Abstract
Transarterial chemoembolization and hepatic arterial infusion chemotherapy are recommended for the treatment in patients with intermediate stage of hepatocellular carcinoma. Impaired liver function was sometime observed in patients with hepatocellular carcinoma after transarterial chemoembolization or hepatic arterial infusion chemotherapy. However, what kinds of factors deeply influence in impaired liver function are not clear. A retrospective study was performed to evaluate the risk factors of impaired liver function in cisplatin-naïve patients treated with these therapies using cisplatin. Prior to and 2 months after these therapies, we analyzed the liver function by Child-Pugh score in these patients. For assessing the severity of chemotherapy-induced nausea and vomiting, we utilized the Common Terminology Criteria for Adverse Events ver. 4.0. In hepatocellular carcinoma patients received these therapies using cisplatin, the cancer stage and treatment without neurokinin-1 (NK1) antagonist were found to be independent risk factors of the impaired liver function. The treatment with NK1 antagonist was effective in reducing chemotherapy-induced nausea and vomiting and patients treated with NK1 antagonist kept their liver functions after cisplatin-used these therapies. The treatment with NK1 antagonist was effective in chemotherapy-induced nausea and vomiting and prevented the impaired liver function associated with cisplatin-used these therapies in hepatocellular carcinoma patients.
Keywords: HCC, TACE, HAIC, CINV, NK1 antagonist
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer, and major clinical risk factors for HCC include infection with hepatitis B virus (HBV) or hepatis C virus (HCV), alcoholic liver disease, non-alcoholic fatty liver disease, and oxidative stress.(1) Most of these risk factors cause inflammation of the liver and lead to cirrhosis, which is present in 80 to 90% of patients with HCC.(2) The prognosis of patients with HCC depends on both residual liver function and tumor burden.(3) There are several approaches to the treatment of HCC, and the selection of treatment is driven by residual liver function, the cancer stage and resources available.(4,5) The Barcelona Clinic Liver Cancer (BCLC) staging is a useful assessment tool that incorporates data on the liver function as determined by the Child-Pugh classification system, number and size of nodules, cancer symptoms, and patient’s performance status.(6) Transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC) are recommended for HCC patients with intermediate-stage BCLC and improve the 2-year survival rate compared with that of more conservative therapy. TACE or HAIC increase the risk for ischemic necrosis of the non-tumoral liver, and this adverse event may lead to severe liver failure. Therefore, maintaining liver function is important for patients receiving these treatments.(7–9)
Chemotherapy-induced nausea and vomiting (CINV) have been reported as the most distressing adverse side effects in more than 90% of the patients treated with highly emetogenic antitumor agents, especially cisplatin.(10,11) After the 1990s, cisplatin, based on guidance for the prevention of CINV, has been used for TACE or HAIC.(12) CINV sometimes leads the malnutrition in patients.(10) The malnutrition is very common in patients with advanced hepatic disease and gets worse with the severity of liver dysfunction because of inadequate and/or quality oral intake, maldigestion, malabsorption, increased energy expenditure and altered substrate demands.(13–15) And the malnutrition which leads impaired liver function, is an independent risk factor for increased morbidity and mortality in these patients.(16) Therefore, the treatment for the CINV which leads the malnutrition, is very important for the patients with HCC after TACE or HAIC.
CINV is differentiated into two categories: acute CINV (mostly serotonin related), occurring within 24 h of initial administration of the chemotherapy; delayed CINV (in part substance P related), occurring 24 h to several days after the initial treatment.(17) Introduction of serotonin (5-hydroxytryptamine-3) receptor antagonist (5-HT3 antagonist) in the early 1990s represented a major advance in the management of acute CINV.(18) Substance P is a regulatory peptide found in areas of the central nerve system and in the gastrointestinal tract and is believed to be an essential component of the emetic reflex. The actions of substance P are mediated through the neurokinin-1 (NK1) receptor.(19) Selective antagonist of the NK1 receptor have demonstrated antiemetic activity against central, peripheral, and combined emetic stimuli.(20) The combination therapy with NK1 antagonist reduces the CINV associated with the highly emetogenic cisplatin-based chemotherapy according to the American Society of Clinical Oncology Clinical Practice Guideline.(21) However, what kinds of factors deeply influence in impaired liver function and the effect of NK1 antagonist against CINV on the impaired liver function is unclear in HCC patients who are treated with either TACE or HAIC. The present study is the first report on the effect on liver function of the antiemetic therapy in HCC patients treated with TACE or HAIC.
