Exploration of the association between eating disorders and autoimmune and autoinflammatory disease in the Danish population.
Abstract
OBJECTIVES:
Identifying factors associated with risk for eating disorders is important for clarifying etiology and for enhancing early detection of eating disorders in primary care. We hypothesized that autoimmune and autoinflammatory diseases would be associated with eating disorders in children and adolescents and that family history of these illnesses would be associated with eating disorders in probands.
METHODS:
In this large, nationwide, population-based cohort study of all children and adolescents born in Denmark between 1989 and 2006 and managed until 2012, Danish medical registers captured all inpatient and outpatient diagnoses of eating disorders and autoimmune and autoinflammatory diseases. The study population included 930 977 individuals (48.7% girls). Cox proportional hazards regression models and logistic regression were applied to evaluate associations.
RESULTS:
We found significantly higher hazards of eating disorders for children and adolescents with autoimmune or autoinflammatory diseases: 36% higher hazard for anorexia nervosa, 73% for bulimia nervosa, and 72% for an eating disorder not otherwise specified. The association was particularly strong in boys. Parental autoimmune or autoinflammatory disease history was associated with significantly increased odds for anorexia nervosa (odds ratio [OR] = 1.13, confidence interval [CI] = 1.01–1.25), bulimia nervosa (OR = 1.29; CI = 1.08–1.55) and for an eating disorder not otherwise specified (OR = 1.27; CI = 1.13–1.44).
CONCLUSIONS:
Autoimmune and autoinflammatory diseases are associated with increased risk for eating disorders. Ultimately, understanding the role of immune system disturbance for the etiology and pathogenesis of eating disorders could point toward novel treatment targets.
What’s Known on This Subject:
Research evidence suggests that psychiatric disorders are associated with immune system disturbance. Autoimmune disease in adults is associated with a significantly increased hazard for subsequent eating disorders and vice versa.
What This Study Adds:
In this study of ∼1 million children and adolescents, we found associations between autoimmune and autoinflammatory disease and eating disorders. Parental history of autoimmune and autoinflammatory disease is associated with significantly increased risk for all eating disorders in their offspring.
Compelling evidence1 suggests that immune system disturbance is associated with psychiatric disorders and contributes to the risk for their onset. Autoimmune processes are implicated in a number of psychiatric disorders.2–5 Transdiagnostic psychiatric genomic investigations implicate immune-neuronal signaling pathways and suggest that autoimmune diseases and psychiatric disorders share etiological factors or pathogenic mechanisms.6
Research on immunologic and inflammatory markers associated with eating disorders has been inconclusive, with some researchers reporting a proinflammatory state,7–12 including the presence of autoantibodies against α-melanocyte-stimulating hormone (a neuropeptide involved in appetite control) and autoantibodies that react with the neurohormones oxytocin and vasopressin,13,14 and others revealing few abnormalities.15,16 Acute anorexia nervosa (AN) onset after a streptococcal infection and/or an autoimmune reaction (eg, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection leading to onset AN) has been documented, yielding recommendations for examining measures of antineuronal antibodies, such as AnAb and D8/17, to assess suspected pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection leading to AN onset.17,18 Population studies in Finland and the United Kingdom19,20 associated antecedent autoimmune diseases with a significantly increased risk for eating disorders, and data from a nationwide Swedish study revealed a bidirectional association between celiac disease and AN.21 In our previous work,22 we revealed that adults with AN, perhaps because of low adipose tissue and high physical activity, have a distinct pattern of immune mediator expression (ie, low cytokines, elevated high-mobility group box 1 protein) that differs from other somatic and psychiatric illnesses.23
We investigated associations between autoimmune and autoinflammatory diseases and eating disorders in youth in a nationwide, population-based cohort. Adolescence is the highest risk period for eating disorders, with a cumulative incidence of ∼1% for AN, ∼1% for bulimia nervosa (BN), and ∼1% for an eating disorder not otherwise specified (EDNOS) detected through medical surveillance.24 Early identification and intervention are critical and are associated with improved outcomes and decreased morbidity and mortality. Yet, eating disorders are often undetected in pediatric care. Even in European countries, only ∼25% of those with eating disorders ever receive treatment.25 Understanding which children and adolescents are at an increased risk for eating disorders by examining antecedent medical status may aid in both detection and treatment.
