ABSTRACT
A novel GII.17 norovirus variant caused major gastroenteritis epidemics in China in 2014 to 2016. To explore the host immune factors in selection of the emergence of this new variant, we characterized its antigenic relatedness with the GII.4 noroviruses that have dominated in China for decades. Through an enzyme-linked immunosorbent assay (ELISA) and a histo-blood group antigen (HBGA) blocking assay using sera from GII.4 and the GII.17 variant-infected patients, respectively, we observed limited cross-immune reactivity by the ELISA but little reactivity by the HBGA blocking assay between GII.4 norovirus and the new GII.17 variant. Our data suggest that, among other possible factors, GII.4-specific herd immunity had little role in the emergence of the new GII.17 variant. Thus, GII.17 may be an important active antigenic type or immunotype that needs to be considered for future vaccine strategies against human noroviruses.
KEYWORDS: norovirus, cross-reactivity, cross-blockade, GII.17 variants, GII.4 variants, noroviruses, vaccine strategy
INTRODUCTION
The genus Norovirus comprises seven genogroups (G), which can be subdivided into more than 30 genotypes (1). Genogroups GI, GII, and GIV noroviruses can infect humans, with GII.4 viruses causing the majority (70 to 80%) of all norovirus-associated gastroenteritis outbreaks worldwide (2, 3). However, it was noted that a novel GII.17 variant (GII.P17-GII.17) emerged as a predominant norovirus, causing major epidemics in China and several other Asian countries during the epidemic seasons of 2014/2015 and 2015/2016 (4–7), replacing the previously predominant GII.4/Sydney 2012 variant. Although it has been suggested that the predominance of GII.17 strains during 2014 to 2016 was associated with the ability to infect a broader range of susceptible individuals and/or a better polymerase that improved viral fitness (8, 9), the precise mechanisms, particularly the host immune selection factor, of this sudden predominance have not been entirely elucidated. Thus, it is highly significant and urgent to explore such mechanisms leading to this sudden epidemic increase, as the GII.17 norovirus was almost undetected in the previous decades. To this end, we measured the cross-reactivity and cross-blockade activity between GII.4 and GII.17 noroviruses using serum samples from norovirus-infected patients to determine the antigenic relatedness between the two predominant norovirus genotypes. Our data help to explain the sudden increase in epidemics caused by the new GII.17 variant and provide valuable information for future vaccine strategies against noroviruses.
RESULTS
Gastroenteritis outbreak caused by GII.17 norovirus.
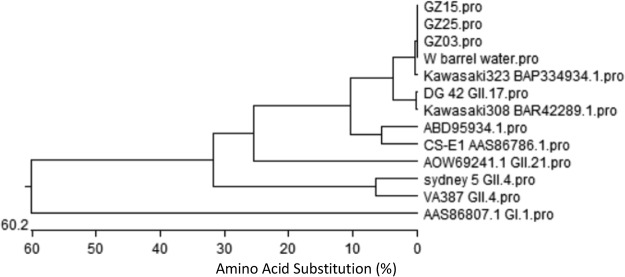
A total of 146 medical staff, 5 inpatients, and 12 food workers were reported sick with acute gastroenteritis in the 10 days of outbreak. Among 58 stool swabs or stool samples tested, 33 samples were found positive for the new GII.17 variant. An inwall swab sample from barrel water was also positive, with 99.8 to 100% shared nucleotide sequence identity with the stool samples (Fig. 1). Outbreak investigation also showed that the barrel water was an important risk factor of the outbreak (χ2 = 59.8, P < 0.001; relative risk [RR] = 12.5, 95% confidence interval [CI] = 7.1 to 27.0). In addition, the same lot of barrel water also caused 3 other school gastroenteritis outbreaks due to the same GII.17 norovirus contamination during the same period in Guangdong Province (unpublished data).
FIG 1.
Phylogenetic analysis based on P-domain sequences of noroviruses detected from stool specimens and water samples during the outbreak. Sequenced nucleotides of case and barrel water samples are shown. P particles of GII.17/DG 42, GII.4/Sydney 5, and GII.4/VA387 were used in cross-reaction and cross-blockade assays.
HBGA phenotyping of saliva samples.
