ABSTRACT
A study comparing the ICT (immunochromatography technology) Toxoplasma IgG and IgM rapid diagnostic test (LDBio Diagnostics, France) with a fully automated system, Architect, was performed on samples from university hospitals of Marseille and Saint-Etienne. A total of 767 prospective sera and 235 selected sera were collected. The panels were selected to test various IgG and IgM parameters. The reference technique, Toxoplasma IgGII Western blot analysis (LDBio Diagnostics), was used to confirm the IgG results, and commercial kits Platelia Toxo IgM (Bio-Rad) and Toxo-ISAgA (bioMérieux) were used in Saint-Etienne and Marseille, respectively, as the IgM reference techniques. Sensitivity and specificity of the ICT and the Architect IgG assays were compared using a prospective panel. Sensitivity was 100% for the ICT test and 92.1% for Architect (cutoff at 1.6 IU/ml). The low-IgG-titer serum results confirmed that ICT sensitivity was superior to that of Architect. Specificity was 98.7% (ICT) and 99.8% (Architect IgG). The ICT test is also useful for detecting IgM without IgG and is both sensitive (100%) and specific (100%), as it can distinguish nonspecific IgM from specific Toxoplasma IgM. In comparison, IgM sensitivity and specificity on Architect are 96.1% and 99.6%, respectively (cutoff at 0.5 arbitrary units [AU]/ml). To conclude, this new test overcomes the limitations of automated screening techniques, which are not sensitive enough for IgG and lack specificity for IgM (rare IgM false-positive cases).
KEYWORDS: toxoplasmosis, Toxoplasma gondii, immunoglobulin G, immunoglobulin M, serology, immunochromatography test, Architect
INTRODUCTION
Toxoplasmosis, which is caused by Toxoplasma gondii, is usually asymptomatic and benign in immunocompetent humans. In pregnant women, maternal transmission may result in congenital toxoplasmosis, which may cause severe disease or sequelae (1). Medical follow-up of obstetrical toxoplasmosis is essential for seronegative women. In severe cellular immunodeficiency, reactivation of the infection causes acute neurological damage and can be lethal if not successfully treated. For these patients, detection of Toxoplasma-specific antibodies showing serological reactivation or primary infection is essential to properly diagnose and prevent severe toxoplasmosis.
The follow-up of obstetrical toxoplasmosis primarily involves the detection of anti-Toxoplasma-specific immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies (2–4). Detection of Toxoplasma-specific IgM with the first blood test is usually cause for concern. The presence of Toxoplasma-specific IgG without IgM confirms the immunization of the patient, thus avoiding unnecessary and expensive follow-up.
As the prevalence of the disease has declined in Europe and France in particular (5), the number of women to be controlled during pregnancy has increased (46% to 63% between 1995 and 2010 in France), which influences the cost-benefit aspect of a mass systematic screening program.
In this study, we assessed the performance of the immunochromatography technology (ICT) Toxoplasma IgG and IgM rapid diagnostic test (LDBio Diagnostics, France) and compared the results with those of the Architect system. This automated technique is reliable for first-line serodiagnosis (6) and was chosen as the screening technique in Saint-Etienne and Marseille. This assessment is critical to define a good serological strategy based on the specificity and sensitivity of the two techniques. The aim of this study was to determine whether the ICT test can overcome the limits of the screening technique and ultimately be used as a second-line test.
RESULTS
The evaluation of the ICT Toxoplasma IgG and IgM test performance was performed as described below.
Panel 1: 767 nonselected prospective sera. (i) IgG analysis.
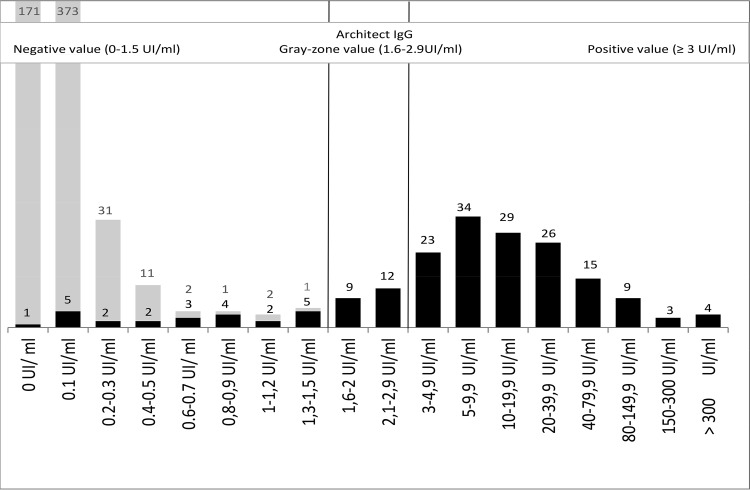
The IgG value distribution provided by the Architect system and ICT test are shown in Fig. 1. The IgG concordance analysis between the two techniques is also detailed in Table 1. Of the 767 serum samples tested, 582 sera were negative according to both the Architect and ICT assays. All of the 143 sera with a positive result according to the Architect (≥3.0 IU/ml) system were also positive with the ICT. Regarding 20 sera that were in the gray zone using the Architect system (1.6 to 2.9 IU/ml), these samples were positive according to the ICT test, which was confirmed by the IgGII Western blot. Additionally, 14 negative samples that fell between 0.6 and 1.5 IU/ml on the Architect system were positive with the ICT test and confirmed by the IgGII Western blot. We also found eight false-positive results using the ICT test (7 negative sera with negative IgM and IgG between 0.1 and 0.3 IU/ml on Architect, one negative IgM with IgG at 2.5 IU/ml on Architect).