Our aims were fourfold. First, to replicate and extend the Finnish findings,19 we examined whether (1) autoimmune diseases (diseases of the adaptive immune system marked by aberrant B- or T-cell responses that lead to autoreactivity demonstrated by autoantibodies or T-cell reactivity to a self-antigen) were associated with increased hazard for subsequent eating disorders, and (2) whether eating disorders were associated with increased hazard for subsequent autoimmune disease. Second, we examined novel associations between autoinflammatory diseases (diseases of the innate immune system marked by activation of the inflammasome and elevated cytokine expression, especially interleukin-1) and eating disorders.26 Third, sex-stratified analyses were performed when feasible. Finally, we examined whether the odds for eating disorders were elevated for youth with a family history (parents, full and half siblings, and cousins) of autoimmune and autoinflammatory diseases. By comparing family members with varying degrees of genetic relatedness (∼50% shared genes in biological parents and full siblings, ∼25% in half siblings, and ∼12.5% in cousins) and with varying shared environmental exposure, we examined whether genetic or environmental factors might be operative.27
Methods
Registers
Since 1968, every person living in Denmark has been assigned a unique personal identification number in the Danish Civil Registration System,28 enabling linkage across national registers. The Psychiatric Central Research Register (PCRR) and the National Patient Register (NPR) capture all inpatient psychiatric and general hospital admissions and, since 1995, all outpatient and emergency department visits.29 The registers include dates of admission and discharge, reason for contact, and main and auxiliary diagnoses30 following a Danish version of the International Classification of Diseases, Eighth Revision (ICD-8) from 1969 to 1993 and the International Classification of Diseases, 10th Revision (ICD-10) codes from 1994.31 This study was approved by the Danish Data Protection Agency. Because all data were anonymized, informed consent was not required.
Population
All children born in Denmark to Danish-born parents between 1989 and 2006 who were alive and residing in Denmark on their sixth birthday (N = 966 141; 48.7% girls) were included. Because of the limitations of the ICD-8 diagnoses of eating disorders, we included only individuals who could have received an incident ICD-10 eating disorder diagnosis (starting in 1994). Individuals born as part of a multiparous birth were excluded to reduce nesting (N = 35 144; 49.1% girls). The study population included 930 977 children (48.7% girls). The study population and their relatives (parents, siblings, half siblings, and cousins) were followed until their date of death, emigration from Denmark, or December 31, 2012, whichever came first.
Eating Disorder Assessment
We used the ICD-10 diagnoses in the PCRR or NPR as follows: AN (F50.0 AN; F50.1 atypical AN), BN (F50.2 BN; F50.3 atypical BN), and EDNOS (F50.8 other eating disorders; F50.9 eating disorder, unspecified). Diagnoses were not mutually exclusive (eg, AN and BN could be included at different times). Onset was age at first inpatient or outpatient contact with a diagnosis in the PCRR or NPR after age 6.
Autoimmune and Autoinflammatory Disease Assessment
Autoimmune and autoinflammatory diseases were defined by using the Danish ICD-8 and ICD-10 diagnoses in the NPR (Supplemental Table 5). Age of onset was age at first inpatient or outpatient contact with an NPR diagnosis. We identified 50 autoimmune diseases or 10 autoinflammatory diseases (Supplemental Table 5). Because associations between eating disorders and autoimmune diseases could be driven by gastrointestinal factors, the autoimmune disease category also included a subcategory of diseases with a suspected presence of gastrointestinal involvement with no minimum age of onset.
Statistical Analyses
We used Stata version 13 (Stata Corp, College Station, TX) and adjusted for sex by means of separate underlying hazard functions using Cox proportional hazards regression for each of the following eating disorder outcomes: AN, BN, and EDNOS. All estimates were adjusted for calendar-time in the parametric part of the Cox model, and for age in the nonparametric part. Calendar year (categorized as 1995–1999, 2000–2004, 2005–2009, and 2010–2012) and autoimmune disorder status were treated as time-dependent variables, whereas all other variables were considered time-independent. Hazard of autoimmune and autoinflammatory disease were calculated in a similar manner, with eating disorder status as a time-dependent exposure variable. Sex-specific analyses were conducted when cell sizes were adequate. The combined follow-up time was 8.5 million person-years for all survival analyses. The association between eating disorders in the proband and autoimmune and autoinflammatory diseases in family members was evaluated by calculating odds ratios (ORs) by using logistic regressions adjusted for sex and birth order (first, second, third, or fourth–18th). The presence of an eating disorder in the proband and the presence of autoimmune and autoinflammatory disease in family members was defined as a diagnosis at any time before December 31, 2012. Family members were grouped as parents, full siblings, maternal half siblings, paternal half siblings, and cousins. Because the ICD-10 diagnoses were only used to define eating disorders starting in 1994, the reverse analyses (eg, association between autoimmune and autoinflammatory disease in the proband and eating disorders in family members) could not be completed.