The HBGA phenotypes of the saliva samples from 15 individuals were determined for downstream characterization of cross-reactivity and cross-blockade between GII.4 noroviruses and the new GII.17 variant. We found two nonsecretors among 5 asymptomatic controls, but only one nonsecretor among 10 symptomatic cases, indicating that nonsecretors were at lower infection risk, which was consistent with results of our previous study (10). The basic demographics, symptoms, stool test results, and HBGA phenotypes of these 15 individuals are shown in Table 1.
TABLE 1.
Basic demographics, symptoms, stool tests, and HBGA phenotypes of study populationa
| Group (n) | Subject | Age (yrs) | Onset date (yr.mo.day) | No. of patients within 24 h with: |
Stool test results for norovirus |
HBGA phenotypes | Secretor status | ||
|---|---|---|---|---|---|---|---|---|---|
| Vomiting | Diarrhea | PCR positive | Genotype | ||||||
| Asymptomatic controls (5) | GZ01 | 20 | ND | ND | H+, Leb+, Ley+ | S | |||
| GZ02 | 24 | ND | ND | B+, Leb+, Ley+ | S | ||||
| GZ08 | 30 | ND | ND | Lea+, Lex+ | N | ||||
| GZ09 | 54 | ND | ND | H+, Leb+, Ley+ | S | ||||
| GZ10 | 45 | ND | ND | Lea+, Lex+ | N | ||||
| Symptomatic cases (10) | GZ03 | 29 | 2015.11.6 | 3 | 1 | GII | GII.17 | B+, Leb+, Ley+ | S |
| GZ04 | 37 | 2015.11.6 | 4 | 5 | GII | GII.17 | H+, Leb+, Ley+ | S | |
| GZ05 | Unknown | 2015.11.7 | 5 | 4 | GII | NP | A+, Leb+, Ley+ | S | |
| GZ06 | Unknown | 2015.11.8 | 0 | 6 | GII | NP | Lea+, Lex+ | N | |
| GZ07 | Unknown | 2015.11.7 | 5 | 2 | GII | NP | A+, Leb+, Ley+ | S | |
| GZ15 | 38 | 2015.11.5 | 0 | 4 | GII | GII.17 | B+, Leb+, Ley+ | S | |
| GZ16 | 23 | 2015.11.5 | 5 | 3 | GII | GII.17 | H+, Leb+, Ley+ | S | |
| GZ21 | 29 | 2015.11.6 | 1 | 4 | GII | GII.17 | B+, Leb+, Ley+ | S | |
| GZ23 | 23 | 2015.11.6 | 0 | 3 | GII | GII.17 | Lea+, Leb+ | S | |
| GZ25 | 24 | 2015.11.5 | 0 | 4 | GII | GII.17 | A+, Leb+ | S | |
ND, not determined; NP, not performed; S, secretor; N, nonsecretor.
Sera from GII.17-norovirus-infected patients showed limited cross-reactivity to GII.4 noroviruses.
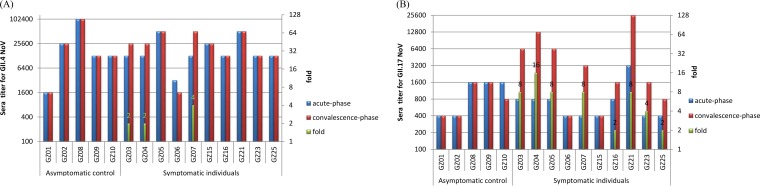
Sera collected during the acute phase of the GII.17-infected patients exhibited high antibody titers against GII.4 (≥1,600, accounting for 86.7%), but low titers against GII.17 (≤1,600, accounting for 93.75%) noroviruses (Fig. 2). These preexisting specific antibody titers to GII.4 and GII.17 noroviruses were significantly different (paired t test; t = 3.775, df = 14, P = 0.002), showing no correlation (correlation = 0.405, P = 0.135). For the asymptomatic controls (n = 5), antibody titers of the sera collected during the convalescent phase did not increase against either GII.4 or GII.17 noroviruses (Fig. 2A and B). In contrast, antibody titers of the convalescent-phase sera from six symptomatic patients (n = 10) exhibited seroconversion (≥4-fold increase) against the new GII.17 variant (Fig. 2B). However, only one had a 4-fold increase in the antibody titers against GII.4 norovirus (Fig. 2A).
FIG 2.