FIG 1.
Distribution of nonselected IgG serum titers with Architect. The dark columns correspond to sera that are positive by ICT IgG-IgM (LDBIO Diagnostics). The gray columns correspond to sera that are negative by ICT IgG-IgM (LDBIO Diagnostics).
TABLE 1.
Analysis of IgG concordant and discrepant sera between ICT and Toxo IgG Architect test by testing of 767 nonselected samples
| IgG analysis | n | Architect valuea (UI/ml) | ICTb | Reference Western blotc (LDBio Toxo II IgG) | Conclusion |
|---|---|---|---|---|---|
| Concordant | 582 | 0–1.3 (Neg) | Neg | NR | Neg IgG |
| Concordant | 143 | ≥3 | Pos | NR | Pos IgG |
| Minor discrepant | 20 | 1.6–2.9 (gray zone) | Pos | Pos | Pos IgG |
| Discrepant | 14 | 0.6–1.5 (Neg) | Pos | Pos | Pos IgG (false-negative Architect) |
| Discrepant | 1 | 2.5 (gray zone) | Pos | Neg | Neg IgG (false-positive ICT) |
| Discrepant | 7 | 0.1–0.3 (Neg) | Pos | Neg | Neg IgG (false-positive ICT) |
IgG concentrations on Architect: negative, ≤1.5 IU/ml; gray zone, 1.6 to 2.9 IU/ml; positive, ≥3 IU/ml. Neg, negative.
Pos, positive.
NR, not realized.
(ii) IgM analysis.
Among 737 IgM-negative sera on Architect (0 to 0.49 arbitrary units [AU]/ml), 571 were also IgG negative and had a negative ICT. For the other 166 negative IgM sera, they were positive for IgG and had a positive ICT (IgG and IgM) (Table 2).
TABLE 2.
Analysis of IgM concordant and discrepant sera between ICT IgG-IgM and Toxo IgM Architect by testing of 767 nonselected samples
| IgM analysis | No. of samplesa (total n = 767, total M = 356, total SE = 411) | Architect IgM value (AU/ml) | ICT (IgM-IgG) | Reference IgM (M = ISAgA, SE = Platelia) | IgG analysis | Conclusion (follow-up) |
|---|---|---|---|---|---|---|
| Concordant with IgG analysis | 737 (M = 340, SE = 397) | 0–0.49 (Neg) | Neg (571), Pos (166) | NR | Neg IgG (571), Pos IgG (166) | Neg IgM |
| Concordant | 15 (M = 8, SE = 7) | >0.60 (Pos) | Pos | Pos | Pos IgG | Pos IgM (residual with IgG) |
| Concordant | 6 (M = 2, SE = 4) | >0.60 (Pos) | Pos | Pos | Neg or equivocal IgG | Beginning primary infection: IgM without IgG |
| Minor discrepant | 4 (M = 3, SE = 1) | 0.50–0.59 (gray zone) | Pos | Pos | Pos IgG | Pos IgM (residual with IgG) |
| Minor discrepant | 1 (M = 1) | 0.50–0.59 (gray zone) | Pos | Pos | Neg IgG | Beginning primary infection: IgM without IgG |
| Minor discrepant | 1 (M = 1) | 0.50–0.59 (gray zone) | Neg | Neg | Neg IgG | Neg IgM |
| Discrepant | 3 (M = 2, SE = 1) | >0.60 (Pos) | Neg | 1 Neg (M), 2 Posb (1 M, 1 SE) | Neg IgG | Neg IgM |
M, sera from Marseille tested with reference technique IgM immunosorbent agglutination assay (ISAgA; bioMérieux, Marcy l'Etoile, France). SE, sera from Saint-Etienne tested with reference technique Platelia Toxo IgM (Platelia; Bio-Rad, Marne la Coquette, France).
These two patients were positive by Architect IgM and immunocapture, but the serological IgG follow-up at 2 weeks and 1 month did not reveal the appearance of IgG; nonspecific IgM was found.
Concerning the positive results on the Architect system (>0.6 AU/ml), 15 were concordant with the ICT test and confirmed by immunocapture, while 6 were positive, but without IgG, according to both the ICT test and immunocapture (indicating early primary infection). The other 9 sera are major or minor discrepant and are detailed in Table 2.
(iii) Sensitivity, specificity, NPV, and PPV.
The concordance between the Architect and ICT systems concerning sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) are shown in Table 3. Considering equivocal samples with Architect as positive (6) (see results described below for low IgG titers), the correlation between the two techniques was 97.2% for IgG and 99.6% for IgM.
TABLE 3.