Results
Hazard of Eating Disorders in Individuals With Autoimmune Diseases
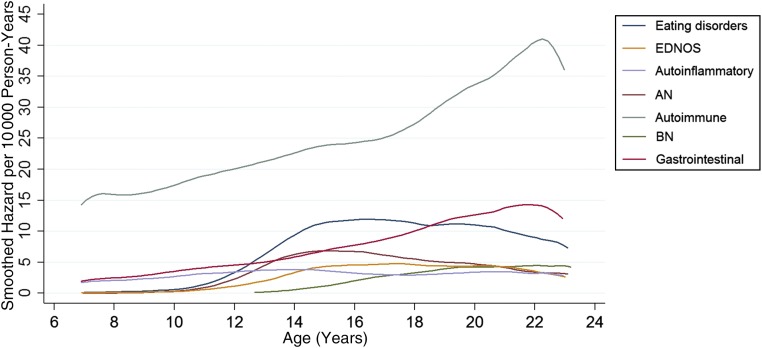
A total of 25 984 children and adolescents (54.2% girls) were diagnosed with an autoimmune or autoinflammatory disease. With the smoothed hazard curve, (Fig 1) we show that the risk for autoimmune but not autoinflammatory disease increases through childhood and adolescence. Of those diagnosed with autoimmune or autoinflammatory disease, 5617 (21.6%) had diseases with gastrointestinal involvement, 3456 individuals (13.3%) had diseases with autoinflammatory processes, and 157 (0.6%) individuals had a subsequent eating disorder diagnosis (Table 1).
FIGURE 1.
Hazard curves for eating disorders and autoimmune and autoinflammatory illnesses.
TABLE 1.
HRs and 95% CIs of Subsequent Eating Disorders in All Children and Adolescents With Autoimmune and Autoinflammatory Diseases
| No. Exposed | AN | BN | EDNOS | Any Eating Disorder | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | HR | 95% CI | No. of Cases | HR | 95% CI | No. of Cases | HR | 95% CI | No. of Cases | HR | 95% CI | ||
| Autoimmune | 25 984 | 78 | 1.36** | 1.09–1.71 | 40 | 1.73*** | 1.26–2.39 | 70 | 1.72*** | 1.36–2.19 | 157 | 1.50*** | 1.27–1.75 |
| Gastrointestinal | 5617 | 17 | 1.74* | 1.08–2.81 | 6 | 1.31 | 0.59–2.93 | 18 | 2.48*** | 1.56–3.96 | 32 | 1.73** | 1.22–2.45 |
| Autoinflammatory | 3456 | 10 | 1.31 | 0.70–2.44 | 5 | 1.67 | 0.69–4.02 | 15 | 2.79*** | 1.68–4.64 | 26 | 1.87** | 1.27–2.75 |
P < .05; ** P < .01; *** P < .0001.
In Cox models, hazard ratios (HRs) >1 indicate a higher probability of illness whereas ratios <1 indicate a lower probability of illness in comparison with unaffected individuals (Table 1). There was a 36% higher hazard for AN, 73% for BN, 72% for EDNOS, and 50% for any eating disorder for children and adolescents with autoimmune or autoinflammatory diseases. Autoimmune diseases with gastrointestinal involvement were significantly associated with higher hazards for AN (74% higher hazard) and for EDNOS (148% higher hazard). Autoinflammatory diseases were associated with significantly increased hazard for EDNOS (179% higher hazard). In sex-stratified analyses (Table 2), male children and adolescents with any autoimmune disease had a 368% higher hazard for BN and 198% for EDNOS. Male children and adolescents with any autoinflammatory disease had a 740% higher hazard for EDNOS. The hazards for male children have wide confidence intervals (CIs) and should be interpreted with caution.
TABLE 2.
HRs and 95% CIs of Subsequent Eating Disorders in Children and Adolescents With Autoimmune and Autoinflammatory Diseases, by Sex
| AN | BN | EDNOS | Any Eating Disorder | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Girl | Boy | Girl | Boy | Girl | Boy | Girl | Boy | |||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Autoimmune | 1.38** | 1.09–1.74 | 1.10 | 0.41–2.97 | 1.68** | 1.21–2.33 | 4.68* | 1.08–20.27 | 1.62*** | 1.25–2.10 | 2.98** | 1.52–5.85 | 1.45*** | 1.23–1.72 | 2.14*** | 1.25–3.65 |
| Gastrointestinal | 1.86* | 1.16–3.00 | — | — | 1.34 | 0.60–2.99 | — | — | 2.39** | 1.46–3.91 | 3.64 | 0.90–14.70 | 1.73** | 1.21–2.48 | 1.70 | 0.42–6.84 |
| Autoinflammatory | 1.11 | 0.55–2.23 | 4.76* | 1.18–19.20 | 1.70 | 0.70–4.09 | — | — | 2.39** | 1.35–4.22 | 8.40*** | 2.68–26.35 | 1.68* | 1.10–2.55 | 5.20** | 1.94–13.94 |
—, not applicable.