Patients and Methods
Subjects
From 2008 to 2015, 141 cisplatin-naïve patients with HCC who received TACE or HAIC treatment using cisplatin were enrolled at Osaka Medical College. 4 patients were excluded for the following reasons: a patient had the heart failure; 2 patients had the bacterial infection; a patient had the renal dysfunction. Some patients were classified as having advanced disease if they were not eligible for or had disease progression after surgical or locoregional therapies. Other patients, who were classified as having early disease, did not choose surgical or locoregional therapies. We used the Barcelona Clinic Liver Cancer (BCLC) Staging System for the determination of HCC stages.(22) These patients were treated with antiemetic drugs; corticosteroid (dexamethasone), selective 5-HT3 antagonist (granisetron), and NK1 antagonist (aprepitant) to relieve the side effects of chemotherapy with either TACE or HAIC. The treatment with NK1 antagonist was identified as combination therapy of corticosteroid, selective 5-HT3 antagonist and NK1 antagonist. The treatment without NK1 antagonist was identified as combination therapy of corticosteroid and selective 5-HT3 antagonist.
Study design
A retrospective study was performed to evaluate the risk factors of impaired liver function and to preventive effects of the impaired liver function for the antiemetic therapy with NK1 antagonist in cisplatin-naïve patients treated with TACE or HAIC using cisplatin. The Child-Pugh score is used as serum albumin, serum total bilirubin and prothrombin time for the assessment of the liver function. Because, some patients with HCC receive TACE or HAIC every 3 or 4 months, we measured the serum albumin, serum total bilirubin and prothrombin time by commercial methods prior to and 2 months after TACE or HAIC treatment using cisplatin. We used the minimum or maximum of the reference values as cut-off values for these parameters. The impaired liver function was identified as a change of Child-Pugh stage 2 months after TACE or HAIC which compared with prior. It is considered that one of the main roles assigned to albumin is as an indicator of the malnutrition.(23) Therefore, the comparison of serum albumin between prior and after TACE was used for the deterioration of malnutrition in this study.
Assessment of CINV
Same medical staffs maintained a diary for patients in which the timing and intensity of nausea and the timing and number of episodes of emesis were recorded. Subjects reported the severity of nausea and the number of emesis episodes from the day of treatment to the day for discharge. For assessing the severity of the nausea, vomiting, and anorexia, we utilized the Common Terminology Criteria for Adverse Events ver. 4.0 (CTCAE ver. 4.0). Acute CINV was identified as adverse events occurring within 24 h of initial administration of the chemotherapy. And delayed CINV was identified as occurring 24 h to several days after the initial chemotherapy.
Statistical Analysis
Statistical analyses were performed using JMP Pro 10 software (SAS Institute Inc., Cary, NC). Differences in patient characteristics between patients with and without impaired liver function were compared using the Pearson’s chi-square test or the Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. To determine the most suitable cut-off level of cisplatin’s dose, we used receiver operating characteristic (ROC) curve analysis. To identify risk factors of impaired liver function in patients treated with TACE or HAIC, multivariate analysis with a multiple logistic regression model were conducted including the variables with significant differences on univariate analysis. A p value of less than 0.05 was considered as a statistically significant.
