network. The Rare Diseases Clinical Research Network is an initiative of the Office of Rare Diseases Research of the National Center for Advancing Translational Sciences and is funded through collaboration between the National Center for Advancing Translational Sciences, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Bebin serves on the scientific advisory board for Novartis Pharmaceuticals Corporation and GW Pharmaceuticals and receives clinical research support from both. Dr Wu serves on the professional advisory board for the Tuberous Sclerosis Alliance, has received honoraria from and serves on the scientific advisory board and the speakers’ bureau for Novartis and H. Lundbeck A/S, and has received research support from the Tuberous Sclerosis Alliance, Novartis, and Today’s and Tomorrow’s Children Fund. Dr Sahin has received research funding from F. Hoffman-La Roche AG, Novartis, Pfizer, and LAM Therapeutics; has served on the scientific advisory board of Sage Therapeutics Inc. and PTEN Research Foundation; and serves on the professional advisory board of the Tuberous Sclerosis Alliance. Dr Krueger has received research funding from Novartis, the Clack Foundation Inc., and the Tuberous Sclerosis Alliance; has received consultant fees from Novartis; and serves on the professional advisory board of the Tuberous Sclerosis Alliance. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Sahin M, Henske EP, Manning BD, et al. ; Tuberous Sclerosis Complex Working Group to Update the Research Plan . Advances and future directions for tuberous sclerosis complex research: recommendations from the 2015 Strategic Planning Conference. Pediatr Neurol. 2016;60:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krueger DA, Northrup H; International Tuberous Sclerosis Complex Consensus Group . Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jülich K, Sahin M. Mechanism-based treatment in tuberous sclerosis complex. Pediatr Neurol. 2014;50(4):290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615(1):125–127 [DOI] [PubMed] [Google Scholar]
- 5.Curatolo P, Maria BL. Tuberous sclerosis. Handb Clin Neurol. 2013;111:323–331 [DOI] [PubMed] [Google Scholar]
- 6.Crino PB. Evolving neurobiology of tuberous sclerosis complex. Acta Neuropathol. 2013;125(3):317–332 [DOI] [PubMed] [Google Scholar]
- 7.Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84(2):275–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51(7):1236–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yates JRW, Maclean C, Higgins JNP, et al. ; Tuberous Sclerosis 2000 Study Group . The Tuberous Sclerosis 2000 Study: presentation, initial assessments and implications for diagnosis and management. Arch Dis Child. 2011;96(11):1020–1025 [DOI] [PubMed] [Google Scholar]
- 10.de Vries PJ, Whittemore VH, Leclezio L, et al. . Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND checklist. Pediatr Neurol. 2015;52(1):25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leclezio L, de Vries PJ. Advances in the treatment of tuberous sclerosis complex. Curr Opin Psychiatry. 2015;28(2):113–120 [DOI] [PubMed] [Google Scholar]
- 12.Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010;14(2):146–149 [DOI] [PubMed] [Google Scholar]
- 13.Kotulska K, Jurkiewicz E, Domańska-Pakieła D, et al. . Epilepsy in newborns with tuberous sclerosis complex. Eur J Paediatr Neurol. 2014;18(6):714–721 [DOI] [PubMed] [Google Scholar]
- 14.Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group . Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta AN, Hahn CD, Sahin M. Clinical presentation and diagnosis of tuberous sclerosis complex in infancy. J Child Neurol. 2008;23(3):268–273 [DOI] [PubMed] [Google Scholar]
- 16.Staley BA, Vail EA, Thiele EA. Tuberous sclerosis complex: diagnostic challenges, presenting symptoms, and commonly missed signs. Pediatrics. 2011;127(1). Available at: www.pediatrics.org/cgi/content/full/127/1/e117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth J, Roach ES, Bartels U, et al. . Subependymal giant cell astrocytoma: diagnosis, screening, and treatment. Recommendations from the International Tuberous Sclerosis Complex Consensus Conference 2012. Pediatr Neurol. 2013;49(6):439–444 [DOI] [PubMed] [Google Scholar]
- 18.Kingswood C, Bolton P, Crawford P, et al. . The clinical profile of tuberous sclerosis complex (TSC) in the United Kingdom: a retrospective cohort study in the Clinical Practice Research Datalink (CPRD). Eur J Paediatr Neurol. 2016;20(2):296–308 [DOI] [PubMed] [Google Scholar]
- 19.Capal JK, Bernardino-Cuesta B, Horn PS, et al. . Influence of seizures on early development in tuberous sclerosis complex. Epilepsy Behav. 2017;70(pt A):245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost JD Jr, Hrachovy RA. Pathogenesis of infantile spasms: a model based on developmental desynchronization. J Clin Neurophysiol. 2005;22(1):25–36 [DOI] [PubMed] [Google Scholar]