P < .05; ** P < .01; *** P < .0001.
Hazard of Autoimmune or Autoinflammatory Diseases in Individuals With Eating Disorders
A total of 3914 adolescents received at least 1 eating disorder diagnosis (91.9% girls) during the study period. With the smoothed hazard curve (Fig 1), we show that the risk for all eating disorders increases through age 14 and then remains high through the remainder of adolescence. Of those diagnosed with eating disorders, 2223 (56.3%) received an AN diagnosis, 727 (18.4%) a BN diagnosis, 1520 (38.5%) an EDNOS diagnosis, and 82 (2.1%) had a subsequent autoimmune or autoinflammatory diagnosis (Table 3). The percentages add to >100 because of overlapping categories.
TABLE 3.
HRs and 95% CIs of Subsequent Autoimmune and Autoinflammatory Disease in Individuals With Eating Disorders
| No. Exposed | Autoimmune Disease | Gastrointestinal | Autoinflammatory Disease | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | HR | 95% CI | No. of Cases | HR | 95% CI | No. of Cases | HR | 95% CI | ||
| AN | 2223 | 47 | 1.64*** | 1.23–2.18 | 13 | 1.34 | 0.77–2.30 | ≤3 | 0.26 | 0.04–1.88 |
| BN | 727 | 11 | 1.31 | 0.73–2.37 | ≤3 | 0.99 | 0.32–3.08 | ≤3 | 1.07 | 0.15–7.64 |
| EDNOS | 1520 | 39 | 2.21*** | 1.62–3.03 | 12 | 1.97* | 1.12–3.48 | ≤3 | 0.87 | 0.22–3.50 |
| Any eating disorder | 3946 | 82 | 1.68*** | 1.35–2.09 | 24 | 1.43 | 0.96–2.15 | 4 | 0.63 | 0.24–1.69 |
P < .05; *** P < .001.
AN was significantly associated with any subsequent autoimmune or autoinflammatory disease (64% higher hazard). EDNOS was significantly associated with any subsequent autoimmune or autoinflammatory disease (121% higher hazard) and autoimmune diseases with gastrointestinal involvement (97% higher hazard). In analyses stratified by sex, there were no male patients with eating disorders who had subsequent diagnoses of autoimmune or autoinflammatory disease.
Hazard of Eating Disorders in Individuals With a Family History of Autoimmune or Autoinflammatory Diseases
Any family history of autoimmune or autoinflammatory disease was associated with significantly increased odds for AN, BN, and EDNOS (Table 4). Adolescents with parents who had autoimmune or autoinflammatory disease diagnoses had 13% higher odds of developing AN, 29% higher odds of developing BN, 27% higher odds of developing EDNOS, and 19% higher odds of developing any eating disorder. Individuals with full siblings who had autoimmune or autoinflammatory disease diagnoses had 48% higher odds of developing AN, 47% higher odds of developing EDNOS, and 37% higher odds of developing any eating disorder. Autoimmune or autoinflammatory disease in maternal or paternal half siblings were not found to be related to eating disorder status in probands. Individuals with cousins who had autoimmune or autoinflammatory disease also had 20% higher odds of developing EDNOS and 17% higher odds of developing any eating disorder.
TABLE 4.
OR (95% CI) of Eating Disorders in Individuals With Family History of Autoimmune and Autoinflammatory Diseases
| AN | BN | EDNOS | Any Eating Disorder | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Parents | 1.13* | 1.01–1.25 | 1.29** | 1.08–1.55 | 1.27*** | 1.13–1.44 | 1.19*** | 1.10–1.28 |
| Full siblings | 1.48*** | 1.21–1.80 | 1.03 | 0.69–1.54 | 1.47** | 1.16–1.86 | 1.37*** | 1.18–1.60 |
| Maternal half siblings | 0.92 | 0.55–1.53 | 1.37 | 0.68–2.76 | 1.26 | 0.76–2.11 | 1.19 | 0.86–1.66 |
| Paternal half siblings | 1.13 | 0.77–1.67 | 1.18 | 0.61–2.29 | 1.33 | 0.86–2.05 | 1.20 | 0.90–1.59 |
| Cousins | 1.11 | 0.99–1.25 | 1.10 | 0.89–1.36 | 1.20* | 1.04–1.38 | 1.17** | 1.07–1.27 |
P < .05; ** P < .01; *** P < .001.
