ABSTRACT
Edwardsiella spp. are responsible for significant losses in important wild and cultured fish species worldwide. Recent phylogenomic investigations have determined that bacteria historically classified as Edwardsiella tarda actually represent three genetically distinct yet phenotypically ambiguous taxa with various degrees of pathogenicity in different hosts. Previous recognition of these taxa was hampered by the lack of a distinguishing phenotypic character. Commercial test panel configurations are relatively constant over time, and as new species are defined, appropriate discriminatory tests may not be present in current test panel arrangements. While phenobiochemical tests fail to discriminate between these taxa, data presented here revealed discriminatory peaks for each Edwardsiella species using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) methodology, suggesting that MALDI-TOF can offer rapid, reliable identification in line with current systematic classifications. Furthermore, a multiplex PCR assay was validated for rapid molecular differentiation of the Edwardsiella spp. affecting fish. Moreover, the limitations of relying on partial 16S rRNA for discrimination of Edwardsiella spp. and advantages of employing alternative single-copy genes gyrB and sodB for molecular identification and classification of Edwardsiella were demonstrated. Last, sodB sequencing confirmed that isolates previously defined as typical motile fish-pathogenic E. tarda are synonymous with Edwardsiella piscicida, while atypical nonmotile fish-pathogenic E. tarda isolates are equivalent to Edwardsiella anguillarum. Fish-nonpathogenic E. tarda isolates are consistent with E. tarda as it is currently defined. These analyses help deconvolute the scientific literature regarding these organisms and provide baseline information to better facilitate proper taxonomic assignment and minimize erroneous identifications of Edwardsiella isolates in clinical and research settings.
KEYWORDS: Edwardsiella, MALDI-TOF, multiplex PCR, sequencing, FAME, aquaculture, gyrB, rep-PCR, sodB
INTRODUCTION
The Edwardsiella genus was first recognized in the 1960s to describe a group of isolates that did not fit within any known group of Enterobacteriaceae. Initially referred to simply as “bacterium 1483-1459,” this group included representatives of the “Bartholomew” group first isolated from a human patient with enteric fever and acute gastroenteritis (1) and possessed many similarities to the “Asakusa” group reported from snakes in Japan (2–4). Based on phenotypic differences between the 1483-1459 strains and other groups of Enterobacteriaceae, the genus was designated Edwardsiella and the species E. tarda was adopted to represent this previously undescribed group (5).
Prior to 2013, the genus consisted of only 3 taxa, E. tarda, E. ictaluri, and E. hoshinae (6), which represented a diverse group of Gram-negative bacteria infecting a wide range of piscine, reptilian, avian, and mammalian hosts (7). There are limited reports of E. hoshinae from a small number of avian and reptilian hosts (8, 9). Conversely, E. ictaluri is well studied as the causative agent of enteric septicemia of catfish (ESC) in catfish aquaculture in the southeastern United States (10). Although it is predominantly considered a pathogen of U.S. farm-raised channel catfish, reports have implicated E. ictaluri in mortality events in catfish aquaculture in Asia (11–14) and Pangasius catfish imported into the Caribbean (15). Moreover, E. ictaluri was reported from mortality events in tilapia pond culture in Central America, laboratory populations of zebrafish in the United States, and wild populations of ayu (Plecoglossus altivelis) in Japan (16–18).
Comparatively, Edwardsiella tarda is cited as the causative agent of edwardsiellosis in fish and has been reported from over 25 fish species and on all seven continents (7, 19, 20). It has also been isolated from reptiles, birds, and mammals; it has moderate zoonotic potential and is likely an opportunistic pathogen in young, elderly, and immunocompromised individuals (7, 21–25). Despite its wide host range, E. tarda has mostly been implicated in disease outbreaks in cultured fish and is considered one of the most important bacterial pathogens in global aquaculture (26–28).
Primarily viewed as a pathogen of marine and freshwater fish, E. tarda has extensive phenotypic and genetic diversity. In 2012, a comparative phylogenomic study demonstrated that isolates phenotypically identified as E. tarda comprised two genetically distinct, polyphyletic groups (29). This work was supported by concurrent investigations utilizing multilocus sequence analysis (MLSA) of E. tarda isolates in Asia and Europe, as well as genotypic and phenotypic analyses of E. tarda isolates from fish in the United States (30, 31). These studies concluded that isolates historically classified as E. tarda actually represented three genetically distinct yet phenotypically indistinguishable species. Further phenotypic characterization, DNA-DNA hybridization, and phylogenetic analyses led to the adoption of E. piscicida as a fourth member of the genus in 2013 (6). Expanding on these analyses, polyphasic phenotypic and genomic characterization of Edwardsiella isolates from diseased eels and other fishes led to the addition of a fifth species of Edwardsiella, E. anguillarum, in 2015 (32).
Prior to this recent segregation, research documenting phenotypic and genotypic diversity of E. tarda resulted in multiple generalized designations to account for the extensive intraspecific variability (28, 33–36). As a result, isolates primarily fell into one of three different categories: (i) typical motile fish-pathogenic E. tarda; (ii) atypical nonmotile fish-pathogenic E. tarda; and (iii) fish-nonpathogenic E. tarda (33, 37). The recent separation of E. tarda into three discrete taxa suggests that these designations likely correspond with the recent segregation (32, 38). The work described here employed routine phenotypic and genotypic analyses, coupled with popular microbial identification systems and molecular confirmatory methods, to evaluate current procedures for differentiating the Edwardsiella spp. and to link historical records and former E. tarda designations to current phylogenomic assignments and contemporary taxonomic nomenclature. In addition, the diverse collection of isolates provided a unique opportunity to characterize plasmids from a variety of temporally and geographically discrete congeners and identify potential commonalities in plasmids carried by Edwardsiella spp. from different origins.
RESULTS
Motility and TSI.
The motility and triple sugar iron medium (TSI) results for each isolate are listed in Table 1. The E. hoshinae isolate and all E. piscicida isolates were motile. Motility was also observed for the three E. ictaluri isolates, although dispersion was not as widespread. The observed motility of E. anguillarum and E. tarda isolates varied by isolate. All Edwardsiella isolates tested positive for glucose fermentation. No hydrogen sulfide production was observed in the E. hoshinae or E. ictaluri isolates; production from E. anguillarum isolates was weak. All E. piscicida and E. tarda isolates were positive for hydrogen sulfide production. Gas production was present in 6/7 E. anguillarum, 1/1 E. hoshinae, 0/3 E. ictaluri, 25/25 E. piscicida, and 10/11 E. tarda isolates.
TABLE 1.
Motility and TSI analysis of the isolates used in the current study
| Isolate | Motilitya,b | TSIb |
|---|---|---|
| E. anguillarum | ||
| EA181011 | − | K/A + gas + H2S (weak) |
| LADL05-105 | + | K/A + gas + H2S (weak) |
| 43472 | + (weak) | K/A + gas + H2S (weak) |
| 43664 | + (weak) | K/A + H2S (weak); no gas |
| 43473 | + | K/A + gas + H2S (weak) |
| 43659 | + | K/A + gas + H2S (weak) |
| 43651 | + | K/A + gas + H2S (weak) |
| E. hoshinae ATCC 35051 | + | A/A + gas |
| E. ictaluri | ||
| 11-149A | + (weak) | K/A |
| RUSVM-1 | + (weak) | K/A |
| S97-773 | + (weak) | K/A |
| E. piscicida | ||
| PB 07-309 | + | K/A + gas |
| NFAVS-1 | + | K/A + gas + H2S |
| Fr373.2 | + | K/A + gas + H2S |
| HL1.1 | + | K/A + gas + H2S |
| HL25.1 | + | K/A + gas + H2S |
| HL32.1 | + | K/A + gas + H2S |
| WFE1 | + | K/A + gas + H2S |
| S11-285 | + | K/A + gas + H2S |
| C1490 | + | K/A + gas + H2S |
| CMT 8211-1 | + | K/A + gas + H2S |
| REDS 81911-E | + | K/A + gas + H2S |
| RBR8.1 | + | K/A + gas + H2S |
| SC 09-03 | + | K/A + gas + H2S |
| ACC69.1 | + | K/A + gas + H2S |
| CAQ 8.10 | + | K/A + gas + H2S |
| CAQ 10.10 | + | K/A + gas + H2S |
| CAQ 3.9 | + | K/A + gas + H2S |
| A15-02670 | + | K/A + gas + H2S |
| 43628 | + | K/A + gas + H2S |
| 43662 | + | K/A + gas + H2S (weak) |
| 43644 | + | K/A + gas + H2S |
| 43475 | + | K/A + gas + H2S |
| 43658 | + | K/A + gas + H2S |
| 43468 | + | K/A + gas + H2S |
| 43656 | + | K/A + gas + H2S |
| E. tarda | ||
| Edwardsiella 9.1 | + | K/A + gas + H2S |
| Edwardsiella 9.2 | + | K/A + gas + H2S |
| Edwardsiella 9.3 | + | K/A + gas + H2S |
| Edwardsiella 9.4 | + | K/A + gas + H2S |
| FL95-01 | + | K/A + gas + H2S |
| 070720-1 3A | + | K/A + gas + H2S |
| 070720-1 2HLDOM | + | K/A + gas + H2S |
| 43657 | − | K/A + gas + H2S |
| 43650 | + | K/A + gas + H2S |
| 43627 | + | K/A + gas + H2S |
| 43663 | − | K/A + H2S |
+, positive result; −, negative result.
K/A, glucose fermentation only; A/A, glucose and lactose and/or sucrose fermentation; gas, gas production; H2S, sulfur reduction; (weak), positive result less robust than that observed in other samples.
Microbial identification systems.
The API 20E system correctly identified all PCR-confirmed E. tarda and E. hoshinae isolates with ≥99% confidence. The three E. ictaluri isolates from three different fish hosts all produced an identical API code, in line with previous reports (17, 18), which resulted in an identification of Escherichia coli with a 52.7% confidence level (CL). Of the E. piscicida isolates tested, 64% (16/25) were identified as E. tarda (CL, 96.7% to 99.9%). The remaining 36% (9/25) of E. piscicida isolates produced codes that were nondefinitive as they represented multiple species. Similarly, 29% (2/7) of E. anguillarum isolates produced nondefinitive ambiguous codes. Of the remaining E. anguillarum isolates, 4/7 (57%) were identified as E. tarda (CL, 96.7% to 99.4%) and 1/7 (14%) was identified as Vibrio parahaemolyticus (CL, 53.2%). API 20E results are consistent with those reported previously for Edwardsiella spp. (27, 33, 39) and can be found in Table 2.
TABLE 2.