Concordance, Se, Sp, PPV, NPV, Youden index, and Yule Q by testing 767 nonselected samples for anti-Toxoplasma IgG and/or IgMd
| Test | Concordance with ICT (%) | Sea (%) | NPV (%) | Spb (%) | PPV (%) | Youden index (Se + Sp − 1) | Yule Q (%) |
|---|---|---|---|---|---|---|---|
| ICT for IgG | NAc | 100 (98.5–100) | 100 (98.5–100) | 98.7 (97.8–99.6) | 95.8 (94.2–97.4) | 0.99 | 100 |
| Architect IgG (cutoff, 1.6 IU/ml) | 97.2 (96.0–98.4) | 92.1 (88.1–96.1) | 97.7 (95.5–99.8) | 99.8 (99.4–100) | 99.4 (98.8–100) | 0.92 | 100 |
| Architect IgG (cutoff, 3.0 IU/ml) | 94.5 (92.9–96.1) | 80.7 (74.9–86.5) | 94.6 (91.3–97.9) | 100 (99.6–100) | 100 (99.6–100) | 0.81 | 100 |
| ICT for IgM | NA | 100 (92.6–100) | NA | 100 (98.3–100) | NA | NA | NA |
| Architect IgM (cutoff, 0.5 AU/ml) | 99.6 (99.1–100) | 96.1 (88.7–100) | NA | 99.6 (98.3–100) | NA | NA | NA |
| Architect IgM (cutoff, 0.6 AU/ml) | 99.1 (98.4–99.8) | 80.8 (65.7–95.9) | NA | 99.7 (98.3–100) | NA | NA | NA |
Se, sensitivity defined as true positive/(true positive + false negative).
Sp, specificity defined as true negative/(true negative + false positive).
NA, not applicable.
Values in parentheses are 95% confidence intervals.
Taking reference techniques and clinical data together, the ICT assay shows a sensitivity of 100% and a specificity of 98.7% for IgG, 100% sensitivity and specificity for IgM, and an NVP and PPV for IgG of 100% and 95.8%, respectively.
Analysis of the 235 selected sera in panel 2. (i) Panel 2.1: low IgG titers (n = 92).
The results of 92 selected sera with low IgG titers (0.6 to 3.4 IU/ml) are shown in Table 4. IgG-positive results on the Architect system were set to 3 IU/ml. We found that for values between 2.1 and 3.4 IU/ml, both the ICT and IgGII Western blot results were positive for 100% of the 38 sera tested. For 47 cases in the large range from 0.8 to 2.0 IU/ml (31 IgG negative and 16 IgG Architect gray zone), the concordance between the ICT and the IgGII Western blot was perfect. Most of the cases were positive (41/47), whereas some were IgG negative with Architect (31/47). For the 7 sera with IgG values ranging from 0.6 to 0.7 IU/ml (negative on Architect), 3 ICT results were positive while 4 sera subjected to IgGII Western blot were positive.
TABLE 4.
Analysis of IgG concordant and discrepant sera between ICT and Toxo IgG Architect for ninety-two selected sera with low IgG titer
| Architect IgG valuea (IU/ml) | No. of sera | Positive ICT [no. positive/total no. (%)] | Conclusion [no. with positive IgGII Western blot/total no. (%)] |
|---|---|---|---|
| 0.6–0.7 | 7 | 3/7 (43) | 4/7 (57) |
| 0.8–0.9 | 7 | 5/7 (71) | 5/7 (71) |
| 1–1.2 | 12 | 11/12 (92) | 11/12 (92) |
| 1.3–1.5 | 12 | 10/12 (83) | 10/12 (83) |
| 1.6–2 | 16 | 15/16 (94) | 15/16 (94) |
| 2.1–2.9 | 27 | 27/27 (100) | 27/27 (100) |
| 3–3.4 | 11 | 11/11 (100) | 11/11 (100) |
IgG concentrations on Architect: negative, ≤1.5 IU/ml; gray zone, 1.6 to 2.9 IU/ml; positive, ≥3 IU/ml.
(ii) Panel 2.2: high IgG titers (n = 20).
Regarding the sera with high IgG titers (range, 125 to 2,000 IU/ml), all ICT were positive (weakly or strongly positive depending on strip intensity), but there was no correlation between the titer and the intensity of the test band.
(iii) Panel 2.3: sera followed by seroconversion (n = 50).
For 33 sera, IgG and IgM were associated. Regarding 14 sera with IgM of >0.5 AU/ml without IgG (IgG of <1.5 IU/ml), both the immunocapture test and the ICT were positive. For the last 3 sera corresponding to the first sample of a proved seroconversion, 2 were positive with ICT and immunosorbent agglutination assay (ISAgA), whereas only one was negative with all techniques.
(iv) Panel 2.4: sera without seroconversion (n = 33) and with nonspecific IgM on Architect (n = 23).
The 10 negative or equivocal sera (0.35 < IgM < 0.59 AU/ml) were negative according to the ICT and immunocapture assays. Concerning the 23 other sera (IgM positive by Architect), we conclude that all of these corresponded to false-positive results, since the IgG follow-up did not show the appearance of IgG and increasing IgM. Ten out of 23 sera tested positive on the Architect system and negative by immunocapture and ICT test, which indicates a false-positive result on Architect. Eleven out of 23 samples yielded positive immunocapture and Architect results but negative ICT results and corresponded to false-positive results on both the Architect system and immunocapture assay. We found that 2/23 sera tested positive for IgM with the three techniques.
(v) Panel 2.5: false-positive IgG (n = 11).