Discussion
Autoimmune and autoinflammatory diseases are associated with significantly increased hazard for subsequent eating disorders, and eating disorders are associated with a significantly increased hazard for subsequent autoimmune disease. These results confirm previous findings19; however, different measurement of risk, younger age of participants, and different follow-up times preclude direct comparison. We extend previous science by demonstrating that youth with EDNOS also have an increased hazard for autoimmune and autoinflammatory disease,19 and we present the first documented association between eating disorders and autoinflammatory disease and between familial autoimmune disease and eating disorders in offspring.
What mediates the association between autoimmune or autoinflammatory disease and eating disorders remains unknown. Eating disorders are commonly associated with reward dysfunction, and neural inflammation can lead to changes in reward functioning including heightened reactivity to threat, increased sensitivity to punishment, and decreased connectivity in reward pathways.32–35 Why autoimmune illness is so strongly associated with increased hazard for eating disorders in male youth is unclear; although our results are preliminary, this finding has been documented previously.19 The development of eating disorders in boys may be more dependent on biological vulnerability than for girls.36
Gastrointestinal Autoimmune Disease
With our evidence, we suggest that eating disorders are associated with autoimmune disease with gastrointestinal involvement. EDNOS predicted a higher hazard of a subsequent autoimmune disease with gastrointestinal involvement. Moreover, autoimmune disease with gastrointestinal involvement predicted a higher hazard of subsequent AN and EDNOS. This association may be a diagnostic artifact caused by the differential diagnosis between eating disorders and gastrointestinal autoimmune diseases. AN and EDNOS may be misdiagnosed in individuals whose weight loss is due to malabsorption from gastrointestinal autoimmune illness such as Crohn disease or celiac disease. Gastrointestinal autoimmune diseases may also be misdiagnosed in patients with AN and EDNOS because of a misinterpretation of the underlying causes of food reactivity and delayed gastric emptying that are often a consequence of fasting behaviors.37,38
Alternatively, proinflammatory cytokines produced by autoimmune diseases such as Crohn disease (eg, tumor necrosis factor α) lead to appetite suppression and weight loss, which may trigger eating disorder symptoms in vulnerable individuals.39,40 In a case study,39 administration of an antitumor necrosis factor α treatment of a woman comorbid for Crohn disease and AN led to weight gain and decreased eating disorder symptomatology. Behaviorally, pain in response to eating and avoidance of foods that cause flare-ups for gastrointestinal autoimmune disease could elicit eating disorder behavior or exacerbate symptoms in individuals with eating disorders. The associations between autoimmune disease and eating disorders are also not limited to gastrointestinal disorders, making misdiagnosis unlikely to be the sole explanation for the documented associations.
Autoinflammatory Disease
To our knowledge, this is the first study in which researchers document an association between autoinflammatory diseases and eating disorders. This fairly new classification of diseases is marked by diseases of the innate immune system and high levels of clinical inflammation. Genetic factors have been implicated in many autoinflammatory diseases,41,42 and researchers who conducted animal research suggest that the interplay between nutritional composition and intestinal microbiota may contribute to their onset. In these studies,43 mice genetically predisposed to autoinflammatory disease and exposed to a low-fat diet experienced changes in their intestinal bacteria (increases in Prevotella), which led to the onset of autoinflammatory disease. Although little is known about the intestinal microbiota in patients with EDNOS, we44 have found that the intestinal microbiota of patients with acute AN is characterized by significantly lower taxa diversity than controls. Future research in this area should also include measures of nutritional composition and intestinal microbiota.
Family History: Genetic and Environmental Factors
Autoimmune and autoinflammatory disease in parents is also associated with significantly increased odds for all eating disorders (AN, BN, EDNOS). Autoimmune and autoinflammatory disease in full siblings is also associated with significantly increased odds for eating disorders (AN and EDNOS in particular) and autoimmune and autoinflammatory disease in cousins is also associated with significantly increased odds for eating disorders (EDNOS in particular). Although the pattern of the association across varying degrees of relatedness can assist in making inferences about whether genetic or environmental factors explain the association,27 we found no clear patterns to make this distinction, potentially caused by low power because of the small number of half siblings. That autoimmune disease in cousins who share fewer environmental exposures was associated with eating disorders may, however, point to shared genetic risk. Supporting this interpretation is the discovery of the first genome-wide significant locus for AN on chromosome 12 (rs4622308) in a region harboring a previously reported type 1 diabetes and autoimmune disease locus.45 In addition, linkage disequilibrium score genetic correlations revealed intriguing negative genetic correlations (albeit nonsignificant) between AN and some autoimmune diseases (rheumatoid arthritis, ulcerative colitis, and Crohn disease), suggesting shared genetic effects with opposite directions of effect.46 As sample sizes increase, we expect greater clarity in the association of eating disorders and autoimmune diseases on the genomic level. Moreover, genome-wide association data are not yet available for BN and EDNOS.