Antimicrobial identification system results for isolates analyzed in the current studya
| Isolate | API 20E |
Biolog |
BBL Crystal Enteric/Nonfermentor |
MALDI-TOF |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Code | ID | CL (%) | ID | CL (%) | Code | ID | CL (%) | ID | CS | |
| E. piscicida | ||||||||||
| PB 07-309 | 6364000b | E. tarda | 65 | 2403010113 | E. tarda | 99.9 | E. tarda | 2.23 | ||
| NFAVS-1 | 6764000b | E. tarda | 81 | 2002010113 | E. tarda | 99.5 | E. tarda | 2.12 | ||
| Fr373.2 | 4744000 | E. tarda | 99.4 | E. tarda | 87 | 2003010113 | E. tarda | 99.2 | E. tarda | 2.18 |
| HL1.1 | 4744000 | E. tarda | 99.4 | E. tarda | 83 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.25 |
| HL25.1 | 4344000 | E. tarda | 99.4 | E. ictaluri | 67 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.21 |
| HL32.1 | 4744000 | E. tarda | 99.4 | E. tarda | 58 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.24 |
| WFE1 | 4764000 | E. tarda | 96.7 | E. tarda | 59 | 2003100113 | E. tarda | 98.7 | E. tarda | 2.23 |
| S11-285 | 4744000 | E. tarda | 99.4 | E. tarda | 95 | 2403110113 | E. tarda | 99.9 | E. tarda | 2.25 |
| C1490 | 6764000b | E. tarda | 68 | 2002000113 | E. tarda | 99.9 | E. tarda | 2.13 | ||
| CMT 8211-1 | 6764000b | E. tarda | 86 | 2003000113 | E. tarda | 99.7 | E. tarda | 2.24 | ||
| REDS 81911-E | 4764000 | E. tarda | 96.7 | E. tarda | 58 | 2003100113 | E. tarda | 98.7 | E. tarda | 2.21 |
| RBR8.1 | 6564000b | E. ictaluri | 62 | 2003000113 | E. tarda | 99.7 | E. tarda | 2.26 | ||
| SC 09-03 | 4764000 | E. tarda | 96.7 | E. tarda | 94 | 0403010113 | E. tarda | 99.9 | E. tarda | 2.20 |
| ACC69.1 | 6564000b | E. tarda | 62 | 2003000113 | E. tarda | 99.7 | E. tarda | 2.18 | ||
| CAQ 8.10 | 6565000b | E. ictaluri | 80 | 2002000113 | E. tarda | 99.9 | E. tarda | 2.22 | ||
| CAQ 10.10 | 4564000 | E. tarda | 97.4 | E. ictaluri | 81 | 2003000113 | E. tarda | 99.7 | E. tarda | 2.18 |
| CAQ 3.9 | 4544000 | E. tarda | 99.9 | E. ictaluri | 62 | 2002000113 | E. tarda | 99.9 | E. tarda | 2.25 |
| A15-02670 | 4344000 | E. tarda | 99.4 | E. tarda | 81 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.24 |
| 43628 | 4764000 | E. tarda | 96.7 | E. tarda | 83 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.24 |
| 43662 | 6364000b | E. tarda | 83 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.24 | ||
| 43644 | 6764000b | E. tarda | 74 | 2003100113 | E. tarda | 98.7 | E. tarda | 2.18 | ||
| 43475 | 4744000 | E. tarda | 99.4 | E. tarda | 62 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.28 |
| 43658 | 4744000 | E. tarda | 99.4 | E. ictaluri | 88 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.14 |
| 43468 | 6744000 | E. tarda | 99.4 | E. ictaluri | 69 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.23 |
| 43656 | 4344000 | E. tarda | 99.4 | E. ictaluri | 67 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.17 |
| E. anguillarum | ||||||||||
| EA181011 | 4744000 | E. tarda | 99.4 | E. tarda | 70 | 2002010113 | E. tarda | 99.5 | E. tarda | 2.15 |
| LADL05-105 | 4344100 | V. parahaemolyticus | 53.2 | E. ictaluri | 68 | 2403014113 | E. tarda | 99.9 | E. tarda | 2.17 |
| 43472 | 6744100 | E. tarda | 99.4 | E. tarda | 76 | 2003114113 | B. gladioli | 94.4 | E. tarda | 2.31 |
| 43664 | 4764000 | E. tarda | 96.7 | E. hoshinae | 69 | 2003114113 | B. gladioli | 94.4 | E. tarda | 2.29 |
| 43473 | 6744100 | E. tarda | 99.4 | E. ictaluri | 76 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.23 |
| 43659 | 6745100b | E. ictaluri | 97 | 2003114113 | B. gladioli | 94.4 | E. tarda | 2.20 | ||
| 43651 | 6345100b | E. ictaluri | 86 | 2003114113 | B. gladioli | 94.4 | E. tarda | 2.26 | ||
| E. hoshinae ATCC 35051 | 4744120 | E. hoshinae | 99.9 | E. hoshinae | 98 | 0443014013 | E. hoshinae | 99.9 | E. hoshinae | 2.26 |
| E. ictaluri | ||||||||||
| 11-149A | 4004000 | E. coli | 52.7 | E. ictaluri | 97 | 2003010023c | E. ictaluri | 2.31 | ||
| RUSVM-1 | 4004000 | E. coli | 52.7 | E. ictaluri | 72 | 2002000103c | E. ictaluri | 2.02 | ||
| S97-773 | 4004000 | E. coli | 52.7 | E. ictaluri | 70 | 2002000113 | E. tarda | 78.6 | E. ictaluri | 2.32 |
| E. tarda | ||||||||||
| Edwardsiella 9.1 | 4744000 | E. tarda | 99.4 | E. tarda | 62 | 2002000113 | E. tarda | 99.9 | E. tarda | 2.33 |
| Edwardsiella 9.2 | 4744000 | E. tarda | 99.4 | E. ictaluri | 67 | 0403110013 | E. tarda | 99.9 | E. tarda | 2.33 |
| Edwardsiella 9.3 | 4744000 | E. tarda | 99.4 | E. tarda | 94 | 0402000013 | E. tarda | 99.9 | E. tarda | 2.29 |
| Edwardsiella 9.4 | 4744000 | E. tarda | 99.4 | E. tarda | 71 | 2402000013 | E. tarda | 99.9 | E. tarda | 2.49 |
| FL95-01 | 4744000 | E. tarda | 99.4 | E. tarda | 96 | 2002010113 | E. tarda | 99.5 | E. tarda | 2.39 |
| 070720-1 3A | 6744000 | E. tarda | 99.4 | E. tarda | 72 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.43 |
| 070720-1 2HLDOM | 6744000 | E. tarda | 99.4 | E. tarda | 72 | 2002000113 | E. tarda | 99.9 | E. tarda | 2.26 |
| 43657 | 6744000 | E. tarda | 99.4 | E. tarda | 96 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.34 |
| 43650 | 4744000 | E. tarda | 99.4 | E. tarda | 96 | 2003110113 | E. tarda | 70.3 | E. tarda | 2.34 |
| 43627 | 4744000 | E. tarda | 99.4 | E. tarda | 96 | 2003010113 | E. tarda | 99.2 | E. tarda | 2.47 |
| 43663 | 6744000 | E. tarda | 99.4 | E. tarda | 94 | 2003010113 | E. tarda | 99.2 | E. tarda | 2.30 |
Abbreviations: CL, confidence level; CS, confidence score; ID, identification.
Unacceptable profile in API, multiple species identifications possible.
Profile not in BBL database, unable to provide an identification.
The Biolog microbial identification system identified all study isolates as members of Edwardsiella (E. hoshinae, E. ictaluri, or E. tarda) (Table 2) with various levels of confidence. The E. hoshinae isolate (CL, 98%) and the E. ictaluri isolates (CL, 70% to 97%) were both correctly identified. E. tarda isolate Edwardsiella 9.2 was identified as E. ictaluri (CL, 67%). All other E. tarda isolates (10 of 11; 91%) were identified in agreement with PCR results (CL, 62% to 96%). Similarly, E. piscicida isolates were identified as either E. tarda (17 of 25; 68%; CL, 58% to 95%) or E. ictaluri (8 of 25; 32%; CL, 62% to 88%). The E. anguillarum isolates also generated multiple codes, resulting in identifications of E. ictaluri (4/7; 57%; CL, 68% to 97%), E. tarda (2/7; 29%; CL, 70% to 76%), or E. hoshinae (1/7; 14%; CL, 69%).
The BBL Crystal Enteric/Nonfermentor identification kit also correctly identified the E. hoshinae isolate (CL, 99.9%) and all E. tarda isolates (CL, 70.3% to 99.9%). Of the three E. ictaluri isolates, only S97-773 (isolated from a diseased catfish) produced a code present in the BBL database, which identified it as E. tarda (78.6%). The E. piscicida isolates produced a variety of codes, all resulting in an identification of E. tarda from the BBL database with confidence levels ranging between 70.3% and 99.9%. Of the seven E. anguillarum isolates, four (57%) produced identical codes, which resulted in an identification of Burkholderia gladioli (CL, 94.4%). The remaining three E. anguillarum isolates produced similar codes resulting in an identification of E. tarda (CL, 70.3% to 99.9%). BBL Crystal codes are consistent with those reported previously for Edwardsiella (31, 38) and listed in Table 2.
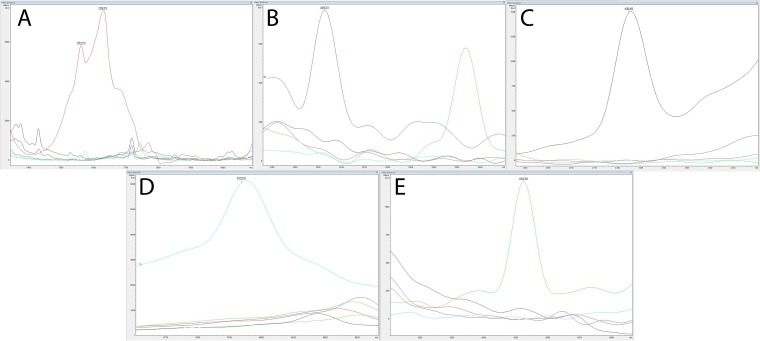
The matrix-assisted laser desorption ionization (MALDI) identification score for each isolate, based on the Bruker Biotyper RTC v. 3.1 and microbial peptide mass spectrum database V5.0.0.0 (Bruker Daltonics, Billerica, MA), is displayed in Table 2. The Bruker MALDI-time of flight (TOF) method correctly identified all the E. tarda, E. ictaluri, and E. hoshinae isolates examined with an identification score above 2.0. All E. piscicida and E. anguillarum isolates tested were identified as Edwardsiella tarda with a score above 2.0. However, unique species-specific peptide mass peaks (m/z) at 7,628, 8,793, and 4,252 were observed in the spectral profiles for E. anguillarum, E. piscicida, and E. tarda, respectively (Fig. 1).
FIG 1.
Unique peptide mass peaks generated from E. anguillarum (red) (A), E. hoshinae (purple) (B), E. ictaluri (black) (C), E. piscicida (blue) (D), and E. tarda (green) (E) using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) technology.
Fatty acid methyl ester (FAME) analysis.
The major fatty acid constituents of the Edwardsiella isolates were 14:0, 16:0, 17:0 cyclo, summed feature 3 (16:1 w7c/16:1 w6c and 16:1 w6c/16:1 w7c), and summed feature 8 (18:1 w7c and 18:1 w6c). Fatty acid analysis results are displayed in Table 3.
TABLE 3.
Mean percent fatty acid composition for Edwardsiella spp. analyzed in the current study
| Fatty acid | Mean (SD) fatty acid content of species: |
||||
|---|---|---|---|---|---|
| E. hoshinaea | E. ictaluri | E. piscicida | E. anguillarum | E. tarda | |
| 12:0 | 1.80 | 1.62 (0.08) | 0.97 (0.18) | 1.05 (0.16) | 2.45 (0.18) |
| 13:0 | 2.21 | 0.41 (0.15) | 1.23 (0.49) | 0.67 (0.18)b | 0.69 (0.22) |
| 14:0 | 9.41 | 11.15 (0.40) | 15.06 (1.13) | 14.10 (1.01) | 11.22 (0.98) |
| 16:0 | 22.51 | 28.29 (2.07) | 26.65 (1.49) | 30.83 (1.91) | 29.24 (0.85) |
| 17:0 cyclo | 11.12 | 8.84 (3.91) | 21.47 (5.02) | 15.07 (3.11) | 13.83 (2.79) |
| 17:0 | 1.79 | 0.48 (0.17) | 1.24 (0.36) | 0.72 (0.37) | 0.86 (0.27) |
| 18:1 w9c | 1.14 | 1.57 (0.16) | 1.47 (0.22) | 1.36 (0.16) | 1.52 (0.12) |
| 18:0 | 1.24 | 1.65 (0.44) | 1.12 (0.21) | 1.10 (0.17) | 1.28 (0.16) |
| 19:0 cyclo w8c | 0.61 | 2.02 (1.44) | 1.12 (0.50) | 1.37 (0.40) | 1.52 (0.44) |
| Summed feature 2 | 4.00 | 4.57 (0.52) | 4.83 (0.46) | 4.54 (0.35) | 4.35 (0.26) |
| Summed feature 3 | 27.40 | 31.07 (3.58) | 15.68 (5.70) | 22.89 (4.29) | 23.71 (3.43) |
| Summed feature 5 | 0.90 | 1.28 (0.08) | 1.10 (0.21) | 0.85 (0.16) | 0.80 (0.06) |
| Summed feature 8 | 11.75 | 5.67 (1.97) | 5.99 (1.01) | 4.54 (0.56) | 7.61 (1.09) |
Standard deviation could not be calculated; only 1 E. hoshinae isolate was analyzed.
Fatty acid was present in only 5 of 7 E. anguillarum isolates analyzed.
Antimicrobial susceptibility profiles.
The MICs of 39 antimicrobial compounds were tested for all 47 Edwardsiella isolates in the current study, resulting in a range of intraspecific and interspecific variation for each antimicrobial compound (Tables 4 and 5). However, no discriminatory antimicrobial compound was identified. For many of the carbapenems, cephalosporins, and macrolides, the MICs for different isolates within each Edwardsiella species were largely consistent. Greater variation among MICs was present for aminoglycosides and tetracyclines. The susceptibility of E. piscicida isolates to amoxicillin was more variable than that of the other Edwardsiella spp., with MICs ranging from ≤0.5 to 4 mg/liter. Similarly, the patterns of susceptibility of E. anguillarum isolates to penicillin displayed a greater degree of intraspecific variation than did those of the other Edwardsiella spp. The antimicrobial susceptibility profiles generated from this analysis were generally consistent with the putative antibiotic resistance function of plasmid-carried open reading frames (ORFs) (see Fig. 5, 6, and 7 and also Tables S1 and S2 in the supplemental material).
TABLE 4.