IgG titers specific to GII.4 (A) and GII.17 (B) of human sera collected from a GII.17 outbreak. Acute- and convalescent-phase serum samples (5 asymptomatic controls and 10 symptomatic cases) were collected during an outbreak. The fold increases from acute- to convalescent-phase serum in IgG titers (green columns) were determined (right-hand y axis).
Sera from GII.17 norovirus-infected patients did not cross-block against GII.4 norovirus-attachment factor interaction.
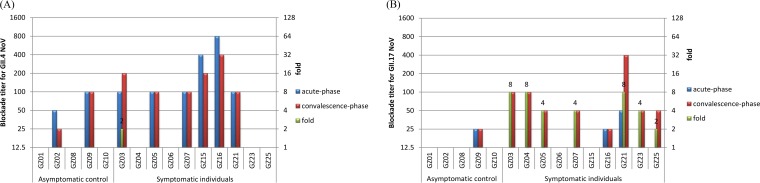
Our data showed that 8 out of 15 (53.3%) acute-phase serum samples showed blockade antibody titers of ≥50 against GII.4 norovirus-attachment factor interaction (Fig. 3A), while only three (20%) had low blockade antibody titers (≥25 but ≤50) against the new GII.17 variant (Fig. 3B). Similarly, for the asymptomatic controls (n = 5), blockade antibody titers of convalescent-phase sera against GII.4 and GII.17 noroviruses did not increase (Fig. 3A and B). In contrast, six convalescent-phase sera from the symptomatic individuals (n = 10) showed increased antibody titers against the new GII.17 variant with a ≥4-fold increase (Fig. 3B). It was noted that no blockade antibody titers against GII.4 norovirus were seen among the convalescent-phase sera with a ≥4-fold increase (Fig. 3A).
FIG 3.
Blockade titers against GII.4 P domain-HBGA (A) and GII.17 P domain-HBGA attachments (B) using human sera collected from a GII.17 outbreak. Acute- and convalescent-phase serum samples (5 asymptomatic controls and 10 symptomatic cases) were collected during an outbreak. The fold increases from acute- to convalescent-phase serum in blockade titers (green columns) were determined (right-hand y axis).
None of the three acute-phase serum samples from nonsecretor individuals showed blockade titers against GII.4 norovirus-HBGA attachment. Interestingly, the convalescent-phase sera from patient GZ06 who is a nonsecretor but showed typical symptom of 6 diarrhea episodes within 24 h after onset, exhibited no increased blockade titer either.
Sera from GII.4 norovirus-infected patients did not cross-block against GII.17 norovirus-attachment factor interaction.
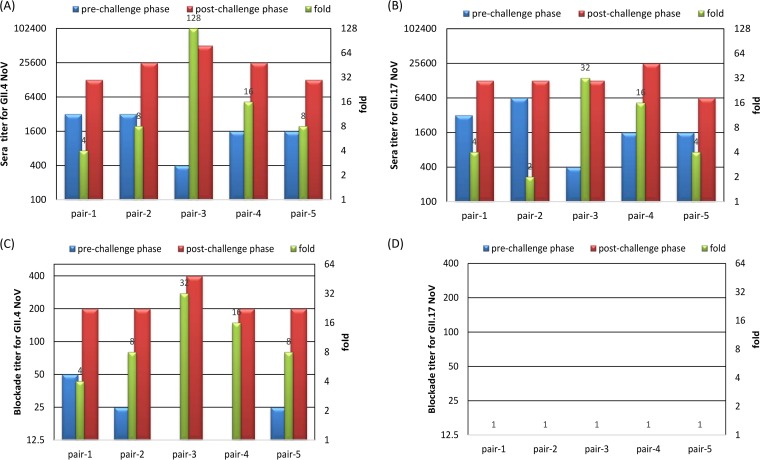
All five convalescent-phase sera that were collected from GII.4 norovirus-infected volunteers at day 30 after GII.4 challenge/infection exhibited both IgG and blockade antibody titers against GII.4 norovirus with ≥4-fold increases compared with those of the sera from the same volunteers before the challenge (Fig. 4A and C). A total of 4 of the 5 sera also showed seroconversion (IgG) (≥4-fold increase; Fig. 4B) against the new GII.17 variant. However, no specific blockade (<25, set as 12.5) against the GII.17 variant attachment to HBGAs was seen for acute- and convalescent-phase serum samples (Fig. 4D).