Importantly, unmeasured environmental mediators could explain the observed associations. These could be specific, shared environmental exposures (eg, nutrition, smoking exposure) that place family members at risk for both classes of illness or general, such as increases in familial stress because of illness or greater illness detection because of increased healthcare contact. Adequately-powered molecular genetic and twin designs are necessary to explicate these genetic and environmental pathways.
Strengths and Limitations
No private psychiatric hospitals exist in Denmark; hospital-based treatment is free of charge, and the nationwide registration of psychiatric disorders is almost complete. However, psychiatrists and psychologists do work in private practice and treat up to 20% of individuals with eating disorders.30 These individuals may not be captured in the current study. The use of medical surveillance may have led to increased documented relative prevalence of AN in this sample in comparison with BN and EDNOS. Of those with eating disorders in the current study, 2233 (57.1%) received an AN diagnosis, 727 (18.6%) received a BN diagnosis, and 1520 (38.8%) received an EDNOS diagnosis, which differs from population-based surveys. It is likely that individuals with BN and EDNOS were under-detected, which could have affected our conclusions in 2 ways. First, patients with eating disorders who had inpatient and outpatient hospital-based contacts may be more medically compromised and more likely to also suffer from an autoimmune disease, thereby elevating the observed association. Second, undetected individuals with BN and EDNOS could have increased our statistical power to examine this association and thus, the observed associations are attenuated.
In addition, we only examined eating disorders in children and adolescents who were <24 years using the Danish registers because of the limitations of the ICD-8 diagnoses, which may have attenuated HRs for both the antecedent and subsequent autoimmune and autoinflammatory diseases. The peak age of incidence for eating disorders occurs during adolescence,24 but the period of incident risk for eating disorders extends across the lifespan. Although some autoimmune and autoinflammatory diseases onset in childhood and adolescence (eg, type 1 diabetes, Crohn disease) and would be captured in these analyses,47 others typically do not onset until midlife (eg, rheumatoid arthritis).48 As this study population ages, the HRs may intensify or attenuate commensurably. Like eating disorders, some autoimmune and autoinflammatory diseases may be under-detected in primary care, which may also have attenuated these associations.
Because of the limitations of the ICD-8 diagnoses, we were unable to examine potential associations between parental eating disorders and autoimmune and autoinflammatory diseases in offspring. Although we could have included parental diagnoses of eating disorders after the PCRR deployed the ICD-10 in 1994, we would have missed a large number of parents with an eating disorder history before 1994.
Conclusions
The biological associations between autoimmune and autoinflammatory disease and eating disorders are becoming increasingly clear. Our findings support compelling lines of evidence from researchers suggesting that immune system disturbance is both comorbid with psychiatric disorders and can increase risk for illness.3–5,19 Research into immune system dysfunction represents a novel lens to examine risk for eating disorders and could inform new treatments. Novel ideas are desperately needed in eating disorders; only 10% of adolescents receive treatment, the average duration of most eating disorders is >7 years, ∼25% of adolescents develop chronic illness, and there are no medications specifically for AN.49 Supported by our findings, we suggest further examination into the complex interplay between immune system disturbance, nutritional, and neuropsychiatric processes. Understanding the role of the immune system could explicate the etiology of eating disorders, aid early detection, personalize intervention, and ultimately improve outcomes.
Acknowledgment
Thanks to Prof Kerstin von Plessen for additional assistance with the Danish ICD diagnostic codes.
Glossary
- AN
anorexia nervosa
- BN
bulimia nervosa
- CI
confidence interval
- EDNOS
eating disorder not otherwise specified
- HR
hazard ratio
- ICD-8
International Classification of Diseases, Eighth Revision
- ICD-10
International Classification of Diseases, 10th Revision
- NPR
National Patient Register
- OR
odds ratio
- PCRR
Psychiatric Central Research Register
Footnotes
Drs Zerwas, Thornton, Quaranta, Koch, Pisetsky, and Bulik conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Larsen and Drs Petersen and Mortensen conceptualized and designed the study, conducted the data analysis, drafted the initial manuscript, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work and ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by The Anorexia Nervosa Genetics Initiative, an initiative of the Klarman Family Foundation and the Foundation of Hope: Research and Treatment of Mental Illness. Dr Zerwas is supported by a National Institute of Mental Health career development grant (K01MH100435). Dr Bulik acknowledges funding from the Swedish Research Council (VR Dnr: 538-2013-8864). The funders of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Bulik is a grant recipient and consultant for Shire. Dr Zerwas has consulted for L.E.K. consulting. Dr Pisetsky is a grant recipient from Pfizer and is a member of a Data and Safety Monitoring Board for Celgene. Dr Thornton is an investigator on a grant from Shire; the other authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2017-3060.