Results
Comparison of clinical background of HCC patients after TACE or HAIC between with impaired liver function and without
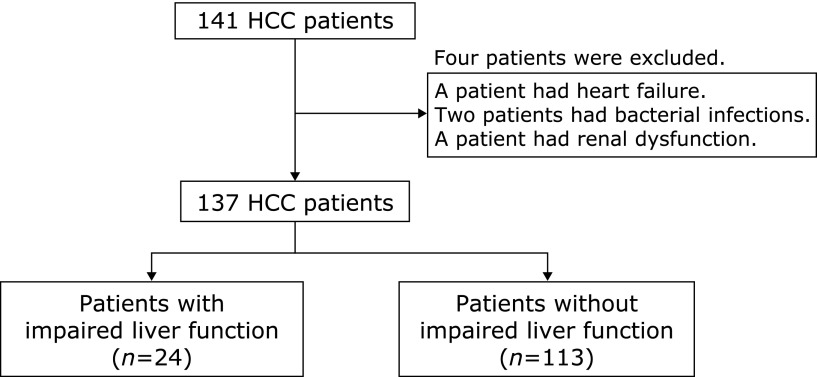
A total of 137 cisplatin-naïve patients with HCC, who were received TACE or HAIC treatment using cisplatin, were enrolled in this study at Osaka Medical College. The impaired liver function was detected in 24 of these patients (Fig. 1). In comparing the clinical background of between these patients with impaired liver function or without, we found that there were no differences in age, gender, etiology, Child-Pugh score, performance status by Eastern Cooperative Oncology Group, history of angiography and embolization for HCC. Although some of the patients’ characteristics were known as risk factors for CINV (female, young and history of alcohol use), there were no differences between the two groups. The HCC stage by BCLC staging system was different between two groups and the impaired liver function in patients with intermediate or advanced stages of HCC were significantly more than in patients with very early or early stages. The dose of cisplatin was higher than the group with impaired liver function. Comparing for the treatment against CINV, liver dysfunction in the group of the treatment with NK1 antagonist was significantly less than without (Table 1). By univariate analysis of these patients, BCLC stage (intermediate or advanced), dose of cisplatin and treatment without NK1 antagonist were suggested as risk factors. As a result of multivariate analysis with a multiple logistic regression model, BCLC stage and treatment without NK1 antagonist were found to be independent risk factors of the impaired liver function in HCC patients after TACE or HAIC (Table 2). It is considered that liver function in patients with advanced stages of HCC will be deteriorated, regardless of the TACE or HAIC. Next, we studied the effect of treatment with NK1 antagonist against CINV on the liver function.
Fig. 1.
Flow chart of sample size.
Table 1.
Clinical background of these patients
| Patients with impaired liver function (n = 24) | Patients without impaired liver function (n = 113) | p value | |
|---|---|---|---|
| Age (years) | |||
| Mean (range) | 70.9 ± 7.88 | 68.3 ± 9.97 | 0.293 |
| Gender | 0.624 | ||
| Male | 13 | 55 | |
| Female | 10 | 58 | |
| Etiology | 0.133 | ||
| HBV | 5 | 17 | |
| HCV | 18 | 75 | |
| Others | 1 | 21 | |
| Child-Pugh Score | 5.79 ± 0.93 | 5.81 ± 1.12 | 0.74 |
| HCC stage (BCLC) | <0.001 | ||
| Very early or early | 2 | 59 | |
| Intermediate or advanced | 22 | 54 | |
| Performance status | 0.157 | ||
| 0 | 23 | 97 | |
| 1 | 1 | 15 | |
| Alcohol drink/week (times) | 0.858 | ||
| 0 | 19 | 87 | |
| 1–7 | 4 | 18 | |
| >7 | 1 | 8 | |
| History of angiography (+/–) | 7/17 | 47/66 | 0.12 |
| Serum albumin | 3.41 ± 0.48 | 3.53 ± 0.50 | 0.209 |
| Total bilirubin | 1.16 ± 0.67 | 0.91 ± 0.47 | 0.106 |
| Prothrombin time (%) | 85.8 ± 12.0 | 89.0 ± 12.0 | 0.388 |
| Dose of cisplatin (mg/body) | 52.2 ± 27.6 | 34.6 ± 27.0 | 0.002 |
| Emblization (+/–) | 12/12 | 71/42 | 0.247 |
| Treatment | 0.004 | ||
| with NK1 antagonists | 10 | 87 | |
| without NK1 antagonists | 14 | 26 |
Table 2.