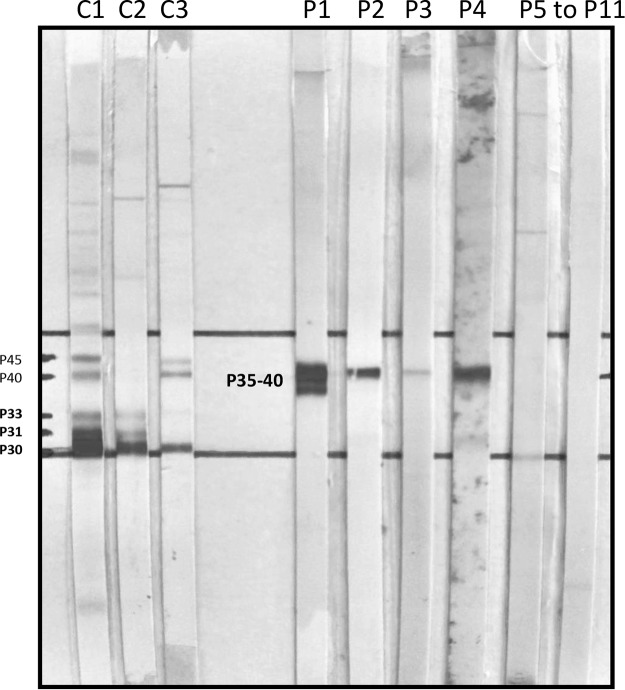
The 11 sera with IgG between 3.2 and 21.6 IU/ml on the Architect system had a negative ICT. The Western blot (Toxo II IgG LDBio Diagnostics) results were negative with different types of profiles (Fig. 2). Profile 1 displayed IgG at 3.2 IU/ml on Architect with 2 bands (negative) (P35 to P40). Profiles 2 to 4 displayed IgG of 3.2 to 21.6 IU/ml on the Architect system with a single band (negative) (P40). Profiles 5 to 11 displayed no bands.
FIG 2.
Toxoplasma IgGII Western blot (LDBIO Diagnostics) profiles. These profiles were obtained from 11 sera of the panel corresponding to nonspecific IgG on Architect with a negative ICT. C, control; C1, positive control with IgG of 5 UI/ml on Architect; C2, positive control with IgG of 1.2 UI/ml on Architect; C3, positive control of a confirmed seroconversion with IgG of 0.6 UI/ml on Architect; P, panel. Eleven sera were used: P1, IgG of 3.2 UI/ml on Architect, negative ICT, 2 bands (i.e., negative) with Toxo II IgG Western blot analysis; P2 to P4, IgG of 3.2 to 21.6 UI/ml on Architect, negative ICT, 1 band (i.e., negative) with Toxo II IgG Western blot analysis; P5 to P11, IgG of 3.8 to 6.7 UI/ml on Architect, negative ICT, 0 band (i.e., negative) with Toxo II IgG Western blot analysis; P6 to P10, not presented.
(vi) Panel 2.6: analysis of selected sera of toxoplasmosis-seronegative patients and with potentially cross-reacting sera for CMV infection, EBV infection, HIV infection, and presence of RF.
The panel of 6 sera from patients with anti-CMV (cytomegalovirus) antibodies included 4 sera with IgG- and IgM-specific anti-CMV antibodies, which probably corresponded to acute infection. Among 13 sera with positive Epstein-Barr virus (EBV) serology, 8 had both IgM and VCA-IgG; these results corresponded to a probable primary EBV infection. We selected 6 HIV-positive sera that corresponded to a recent primary infection (positive P24 and antigen viral load between 3.5 and 5 log copies/ml). All sera were seronegative for Toxoplasma, but 4 ICT were false-positive tests, indicating probable HIV serology, EBV infection, and rheumatoid factor (RF). To summarize, among these 29 sera, we found the following nonspecific reactions: one positive for IgG on the Architect system, three equivocal or positive for IgM on the Architect system, and four equivocal or positive for IgG-IgM according to the ICT test (Table 5).
TABLE 5.
Twenty-nine selected sera from toxoplasmosis-seronegative patients with CMV, EBV, HIV, and the presence of RF
| Toxoplasmosis-seronegative sera | No. of sera | No. positive by: |
||
|---|---|---|---|---|
| IgG Architect | IgM Architect | ICT | ||
| CMV infection | 6 | 0/6 | 0/6 | 0/6 |
| EBV infection | 13 | 0/13 | 3/13 (gray zone or positive) | 1/13 (weakly positive) |
| HIV infection | 5 | 0/5 | 0/5 | 2/5 |
| RF | 5 | 1/5 (gray zone) | 0/5 | 1/5 |
| Total | 29 | 1/29 | 3/29 | 4/29 |
DISCUSSION
Serological diagnosis of toxoplasmosis requires quantitative tests, most of which are automated techniques. The automated techniques have several advantages: they are adapted to the routine analysis of large quantities of samples and produce quantitative and reproducible IgG and IgM results, particularly for the Architect technique, with excellent specificity for IgG (near 100%) and excellent sensitivity for IgM (6–8).
However, automated techniques have drawbacks: concerning the IgG parameter, most of these techniques lack sensitivity (Vidas, Access, Centaur, Immulite, AxSym, Platelia, and Enzygnost) (6, 7) found with the Architect system. Concerning IgM, some automated techniques can display an imperfect sensitivity, except the Platelia system, which is well correlated with the ISAgA system (6).