FIG 4.
IgG and blockade titers against GII.4 (A and C) and GII.17 (B and D) norovirus from human sera from a GII.4 challenge study. Five pairs of prechallenge (day 0) and postchallenge (day 30) serum samples were used. The fold increases from acute- to convalescent-phase serum in IgG or blockade titers (green columns) were determined (right-hand y axis). No specific blockade (<25, set as 12.5) against the GII.17 variant attachment to the HBGAs was seen for either phase serum samples (D).
DISCUSSION
Noroviruses, members of the Norovirus genus, are the major viral cause of acute gastroenteritis in both children and adults worldwide (11). While noroviruses of genogroups GI, GII, and GIV infect humans, GII.4 noroviruses were the predominantly circulating genotype, causing 70 to 80% of all norovirus epidemics (2, 3, 12) over the past 3 decades. Thus, GII.4 noroviruses are the major targets for ongoing norovirus vaccine development (13–15). However, the recent emergence and predominance of the new GII.17 variant causing major gastroenteritis outbreaks in China (4–7) suggest a need for inclusion of other non-GII.4 viruses in the vaccine strategy.
Our group and others have previously demonstrated that the new GII.P17-GII.17 variant gained a broad host spectrum of A, B, and H HBGAs of the general population (4, 6, 10), explaining the predominance of the new GII.17 variant. In this study, we further demonstrated that there is no cross-blockade between GII.17 and GII.4 noroviruses, using serum samples from patients infected with a new GII.17 variant collected from an outbreak study and patients infected with GII.4 norovirus collected during a previous GII.4 challenge study (16). Our data strongly suggested that an individual with a high blockade antibody titer against GII.4 norovirus did not block the new GII.17 variant attachment to HBGAs, suggesting no protection against infection by the new GII.17 variant. In other words, the host herd immunity against the predominant GII.4 noroviruses played little role in the emergence of the new GII.17 variant. Thus, in addition to the broadened host spectrum and increased polymerase activity proposed in previous studies (6–8, 10, 17, 18), lack of specific herd immunity among susceptible populations may also contribute to the emergence and rapid expansion of the new GII.17 variant causing major epidemics in China in recent years.
The lack of immune protection by previous norovirus infection has been shown by several sequential infection studies on different norovirus genotypes. For example, two children in Hong Kong that were infected with GII.4 noroviruses were reinfected with GII.17 viruses (6); a child was infected sequentially by three different noroviruses (GII.4, GII.6, and GII.17) during a 3-year period (19); and another young child was infected sequentially by a GII.4 and then a GII.6 norovirus (20). These case study results suggest little heterologous protection among different norovirus genotypes, indicating that host herd immunity against the predominant GII.4 viruses played little role in the emergence of the new GII.17 variant. A similar case of sudden emergence of GII.2 viruses occurred in different countries during the 2016 to 2017 season (21–23).
In an effort to optimize the vaccine development against norovirus, Parra et al. provided a new perspective recently on the genetic and antigenic diversity of noroviruses, describing two patterns of diversification, “static” versus “evolving” (19). They proposed a new clustering of human norovirus genotypes into “immunotypes," in which GII.4 belongs to immunotype G as an “evolving” pattern, while GII.17 belongs to immunotype J as a “static” pattern. They also suggested that there may be little to no protection between different immunotypes (19). In our present study, we provide further support for their hypotheses. Thus, the GII.17 noroviruses may represent a new target that needs to be considered for future vaccine strategies against noroviruses (24).
A recurrent infection by the new GII.17 variant was observed in an elderly patient (25), suggesting a short-duration homogeneous immunity against the new GII.17 Kawasaki variant in some individuals with impaired immunity. In the present study, we noted that a blockade titer at 50 (GZ21) against the new GII.17 variant did not provide effective protection in the studied outbreak (Fig. 3), which indicated that a preexisting low antibody titer may not protect against infection by the homologous GII.17 variant. We also noted that 2 (GZ06 and GZ15, Fig. 2) out of 10 sera from patients did not show an increase in antibody titers after GII.17 infection, suggesting a lack of an effective homogenous immune response in some individuals. Finally, we noted that nonsecretor GZ06 was infected, indicating that the nonsecretor status does not absolutely protect an individual against infection by the new GII.17 variant. Further study is warranted to clarify these issues.