Hazard ratios of impaired liver function in patients after TACE or HAIC
| Hazard ratio | 95%CI | p value | |
|---|---|---|---|
| BCLC (intermediate or advanced) | 12.7 | 3.32–84.29 | <0.001 |
| Dose of cislatin (mg/body) | 1.01 | 0.99–1.03 | 0.372 |
| Treatment without NK1 antagonist | 3.36 | 1.14–10.23 | 0.002 |
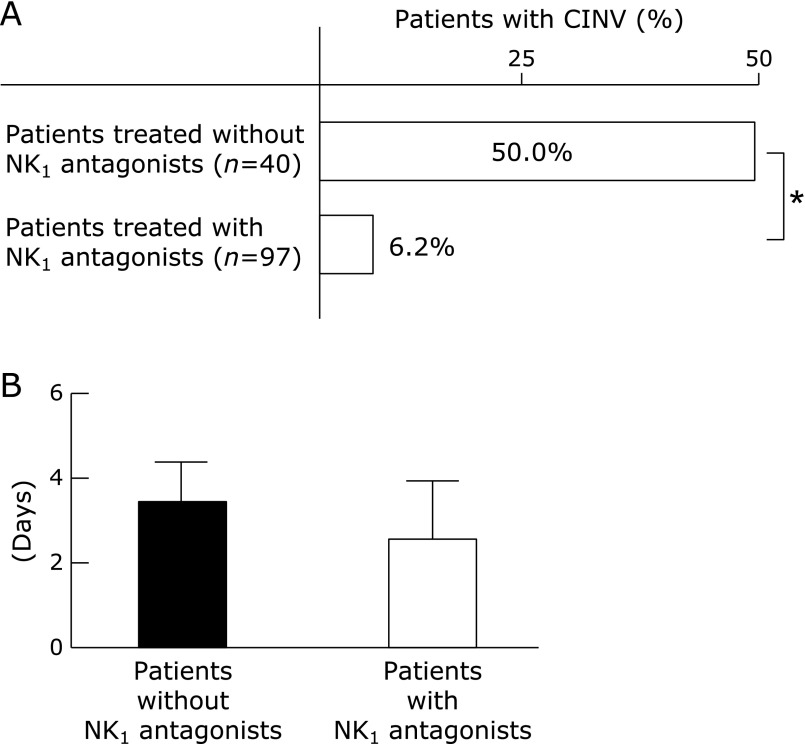
The frequency of CINV in HCC patients after TACE or HAIC
We compared the incidence of CINV after TACE or HAIC between HCC patients treated with NK1 antagonist and without. Although, CINV was reported in 50% of patients treated without NK1 antagonist, only 6.2% of patients treated with NK1 antagonist were developed CINV after TACE or HAIC (Fig. 2A). We compared for the phases of CINV of these patients. Total 26 of patients had CINV in all patients and 11 of those patients had both CINV. A ratio of acute CINV was more than delayed CINV in patients without NK1 antagonist. On the contrary, a ratio of delayed CINV was more than acute CINV in patients with NK1 antagonist (Table 3A). Sixteen patients experienced delayed CINV with an average duration of 3.8 ± 1.7 days. There was no difference in an average duration between in patients with NK1 antagonist and without (Fig. 2B).
Fig. 2.
(A) Percentages of CINV in patients with NK1 antagonist or without after TACE or HAIC. The Pearson’s chi-square test was used to compare the percentages in each group. *p<0.05. (B) Average duration of CINV in HCC patients by treatment group. The Mann-Whitney U test was used to compare the average duration in each group.
Table 3.
The severity and frequency of CINV in HCC patients after TACE or HAIC
| (A) Number of CINV patients by treatment groups | |||
|---|---|---|---|
| Acute CINV | Delayed CINV | Both CINV | |
| Patients without NK1 antagonist (n = 40) | 18 (45.0%) | 11 (27.5%) | 9 (22.5%) |
| Patients with NK1 antagonist (n = 97) | 3 (3.1%) | 5 (5.1%) | 2 (2.0%) |
| (B) Number and severity of adverse events in patients by treatment groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Nausea (n = 20) |
Vomiting (n = 14) |
Anorexia (n = 22) |
||||||
| Grade 1 | Grade 2 | Grade 1 | Grade 2 | Grade 1 | Grade 2 | |||
| Patients without NK1 antagonists | 10 | 6 | 11 | 3 | 9 | 7 | ||
| Patients with NK1 antagonists | 3 | 1 | 0 | 0 | 4 | 2 | ||
| (C) Number and severity of adverse events in CINV patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Nausea (n = 20) |
Vomiting (n = 14) |
Anorexia (n = 22) |
||||||
| Grade 1 | Grade 2 | Grade 1 | Grade 2 | Grade 1 | Grade 2 | |||
| Acute CINV only (n = 10) | 5 | 2 | 6 | 2 | 4 | 3 | ||
| Delayed CINV only (n = 5) | 3 | 0 | 0 | 0 | 2 | 2 | ||
| Both CINV (n = 11) | 5 | 5 | 5 | 1 | 7 | 4 | ||
The severity of CINV in HCC patients after TACE of HAIC
We compared the number and severity of CINV in patients with NK1 antagonist and without. The severity of adverse events in the patients was assessed by CTCAE ver. 4.0 and three adverse events (nausea, vomiting and anorexia) were reported in these patients. The prevalence of adverse events in both groups was shown in Table 3B. The number of patients with nausea was 20 and the number of anorexia was 22 in these patients. These adverse events were observed in both groups of with NK1 antagonist and without. However, 14 of vomiting were only reported in patients without NK1 antagonist. All of the CINV in these patients were grade 1 or 2 for CTCAE ver. 4.0 and no grade 3 CINV was observed.