Antimicrobial susceptibilities to single compounds of Edwardsiella isolates analyzed in the current studya
| Antibiotic (range, mg/liter) | Taxon | No. of strains with MIC (mg/liter): |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ≥256 | ||
| Aminoglycosides | |||||||||||
| Amikacin (8–32) | All strains | 47 | |||||||||
| Gentamicin (0.5–8) | E. anguillarum | 5 | 1 | ||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 2 | 1 | |||||||||
| E. piscicida | 23 | 2 | |||||||||
| E. tarda | 5 | 6 | |||||||||
| Neomycin (2–32) | All strains | 47 | |||||||||
| Spectinomycin (8–64) | E. anguillarum | 7 | |||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 1 | 1 | 1 | ||||||||
| E. piscicida | 22 | 3 | |||||||||
| E. tarda | 8 | 2 | 1 | ||||||||
| Streptomycin (8–1,024) | All strains | 45 | 1 | 1 | |||||||
| Tobramycin (2–8) | All strains | 47 | |||||||||
| Carbapenems | |||||||||||
| Doripenem (0.5–4) | All strains | 47 | |||||||||
| Ertapenem (0.25–8) | All strains | 47 | |||||||||
| Imipenem (0.5–8) | All strains | 47 | |||||||||
| Meropenem (0.5–8) | All strains | 47 | |||||||||
| Cephalosporins | |||||||||||
| Cefepime (4–32) | All strains | 47 | |||||||||
| Cefazolin (1–16) | E. anguillarum | 4 | 3 | ||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 13 | 10 | 2 | ||||||||
| E. tarda | 9 | 2 | |||||||||
| Ceftazidime (1–16) | All strains | 46 | 1 | ||||||||
| Ceftiofur (0.25–4) | All strains | 47 | |||||||||
| Ceftriaxone (0.5–32) | All strains | 47 | |||||||||
| Macrolides | |||||||||||
| Erythromycin (0.12–4) | E. anguillarum | 1 | 6 | ||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 1 | 24 | |||||||||
| E. tarda | 1 | 10 | |||||||||
| Tylosin tartrate (2.5–20) | All strains | 1 | 1 | 45 | |||||||
| Penicillins | |||||||||||
| Amoxicillin (0.25–16) | E. anguillarum | 1 | 6 | ||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 1 | 9 | 11 | 4 | |||||||
| E. tarda | 10 | 1 | |||||||||
| Ampicillin (8–16) | All strains | 47 | |||||||||
| Penicillin (0.06–8) | E. anguillarum | 1 | 3 | 2 | 1 | ||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 2 | 1 | |||||||||
| E. piscicida | 4 | 10 | 11 | ||||||||
| E. tarda | 6 | 3 | 2 | ||||||||
| Piperacillin (16–32) | All strains | 46 | 1 | ||||||||
| Quinolones | |||||||||||
| Ciprofloxacin (0.5–2) | All strains | 46 | 1 | ||||||||
| Enrofloxacin (0.12–2) | All strains | 46 | 1 | ||||||||
| Levofloxacin (1–8) | All strains | 47 | |||||||||
| Tetracyclines | |||||||||||
| Minocycline (1–8) | E. anguillarum | 1 | 3 | 3 | |||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 5 | 10 | 6 | 1 | 3 | ||||||
| E. tarda | 11 | ||||||||||
| Oxytetracycline (0.25–8) | E. anguillarum | 2 | 4 | 1 | |||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 10 | 8 | 2 | 1 | 4 | ||||||
| E. tarda | 2 | 6 | 1 | 2 | |||||||
| Tetracycline (0.25–8) | E. anguillarum | 2 | 5 | ||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 2 | 1 | |||||||||
| E. piscicida | 18 | 2 | 1 | 4 | |||||||
| E. tarda | 6 | 3 | 2 | ||||||||
| Other | |||||||||||
| Aztreonam (1–16) | All strains | 46 | 1 | ||||||||
| Clindamycin (0.5–4) | E. anguillarum | 1 | 6 | ||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 1 | 5 | 19 | ||||||||
| E. tarda | 7 | 4 | |||||||||
| Florfenicol (1–8) | All strains | 47 | |||||||||
| Nitrofurantoin (32–64) | All strains | 46 | 1 | ||||||||
| Novobiocin (0.5–4) | E. anguillarum | 2 | 5 | ||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 1 | 2 | |||||||||
| E. piscicida | 1 | 5 | 19 | ||||||||
| E. tarda | 3 | 3 | 1 | 1 | 3 | ||||||
| Sulfadimethoxine (32–256) | E. anguillarum | 7 | |||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 25 | ||||||||||
| E. tarda | 4 | 1 | 6 | ||||||||
| Sulfathiazole (32–256) | E. anguillarum | 7 | |||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 25 | ||||||||||
| E. tarda | 1 | 1 | 6 | ||||||||
| Tigecycline (1–8) | E. anguillarum | 6 | 1 | ||||||||
| E. hoshinae | 1 | ||||||||||
| E. ictaluri | 3 | ||||||||||
| E. piscicida | 22 | 3 | |||||||||
| E. tarda | 11 | ||||||||||
Antimicrobial susceptibilities were determined by the broth microdilution method using the Sensititre GN4F and Avian1F plates, according to the manufacturer's protocol. Numbers in the lowest concentration of the antibiotic represent the maximal MIC at this concentration. An MIC higher than the highest concentration tested is cited in the subsequent higher concentration step.
TABLE 5.
Antimicrobial susceptibilities to combinatory compounds of Edwardsiella isolates analyzed in the current studya
| Antibiotic and MIC (mg/liter) | No. of strains with MIC by taxon: |
|||||
|---|---|---|---|---|---|---|
| All strains | E. anguillarum | E. hoshinae | E. ictaluri | E. piscicida | E. tarda | |
| Ampicillin-sulbactam | ||||||
| ≤4/2 | 47 | |||||
| 8/4 | ||||||
| ≥16/8 | ||||||
| Trimethoprim-sulfamethoxazole | ||||||
| ≤0.5/9.5 | 4 | 1 | 1 | 10 | ||
| 1/19 | ||||||
| 2/38 | 2 | 9 | ||||
| ≥4/76 | 1 | 8 | 1 | |||
| Ticarcillin-clavulanic acid | ||||||
| ≤8/2 | 47 | |||||
| 16/2 | ||||||
| 32/2 | ||||||
| ≥64/2 | ||||||
| Piperacillin-tazobactam | ||||||
| ≤8/4 | 47 | |||||
| 16/4 | ||||||
| 32/4 | ||||||
| 64/4 | ||||||
| ≥128/4 | ||||||
Antimicrobial susceptibilities were determined by the broth microdilution method using the Sensititre GN4F and Avian1F plates, following the manufacturer's protocol. Numbers in the lowest concentration of the antibiotic represents the maximal MIC at this concentration. An MIC higher than the highest concentration tested is cited in the subsequent higher concentration step.
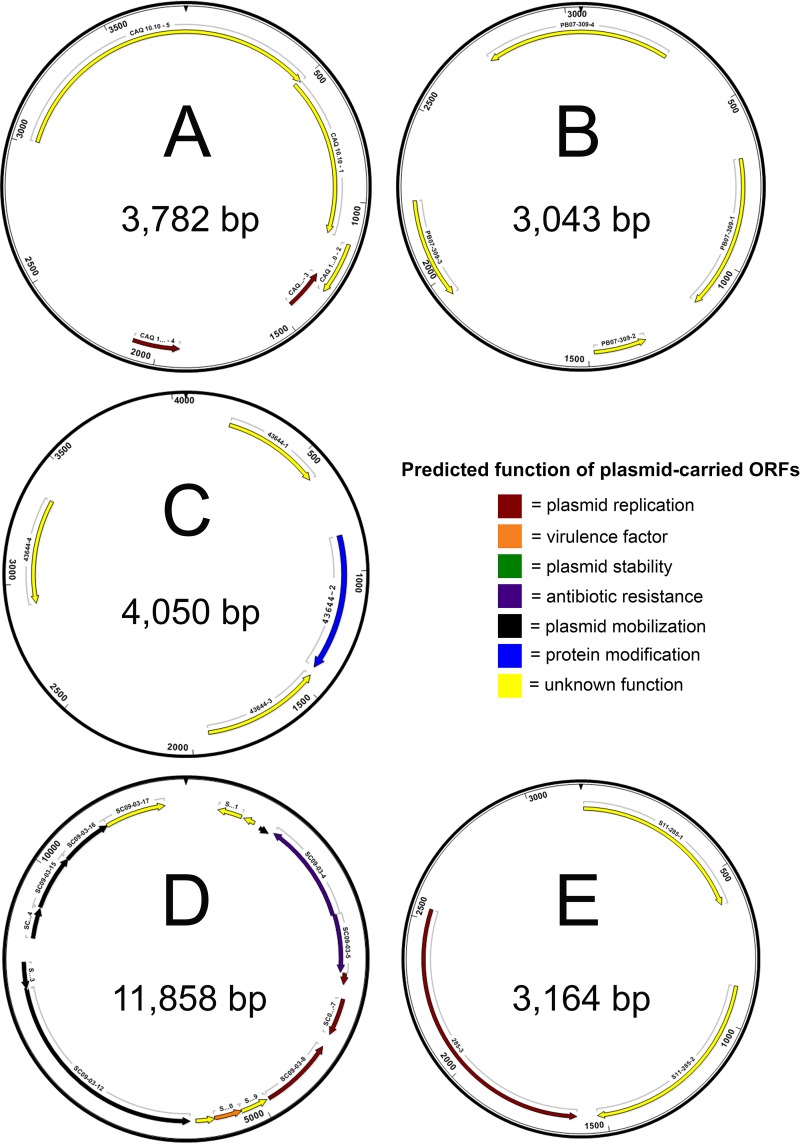
FIG 5.
Physical maps of complete nucleotide sequences of plasmids harvested from E. piscicida isolates CAQ 10.10, CAQ 3.9, HL1.1, HL25.1, HL32.1, ACC69.1, CAQ 8.10, Fr373.2, and RBR8.1 (A), PB 07-309 (B), 43644 (C), SC 09-03 (D), and S11-285 (E). Maps indicate locations of predicted open reading frames (ORFs), which are color coded according to predicted function. Predicted products and putative functions of ORFs are provided in Table S1.
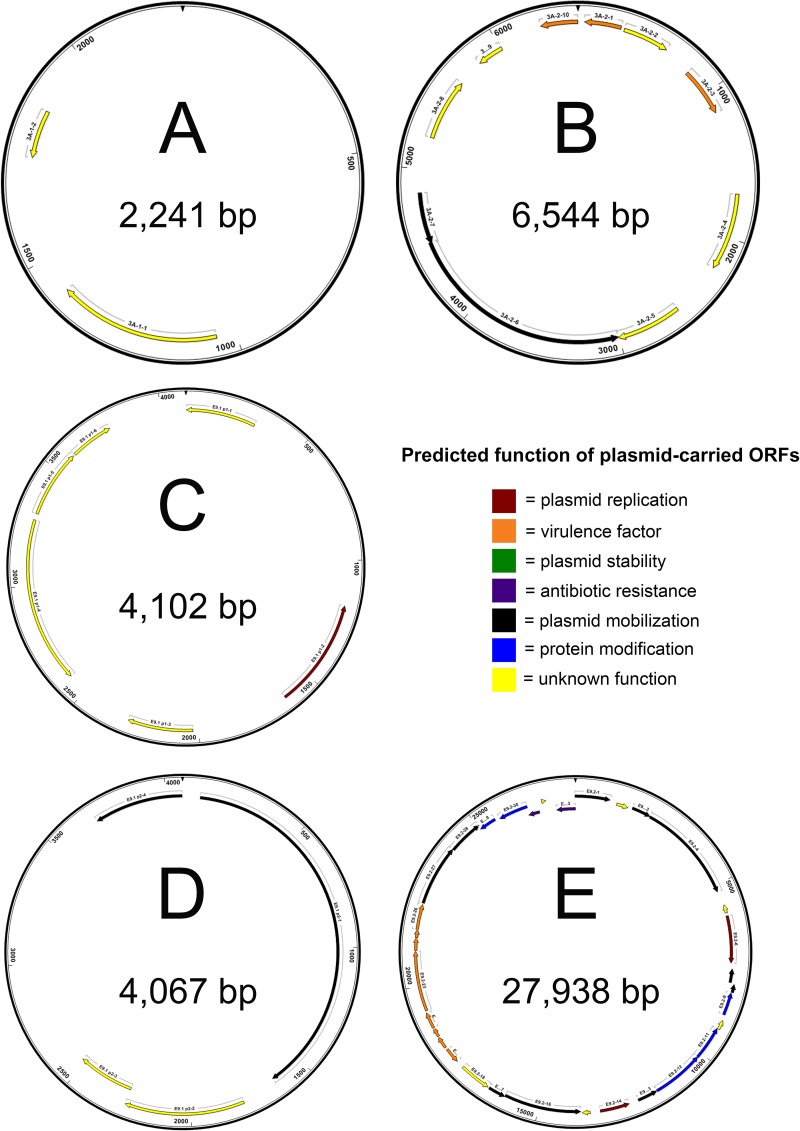
FIG 6.
Physical maps of complete nucleotide sequences of plasmids harvested from E. tarda isolates 070720-1 3A (A and B), Edwardsiella 9.1 (C and D), and Edwardsiella 9.2 (E). Maps indicate locations of predicted open reading frames (ORFs), which are color coded according to predicted function. Predicted products and putative functions of ORFs are provided in Table S2.