Conclusion.
We found limited cross-reactivity but no cross-blockade activity between GII.4 and GII.17, using human sera from patients after noroviruses infection, providing new evidence explaining the emergence and rapid expansion of the new GII.17 variant causing major epidemics. Our data indicated that the GII.17 variant needs to be included in future vaccine strategies against human noroviruses.
MATERIALS AND METHODS
Outbreak investigation and sample collection.
From 2 to 11 November 2015, a norovirus gastroenteritis outbreak occurred at a hospital in the city of Guangzhou, Guangdong Province, China. Cases of norovirus infection and disease were defined by having at least one of the following symptoms after 2 November (26): vomiting, diarrhea, or nausea combined with stomach cramps. A total of 58 stool swabs or stool samples from patients with infection and one inwall swab from barrel water were collected. The inwall swab was taken because field investigation often showed that drinking barrel water was a high-risk source, leading to norovirus outbreaks. A total of 15 saliva samples and paired serum samples were collected, of which acute-phase sera were collected on 11 November, while convalescent-phase sera were collected on 11 December. Among these 15 sera, 10 were taken from symptomatic patients at onset time on or after 5 November, and 5 were collected from asymptomatic individuals as controls in the same setting. Sample collection was approved by the ethics committee of the Guangdong Provincial Center for Disease Control and Prevention (GDCDC-W96-027B-2014.100) and informed consent was obtained from each involved individual.
Detection of histo-blood group antigens in saliva.
The HBGA phenotypes of A (Z2A), B (Z5H-2), H (87-N) (Santa Laboratories, Inc., CA), Lea (BG-5), Leb (BG-6), Lex (BG-7), and Ley (BG-8) (Signet Dedham, MA) antigens of the saliva samples were determined using the corresponding monoclonal antibodies, as described previously (27). Briefly, boiled saliva (1:1,000) was coated on high-binding ELISA plates (Costar, Corning, NY, USA). After blocking with 5% nonfat milk-phosphate-buffered saline (PBS), 100 μl diluted (1:300) monoclonal antibodies specific for A, B, H (Santa Cruz, CA), Lea, Leb, Lex, and Ley (Signet Laboratories Inc., Dedham, MA) antigens were added. Then horseradish peroxidase (HRP)-conjugated goat anti-mice IgG or IgM (1:3,000) (Boster Biological Technology, Pleasanton, CA) was added. The signal intensities were displayed by adding HRP substrate reagents for 10 min (Beyotime Biotechnology Co., Ltd., Shanghai, China). The cutoff for a positive signal was optical density at 450 nm (OD450) = 0.2. Well-characterized positive and negative saliva samples were added in each plate as quality control.
Norovirus genotyping.
Viral RNA was extracted from 10% stool suspension, detected by one-step real-time reverse transcription-PCR assay (RT-PCR; using GI and GII primers). For further genotyping, One-Step RT-PCR (Qiagen, CA, USA) was performed with region-C-specific primers. The positive PCR products were sequenced and genetic identity of the viruses was determined using the NoV Automated Genotyping Tool and genotyped using the region-C-specific primers (17, 28, 29).
Preparation of norovirus P proteins.
The cDNA encoding the P proteins of the new GII.17 variant, collected from a previous GII.17 outbreak (10), was cloned into the expression vector pGEX-4T-1 (Amersham Biosciences, Piscataway, NJ) between the SalI and NotI sites. After sequence confirmation, the P proteins were expressed in Escherichia coli. Briefly, the BL21 cultures were induced by IPTG (isopropyl-β-d-thiogalactopyranoside) (0.5 mM) at room temperature (22°C) overnight. The recombinant capsid fusion proteins were purified using Glutathione Sepharose 4 Fast Flow resin (7 Sea Pharmatech Co., Ltd., China) according to the manufacturer's instructions. Glutathione S-transferase (GST) was removed from the P proteins by thrombin (GE Healthcare Life Sciences, NJ, USA) cleavage on beads at room temperature overnight (30, 31). P particles of GII.4/Sydney and GII.4/VA387 variants were used as GII.4 antigens (32).