The number and severity of adverse events in CINV patients were shown in Table 3C. Three adverse events were reported in the group of only acute CINV patients and in the group of both CINV. However, vomiting was not reported in the group of only delayed CINV patients. These results indicated that the treatment with NK1 antagonist was effective in reducing the frequency of both phases of CINV and vomiting in patients after TACE or HAIC.
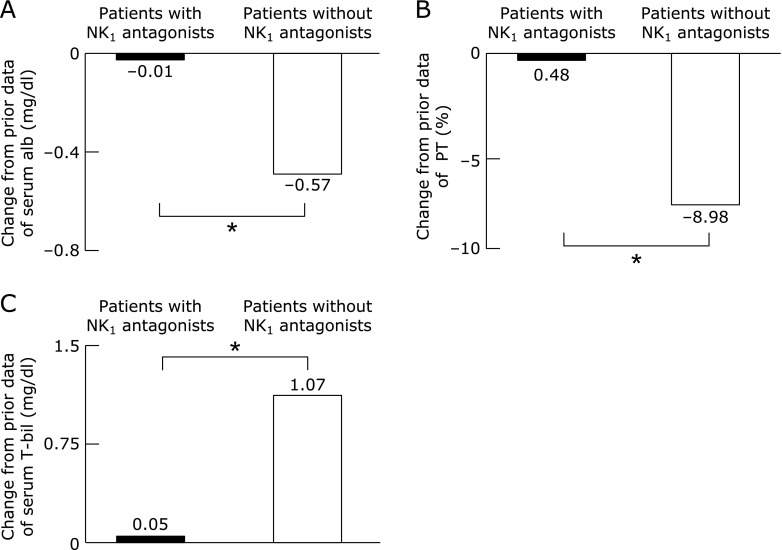
The preventive effect of the impaired liver function for treatment of NK1 antagonist
The laboratory data which were evaluated in Child-Pugh score prior and 2 months after TACE or HAIC, were compared in patients with NK1 antagonist and without. Changes from prior data of serum albumin, prothrombin time and serum total bilirubin were shown in Fig. 3. Change in serum albumin from prior showed significantly reductions in patients without NK1 antagonist versus patients with NK1 antagonist. Because, serum albumin is the indicator of nutritional status, the malnutrition was deteriorated in patients without NK1 antagonist after TACE or HAIC. Furthermore, the change in prothrombin time from prior showed significantly reductions with patients without NK1 antagonist versus patients with NK1 antagonist and the change in serum total bilirubin was significantly increased on patients without NK1 antagonist. These data were little change in patients with NK1 antagonist. The deterioration of malnutrition which leads the impaired liver function, was not detected in patients with NK1 antagonist after TACE or HAIC. These results indicate that the treatment with NK1 antagonist may has a preventive effect on liver dysfunction caused by TACE or HAIC in HCC patients.
Fig. 3.
Change in serum albumin (A), prothrombin time (B) and serum total bilirubin (C) from prior to 2 months after TACE or HAIC in HCC patients. The Mann-Whitney U test was used to compare the data in patients with NK1 antagonist or without, respectively. *p<0.05.