This study was carried out to evaluate the ICT Toxoplasma IgG and IgM rapid diagnostic test. We aimed to determine whether the ICT test is able to overcome the sensitivity limits of automated techniques, particularly those of the Architect system, which is used as a first-line diagnostic approach. A similar recent study conducted in Lyon by Chapey et al. also evaluated the ICT test (8) and found sensitivity of 97% and specificity of 96%, which are similar to results of our study. However, in this study, the reliability of the ICT test has been compared to that of the Architect system and not to the reference technique. Therefore, the expertise of discrepant cases is uncertain and does not highlight the interests and the limits of this new test. Our study cohort was larger than that of the Lyon study, as it included 1,002 sera from two centers (Marseille and Saint-Etienne). Furthermore, we selected various categories of sera to evaluate the relevance of the test in critical situations (e.g., low IgG titers, seroconversion, nonspecific IgM titers, etc.).
Another recent study has evaluated this new technique (9). The methodological approach is different and complementary (in relation to our work). This study uses 180 sera: 115 chronically infected persons with known serotype (48 serotype II, 14 serotype I-III, serotype I-IIIa, and 28 serotype atypical, haplogroup 12), 51 seronegative samples, and 13 samples from recently infected persons. In this study, the ICT test had 100% sensitivity and specificity. The authors also developed the theme of economic considerations, and we agree that the ICT test offers new options for improved prenatal care in low- and middle-income countries; it facilitates early identification and diagnosis, with similar or better sensitivity and specificity than automated techniques.
The ICT is a qualitative test that simultaneously detects specific Toxoplasma IgG and IgM. A positive result can be caused by the presence of IgG and/or IgM anti-Toxoplasma. Regarding nonselected sera, our study revealed that sensitivity for IgG was 100% with the ICT test (Table 3), which correlated with the Western blot results. This is not surprising, as the ICT test is calibrated with the Toxoplasma IgGII Western blot assay marketed by the same company (LDBio Diagnostics). All samples with IgG values in the gray zone and 14 negative samples according to the Architect system were positive with the ICT test (Table 1).
To better explore the sensitivities of the ICT and Architect IgG system, we selected a panel of 92 sera with IgG titers close to the threshold (Table 4). The analysis of these results clearly shows a higher sensitivity of the ICT than the Architect IgG technique. This observation confirms that the IgG cutoffs chosen by Abbott are too high and stringent. Figure 1 highlights the gain in sensitivity of the ICT test, which deciphers the equivocal results of the Architect system. These results are in line with those of Chapey et al. (8) and Villard et al. (6), who have worked on a similar but smaller panel (21 and 35 low-IgG-titer sera, respectively). However, our results show the limits of Chapey's study, which resulted in 8/400 false-positive ICT tests. For these specific cases, only a sensitive enough confirmatory technique (Western blot or dye test) would lead to a reliable conclusion.
Overall, with this sensitive rapid test (IgG), it is possible to avoid performing large numbers of unnecessary serological follow-up tests, thus providing significant economic savings. Concerning test specificity, false-positive IgG results on the Architect system in routine practice are rare (10), which is due to the use of T. gondii recombinant P30 (SAG-1) and P35 (GRA-8) antigens in the Architect Toxoplasma IgG assay. False-positive results often are associated with nonspecific anti-p35 antibodies. Systematic confirmation of positive IgG results is critical in parasitology laboratories. Using the ICT test as a second-line diagnostic approach enables the identification of sera that yield a false-positive result on the Architect system. Indeed, all 11 false-positive IgG selected sera were negative according to the ICT test and confirmed via Toxoplasma IgGII Western blot analysis, which showed different types of profiles (0, 1, or 2 bands) but did not correspond to serological profiles of toxoplasmosis.
False-positive IgG results with the ICT test occurred at low frequency (specificity of 98.7%), and discrepancies with the Architect system corresponded to negative IgG values (7/767) or a gray-zone IgG value (1/767) and need complementary expertise with reference techniques. Therefore, in rare cases of discrepant results between the Architect and ICT tests, we systematically confirmed the results using the Toxoplasma IgGII Western blot assay.
Concerning IgM detection, the ICT test displayed outstanding sensitivity and specificity. This technique was similar to the Architect system in cases of seroconversion. Furthermore, for two cases the ICT test exhibited better sensitivity and specificity for IgM than the Architect technique (Table 6). The ICT test is very useful for detecting IgM without IgG, and as has been mentioned, it also can distinguish nonspecific IgM (i.e., natural IgM) from specific Toxoplasma IgM.
TABLE 6.