Cross-reactivity between GII.17 noroviruses with GII.4 using sera from norovirus-infected patients.
The paired serum samples were used to study antigenic cross-reactivity between GII.4 and GII.17 norovirus by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated with 0.5 μg/ml diluted P particles of GII.4/Sydney strain or GII.17/DG42 (10). After blocking, paired patient serum samples were added at 2-fold serial dilution. Then horseradish peroxidase (HRP)-conjugated rabbit anti-human IgG was used to detect the bound norovirus antibodies. The signals were developed using a tetramethylbenzidine (TMB) substrate kit. The cutoff for a positive signal was set as OD450 = 0.2.
Cross-blockade between GII.17 and GII.4 using sera from norovirus-infected patients.
Due to lack of cell culture and small animal infection models, an HBGA-based blockade assay served as a surrogate for neutralization (33). A blockade assay to measure the ability of serum antibodies to block NoV P particles binding to saliva was developed and optimized. A saliva sample (HBGA phenotypes: B+, Leb+, and Ley+), which showed good binding to the following P particles, was diluted (1:1,000) and coated on a microtiter plate. After blocking with the dry milk, the GII.4 or GII.17 P particles (0.2 to 0.5 μg/ml) that were preincubated with paired sera from GII.17 norovirus-infected patients at 37°C for 1 h were added to the saliva-coated plates. The bound P particles were detected using our in-house anti-GII.4 or anti-GII.17 norovirus (1:3,000) mice sera, followed by incubation with the HRP-conjugated goat anti-mice IgG. P particles without preincubation with a serum sample were used as a positive control. Results were accepted if positive-control optical density (OD) values were within the range of 1.0 ± 0.3 (33). The 50% blocking titer (BT50), defined as the maximal dilution (folds) of a serum sample that showed at least 50% blockade (OD) compared with the positive control, was determined for each serum sample. A value of 12.5 was assigned to a serum sample with a BT50 <25.
Five paired sera from GII.4 norovirus-infected patients collected in a previous GII.4 challenge study (16, 34) were used to determine cross-blockade against the HBGA attachment of the new GII.17 variant as described above.
Statistical analysis.
Paired t test and correlation analysis were used for the mean preexisting antibody titers specific to GII.4 and GII.17 noroviruses using SPSS 20 for Windows 7 (SPSS Inc., IL, USA). Statistical significance was set as a P value of <0.05.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grants no. 31771007, 81773975, and 81473402), the Natural Science Foundation of Guangdong Province (grant no. 2014A030313332), a collaborative innovation major project of the Science and Technology Program of Guangzhou (grant no. 201604020111) and a scientific research initiation plan of Southern Medical University (grants no. QD2015N006 and CX2015N001).
We declare no conflict of interest.
Y.-C.D., X.J., and X.-F.Z. conceived and designed the experiments; Y.-C.D., Q.H., L.Q., and Y.-L.Z. coordinated outbreak investigation; Y.-C.D., Q.H., L.Q., Y.-L.Z., and Y.L. collected samples; Y.-C.D., M.X., L.Q., Y.-L.Z., and J.-D.L. performed experiments; Y.-C.D., M.T., L.Q., Y.-L.Z., X.J., and X.-F.Z. analyzed and interpreted the data; Y.-C.D., M.T., X.J., and X.-F.Z. wrote the manuscript; and Y.-C.D., M.X., Q.H., M.T., L.Q., Y.-L.Z., J.-D.L., X.J., and X.-F.Z approved the final version.