- 21.Paciorkowski AR, Thio LL, Rosenfeld JA, et al. . Copy number variants and infantile spasms: evidence for abnormalities in ventral forebrain development and pathways of synaptic function. Eur J Hum Genet. 2011;19(12):1238–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavone P, Striano P, Falsaperla R, Pavone L, Ruggieri M. Infantile spasms syndrome, West syndrome and related phenotypes: what we know in 2013. Brain Dev. 2014;36(9):739–751 [DOI] [PubMed] [Google Scholar]
- 23.Wu JY, Peters JM, Goyal M, et al. . Clinical electroencephalographic biomarker for impending epilepsy in asymptomatic tuberous sclerosis complex infants. Pediatr Neurol. 2016;54:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Go CY, Mackay MT, Weiss SK, et al. ; Child Neurology Society; American Academy of Neurology . Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78(24):1974–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, McDaniel SS, Rensing NR, Wong M. Vigabatrin inhibits seizures and mTOR pathway activation in a mouse model of tuberous sclerosis complex. PLoS One. 2013;8(2):e57445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jóźwiak S, Kotulska K, Domańska-Pakieła D, et al. . Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Paediatr Neurol. 2011;15(5):424–431 [DOI] [PubMed] [Google Scholar]
- 27.Bolton PF, Clifford M, Tye C, et al. ; Tuberous Sclerosis 2000 Study Group . Intellectual abilities in tuberous sclerosis complex: risk factors and correlates from the Tuberous Sclerosis 2000 Study. Psychol Med. 2015;45(11):2321–2331 [DOI] [PubMed] [Google Scholar]
- 28.Curatolo P, Bjørnvold M, Dill PE, et al. . The role of mTOR inhibitors in the treatment of patients with tuberous sclerosis complex: evidence-based and expert opinions. Drugs. 2016;76(5):551–565 [DOI] [PubMed] [Google Scholar]
- 29.Krueger DA, Wilfong AA, Holland-Bouley K, et al. . Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74(5):679–687 [DOI] [PubMed] [Google Scholar]
- 30.French JA, Lawson JA, Yapici Z, et al. . Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10056):2153–2163 [DOI] [PubMed] [Google Scholar]
- 31.Crino PB. The mTOR signaling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12(7):379–392 [DOI] [PubMed] [Google Scholar]
- 32.Kotulska K, Chmielewski D, Borkowska J, et al. . Long-term effect of everolimus on epilepsy and growth in children under 3 years of age treated for subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Eur J Paediatr Neurol. 2013;17(5):479–485 [DOI] [PubMed] [Google Scholar]
- 33.Jóźwiak S, Kotulska K, Berkowitz N, Brechenmacher T, Franz DN. Safety of everolimus in patients younger than 3 years of age: results from EXIST-1, a randomized, controlled clinical trial. J Pediatr. 2016;172:151.e1–155.e1 [DOI] [PubMed] [Google Scholar]
- 34.Capal JK, Franz DN. Profile of everolimus in the treatment of tuberous sclerosis complex: an evidence-based review of its place in therapy. Neuropsychiatr Dis Treat. 2016;12:2165–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curatolo P, Aronica E, Jansen A, et al. . Early onset epileptic encephalopathy or genetically determined encephalopathy with early onset epilepsy? Lessons learned from TSC. Eur J Paediatr Neurol. 2016;20(2):203–211 [DOI] [PubMed] [Google Scholar]
- 36.Jóźwiak S, Kotulska K, Kasprzyk-Obara J, et al. . Clinical and genotype studies of cardiac tumors in 154 patients with tuberous sclerosis complex. Pediatrics. 2006;118(4). Available at: www.pediatrics.org/cgi/content/full/118/4/e1146 [DOI] [PubMed] [Google Scholar]
- 37.Józwiak S, Schwartz RA, Janniger CK, Bielicka-Cymerman J. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol. 2000;15(10):652–659 [DOI] [PubMed] [Google Scholar]
- 38.Curatolo P, Moavero R, Roberto D, Graziola F. Genotype/phenotype correlations in tuberous sclerosis complex. Semin Pediatr Neurol. 2015;22(4):259–273 [DOI] [PubMed] [Google Scholar]
- 39.Jones AC, Shyamsundar MM, Thomas MW, et al. . Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet. 1999;64(5):1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dabora SL, Jóźwiak S, Franz DN, et al. . Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68(1):64–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi J-E, Chae J-H, Hwang YS, Kim KJ. Mutational analysis of TSC1 and TSC2 in Korean patients with tuberous sclerosis complex. Brain Dev. 2006;28(7):440–446 [DOI] [PubMed] [Google Scholar]
- 42.Au KS, Williams AT, Roach ES, et al. . Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med. 2007;9(2):88–100 [DOI] [PubMed] [Google Scholar]
- 43.Davis PE, Peters JM, Krueger DA, Sahin M. Tuberous sclerosis: a new frontier in targeted treatment of autism. Neurotherapeutics. 2015;12(3):572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]