Discussion
Cancer is one of the leading causes of death in the world, and its incidence has increased rapidly as the worldwide population continues to grow and age. Despite truly meaningful progress in targeted chemotherapy, many cancer treatment regimens still use cytotoxic chemotherapeutic agents that produce profound emesis and nausea that begin acutely on the day of chemotherapy and then reappear some days later.(10,18) Patients beginning cancer treatment consistently list CINV as one of their greatest fears, and sometimes completely refuse or withdraw from therapy that has been prescribed to prolong or save their lives.(24) And, CINV leads the deterioration of malnutrition in patients.(10) Therefore, the control of CINV is a significant factor in ensuring patients’ quality of life during their treatment regiments to obtain the full benefit of chemotherapy.(25) In this study, grade 1 and 2 adverse events were reported in our groups of HCC patients. However, grade 3 adverse event was not observed, whether patients did treat with NK1 antagonist or not. In TACE of HAIC, cisplatin was injected to the HCC selectively and it was retained in the tumor along with iodinated poppy seed oil. Because, that iodinated poppy oil works to keep the cisplatin in the tumor, cisplatin do not spread in whole body after TACE or HAIC. Therefore, the severe adverse events were reduced in these patients while increasing the antitumor effect.
The BCLC classification divides HCC patients in 5 stages according to the patient’s performance status, number and size of nodules, cancer symptoms, and liver function as determined by the Child-Pugh classification system.(26) In this study, advanced stages of HCC (intermediate and advanced stage) was shown to be independent risk factors for the patients with impaired liver function after TACE or HAIC. BCLC staging system is one of a number of efforts to understand the prognosis for patients with HCC.(6) It is also considered that liver function in patients with advanced stages of HCC will be deteriorated, regardless of the TACE or HAIC.(3) Liver function was maintained with the treatment with NK1 antagonist during chemotherapy. Because the treatment with NK1 antagonist was very effective for CINV in these patients, the deterioration of malnutrition was not seen in patients after chemotherapy. The preventive effect of the treatment with NK1 antagonist retained liver function in HCC patients after TACE or HAIC.
For the treatment of HCC by TACE or HAIC, some antineoplastic agents are used in Japan. Epirubicin, commonly used for treatment of HCC in both TACE and HAIC since the 1990s, is categorized as having a moderate risk for CINV in guidelines for the prevention of CINV.(27) In our hospital, the number of HCC patients who were treated with epirubicin by either TACE or HAIC was approximately fifteen per year, and the patients with CINV was less than those who were treated with cisplatin. In 2009 miriplatin, a new antineoplastic agent of platinum complexes was approved for the treatment of HCC by HAIC in Japan. This agent has a high affinity toward the iodinated poppy seed oil, and platinum components are released slowly from miriplatin suspended in iodinated poppy seed oil.(28) Therefore, the total platinum concentration in the plasma shows a gradual change at a constant low concentration. There were few patients who were treated with miriplatin, and none had CINV in our hospital. Further study will be needed to assess this new drug.
NK1 receptor antagonist which developed as a treatment for CINV, acts by inhibiting the binding of substance P to the NK1 receptor in the vomiting center. Furthermore, it has reported that aprepitant is effective in the delayed phase of CINV.(18,20,21) The treatment with NK1 antagonist in HCC patients treated with TACE or HAIC, was effective in both phases of CINV in our study. Nausea, anorexia, and vomiting were strongly suppressed by this therapy. This result is similar to that of aprepitant-containing antiemetic studies for other cancers.(29,30) Although, the benefit of NK1 antagonist becomes apparent approximately 12 to 16 h after initiation of treatment, the therapeutic effect of NK1 antagonist was evident during both the acute and the delayed phases in this study.
In conclusion, BCLC stage and treatment without NK1 antagonist were found to be independent risk factors of the impaired liver function in HCC patients after TACE or HAIC. The treatment with NK1 antagonist was very effective in both phases of CINV associated with cisplatin-used TACE or HAIC in HCC patients.