REFERENCES
- 1.Vinje J. 2015. Advances in laboratory methods for detection and typing of norovirus. J Clin Microbiol 53:373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siebenga JJ, Lemey P, Kosakovsky Pond SL, Rambaut A, Vennema H, Koopmans M. 2010. Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathog 6:e1000884. doi: 10.1371/journal.ppat.1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai YC, Hu GF, Zhang XF, Song CL, Xiang WL, Wu XB, Wang LY, Jiang X, Nie J. 2011. Molecular epidemiology of norovirus gastroenteritis in children in Jiangmen, China, 2005–2007. Arch Virol 156:1641–1646. doi: 10.1007/s00705-011-1010-3. [DOI] [PubMed] [Google Scholar]
- 4.Jin M, Zhou YK, Xie HP, Fu JG, He YQ, Zhang S, Jing HB, Kong XY, Sun XM, Li HY, Zhang Q, Li K, Zhang YJ, Zhou DQ, Xing WJ, Liao QH, Liu N, Yu HJ, Jiang X, Tan M, Duan ZJ. 2016. Characterization of the new GII.17 norovirus variant that emerged recently as the predominant strain in China. J Gen Virol 97:2620–2632. doi: 10.1099/jgv.0.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J, Sun L, Fang L, Yang F, Mo Y, Lao J, Zheng H, Tan X, Lin H, Rutherford S, Guo L, Ke C, Hui L. 2015. Gastroenteritis outbreaks caused by norovirus GII.17, Guangdong Province, China, 2014–2015. Emerg Infect Dis 21:1240–1242. doi: 10.3201/eid2107.150226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan MC, Lee N, Hung TN, Kwok K, Cheung K, Tin EK, Lai RW, Nelson EA, Leung TF, Chan PK. 2015. Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014. Nat Commun 6:10061. doi: 10.1038/ncomms10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu J, Ai J, Jin M, Jiang C, Zhang J, Shi C, Lin Q, Yuan Z, Qi X, Bao C, Tang F, Zhu Y. 2015. Emergence of a new GII.17 norovirus variant in patients with acute gastroenteritis in Jiangsu, China, September 2014 to March 2015. Euro Surveill 20(24): pii=21178. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21178. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Fang L, Zheng H, Lao J, Yang F, Sun L, Xiao J, Lin J, Song T, Ni T, Raghwani J, Ke C, Faria NR, Bowden TA, Pybus OG, Li H. 2016. The evolution and transmission of epidemic GII.17 noroviruses. J Infect Dis 214:556–564. doi: 10.1093/infdis/jiw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushima Y, Ishikawa M, Shimizu T, Komane A, Kasuo S, Shinohara M, Nagasawa K, Kimura H, Ryo A, Okabe N, Haga K, Doan YH, Katayama K, Shimizu H. 2015. Genetic analyses of GII.17 norovirus strains in diarrheal disease outbreaks from December 2014 to March 2015 in Japan reveal a novel polymerase sequence and amino acid substitutions in the capsid region. Euro Surveill 20(26): pii=21173. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21173. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XF, Huang Q, Long Y, Jiang X, Zhang T, Tan M, Zhang QL, Huang ZY, Li YH, Ding YQ, Hu GF, Tang S, Dai YC. 2015. An outbreak caused by GII.17 norovirus with a wide spectrum of HBGA-associated susceptibility. Sci Rep 5:17687. doi: 10.1038/srep17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green KY. 2013. Caliciviridae: the noroviruses, p 949–9. In Knipe DM, Howley PM (ed), Fields virology, 6th ed Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 12.Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. 2013. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol 56:185–193. doi: 10.1016/j.jcv.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Tan M, Jiang X. 2014. Vaccine against norovirus. Hum Vaccin Immunother 10:1449–1456. doi: 10.4161/hv.28626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atmar RL, Estes MK. 2012. Norovirus vaccine development: next steps. Expert Rev Vaccines 11:1023–1025. doi: 10.1586/erv.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parra GI, Bok K, Taylor R, Haynes JR, Sosnovtsev SV, Richardson C, Green KY. 2012. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 30:3580–3586. doi: 10.1016/j.vaccine.