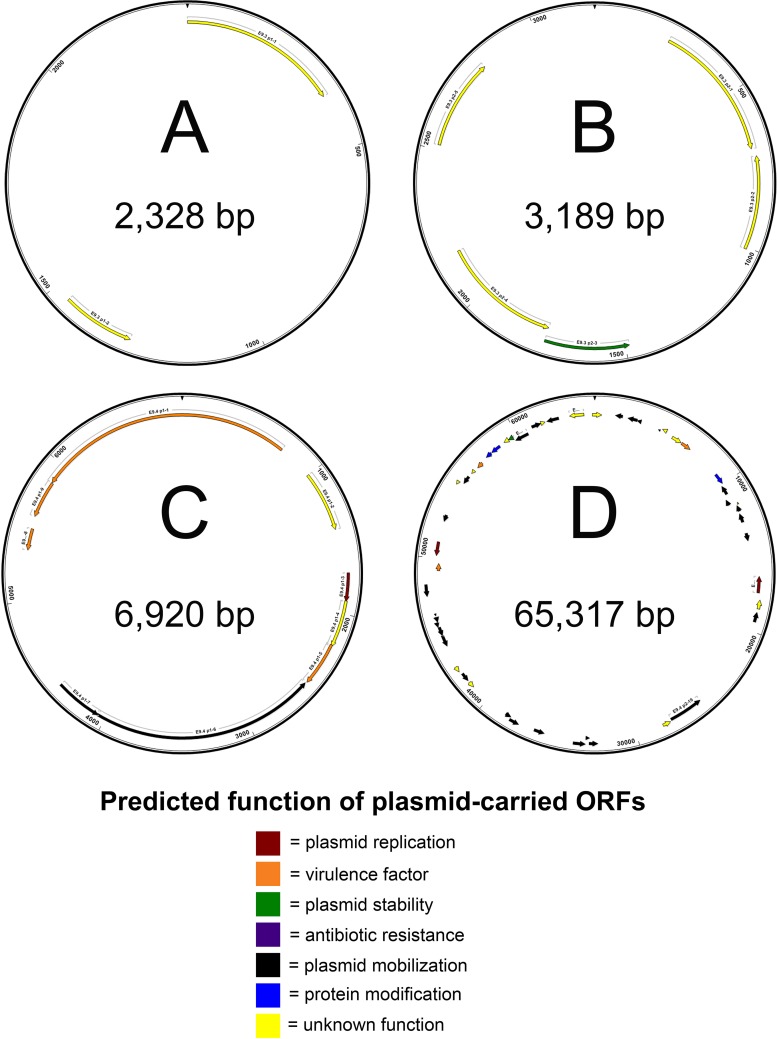
FIG 7.
Physical maps of complete nucleotide sequences of plasmids harvested from E. tarda isolates Edwardsiella 9.3 (A and B) and Edwardsiella 9.4 (C and D). Maps indicate locations of predicted open reading frames (ORFs), which are color coded according to predicted function. Predicted products and putative functions of ORFs are provided in Table S2.
Phylogenetic analysis.
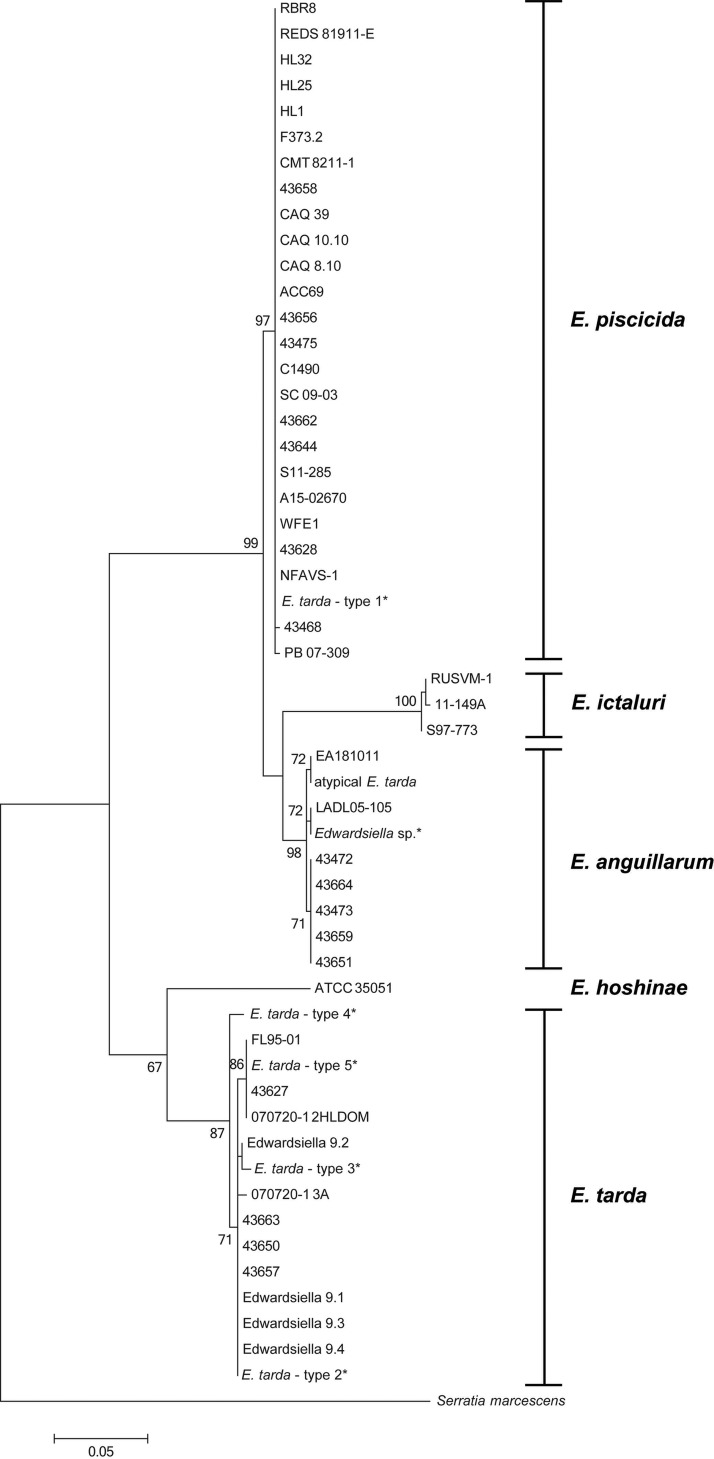
Within groups, partial 16S rRNA sequences (1,062 bp) displayed high intraspecific similarity (99.90% to 100%). However, 16S rRNA had low discriminatory power among Edwardsiella congeners, with 99.15% to 99.91% interspecific similarity among them (Table 6; Fig. S1). Conversely, gyrB (1,800 bp) and sodB (461 bp) displayed high discriminatory power among Edwardsiella congeners (84.02% to 95.88% and 83.95% to 97.16%, respectively) while at the same time maintaining high intraspecific similarity (99.47% to 100% and 99.72% to 100%, respectively) (Fig. 2 and Table 6; also Fig. S2). E. anguillarum and E. piscicida shared the highest similarity with one another, with 95.88% at gyrB and 97.16% at sodB. Conversely, E. hoshinae and E. ictaluri were the most divergent, with 84.02% identity at gyrB and 83.95% at sodB. Intragenomic 16S rRNA gene heterogeneity for each Edwardsiella species ranged from 0.0 to 0.6% (Table 7).
TABLE 6.
Percent similarity matrix between Edwardsiella spp. across 1,062 bp of the 16S rRNA locus, 1,800 bp of the gyrB locus, and 461 bp of the sodB locus
| Locus and species | % similarity for species: |
||||
|---|---|---|---|---|---|
| E. anguillarum | E. hoshinae | E. ictaluri | E. piscicida | E. tarda | |
| 16S rRNA | |||||
| E. anguillarum | 100.00 | ||||
| E. hoshinae | 99.15 | 100.00 | |||
| E. ictaluri | 99.91 | 99.25 | 100.00 | ||
| E. piscicida | 99.72 | 99.05 | 99.63 | 99.99 | |
| E. tarda | 99.34 | 99.81 | 99.26 | 99.24 | 99.90 |
| gyrB | |||||
| E. anguillarum | 99.94 | ||||
| E. hoshinae | 84.05 | 100.00 | |||
| E. ictaluri | 94.61 | 84.02 | 99.73 | ||
| E. piscicida | 95.88 | 84.72 | 94.82 | 99.78 | |
| E. tarda | 85.02 | 88.86 | 84.70 | 85.81 | 99.47 |
| sodB | |||||
| E. anguillarum | 99.81 | ||||
| E. hoshinae | 86.86 | 100.00 | |||
| E. ictaluri | 92.56 | 83.95 | 99.81 | ||
| E. piscicida | 97.16 | 86.99 | 92.39 | 99.97 | |
| E. tarda | 88.54 | 91.38 | 86.16 | 89.12 | 99.72 |
FIG 2.
Phylogenetic relationships of Edwardsiella spp. based on sodB gene sequence. Relatedness was inferred from the maximum likelihood method based and rooted at Serratia marcescens. The percentage of replicate trees in which the associated sequences clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. The isolates described by Yamada and Wakabayashi (33) are marked with asterisks.
TABLE 7.
Intragenomic heterogeneity of 16S rRNA for representative Edwardsiella genomes
| Isolate | No. of 16S copies in genome | No. of differences (bp) | Dissimilarity range (%) |
|---|---|---|---|
| E. anguillarum LADL05-105 | 9 | 0–4 | 0.0–0.3 |
| E. hoshinae ATCC 35051 | 9 | 0–3 | 0.0–0.2 |
| E. ictaluri 93-146 | 8 | 0–3 | 0.0–0.2 |
| E. piscicida S11-285 | 10 | 0–6 | 0.0–0.4 |
| E. tarda FL95-01 | 9 | 0–9 | 0.0–0.6 |
Genetic fingerprinting.
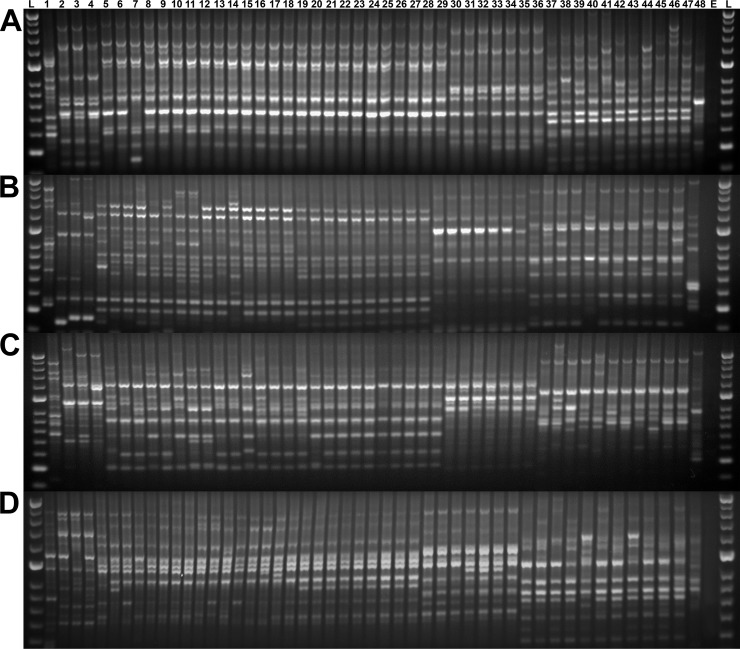
Similar to the phylogenetic analysis, repetitive extragenic palindromic PCR (rep-PCR) profiles for Edwardsiella isolates formed five distinct clusters representing the five taxa of Edwardsiella, regardless of primer set. Of the four primer sets evaluated, the BOX and GTG5 primers demonstrated the smallest amount of intraspecific variability (Fig. 3), with the BOX primer generating the most consistent patterns within groups. UPGMA (unweighted pair group method using arithmetic averages) analysis based on the BOX primer placed these five clusters within two larger phylogroups. In line with previous reports, E. piscicida, E. anguillarum, and E. ictaluri formed one cluster, and the other group contained E. tarda and E. hoshinae isolates. The genetic profiles of E. anguillarum, E. ictaluri, and E. piscicida all shared greater than 90% similarity within their respective taxa. The profiles of E. tarda isolates demonstrated the greatest intraspecific variability, with 60% to 96.4% similarity among isolates (Fig. S3).
FIG 3.
Genetic fingerprints of Edwardsiella spp. generated from repetitive extragenic palindromic PCR amplification of gDNA from Edwardsiella hoshinae (lane 1), E. ictaluri (lanes 2 to 4), E. piscicida (lanes 5 to 29), E. anguillarum (lanes 30 to 36), and E. tarda (lanes 37 to 47) using E. coli as an outlier (ATCC 25922, lane 48), a no-template control (lane E), and concurrently run standards (Hyperladder 50 bp, lanes L). Genetic profiles were generated using BOX (A), ERIC I and II (B), ERIC II (C), and GTG5 (D) primers.
mPCR.