Comparison of results between Toxo IgM Architect and ICT from 50 sera (corresponding to 24 women) with seroconversion and 33 sera with nonspecific IgMa
| No. of sera tested | Architect IgM (AU/ml; range) | ICT (% positive) | IgM reference technique [Platelia or ISAgA (range)] | Architect IgG (IU/ml) | Conclusion |
|---|---|---|---|---|---|
| Proved seroconversion (n = 50) | 98 | ||||
| 1 (1 M) | 0.34 | Neg | Neg; 1 ISAgA (5) | 0 | Beginning of IgM increasing |
| 2 (2 M) | 0.39–0.39 | Pos | Pos; 2 ISAgA (9–9) | <0.2 | Beginning of IgM increasing |
| 14 (2 M, 12 SE) | 0.78–9.04 | Pos | Pos; 2 ISAgA (10–12); 12 Platelia (2.84–9.87) | <1.5 | IgM positive (all techniques) |
| 33 (19 M, 14SE) | 0.55–20.61 | Pos | Pos; 19 ISAgA (9–12); 14 Platelia (1.18–9.38) | ≥1.5 | Proved seroconversion |
| No IgG seroconversion (n = 33), no specific IgM | 6 | ||||
| 8 (8 SE) | 0.30–0.49 | Neg | Neg; 8 Platelia (0.10–0.78) | <0.2 | Absence of IgM |
| 2 (2 SE) | 0.5–0.59 | Neg | Neg; 2 Platelia (0.45–0.65) | <0.2 | Absence of IgM |
| 10 (4 M, 6 SE) | 0.63–1.08 | Neg | Neg; 4 ISAgA (0–2); 6 Platelia (0.23–0.78) | <0.2 | False-positive IgM Architect |
| 11 (7 M, 4 SE) | 0.76–1.80 | Neg | Pos; 7 ISAgA (9–11); 4 Platelia (1.30–2.17) | <0.2 | False-positive IgM Architect and reference technique |
| 2 (2 SE) | 0.69–3.96 | Pos | Pos; 2 Platelia (1.23–2.58) | 1, 0.2 | False-positive Architect, ICT, and reference technique |
The following cutoffs were used: Architect IgM (Abbott Diagnostics, Wiesbaden, Germany), 0.5 to 0.6 AU/ml; Platelia Toxo IgM (Bio-Rad, Marne la Coquette, France), 0.8 to 1.0; ISAgA IgM (bioMérieux, Marcy l'Etoile, France), 6 to 9.
To conclude, the Toxoplasma ICT IgG-IgM rapid test appears quite reliable. ICT sensitivity for IgG is equivalent to that of the reference technique (Toxoplasma IgGII Western blot analysis). ICT is also less expensive than the Western blot approach. The cost of ICT and automated techniques analyses (IgG and IgM) is on the order of $10, whereas it is about $30 for the IgGII Western blot. The ICT test can detect Architect false-positive IgG results and is capable of differentiating between nonspecific IgM and specific IgM in the majority of cases. In contrast, the reference immunocapture techniques (ISAgA and Platelia) often yielded false-positive results. In cases of positive IgM results, the serological follow-up 2 weeks apart is always performed to detect the possible appearance of IgG (11). Combined with the automated technique, the ICT test enables reliable orientation of the diagnostic results.
Lastly, the ICT IgG-IgM test could be promoted as a first-line technique in developing countries and could be particularly interesting for the early follow-up of pregnant women. This indication was also mentioned by Begeman et al. (9). Indeed, the simple determination of the serological status in early pregnancy compared to the serological status at birth seems simple and feasible in developing countries. This would allow to follow-up of the discrepant cases by targeting the cases requiring expertise, by limiting the cost of follow-up, and by increasing accessibility to screening through the simplicity of this technique (i.e., point of care). Obviously, in cases of serological status transition from negative to positive, these patients should be secondarily monitored in a reference laboratory.
MATERIALS AND METHODS
Serological samples.
The study was carried out with 1,002 serum samples from patients tested in the laboratories of the University Hospital of Saint-Etienne (Saint-Etienne, France) and the La Timone University Hospital (Marseille, France). Two panels were performed.
Panel 1 included 767 nonselected prospective sera, 356 from Marseille and 411 from Saint-Etienne. Panel 2 (Table 7) was comprised of 235 sera selected based on results obtained using the Architect system; the serum characteristics were the following. For panel 2.1, 92 sera were derived from patients with low IgG titers (0.6 to 3.4 IU/ml) without IgM. For panel 2.2, 20 sera with high IgG titers, which correspond to serological reactivation, were used. For panel 2.3, 50 samples from 24 patients with acute toxoplasmosis and seroconversion, which then showed IgG appearance, were used. For all of these patients, a previous serum test was negative for IgG and IgM. Seventeen sera had an IgG titer of ≤1.5 IU/ml, and 33 sera had an IgG titer of >1.5 IU/ml (Table 7). For panel 2.4, we used 33 sera from 33 patients with IgM values between 0.35 and 3.96 AU/ml without IgG and not succeeded by seroconversion. Systematic IgG follow-up was done 2 weeks and 1 month later and did not show IgG appearance or increasing of IgM (data not shown). These samples corresponded to 23 false-positive results (IgM of >0.6 AU/ml), 2 equivocal IgM results (0.5 < IgM < 0.6 AU/ml), and 8 negative results close to the threshold (0.3 < IgM < 0.5 AU/ml) using the Architect system. For panel 2.5, 11 false-positive IgG sera from 11 patients not confirmed by the reference technique IgGII Western blot were used. These cases are rare (10) and have been identified thanks to IgG complementary techniques (negative hemagglutination or negative indirect immunofluorescence) and Western blot analysis. The estimated frequency of these cases is 1/10,000. For panel 2.6, we used 29 sera with the absence of IgG and IgM anti-Toxoplasma gondii (reference techniques) and with positive serology (IgG and/or IgM) for cytomegalovirus (CMV), Epstein-Barr virus (EBV), and HIV and the presence of rheumatoid factor (RF), which could interfere with the specificity of the ICT Toxoplasma IgG and IgM tests in the assessment of patients with unrelated antibodies.
TABLE 7.