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai YC, Zhang XF, Xia M, Tan M, Quigley C, Lei W, Fang H, Zhong W, Lee B, Pang X, Nie J, Jiang X. 2015. Antigenic relatedness of norovirus GII.4 variants determined by human challenge sera. PLoS One 10:e0124945. doi: 10.1371/journal.pone.0124945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf S, Williamson WM, Hewitt J, Rivera-Aban M, Lin S, Ball A, Scholes P, Greening GE. 2007. Sensitive multiplex real-time reverse transcription-PCR assay for the detection of human and animal noroviruses in clinical and environmental samples. Appl Environ Microbiol 73:5464–5470. doi: 10.1128/AEM.00572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parra GI, Green KY. 2015. Genome of emerging norovirus GII.17, United States, 2014. Emerg Infect Dis 21:1477–1479. doi: 10.3201/eid2108.150652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra GI, Squires RB, Karangwa CK, Johnson JA, Lepore CJ, Sosnovtsev SV, Green KY. 2017. Static and evolving norovirus genotypes: implications for epidemiology and immunity. PLoS Pathog 13:e1006136. doi: 10.1371/journal.ppat.1006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parra GI, Green KY. 2014. Sequential gastroenteritis episodes caused by 2 norovirus genotypes. Emerg Infect Dis 20:1016–1018. doi: 10.3201/eid2006.131627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ao Y, Wang J, Ling H, He Y, Dong X, Wang X, Peng J, Zhang H, Jin M, Duan Z. 2017. Norovirus GII.P16/GII.2-associated gastroenteritis, China, 2016. Emerg Infect Dis 23:1172–1175. doi: 10.3201/eid2307.170034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bidalot M, Thery L, Kaplon J, De Rougemont A, Ambert-Balay K. 2017. Emergence of new recombinant noroviruses GII.P16-GII.4 and GII.P16-GII.2, France, winter 2016 to 2017. Euro Surveill 22(15): pii=22768. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu J, Fang L, Sun L, Zeng H, Li Y, Zheng H, Wu S, Yang F, Song T, Lin J, Ke C, Zhang Y, Vinje J, Li H. 2017. Association of GII.P16-GII.2 recombinant norovirus strain with increased norovirus outbreaks, Guangdong, China, 2016. Emerg Infect Dis 23:1188–1190. doi: 10.3201/eid2307.170333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan MHY, Chen H, Podkolzin AT, Zaytseva EV, Komano J, Sakon N, Poovorawan Y, Vongpunsawad S, Thanusuwannasak T, Hewitt J, Croucher D, Collins N, Vinjé J, Pang XL, Lee BE, de Graaf M, van Beek J, Vennema H, Koopmans MPG, Niendorf S, Poljsak-Prijatelj M, Steyer A, White PA, Lun JH, Mans J, Hung TN, Kwok K, Cheung K, Lee N, Chan PKS. 2017. Global spread of norovirus GII.17 Kawasaki 308, 2014–2016. Emerg Infect Dis 23:1359–1354. doi: 10.3201/eid2308.161138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan MC, Lee N, Hung TN, Chan LY, Kwok K, Wong RY, Chan PK. 2017. Recurrent infections of emergent norovirus GII.17 in an elderly patient. Clin Infect Dis 64:697–699. doi: 10.1093/cid/ciw822. [DOI] [PubMed] [Google Scholar]
- 26.Nordgren J, Kindberg E, Lindgren PE, Matussek A, Svensson L. 2010. Norovirus gastroenteritis outbreak with a secretor-independent susceptibility pattern, Sweden. Emerg Infect Dis 16:81–87. doi: 10.3201/eid1601.090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang P, Farkas T, Zhong W, Tan M, Thornton S, Morrow AL, Jiang X. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol 79:6714–6722. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima S, Kageyama T, Fukushi S, Hoshino FB, Shinohara M, Uchida K, Natori K, Takeda N, Katayama K. 2002. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J Virol Methods 100:107–114. doi: 10.1016/S0166-0934(01)00404-9. [DOI] [PubMed] [Google Scholar]
- 29.Kroneman A, Vega E, Vennema H, Vinje J, White PA, Hansman G, Green K, Martella V, Katayama K, Koopmans M. 2013. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol 158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan M, Fang P, Chachiyo T, Xia M, Huang P, Fang Z, Jiang W, Jiang X. 2008. Noroviral P particle: structure, function and applications in virus-host interaction. Virology 382:115–123. doi: 10.1016/j.virol.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan M, Jiang X. 2005. The P domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J Virol 79:14017–14030. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Long Y, Zhang XF, GFH, Dai YC. 2016. Expression of NoV GII-4 Sydney strain P particle and analysis of its binding activity with HBGAs receptor in China. Chin J Zoonoses 32:344–348. [Google Scholar]
- 33.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. 2010. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 202:1212–1218. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frenck R, Bernstein DI, Xia M, Huang P, Zhong W, Parker S, Dickey M, McNeal M, Jiang X. 2012. Predicting susceptibility to norovirus GII.4 by use of a challenge model involving humans. J Infect Dis 206:1386–1393. doi: 10.1093/infdis/jis514. [DOI] [PubMed] [Google Scholar]