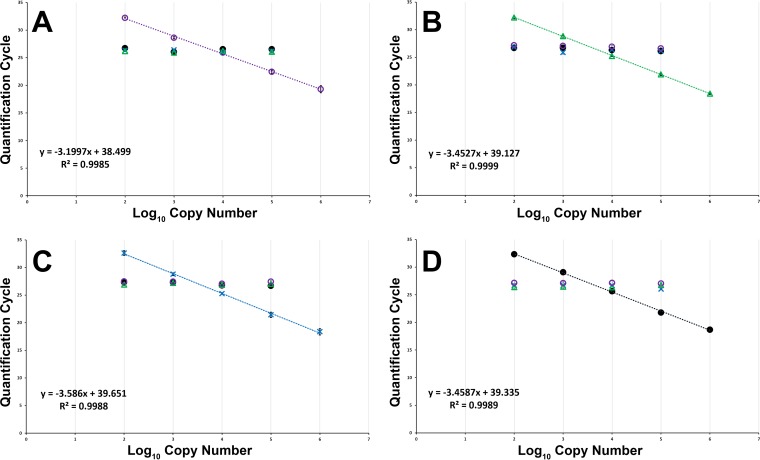
The multiplex real-time PCR (mPCR) assay was repeatable and reproducible, with linear dynamic ranges covering at least 5 orders of magnitude. Disproportionately large quantities of nontarget DNA had no marked effect on amplification efficiency; dilution curves and amplification plots were comparable when run with each Edwardsiella target genomic DNA (gDNA) alone or in the presence of nontarget gDNA (Table 8; Fig. 4) with a quantifiable limit of ∼100 copies of target DNA. Reaction efficiencies were calculated (40) from the slope of the log-linear portion of the serial 10-fold dilutions for each Edwardsiella species and were within the generally accepted range of 90% to 110% (41).
TABLE 8.
Specificity of the mPCR assay for each respective targeta
| Species | Mean (SD) Cq |
||||
|---|---|---|---|---|---|
| gDNA alone from species: |
All Edwardsiella gDNA mixed together | ||||
| E. anguillarum | E. ictaluri | E. piscicida | E. tarda | ||
| E. anguillarum | 23.28 (0.07) | — | — | — | 23.21 (0.09) |
| E. ictaluri | — | 22.61 (0.10) | — | — | 22.49 (0.05) |
| E. piscicida | — | — | 22.63 (0.15) | — | 22.50 (0.09) |
| E. tarda | — | — | — | 22.93 (0.13) | 22.50 (0.12) |
The user-defined fluorescence threshold for Cq determination was set at 50 relative fluorescence units. Dashes indicate no amplification of DNA.
FIG 4.
Mean quantification cycles (Cq) for known serial 10-fold dilutions of E. anguillarum (○) (A), E. ictaluri (△) (B), E. piscicida (×) (C), and E. tarda (●) (D). A dilution series for each assay was performed in the presence of an equal mixture of ∼10,000 copies of each nontarget Edwardsiella sp. gDNA. Error bars indicate standard deviations generated from samples run in triplicate on 3 separate plates. The user-defined baseline threshold for Cq determination was set at 50 relative fluorescence units for all runs.
Plasmid analysis.
Twenty-one (45%) of the Edwardsiella isolates carried plasmids. Summaries of open reading frames and the putative functions of their predicted proteins from E. piscicida and E. tarda plasmids can be found in Tables S1 and S2. Physical maps of isolated plasmids are available in Fig. 5 to 7. Each of the three E. ictaluri isolates carried two plasmids, consistent with previous reports for these isolates (42). Nine of the E. piscicida isolates (Fr373.2, HL1.1, HL25.1, HL32.1, RBR8.1, ACC69.1, CAQ 8.10, CAQ 10.10, and CAQ 3.9) recovered from turbot between 2005 and 2012 in Europe shared an identical plasmid of 3,782 bp. Four additional E. piscicida isolates (PB 07-309, S11-285, SC 09-03, and 43644) from various hosts carried plasmids of different sizes and compositions. The 3,164-bp plasmid identified in isolate S11-285 was in agreement with previous reports (43). Several E. tarda isolates carried two plasmids: 070720 3A (2,241 and 6,544 bp), Edwardsiella 9.1 (4,102 and 4,067 bp), Edwardsiella 9.3 (2,328 and 3,189 bp), and Edwardsiella 9.4 (6,920 and 65,317 bp). Additionally, E. tarda isolate Edwardsiella 9.2 carried one plasmid of 27,938 bp. No plasmids were detected in any of the E. anguillarum isolates or E. hoshinae isolate ATCC 35051, consistent with previous reports (44–46). Alignment of circularized plasmids mapped in this study revealed a variety of locally colinearized blocks (LCBs) shared between some isolates that also matched several Edwardsiella plasmid sequences present in GenBank (data not shown). However, plasmids varied widely in content and arrangement and a conserved LCB or collection of LCBs indicative of a stable plasmid backbone present in all Edwardsiella plasmids was not identified.
DISCUSSION
Recent investigations into the phenotypic and genotypic variation of E. tarda have led to the recognition that isolates previously classified as E. tarda actually represent three distinct taxa: E. tarda, E. piscicida, and E. anguillarum. In light of these findings, the current study was intended to characterize the five Edwardsiella species using common phenotypic and genotypic analyses and demonstrate the importance of updating microbial identification systems to reflect contemporary systematics.
Previous work demonstrated variations in biochemical profiles of Edwardsiella isolates from different fish hosts and geographic origins (31, 38, 47). The work described here is consistent with these previous studies, with extant intraspecific phenotypic variation within some groups. This is not surprising, given the diversity of fish hosts and the broad geographic distribution and wide temporal range of these isolates. Marked inter- and intraspecific variation was also present in fatty acid content; however, no discriminatory fatty acid was identified, in line with previous findings (31). Similarly, antimicrobial susceptibility profiles were variable within groups and no discriminatory antibiotic agent was identified. Although conventional phenotypic methods are user friendly and relatively inexpensive, certain groups of bacteria are difficult to identify using conventional techniques, specifically rare isolates or isolates with ambiguous profiles (48).
The four microbial identification systems in this study correctly identified the Edwardsiella taxa that are recognized and validated for each respective system. However, none of the four databases associated with the systems used here recognize E. anguillarum or E. piscicida. The increasing use of molecular techniques and the growing number of new bacterial taxa identified using genomics technology pose a problem for phenotype database management, resulting in prokaryote databases that lag behind evolving systematics (49). Moreover, commercial test panel configurations are relatively constant over time, and as new species are defined, more appropriate discriminatory metabolic phenotypic tests may not be present in current test panel arrangements (50). Furthermore, many microbial identification databases still consider 16S rRNA the gold standard for taxon identification (50), the limitations of which are discussed below.
Within the species formerly classified as E. tarda, no distinct phenotypic patterns emerged among API 20E and BBL Crystal codes. In addition, no confirmative identifying profile was apparent using the Biolog microbial identification system. It is worth noting, however, that intraspecific variation in phenotypic characters was noted within Edwardsiella species. This is consistent with previous work that failed to identify a discriminatory metabolic fingerprint to differentiate among different E. tarda phylogroups (31). This suggests that isolates identified phenotypically as E. tarda, regardless of the identification system employed, require supplemental confirmation. In light of these findings, and given the rapidly increasing number of representative Edwardsiella genomes available, further work establishing a discriminatory metabolic profile for each Edwardsiella species is warranted.
Similarly, the role of 16S rRNA sequence for differentiation of the Edwardsiella species has recently been called into question (20, 31, 38, 42). The utility of 16S rRNA for bacterial identification has long been a topic of debate, largely due to the high percentage of sequence similarity between closely related species, the lack of a definitive intraspecific dissimilarity value, and the absence of universal guidelines (49–51). Moreover, some organisms possess multiple heterogeneous copies of rRNA, complicating the differentiation between closely related species if intragenomic heterogeneity exceeds interspecific variability (52, 53). As demonstrated in the present research, the intragenomic heterogeneity among Edwardsiella 16S rRNA sequences ranges from 0.0 to 0.6%, which exceeds the interspecific variability previously reported for this group (31, 38, 50).
As a result of these limitations, high 16S rRNA sequence identity (>99%) does not always imply accuracy in microbial identifications, especially in closely related genera (53, 54). This is important to note as many contemporary studies still rely on partial 16S rRNA sequences for molecular confirmation of bacterial identification, often citing 16S rRNA sequences deposited in the National Center for Biotechnology Information's (NCBI's) GenBank and the International Nucleotide Sequence Database (55, 56). These databases are non-peer reviewed and generally accept any listed name and sequence that is submitted. This poses a problem when attempting to identify unknown microorganisms, wherein erroneous identification can occur if archived sequences are inaccurate or misclassified (57). This is further complicated by 16S rRNA searches where inconsistent sequence ends, ambiguous entries, pseudogaps, and insertions can result in misleading sequence matches (54).
Although 16S sequencing is useful in identifying unknown isolates to genus level, the discriminatory power significantly diminishes at the species level, especially in closely related species (48). In these instances, alternative reference genes should be considered. The single-copy gyrB gene, encoding the ATPase domain of DNA gyrase, is essential for DNA replication and is present in all prokaryotes. It contains conserved motifs that facilitate the development of genus-specific or family-specific primers (52). The gyrB gene has been used to explore the diversity of a wide range of bacteria and is more resolute than 16S rRNA in differentiating closely related members of the Enterobacteriaceae, including the Edwardsiella spp. (31, 38, 58). The utility of gyrB in Edwardsiella classification and identification has been demonstrated elsewhere (31, 38, 42), and the work reported here further supports the use of gyrB as an appropriate marker for discrimination of Edwardsiella species.
Similar to gyrB, the iron-cofactored superoxide dismutase gene (sodB) has high discriminatory power among Edwardsiella spp. Prior to the segregation of E. tarda and the identification of E. piscicida and E. anguillarum as discrete taxa, an internal fragment of sodB was used to distinguish between fish-pathogenic and fish-nonpathogenic E. tarda (33). This work raised questions whether fish-pathogenic E. tarda and the fish-nonpathogenic E. tarda type strain from humans (ATCC 15947) were truly monophyletic. The present analysis of sodB sequences found similar groupings and allowed for correlation between these historical analyses and contemporary nomenclature.
The current work confirms that what was defined as typical motile fish-pathogenic E. tarda is synonymous with E. piscicida. E. piscicida isolates in the current study share 99.8% to 100% similarity at sodB to typical motile fish-pathogenic E. tarda isolates described previously (33) (GenBank AB009853). Similarly, sodB sequence analysis showed that atypical nonmotile fish-pathogenic E. tarda is conspecific with E. anguillarum, while isolates identified here as E. tarda were found to be synonymous with fish-nonpathogenic E. tarda (including the E. tarda type strain from humans, ATCC 15947). This agrees with previous genomic assessments demonstrating high genome sequence homology (>97%) between the typical motile (NUF806) and atypical nonmotile (FPC503) E. tarda strains characterized by Matsuyama et al. (34) and the new species E. piscicida and E. anguillarum, respectively (32, 37).
Repetitive extragenic palindromic PCR (rep-PCR) fingerprinting is another common molecular technique used to estimate the relative degrees of similarity between bacterial isolates (59, 60). The rep-PCR analysis in this study produced distinctive banding patterns for each member of the Edwardsiella genus, with some intraspecific variation. This variation was anticipated and congruent with previous research (31, 38, 42), demonstrating the ability of rep-PCR to distinguish among Edwardsiella spp.
Plasmid analysis revealed that slightly less than half of the bacterial isolates in this study carry at least one native plasmid. Plasmid content included several predicted genes associated with replication, antibiotic resistance, and virulence, although this content varied by group and by isolate. The plasmids harvested from E. ictaluri isolates in this study supported previous characterization (42). While these E. ictaluri plasmids are similar in size, they differ in composition and arrangement, which is likely a function of being isolated from different fish hosts in different geographic locales.
Nine of the E. piscicida isolates (Fr373.2, HL1.1, HL25.1, HL32.1, RBR8.1, ACC69.1, CAQ 8.10, CAQ 10.10, and CAQ 3.9) all carried an identical plasmid. This can be expected, however, as these isolates were recovered from a single fish host (turbot) in Europe during a short temporal range (2005 to 2012). This plasmid encodes a replication initiation factor and RNA polymerase, along with several hypothetical proteins.
Plasmids from remaining isolates all vary in size, composition, and arrangement. This is attributed to the diversity of fish hosts, geographic origins, and years of isolation. Of note, 6 of the 13 (46%) remaining plasmids harvested encode mobilization proteins, nucleases, and transposases and carry various resistance genes. Edwardsiella piscicida isolate SC 09-03, recovered from a smallmouth bass in South Carolina, carries an 11,858-bp plasmid with several ORFs containing genes related to tetracycline resistance. During MIC analysis, SC 09-03 demonstrated resistance to the highest concentrations of tetracycline, oxytetracycline, and minocycline analyzed in the current study.