Distribution of 235 selected sera (panel 2) based on the results obtained using the Architect assay and corresponding to different critical serological status
| Panel | Serological status | Result with Architect and confirmatory techniques | No. of sera |
|---|---|---|---|
| 2.1 | Equivocal IgG titers (0.6–3.4 IU/ml) | No IgM | 92 |
| 2.2 | Serological reactivation | High IgG titers of >200 IU/ml | 20 |
| 2.3 | Seroconversion, acute toxoplasmosis | IgG of ≤1.5 IU/ml | 17 |
| IgG of >1.5 IU/ml | 33 | ||
| 2.4 | False-positive IgM | IgM between 0.35 and 0.59 AU/ml | 10 |
| IgM between 0.60 and 3.96 AU/ml | 23 | ||
| 2.5 | False-positive IgG | Negative IgGII Western blot | 11 |
| 2.6 | Crossing reactions with CMV, EBV, HIV, RF | Negative serology | 29 |
Methods and serological diagnostics panel.
In the serological diagnostics panel, diagnosis of T. gondii infection was previously established by testing the samples using routine techniques for the detection of anti-T. gondii IgG and IgM antibodies. In the two centers, IgG and IgM antibodies were determined using Architect Toxo IgG and IgM assays (Abbott Diagnostics, Wiesbaden, Germany) and, if necessary, an IgM immunosorbent agglutination assay (Toxo ISAgA IgM [bioMérieux, Mercy I'Etoile, France] or Platelia Toxo IgM [Bio-Rad, Marne la Coquette, France]) and LDBio Toxo II IgG immunoblotting (LDBio, Lyon, France).
Architect Toxoplasma IgG and IgM (Abbott) is a screening method for the serological diagnosis of toxoplasmosis used at the hospitals in Saint-Etienne and Marseille. The routinely used assay is based on chemiluminescent microparticle immunoassay (CMIA) technology (10). Specimens with IgG concentrations of ≥3.0 IU/ml are considered reactive for IgG antibodies to T. gondii, concentrations ranging from 1.6 to 2.9 IU/ml are considered the gray zone, and concentrations of <1.6 IU/ml are considered nonreactive. For IgM, reactive results were defined as index values of ≥0.60 AU/ml, gray-zone values ranged from 0.50 to 0.60 AU/ml, and nonreactive results were defined as index values of <0.50 AU/ml.
Reference techniques for IgM detection used in each center.
To confirm specificity for IgM, the following routine reference tests were used: the Platelia Toxo IgM (Bio-Rad, Marne la Coquette, France) in Saint-Etienne and the IgM immunosorbent agglutination assay (ISAgA; bioMérieux, Marcy l'Etoile, France) in Marseille. Both techniques, which are based on the immunocapture principle, can be considered reference tests due to their reliability and high sensitivity (12, 13). Platelia Toxo IgM has 97.9% sensitivity and 92.6% specificity and shows 97.6% concordance with ISAgA (6). In cases where IgM was detected without IgG, the secondary detection of IgG was monitored for serum classification (panel 2.3 or 2.4) (reference technique). Serological follow-up revealed the nature of IgM: specific IgM associated with IgG seroconversion, nonspecific IgM, or natural IgM.
Platelia Toxo IgM (Bio-Rad) is a qualitative test used for the detection of IgM antibodies against T. gondii via capture of IgM in the solid phase (the microplate wells are coated with anti-human μ chains). A mixture of antigens and the monoclonal anti-T. gondii antibody labeled with peroxidase is used as the conjugate. In the present study, values ranging from >0.8 to <1 were considered equivocal, while values of ≥1 were considered positive according to the manufacturer's instructions.
ISAgA-IgM (bioMérieux) is based on the agglutination of Toxoplasma antigens by specific IgM antibodies in the patient serum. The technique is based on a combination of two methods: direct agglutination and enzyme-linked immunosorbent assay (ELISA). The commercial kit Toxo-ISAgA (bioMérieux, Mercy I'Etoile, France) uses the IgM monoclonal antibody linked to the solid phase. In the absence of specific antibodies, Toxoplasma precipitates into wells. Agglutination occurs for positive reactions.
Assays were conducted as recommended by the manufacturers, and the cutoffs for the interpretations of the serologic values for adults are the following: positive, ≥9; negative, ≤6; equivocal, between 6 and 9. Those for infants are the following: positive, ≥3; negative, <3.
Reference technique for IgG.
The LDBio Toxoplasma IgGII Western blot test is an immunoenzymatic test that involves immunoblotting to nitrocellulose strips. After standardization, incubation with sera, and fixation of specific IgG onto the band, the anti-Toxoplasma IgG bound to the strip then is detected using an alkaline phosphatase-conjugated antibody and specific substrate. The resulting bands on the patient strip correspond to 30, 31, 33, 40, and 45 kDa (Fig. 2). A positive result is defined by the presence of at least three matching bands on the patient strip, including the specific band at 30 kDa.
The sensitivity and specificity of the Architect Toxo IgG and ICT tests were evaluated by comparing the results with those of the LDBio Toxoplasma IgGII Western blot assay, which is used as a reference confirmatory test for low titers of anti-Toxoplasma IgG (14).
New technique: Toxoplasma ICT IgG-IgM (LDBio Diagnostics).