References
- 1.Eaton WW, Byrne M, Ewald H, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163(3):521–528 [DOI] [PubMed] [Google Scholar]
- 2.Benros ME. Posttraumatic stress disorder and autoimmune diseases. Biol Psychiatry. 2015;77(4):312–313 [DOI] [PubMed] [Google Scholar]
- 3.Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune diseases and psychosis. Biol Psychiatry. 2014;75(4):300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benros ME, Waltoft BL, Nordentoft M, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70(8):812–820 [DOI] [PubMed] [Google Scholar]
- 5.Benros ME, Mortensen PB, Eaton WW. Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci. 2012;1262:56–66 [DOI] [PubMed] [Google Scholar]
- 6.Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways [published corrections appear in Nat Neurosci. 2015;18(6):926 and Nat Neurosci. 2015;18(12):1861]. Nat Neurosci. 2015;18(2):199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack E, Nahmod VE, Emeric-Sauval E, et al. Low lymphocyte interferon-gamma production and variable proliferative response in anorexia nervosa patients. J Clin Immunol. 1993;13(6):445–451 [DOI] [PubMed] [Google Scholar]
- 8.Schattner A, Tepper R, Steinbock M, Hahn T, Schoenfeld A. TNF, interferon-gamma and cell-mediated cytotoxicity in anorexia nervosa; effect of refeeding. J Clin Lab Immunol. 1990;32(4):183–184 [PubMed] [Google Scholar]
- 9.Holden RJ, Pakula IS. Tumor necrosis factor-alpha: is there a continuum of liability between stress, anxiety states and anorexia nervosa? Med Hypotheses. 1999;52(2):155–162 [DOI] [PubMed] [Google Scholar]
- 10.Raymond NC, Dysken M, Bettin K, et al. Cytokine production in patients with anorexia nervosa, bulimia nervosa, and obesity. Int J Eat Disord. 2000;28(3):293–302 [DOI] [PubMed] [Google Scholar]
- 11.Solmi M, Veronese N, Luchini C, et al. Oxidative stress and antioxidant levels in patients with anorexia nervosa after oral re-alimentation: a systematic review and exploratory meta-analysis. Eur Eat Disord Rev. 2016;24(2):101–105 [DOI] [PubMed] [Google Scholar]
- 12.Solmi M, Veronese N, Favaro A, et al. Inflammatory cytokines and anorexia nervosa: A meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237–252 [DOI] [PubMed] [Google Scholar]
- 13.Fetissov SO, Hallman J, Oreland L, et al. Autoantibodies against alpha -MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients. Proc Natl Acad Sci U S A. 2002;99(26):17155–17160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fetissov SO, Harro J, Jaanisk M, et al. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci U S A. 2005;102(41):14865–14870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nova E, Gómez-Martínez S, Morandé G, Marcos A. Cytokine production by blood mononuclear cells from in-patients with anorexia nervosa. Br J Nutr. 2002;88(2):183–188 [DOI] [PubMed] [Google Scholar]
- 16.Kahl KG, Kruse N, Rieckmann P, Schmidt MH. Cytokine mRNA expression patterns in the disease course of female adolescents with anorexia nervosa. Psychoneuroendocrinology. 2004;29(1):13–20 [DOI] [PubMed] [Google Scholar]
- 17.Vincenzi B, O’Toole J, Lask B. PANDAS and anorexia nervosa–a spotters’ guide: suggestions for medical assessment. Eur Eat Disord Rev. 2010;18(2):116–123 [DOI] [PubMed] [Google Scholar]
- 18.Sokol MS, Ward PE, Tamiya H, Kondo DG, Houston D, Zabriskie JB. D8/17 expression on B lymphocytes in anorexia nervosa. Am J Psychiatry. 2002;159(8):1430–1432 [DOI] [PubMed] [Google Scholar]
- 19.Raevuori A, Haukka J, Vaarala O, et al. The increased risk for autoimmune diseases in patients with eating disorders. PLoS One. 2014;9(8):e104845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wotton CJ, James A, Goldacre MJ. Coexistence of eating disorders and autoimmune diseases: record linkage cohort study, UK. Int J Eat Disord. 2016;49(7):663–672 [DOI] [PubMed] [Google Scholar]
- 21.Mårild K, Størdal K, Bulik CM, et al. Celiac disease and anorexia nervosa: a nationwide study. Pediatrics. 2017;139(5):e20164367. [DOI] [PubMed] [Google Scholar]
- 22.Pisetsky DS, Trace SE, Brownley KA, et al. The expression of cytokines and chemokines in the blood of patients with severe weight loss from anorexia nervosa: an exploratory study. Cytokine. 2014;69(1):110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisetsky D, Brownley KA, Trace SE, et al. Cytokine and chemokine expression in acutely-ill patients with anorexia nervosa (AN). In: International Conference on Eating Disorders; May 2–4, 2013; Montreal, Canada [Google Scholar]