The plasmid harvested from Edwardsiella 9.1 (E. tarda), the original isolate from the description of emphysematous putrefactive disease in channel catfish (61), encoded a DNA polymerase, a mobilization protein, and several hypothetical proteins. Plasmids from E. tarda isolates Edwardsiella 9.2 and Edwardsiella 9.4, recovered from channel catfish in the United States, contain ORFs encoding transposases and conjugal transfer proteins. In addition, the 27,938-bp plasmid from Edwardsiella 9.2 contains ORFs encoding mercury resistance.
It is important to note that the methods employed here may be limited in their ability to isolate very large plasmids or plasmids with low copy numbers. For example, multidrug resistance plasmids belonging to the IncA/C family are widely distributed among enterobacterial isolates (62) and have been reported from some E. ictaluri isolates from farm-raised and research channel catfish in the southeastern United States as well as Yersinia ruckeri and Aeromonas salmonicida isolates from salmonids (63–66). The IncA/C plasmids are usually very large and typically present in low copy numbers. While an IncA/C-type plasmid was not observed in any of these isolates, future studies employing more robust techniques suitable for the harvest of very large and/or low-copy-number plasmids are warranted.
The MAUVE program identified several shared LCBs representing regions of homologous sequence shared between different plasmids, although analysis failed to identify a single core region shared across all Edwardsiella plasmids. As plasmids rarely carry fundamental genes required for bacterial growth and replication, but rather an assortment of genes that may be advantageous for survival in specific environmental niches or in response to particular selective pressures, it is unsurprising that a temporally diverse collection of congeneric isolates from an assortment of host and geographic origins would carry plasmids demonstrating a diverse array of organization and function. While not particularly useful in providing confirmatory diagnosis or differentiating between the Edwardsiella species, characterization of plasmid profiles has utility in diagnostics from an epidemiological standpoint. Given the potential dispersal of Edwardsiella-associated plasmids with imported and exported aquaculture products and the capacity of plasmids to be spread across multiple bacterial taxa, the identification and characterization of a “core” set of genes associated with Edwardsiella plasmids warrant further study.
Real-time quantitative PCR (qPCR) assays are becoming more common in fish disease research and diagnostics. Assays are currently available for a variety of bacterial, viral, and parasitic fish pathogens (67). Previous research validated qPCR assays for the detection and quantification of E. anguillarum, E. piscicida, and E. tarda in broth culture, pond water, and catfish tissue (68). The real-time multiplex PCR validated here demonstrated appropriate specificity, sensitivity, reproducibility, and repeatability to reliably discriminate among E. anguillarum, E. ictaluri, E. piscicida, and E. tarda (40). In addition, the presence of large quantities of nontarget DNA had no measurable effect on PCR efficiency, suggesting that this assay could also have application as a research tool for environmental DNA (eDNA) assessments in aquaculture systems, similar to other qPCR assays (69–71), and warrants further study. Still, because no distinguishing phenotypic character has been identified for E. anguillarum, E. piscicida, and E. tarda, this assay is a valuable diagnostic tool, providing a rapid method of confirmatory identification for all Edwardsiella species infecting fish.
Last, matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) is an emerging technology for microbial identification. MALDI-TOF generates protonated ions and uses time of flight to generate a peptide mass fingerprint for each sample (72). It can be used for rapid microbial identification from a pure culture, dramatically improving time to identification (73). The use of MALDI-TOF mass spectrometry (MS) for species and subspecies identification has been reported in several different bacteria (74–76).
Initially, MALDI-TOF classified all E. anguillarum and E. piscicida isolates as E. tarda. This was expected, as E. anguillarum and E. piscicida are not currently recognized by the microbial peptide mass spectrum database v5.0.0.0 (Bruker Daltonics). However, observation of individual spectral profiles revealed that discriminatory peaks were present for each Edwardsiella species. Thus, in spite of deficiencies in the current microbial database, MALDI-TOF can discriminate among the five current Edwardsiella taxa, including the three species formerly classified as E. tarda.
Molecular typing methods described here were all in agreement with taxonomic assignments for all isolates. Despite the lack of a discriminatory metabolic or phenotypic character, MALDI-TOF correlated with multiplex PCR, gyrB, sodB, and rep-PCR identifications and classifications. While molecular confirmation of suspect Edwardsiella isolates is ideal in terms of generating archival data for comparison in future studies, MALDI-TOF offers a dependable, cost-effective alternative for clinical laboratories that require rapid, reliable identification.
Another significant finding resulting from the current research is the confirmation that Edwardsiella isolate 9.1, recovered from the original description of emphysematous putrefactive disease in catfish aquaculture in the 1970s, as well as other suspected E. tarda isolates from catfish aquaculture in the early 1980s, is factually E. tarda. Recent molecular surveys suggest that E. piscicida is far more common in U.S. catfish aquaculture than E. tarda, and it was suspected that these original isolates, in addition to other reports of E. tarda in fish prior to the adoption of E. piscicida, may have been unintentionally misclassified (38). Although this may be the case in some instances, it does not hold true for all historical isolates. In light of recent developments in regard to Edwardsiella systematics, E. piscicida and E. anguillarum appear more commonly associated with disease outbreaks in fish than E. tarda, although comparisons of archived sodB sequences from previous reports to data generated here suggest that E. tarda (as it is currently defined) still occasionally causes disease in fish (20, 31, 33, 38, 68, 77, 97).
Proper identification of bacterial isolates is the foundation on which clinical diagnostics and infectious disease research are built. Consistent taxonomic assignment of bacteria facilitates the definition of host-microbe relationships and the development of therapeutic and preventative strategies, and it is the cornerstone of epidemiological investigations (50). This is especially true for Edwardsiella, as different members of the genus demonstrate various degrees of pathogenicity to different hosts (6, 20, 34, 68, 77). The methodologies described here provide reliable methods of identification of the Edwardsiella species and are consistent with current taxonomic schemes. Moreover, the zoonotic potential of E. tarda and the variable pathogenicity of E. anguillarum, E. piscicida, and E. tarda in different hosts make proper identification of isolates recovered from fish and aquaculture systems extremely important.
Edwardsiella tarda plays an important role in zoonotic infections and is one of the principal pathogens acquired from fish and shellfish, including ornamental pet fish (78–80). The clinical disease that manifests in humans infected with E. tarda may be associated with necrotic skin lesions, gastroenteritis, and, in severe cases, a septicemia leading to osteomyelitis, meningitis, or cholecystitis (81). At present, the zoonotic potential of E. anguillarum and E. piscicida is unknown, and it is unclear if these previous reports are in reference to E. tarda as it is currently defined or to one of the newly recognized species. Consistent methods of identification in line with contemporary systematic nomenclature will limit ambiguity in such reports moving forward. Therefore, it is imperative that nomenclature consistency is applied across different laboratories and throughout different countries. The limitations of databases such as GenBank and the unverified taxon classifications associated with submissions further emphasize the importance for researchers and diagnosticians of remaining attentive to the current literature.
MATERIALS AND METHODS
Bacterial isolates.
Isolates of E. anguillarum, E. hoshinae, E. ictaluri, E. piscicida, and E. tarda were obtained from collaborators and biological collections and expanded in porcine brain heart infusion broth (BHIb) (Bacto; Becton, Dickinson and Company) at optimal growth temperatures for each species (37°C for E. anguillarum, E. hoshinae, E. piscicida, and E. tarda and 28°C for E. ictaluri). Aliquots of broth cultures were stored cryogenically (−80°C) with 15% (vol/vol) glycerol. A collection of 47 representative isolates from 10 countries and 19 host species, isolated over a 47-year period, was chosen for analyses (Table 9). Of note, isolate Edwardsiella 9.1 was recovered during the original description of emphysematous putrefactive disease in channel catfish Ictalurus punctatus in the 1970s (61). For all phenotypic analyses, cryostocks of archived isolates were revived by isolation streaking on Mueller-Hinton II agar (BBL, Becton, Dickinson and Company) supplemented with 5% defibrinated sheep blood (Hemostat Laboratories) and grown for 24 h (E. anguillarum, E. hoshinae, E. piscicida, and E. tarda) or 48 h (E. ictaluri) at temperatures optimal for each respective isolate.
TABLE 9.
Edwardsiella isolates analyzed in the current study
| Isolate | Species | Host | Geographic origin | Yr of isolation |
|---|---|---|---|---|
| EA181011 | E. anguillarum | White grouper | Israel | 2011 |
| LADL05-105 | E. anguillarum | Tilapia | Louisiana, USA | 2005 |
| 43472 | E. anguillarum | Blue striped grunt | Maryland, USA | 2003 |
| 43664 | E. anguillarum | Striped bass | Maryland, USA | 1994 |
| 43473 | E. anguillarum | Tilapia | Maryland, USA | 1997 |
| 43659 | E. anguillarum | Tilapia | Maryland, USA | 1998 |
| 43651 | E. anguillarum | Tilapia | Maryland, USA | 1999 |
| ATCC 35051 | E. hoshinae | Monitor | Chad | 1978 |
| 11-149A | E. ictaluri | Zebrafish | Florida, USA | 2011 |
| S97-773 | E. ictaluri | Channel catfish | Mississippi, USA | 1997 |
| RUSVM-1 | E. ictaluri | Tilapia | Western Hemisphere | 2012 |
| PB 07-309 | E. piscicida | Smallmouth bass | Arkansas, USA | 2007 |
| NFAVS-1 | E. piscicida | Largemouth bass | Florida, USA | 2014 |
| Fr373.2 | E. piscicida | Turbot | France | 2012 |
| HL1.1 | E. piscicida | Turbot | Holland | 2006 |
| HL25.1 | E. piscicida | Turbot | Holland | 2006 |
| HL32.1 | E. piscicida | Turbot | Holland | 2006 |
| WFE1 | E. piscicida | Flounder | Japan | 2002 |
| S11-285 | E. piscicida | Channel catfish | Mississippi, USA | 2011 |
| C1490 | E. piscicida | Largemouth bass | New York, USA | 2014 |
| CMT 8211-1 | E. piscicida | Rainbow trout | North Carolina, USA | 2014 |
| REDS 81911-E | E. piscicida | Rainbow trout | North Carolina, USA | 2014 |
| RBR8.1 | E. piscicida | Turbot | Portugal | 2008 |
| SC 09-03 | E. piscicida | Smallmouth bass | South Carolina, USA | 2009 |
| ACC69.1 | E. piscicida | Turbot | Southern Europe | 2005 |
| CAQ 8.10 | E. piscicida | Turbot | Spain | 2009 |
| CAQ 10.10 | E. piscicida | Turbot | Spain | 2009 |
| CAQ 3.9 | E. piscicida | Turbot | Spain | 2009 |
| A15-02670 | E. piscicida | Blotched fantail stingray | Georgia, USA | 2015 |
| 43628 | E. piscicida | Koi | Maryland, USA | 2000 |
| 43662 | E. piscicida | Seatrout | Maryland, USA | 1988 |
| 43644 | E. piscicida | Striped bass | Maryland, USA | 1994 |
| 43475 | E. piscicida | Striped bass | Pennsylvania, USA | 1996 |
| 43658 | E. piscicida | Striped bass | Pennsylvania, USA | 1996 |
| 43468 | E. piscicida | Striped bass | Maryland, USA | 1999 |
| 43656 | E. piscicida | Striped bass | Maryland, USA | 2000 |
| Edwardsiella 9.1 | E. tarda | Channel catfish | Arkansas, USA | 1969 |
| Edwardsiella 9.2 | E. tarda | Channel catfish | West Virginia, USA | 1977 |
| Edwardsiella 9.3 | E. tarda | Flounder | Virginia, USA | 1984 |
| Edwardsiella 9.4 | E. tarda | Channel catfish | Georgia, USA | 1979 |
| FL95-01 | E. tarda | Channel catfish | Florida, USA | 1995 |
| 070720-1 3A | E. tarda | Tilapia | Michigan, USA | 2007 |
| 070720-1 2HLDOM | E. tarda | Tilapia | Michigan, USA | 2007 |
| 43657 | E. tarda | Bottlenose dolphin | Maryland, USA | 2000 |
| 43650 | E. tarda | Hooded seal | Maryland, USA | 2004 |
| 43627 | E. tarda | Tilapia | Pennsylvania, USA | 2000 |
| 43663 | E. tarda | Toadfish | Maryland, USA | 1988 |
DNA isolation.
Cryostocks were revived as described above, and individual colonies were expanded for 24 to 48 h in static BHIb at appropriate temperatures for each isolate. Cultures were pelleted by centrifugation, and genomic DNA (gDNA) was isolated using a commercial DNA isolation kit according to the manufacturer's suggested protocols for Gram-negative bacteria (Gentra Puregene DNA isolation kit; Qiagen). Isolated gDNA was resuspended in 100 μl of DNA hydration solution (DHS; Gentra Puregene DNA isolation kit; Qiagen), quantified spectrophotometrically (NanoDrop 2000; Thermo Fisher Scientific), diluted with DHS to a final concentration of 10 ng/μl, and cryogenically stored (−80°C) until further use.
Motility and TSI.