Sera were tested by a rapid test in a second step. The test is comprised of a cassette with a nitrocellulose strip with a test band (T. gondii antigens) and a control band (rabbit gamma globulins), as well as a fiberglass support (conjugate pad) impregnated with red latex particles coupled to the toxoplasmic antigen (latex test, or T) and blue latex particles coupled with an anti-rabbit IgG goat antiserum (latex control, or C). The test consists of successively depositing a sample of serum (30 μl) and an eluting solution (3 drops of eluent) in the well provided for this purpose. The reading must be done between 20 and 30 min. The test was considered positive when 2 lines, T and C, appear in the corresponding areas.
ACKNOWLEDGMENTS
We are grateful to Sandra Moore for English language editing and the LDBio Diagnostics team for technical assistance.
This study was sponsored by LDBio Diagnostics. LDBio Diagnostics participated in the study design but did not interfere with the analysis or conclusions reported.
Toxoplasma gondii antigen as well as diverse parasite and fungal antigens sold to LDBio Diagnostics are produced at the institution where C. Mahinc, C. Guillerme, H. Raberin, and P. Flori are currently employed.
REFERENCES
- 1.Montoya J, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Roberts A, Hedman K, Luyasu V, Zufferey J, Bessières MH, Blatz RM, Candolfi E, Decoster A, Enders G, Gross U, Guy E, Hayde M, Ho-Yen D, Johnson J, Lécolier B, Naessens A, Pelloux H, Thulliez P, Petersen E. 2001. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 20:467–474. doi: 10.1007/PL00011289. [DOI] [PubMed] [Google Scholar]
- 3.Remington JS, Thulliez P, Montoya JG. 2004. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol 42:941–945. doi: 10.1128/JCM.42.3.941-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sensini A. 2006. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 12:504–512. [DOI] [PubMed] [Google Scholar]
- 5.Nogareda F, Le Strat Y, Villena I, De Valk H, Goulet V. 2014. Incidence and prevalence of Toxoplasma gondii infection in women in France, 1980-2020: model-based estimation. Epidemiol Infect 142:1661–1670. doi: 10.1017/S0950268813002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villard O, Cimon B, L'Ollivier C, Fricker-Hidalgo H, Godineau N, Houze S, Paris L, Pelloux H, Villena I, Candolfi E. 2016. Help in the choice of automated or semiautomated immunoassays for serological diagnosis of toxoplasmosis: evaluation of nine immunoassays by the French National Reference Center for Toxoplasmosis. J Clin Microbiol 54:3034–3042. doi: 10.1128/JCM.01193-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maudry A, Chene G, Chatelain R, Patural H, Bellete B, Tisseur B, Hafid J, Raberin H, Beretta S, Tran Manh Sung R, Belot G, Flori P. 2009. Bicentric evaluation of six anti-toxoplasma immunoglobulin G (IgG) automated immunoassays and comparison to the Toxo II IgG Western blot. Clin Vaccine Immunol 16:1322–1326. doi: 10.1128/CVI.00128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapey E, Wallon M, Peyron F. 2017. Evaluation of the LDBIO point of care test for the combined detection of toxoplasmic IgG and IgM. Clin Chim Acta Int J Clin Chem 464:200–201. doi: 10.1016/j.cca.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Begeman IJ, Lykins J, Zhou Y, Lai BS, Levigne P, El Bissati K, Boyer K, Withers S, Clouser F, Noble AG, Rabiah P, Swisher CN, Heydemann PT, Contopoulos-Ioannidis DG, Montoya JG, Maldonado Y, Ramirez R, Press C, Stillwaggon E, Peyron F, McLeod R. 2017. Point-of-care testing for Toxoplasma gondii IgG/IgM using Toxoplasma ICT IgG-IgM test with sera from the United States and implications for developing countries. PLoS Negl Trop Dis 11:e0005670. doi: 10.1371/journal.pntd.0005670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gay-Andrieu F, Fricker-Hidalgo H, Sickinger E, Espern A, Brenier-Pinchart M-P, Braun H-B, Pelloux H. 2009. Comparative evaluation of the ARCHITECT Toxo IgG, IgM, and IgG Avidity assays for anti-Toxoplasma antibodies detection in pregnant women sera. Diagn Microbiol Infect Dis 65:279–287. doi: 10.1016/j.diagmicrobio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Villard O, Cimon B, L'Ollivier C, Fricker-Hidalgo H, Godineau N, Houze S, Paris L, Pelloux H, Villena I, Candolfi E. 2016. Serological diagnosis of Toxoplasma gondii infection: recommendations from the French National Reference Center for Toxoplasmosis. Diagn Microbiol Infect Dis 84:22–33. doi: 10.1016/j.diagmicrobio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Meek B, van Gool T, Gilis H, Peek R. 2001. Dissecting the IgM antibody response during the acute and latent phase of toxoplasmosis. Diagn Microbiol Infect Dis 41:131–137. doi: 10.1016/S0732-8893(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 13.Liesenfeld O, Press C, Montoya JG, Gill R, Isaac-Renton JL, Hedman K, Remington JS. 1997. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J Clin Microbiol 35:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franck J, Garin YJ-F, Dumon H. 2008. LDBio-Toxo II immunoglobulin G Western blot confirmatory test for anti-Toxoplasma antibody detection. J Clin Microbiol 46:2334–2338. doi: 10.1128/JCM.00182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]