- 24.Zerwas S, Larsen JT, Petersen L, Thornton LM, Mortensen PB, Bulik CM. The incidence of eating disorders in a Danish register study: associations with suicide risk and mortality. J Psychiatr Res. 2015;65:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preti A, Girolamo Gd, Vilagut G, et al. ; ESEMeD-WMH Investigators . The epidemiology of eating disorders in six European countries: results of the ESEMeD-WMH project. J Psychiatr Res. 2009;43(14):1125–1132 [DOI] [PubMed] [Google Scholar]
- 26.Kastner DL, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory disease reloaded: a clinical perspective. Cell. 2010;140(6):784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health. 2013;103(suppl 1):S46–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish civil registration system. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449 [PubMed] [Google Scholar]
- 29.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(suppl 7):30–33 [DOI] [PubMed] [Google Scholar]
- 30.Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39(suppl 7):54–57 [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2nd ed. Geneva, Switzerland: World Health Organization; 2005 [Google Scholar]
- 32.Frank GK. Advances from neuroimaging studies in eating disorders. CNS Spectr. 2015;20(4):391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muscatell KA, Moieni M, Inagaki TK, et al. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav Immun. 2016;57:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felger JC, Li Z, Haroon E, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21(10):1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol Psychiatry. 2016;80(1):73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strober M, Freeman R, Lampert C, Diamond J, Kaye W. Males with anorexia nervosa: a controlled study of eating disorders in first-degree relatives. Int J Eat Disord. 2001;29(3):263–269 [DOI] [PubMed] [Google Scholar]
- 37.Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372–377 [DOI] [PubMed] [Google Scholar]
- 38.Bern EM, O’Brien RF. Is it an eating disorder, gastrointestinal disorder, or both? Curr Opin Pediatr. 2013;25(4):463–470 [DOI] [PubMed] [Google Scholar]
- 39.Solmi M, Santonastaso P, Caccaro R, Favaro A. A case of anorexia nervosa with comorbid Crohn’s disease: beneficial effects of anti-TNF-α therapy? Int J Eat Disord. 2013;46(6):639–641 [DOI] [PubMed] [Google Scholar]
- 40.Corcos M, Guilbaud O, Paterniti S, et al. Involvement of cytokines in eating disorders: a critical review of the human literature. Psychoneuroendocrinology. 2003;28(3):229–249 [DOI] [PubMed] [Google Scholar]
- 41.Almeida de Jesus A, Goldbach-Mansky R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol. 2013;147(3):155–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aksentijevich I, Kastner DL. Genetics of monogenic autoinflammatory diseases: past successes, future challenges. Nat Rev Rheumatol. 2011;7(8):469–478 [DOI] [PubMed] [Google Scholar]
- 43.Lukens JR, Gurung P, Vogel P, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014;516(7530):246–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleiman SC, Watson HJ, Bulik-Sullivan EC, et al. The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med. 2015;77(9):969–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan L, Yilmaz Z, Gaspar H, et al. ; Eating Disorders Working Group of the Psychiatric Genomics Consortium . Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa [published online ahead of print May 12, 2017]. Am J Psychiatry. doi: 10.1176/appi.ajp.2017.16121402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulik-Sullivan B, Finucane HK, Anttila V, et al. ; ReproGen Consortium; Psychiatric Genomics Consortium; Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3 . An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deal CL, Meenan RF, Goldenberg DL, et al. The clinical features of elderly-onset rheumatoid arthritis. A comparison with younger-onset disease of similar duration. Arthritis Rheum. 1985;28(9):987–994 [DOI] [PubMed] [Google Scholar]
- 49.Berkman ND, Bulik CM, Brownley KA, et al. Management of Eating Disorders. Evidence Report/Technology Assessment Number 135. Rockville, MD: Agency for Healthcare Research and Quality; 2006 [Google Scholar]