Individual colonies of Edwardsiella isolates were stabbed into motility medium (Difco) and evaluated for dispersal after 48 h at 37°C (E. anguillarum, E. hoshinae, E. piscicida, and E. tarda) or 28°C (E. ictaluri). Glucose, sucrose, and/or lactose fermentations, in addition to hydrogen gas and/or hydrogen sulfide production in triple sugar iron medium (TSI; Oxoid Ltd.), were determined using similar incubation conditions.
Microbial identification systems.
The commercial API 20E system (bioMérieux) was used for all bacterial species in accordance with the manufacturer's instructions. Briefly, API 20E strips were inoculated and incubated for 24 h at 37°C for E. anguillarum, E. hoshinae, E. piscicida, and E. tarda and 48 h at 28°C for E. ictaluri. All reagents were added, a seven-digit profile number was generated, and profile numbers were submitted to bioMérieux for microbial identification. Additionally, isolates were analyzed using the Biolog microbial identification system (Biolog) according to the manufacturer's instructions. In short, isolates were streaked for isolation from archived cryostocks on Biolog Universal Growth (BUG; Biolog) agar with 5% sheep blood. After 24 h at 28°C, colonies were picked and added to the inoculating fluid A (IF-A; Biolog) to reach 92% to 98% transmittance (%T). Gen III microplates were inoculated and incubated at 28°C for 24 h, after which reactions were read and identification was performed using OmniLog data collection software (Biolog).
Last, bacterial isolates were subjected to the BBL Crystal Enteric/Nonfermentor identification kit (Becton, Dickinson and Company). Cryostocks were streaked for isolation on Mueller-Hinton II agar (BBL; Becton, Dickinson and Company) supplemented with 5% defibrinated sheep blood (Hemostat Laboratories). Individual colonies were picked using a sterile toothpick and resuspended in inoculating fluid to achieve a 0.5-McFarland-standard turbidity before addition to the assay panel. Panels were incubated at 28°C for 24 to 48 h, and reactions were visualized and recorded.
A commercial matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometer (Bruker MALDI-TOF Biotyper LT) was used for bacterial identification and generation of peptide mass spectral profiles. Bacterial colonies were applied to a spot on the MALDI-TOF target plate and overlaid with freshly made 70% formic acid and α-cyano-4-hydroxycinnamic acid matrix solution according to the manufacturer's recommended protocol. The spectra were captured in positive linear mode in a mass range of 2 to 20 kDa with a laser frequency of 60 Hz (IS1, 20 kV; IS2, 18 kV; lens, 6 kV; extraction delay time, 100 ns). Spectra were acquired in automatic mode by accumulating a maximum of 240 profiles (6 × 40 laser shots from different positions of the target spot). Bacterial identification was performed using the default settings of the software provided with the Bruker MALDI-TOF system. A score of >2 indicated highly probable bacterial genus and species identification. The peptide spectra were collected and analyzed using FlexAnalysis software (Bruker).
FAME analysis.
The 47 Edwardsiella isolates were streaked onto sheep blood agar (SBA; Remel) and incubated for 24 h at 28°C. Following incubation, an average of 35 mg (wet weight) of bacteria was harvested from each plate, placed into individual Pyrex glass tubes, and centrifuged for 1 min at 5,250 × g. Bacteria were saponified by adding 1.0 ml of saponification reagent (150 ml of deionized distilled water combined with 150 ml of high-performance liquid chromatography [HPLC]-grade methanol and 45 g of sodium hydroxide). Sequentially, each tube was vortexed for 5 to 10 s, boiled for 5 min in water at 100°C, vortexed for 10 s, and then boiled for an additional 25 min at 100°C. Samples were methylated by the addition of 2.0 ml methylation reagent (162.5 ml of 6.0 N hydrochloric acid with 137.5 ml of HPLC-grade methanol), vortexed for 10 s, and immediately cooled on ice for 10 min.
Following methylation, FAMEs were extracted by the addition of 1.25 ml extraction solvent (200 ml of HPLC-grade hexane combined with 200 ml of HPLC-grade methyl-tert-butyl ether). Samples were loaded into a circular rotator and centrifuged for 10 min at 3,000 × g to ensure adequate combination of sample and extraction solvent. Tubes were then centrifuged for 1 min at 5,250 × g to separate extraction waste. The bottom phase was removed and discarded using a long-tip Pasteur pipette. Three milliliters of base wash (5.4 g of sodium hydroxide diluted in 45 ml of distilled water) was added to the top phase of each sample and centrifuged for 5 min at 3,000 × g. Samples were held upright at room temperature to complete the separation between the bottom and top phases. The top phase (100 μl) from each sample was removed and transferred into a glass vial (National Scientific Target Vials; C4011-1) fitted with a 100-μl glass insert with polymer feet and lid.
Samples (1 μl) were analyzed on HP-Ultra-2 analytical capillary columns (25 by 0.200 mm, 0.33-μm film thickness; Agilent Technologies, Inc.) using an Agilent Technologies 6850 network gas chromatography system (Agilent Technologies, Inc.). Nitrogen was the carrier gas at a constant flow rate of 0.3 ml/min. The oven was programmed at an initial temperature of 170°C and then heated to 288°C at a ramp rate of 28°C/min. The split ratio was 40:1, and the total run was 6.23 min. Data were analyzed using the Sherlock Microbial Identification System (MIS) RCLIN6 6.2 library (MIDI 2012).
Antimicrobial susceptibility profiles.
MICs of 39 different antimicrobial agents were determined for all 47 Edwardsiella isolates to identify susceptibility patterns that could be exploited to differentiate among the Edwardsiella spp. MICs were evaluated using the Sensititre GN4F and Avian1F plate formats (Trek Diagnostic Systems) using the manufacturer's suggested protocol. Escherichia coli ATCC 25922 was used as the quality control strain. Each inoculum was prepared by suspending individual colonies in sterile distilled water to a 0.5-McFarland-standard turbidity; 30 μl of the suspension was added to 11 ml of cation-adjusted Mueller-Hinton broth (Sigma-Aldrich), and 50 μl of the inoculum was added to each well. Plates were covered with an adhesive seal (provided by the manufacturer) and incubated (24 h at 37°C for E. anguillarum, E. hoshinae, E. piscicida, and E. tarda and 48 h at 28°C for E. ictaluri). Following incubation, plates were checked visually, and MIC values were defined as the lowest drug concentration exhibiting no visible growth.
Phylogenetic analysis.
Three different gene targets were chosen for amplification and sequencing to link historical E. tarda isolates to contemporary phylogenomic assignments. Primers used for amplification and sequencing of the 16S rRNA, gyrB, and sodB genes are listed in Table 10. Amplification reactions (50 μl) were performed using 43 μl of Platinum High-Fidelity PCR SuperMix (Invitrogen), 20 pmol of each primer, ∼50 ng of gDNA, and nuclease-free water to volume. Amplifications were performed using a C1000 Touch thermal cycler (Bio-Rad Laboratories, Inc.). For 16S rRNA and gyrB, the following cycling conditions were used: 3 min of denaturation at 94°C; 45 cycles of 30 s at 94°C, 30 s at 52°C, and 2 min at 68°C; and 7 min of extension at 68°C. For sodB, the following cycling conditions were used: 2 min of denaturation at 94°C; 35 cycles of 30 s at 94°C, 30 s at 42°C, and 30 s at 72°C; and 7 min of extension at 72°C. Amplicons were visualized with UV light after electrophoretic passage through a 0.8% agarose gel containing ethidium bromide (0.5 μg ml−1), excised, and purified using QIAquick columns (Qiagen). Purified PCR products were sequenced commercially using the same primers employed to generate the amplicons (Eurofins Genomics, Louisville, KY). Contiguous sequences were assembled, and ambiguous base calls were manually annotated from corresponding chromatograms using Geneious v10.0.7 (Biomatters, Ltd.) (82).
TABLE 10.
Primers and probes used in the current study
| Purpose and primer | Sequence (5′–3′) | Reference(s) |
|---|---|---|
| 16S sequencing | 95 | |
| 27F | GAGTTTGATCCTGGCTCAG | |
| 1525R | AGAAAGGAGGTGATCCAGCC | |
| gyrB sequencing | 37 | |
| GyrB630F | GGATAACGCGATTGACGAAG | |
| GyrB1245R | ATCRTCYTTCATGGTCGARA | |
| GyrB2198F | TAAAGACGATGAGGCGATGG | |
| GyrB2540R | GCCGTGARCAAARTCRAA | |
| sodB sequencing | 32 | |
| E1F | ATGTCRTTCGAATTACCTGC | |
| 497R | TCGATGTARTARGCGTGTTCCCA | |
| Repetitive sequence-mediated PCR | ||
| BOX | CTACGGCAAGGCGACGCTGACG | 87 |
| ERIC I | ATGTAAGCTCCTGGGGATTCAC | 87 |
| ERIC II | AAGTAAGTGACTGGGGTGAGCG | 59 |
| GTG5 | GTGGTGGTGGTGGTG | 59 |
| Multiplex real-time PCR | ||
| E. tarda | 89 | |
| ET3518F | CAGTGATAAAAAGGGGTGGA | |
| ET3632R | CTACACAGCAACGACAACG | |
| ET3559P | AGACAACAGAGGACGGATGTGGC | |
| E. piscicida | 89 | |
| EP14529F | CTTTGATCATGGTTGCGGAA | |
| EP14659R | CGGCGTTTTCTTTTCTCG | |
| EP14615P | CCGACTCCGCGCAGATAACG | |
| E. anguillarum | 89 | |
| EA1583F | GATCGGGTACGCTGTCAT | |
| EA1708R | AATTGCTCTATACGCACGC | |
| EA1611P | CCCGTGGCTAAATAGGACGCG | |
| E. ictaluri | 70, 96 | |
| EI481F | ACTTATCGCCCTCGCAACTC | |
| EI658R | CCTCTGATAAGTGGTTCTCG | |
| EI561P | CCTCACATATTGCTTCAGCGTCGAC |
Sequences from 16S rRNA, gyrB, and sodB were trimmed and aligned using the MUSCLE (83) application of MEGA v6 (84), and pairwise sequence similarities were determined. Moreover, sodB sequences of Edwardsiella spp. were compared to sodB sequences of typical motile fish-pathogenic E. tarda (GenBank accession no. AB009853), atypical nonmotile fish-pathogenic E. tarda (GenBank accession no. AB009584), and fish-nonpathogenic E. tarda (GenBank accession no. AB009850) (33). The Bayesian inference criterion identified the Kimura 2-parameter model with gamma distribution (16S rRNA), the Tamura-Nei model with gamma distribution (gyrB), and the Tamura 3-parameter model with gamma distribution (sodB) as the best-fit nucleotide substitution model for maximum likelihood analysis (85). All positions containing gaps and missing data were eliminated. The final trees were constructed from 1,000 bootstrap replicates. Additionally, intragenomic heterogeneity of the 16S rRNA was evaluated by BLASTN searches of 16S rRNA sequences against the complete genomes of isolates E. anguillarum LADL05-105, E. hoshinae ATCC 35051, E. piscicida S11-285, and E. tarda FL95-01, which were closed ancillary to the current project (43–45, 86), as well as E. ictaluri 93-146 (87).
Genetic fingerprinting.
Repetitive extragenic palindromic PCR (rep-PCR) fingerprinting was performed on isolates using previously published primer sets (Table 10) and modifications to existing protocols (31, 38, 59, 60, 88). Briefly, 50-μl reaction mixtures comprised 25 μl of IQ Supermix (Bio-Rad; Hercules, CA), 20 pmol (ERIC I and II) or 40 pmol (ERIC II, BOX, and GTG5) of primer, 10 ng of DNA template, and nuclease-free water to volume. Amplifications were performed on a C1000 Touch thermal cycler (Bio-Rad Laboratories, Inc.) with the following temperature profiles: BOX, ERIC II, and ERIC I and II, 1 cycle at 95°C for 10 min; 5 cycles of 95°C for 1 min, 40°C for 1 min, and 72°C for 5 min; and 35 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 5 min; GTG5, 1 cycle at 95°C for 10 min; 45 cycles of 95°C for 1 min, 46°C for 1 min, and 72°C for 3 min with a final extension at 72°C for 10 min. Aliquots of each amplification reaction mixture (10 μl each) and a molecular weight standard (Hyperladder 50 bp; Bioline) were electrophoresed through a 1.5% (wt/vol) agarose gel with ethidium bromide (0.5 μg ml−1) and visualized under UV light. Genetic fingerprints generated by the BOX primer were analyzed using Quantity One software v.4.6.9 (Bio-Rad Laboratories, Inc.). Band sizes were estimated by comparison with concurrently run standards, and distinct bands were manually annotated to calculate Dice coefficients and generate a dendrogram based on the unweighted pair group method using arithmetic averages (UPGMA).
Multiplex real-time PCR.