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer
- CINV
chemotherapy-induced nausea and vomiting
- HACE
transarterial chemoembolization
- HAIC
hepatic arterial infusion chemotherapy
- HCC
hepatocellular carcinoma
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Mutimer DJ, Lok A. Management of HBV- and HCV-induced end stage liver disease. Gut. 2012;61 Suppl 1:59–67. doi: 10.1136/gutjnl-2012-302076. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sheman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong R, Frenette C. Updates in the management of hepatocellular carcinoma. Gastroenterol Hepatol (N. Y.) 2011;7:16–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto N, Yamasaki T, Takami T, et al. Deferasirox, and oral iron chelator, prevents hepatocarcinogenesis and adverse effects of sorafenib. J Clin Biochem Nutr. 2016;58:202–209. doi: 10.3164/jcbn.15-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neil BH, Venook AP. Hepatocellular carcinoma: the role of North American GI steering Committee Task Force and the advent of effective drug therapy. Oncologist. 2007;12:1425–1432. doi: 10.1634/theoncologist.12-12-1425. [DOI] [PubMed] [Google Scholar]
- 7.Cammá C, Schepis F, Oriando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 8.Inada Y, Yamamura M, Kwon M, Uetsuji S, Yamada T, Yamamoto M. Nutritional status in hepatocellular carcinoma associated with liver cirrhosis: effect of intravenous administration of branched-chain amino acid-enriched solution. J Clin Biochem Nutr. 1989;7:251–262. [Google Scholar]
- 9.Tsuda Y, Fukui H, Sujishi T, et al. Is administrating branched-chain amino acid-enriched nutrition achieved symptom-free in malnourished cirrhotic patients? J Clin Biochem Nutr. 2014;54:51–54. doi: 10.3164/jcbn.13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 11.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21 Suppl 5:232–243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 12.Fujiyama S, Shibata J, Maeda S, et al. Phase I clinical study of a novel lipophilic platinum complex (SM-11355) in patients with hepatocellular carcinoma refractory to cisplatin/lipiodol. Br J Cancer. 2003;89:1614–1619. doi: 10.1038/sj.bjc.6601318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teiusanu A, Andrei M, Arbanas T, Nicolaie T, Diculescu M. Nutritional status in cirrhotic patients. Maedica (Buchar) 2012;7:284–289. [PMC free article] [PubMed] [Google Scholar]
- 14.Alberino F, Gatta A, Amodio P, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445–450. doi: 10.1016/s0899-9007(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 15.Saunders J, Brian A, Wright M, Stroud M. Malnutrition and nutrition support in patients with liver disease. Front Gastroenterol. 2010;1:105–111. doi: 10.1136/fg.2009.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: Exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27:430–441. doi: 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 17.Hargreaves R, Ferreira JC, Hughes D, et al. Development of aprepitant, the first neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Ann N Y Acad Sci. 2012;1222:40–48. doi: 10.1111/j.1749-6632.2011.05961.x. [DOI] [PubMed] [Google Scholar]
- 18.Geling O, Eichler HG. Should 5-hydroxytryptamine-3 receptor antagonist be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systemic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005;23:1289–1294. doi: 10.1200/JCO.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Bergstöm M, Hargreaves RJ, Burns HD, et al. Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol Psychiatry. 2004;55:1007–1012. doi: 10.1016/j.biopsych.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 21.Johdan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist. 2007;12:1143–1150. doi: 10.1634/theoncologist.12-9-1143. [DOI] [PubMed] [Google Scholar]
- 22.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 23.Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marín-Ciancas F, Malafarina V. Serum albumin and health in older people: review and meta analysis. Maturitas. 2015;81:17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–4478. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 25.Herrstedt J, Roila F, On behalf of the EMSO Guidelines Working Group. Chemotherapy-induced nausea and vomiting: EMSO clinical recommendations for prophylaxis. Ann Oncol. 2009;20 (Suppl 4):iv156–iv158. doi: 10.1093/annonc/mdp160. [DOI] [PubMed] [Google Scholar]
- 26.European Association for the Study of the Liver; European Organization for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Shimada H, Togo S, et al. Use of transcatheter arterial infusion of anticancer agents with lipiodol to prevent recurrence of hepatocellular carcinoma hepatic resection. Hepatogastroenterology. 1999;46:1083–1088. [PubMed] [Google Scholar]
- 28.Maeda M, Uchida NA, Sasaki T. Liposoluble platinum(II) complexes with antitumor activity. Jpn J Cancer Res. 1986;77:523–525. [PubMed] [Google Scholar]
- 29.Takahashi T, Hoshi E, Takagi M, Katsumata N, Kawahara M, Eguchi K. Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci. 2010;101:2455–2461. doi: 10.1111/j.1349-7006.2010.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.dos Santos LV, Souza FH, Brunetto AT, Sasse AD, da Silveira Nogueira Lima JP. Neurokinin-1 receptor antagonist for chemotherapy-induced nausea and vomiting: a systemic review. J Natl Cancer Inst. 2012;104:1280–1292. doi: 10.1093/jnci/djs335. [DOI] [PubMed] [Google Scholar]