A real-time multiplex PCR (mPCR) specific to E. anguillarum, E. ictaluri, E. piscicida, and E. tarda was developed based on previously published primers, probes, and protocols (38, 68, 89). Primers and probes (Table 10) were synthesized commercially (Eurofins MWG; Louisville, KY); each probe was labeled with a fluorescent reporter dye (E. anguillarum, Texas Red; E. ictaluri, hexachlorofluorescein [HEX]; E. piscicida, 6-carobxyfluorescein [6-FAM]; E. tarda, Cy5) on the 5′ end and appropriate quencher dye (black hole quencher 1 for HEX and 6-FAM; black hole quencher 2 for Texas Red and Cy5) on the 3′ end. The 25-μl reaction mixture contained 12 μl of PCR master mix (TaqMan Environmental Mastermix 2.0; Applied Biosystems), 5 pmol of each primer, 0.5 pmol of each probe, DNA template, and nuclease-free water to volume. Amplifications were performed on a CFX96 thermal cycler (Bio-Rad Laboratories, Inc.) with the following temperature profile: 1 cycle of 95°C for 15 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Data collection occurred following the 60°C annealing/extension step at the end of each cycle.
The specificity of the mPCR assay was tested against both target and nontarget Edwardsiella gDNA. Additionally, the specificity and sensitivity of the assay were tested using serial 10-fold dilutions of target gDNA, supplementing each reaction mixture with an equal mixture of ∼10,000 copies of each nontarget Edwardsiella sp. gDNA to ensure that large quantities of nontarget DNA did not impair reaction efficiency. Samples, as well as no-template controls, were run in triplicate under the reaction conditions described above. Each plate was run in triplicate on three separate occasions to assess the repeatability and reproducibility of the assay. Quantification cycles (Cq) for each reaction were based on a user-defined baseline threshold of 50 relative fluorescence units (RFU).
Plasmid analysis.
For all isolates, plasmid DNA was harvested from 3 ml of expanded BHIb cultures using the QIAprep Spin miniprep kit (Qiagen). Plasmids were identified by separation on a 0.8% agarose gel. When present, plasmid sizes were approximated with concurrently run standards (Supercoiled DNA Ladder; New England BioLabs). Harvested plasmids were submitted to the complete plasmid sequencing service of the DNA Core Facility of the Center for Computational and Integrative Biology at Massachusetts General Hospital (Boston, MA, USA) for sequencing. Open reading frames (ORFs) were predicted using GeneMark.hmm prokaryotic v3.25 (90, 91) and Glimmer v3.02 (92, 93). Putative functions of plasmid ORFs were predicted using a BLASTX search of the NCBI nonredundant protein database using the Bacteria and Archaea code with E values of ≥1e−2 considered insignificant. Last, circularized plasmids were aligned using the progressiveMauve algorithm utility in Geneious, with seed weights and minimum locally colinear block (LCB) scores calculated automatically (94).
Accession number(s).
16S, sodB, and gyrB sequences have been submitted to GenBank under the accession numbers MG225458 to MG225535 and MG230270 to MG230308. Annotated plasmid sequences have been submitted to GenBank under the accession numbers MG212496 to MG212499 and MG225254 to MG228262.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the generosity of Al Camus, D. Barbara Petty, Roy Yanong, Esteban Soto, and Ronald Thune for sharing bacterial isolates that were used in this analyses.
This research was supported by the Mississippi Center for Food Safety and Postharvest Technology (U.S. Department of Agriculture-Agricultural Research Service project no. 58-6402-2-729), the National Institute of Food and Agriculture (U.S. Department of Agriculture project no. 1004524), the U.S. Department of Agriculture-Catfish Health Research Initiative (MIS-371660), the Mississippi State University College of Veterinary Medicine, and the Mississippi Agricultural and Forestry Experiment Station (MAFES).
The views expressed in this article are those of the author(s) and may not reflect the official policy of the Department of Health and Human Services, the U.S. Food and Drug Administration, the USDA, or the U.S. Government. Mention of trade names is for descriptive purposes only and does not imply endorsement by the authors or their respective institutions.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00970-17.
REFERENCES
- 1.King BM, Adler DL. 1964. A previously undescribed group of Enterobacteriaceae. Am J Clin Pathol 41:230–232. [PubMed] [Google Scholar]
- 2.Sakazaki R, Murata Y. 1962. The new group of the Enterobacteriaceae, the Asakusa group. Jpn J Bacteriol 17:616. [Google Scholar]
- 3.Sakazaki R. 1965. A proposed group of the family Enterobacteriaceae, the Asakusa group. Int J Syst Bacteriol 15:45–47. doi: 10.1099/00207713-15-1-45. [DOI] [Google Scholar]
- 4.Sakazaki R. 1967. Studies on the Asakusa group of Enterobacteriaceae (Edwardsiella tarda). Jpn J Med Sci Biol 20:205–212. doi: 10.7883/yoken1952.20.205. [DOI] [PubMed] [Google Scholar]
- 5.Ewing WH, McWhorter AC, Escobar MR, Lubin AH. 1965. Edwardsiella, a new genus of Enterobacteriaceae based on a new species, E. tarda. Int J Syst Bacteriol 15:33–38. doi: 10.1099/00207713-15-1-33. [DOI] [Google Scholar]
- 6.Abayneh T, Colquhoun DJ, Sorum H. 2013. Edwardsiella piscicida sp. nov., a novel species pathogenic to fish. J Appl Microbiol 114:644–654. doi: 10.1111/jam.12080. [DOI] [PubMed] [Google Scholar]
- 7.Mohanty BR, Sahoo PK. 2007. Edwardsiellosis in fish: a brief review. J Biosci 32:1331–1344. doi: 10.1007/s12038-007-0143-8. [DOI] [PubMed] [Google Scholar]
- 8.Grimont PD, Grimont F, Richard C, Sakazaki R. 1980. Edwardsiella hoshinae, a new species of Enterobacteriaceae. Curr Microbiol 4:347–351. doi: 10.1007/BF02605375. [DOI] [Google Scholar]
- 9.Singh IM, Singh S, McMurphy MA, Crupper SS, Mills-Robertson F, Applegate RD. 2004. Antibiotic susceptibility of Edwardsiella hoshinae isolated from northern bobwhite quail (Colinus virginianus). Vet Rec 155:29–29. doi: 10.1136/vr.155.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Hawke JP, McWhorter AC, Steigerwalt AG, Brenner DJ. 1981. Edwardsiella ictaluri sp. nov., the causative agent of enteric septicemia of catfish. Int J Syst Bacteriol 31:396–400. doi: 10.1099/00207713-31-4-396. [DOI] [Google Scholar]
- 11.Crumlish M, Dung TT, Turnbull JF, Ngoc NTN, Ferguson HW. 2002. Identification of Edwardsiella ictaluri from diseased freshwater catfish, Pangasius hypophthalmus (Sauvage), cultured in the Mekong Delta, Vietnam. J Fish Dis 25:733–736. doi: 10.1046/j.1365-2761.2002.00412.x. [DOI] [Google Scholar]
- 12.Yuasa K, Kholidin EB, Panigoro N, Hatai K. 2003. First isolation of Edwardsiella ictaluri from cultured striped catfish Pangasius hypophthalmus in Indonesia. Fish Pathol 38:181–183. doi: 10.3147/jsfp.38.181. [DOI] [Google Scholar]
- 13.Ye S, Li H, Qiao G, Li Z. 2009. First case of Edwardsiella ictaluri infection in China farmed yellow catfish Pelteobagrus fulvidraco. Aquaculture 292:6–10. doi: 10.1016/j.aquaculture.2009.03.036. [DOI] [Google Scholar]
- 14.Suanyuk N, Rogge M, Thune R, Watthanaphiromsakul M, Champhat N, Wiangkum W. 2014. Mortality and pathology of hybrid catfish, Clarias macrocephalus (Gunther) × Clarias gariepinus (Burchell), associated with Edwardsiella ictaluri infection in southern Thailand. J Fish Dis 37:385–395. doi: 10.1111/jfd.12127. [DOI] [PubMed] [Google Scholar]
- 15.Phillips AC, Reichley SR, Ware C, Griffin MJ. 2017. Edwardsiella ictaluri infection in Pangasius catfish imported from West Bengal into the Southern Caribbean. J Fish Dis 40:743–756. doi: 10.1111/jfd.12552. [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Kamaishi T, Sano M, Tensha K, Arima T, Iida T, Nagai T, Nakai T, Iida T. 2008. Outbreaks of Edwardsiella ictaluri infection in ayu Plecoglossus altivelis in Japanese rivers. Fish Pathol 43:152–157. doi: 10.3147/jsfp.43.152. [DOI] [Google Scholar]
- 17.Soto E, Griffin M, Arauz M, Riofrio A, Martinez A, Cabrejos ME. 2012. Edwardsiella ictaluri as the causative agent of mortality in cultured Nile tilapia. J Aquat Anim Health 24:81–90. doi: 10.1080/08997659.2012.675931. [DOI] [PubMed] [Google Scholar]
- 18.Hawke JP, Kent M, Rogge M, Baumgartner W, Wiles J, Shelley J, Savolainen LC, Wagner R, Murray K, Peterson TS. 2013. Edwardsiellosis caused by Edwardsiella ictaluri in laboratory populations of zebrafish Danio rerio. J Aquat Anim Health 25:171–183. doi: 10.1080/08997659.2013.782226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawke JP, Khoo LH. 2004. Infectious diseases, p 387–443. In Tucker CS, Hargreaves JA (ed), Biology and culture of the channel catfish. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 20.Griffin MJ, Greenway TE, Wise DJ. 2017. Edwardsiella spp., p 190–210. In Woo PTK, Cipriano RC (ed), Fish viruses and bacteria: pathobiology and protection. CABI, Wallingford, United Kingdom. [Google Scholar]
- 21.Sharma VK, Kaura YK, Singh P. 1974. Frogs as carriers of Salmonella and Edwardsiella. Antonie Van Leeuwenhoek 40:171–175. doi: 10.1007/BF00394564. [DOI] [PubMed] [Google Scholar]
- 22.Tan RJS, Lim EW. 1977. Occurrence of Edwardsiella tarda in the household lizard, Gecko gecko. Jpn J Med Sci Biol 30:321–323. doi: 10.7883/yoken1952.30.321. [DOI] [PubMed] [Google Scholar]
- 23.Nucci C, da Silveira WD, da Silva Correa S, Nakazato G, Bando SY, Ribeiro MA, Pestana de Castro AF. 2002. Microbiological comparative study of isolates of Edwardsiella tarda isolated in different countries from fish and humans. Vet Microbiol 89:29–39. doi: 10.1016/S0378-1135(02)00151-7. [DOI] [PubMed] [Google Scholar]
- 24.Leotta GA, Piñeyro P, Serena S, Vigo GB. 2009. Prevalence of Edwardsiella tarda in Antarctic wildlife. Polar Biol 32:809–812. doi: 10.1007/s00300-009-0610-9. [DOI] [Google Scholar]
- 25.Wang X, Yan M, Wang Q, Ding L, Li F. 2012. Identification of Edwardsiella tarda isolated from duck and virulence genes detection. Afr J Microbiol Res 6:4970–4975. [Google Scholar]
- 26.Kodama H, Murai T, Nakanishi Y, Yamamoto F, Mikami T, Izawa H. 1987. Bacterial infection which produces high mortality in cultured Japanese flounder (Paralichthys olivaceus) in Hokkaido. Jpn J Vet Res 35:227–234. [PubMed] [Google Scholar]
- 27.Castro N, Toranzo AE, Barja JL, Nunez S, Magarinos B. 2006. Characterization of Edwardsiella tarda strains isolated from turbot, Psetta maxima (L). J Fish Dis 29:541–547. doi: 10.1111/j.1365-2761.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu T, Zhang X-H. 2014. Edwardsiella tarda: an intriguing problem in aquaculture. Aquaculture 431:129–135. doi: 10.1016/j.aquaculture.2013.12.001. [DOI] [Google Scholar]
- 29.Yang M, Lv Y, Xiao J, Wu H, Zheng H, Liu Q, Zhang Y, Wang Q. 2012. Edwardsiella comparative phylogenomics reveal the new intra/inter-species taxonomic relationships, virulence evolution and niche adaptation mechanisms. PLoS One 7:e36987. doi: 10.1371/journal.pone.0036987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abayneh T, Colquhoun DJ, Sorum H. 2012. Multi-locus sequence analysis (MLSA) of Edwardsiella tarda isolates from fish. Vet Microbiol 158:367–375. doi: 10.1016/j.vetmic.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Griffin MJ, Quiniou SM, Cody T, Tabuchi M, Ware C, Cipriano RC, Mauel MJ, Soto E. 2013. Comparative analysis of Edwardsiella isolates from fish in the eastern United States identifies two distinct genetic taxa amongst organisms phenotypically classified as E. tarda. Vet Microbiol 165:358–372. doi: 10.1016/j.vetmic.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Shao S, Lai Q, Liu Q, Wu H, Xiao J, Shao Z, Wang Q, Zhang Y. 2015. Phylogenomics characterization of a highly virulent Edwardsiella strain ET080813(T) encoding two distinct T3SS and three T6SS gene clusters: propose a novel species as Edwardsiella anguillarum sp. nov. Syst Appl Microbiol 38:36–47. doi: 10.1016/j.syapm.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Yamada Y, Wakabayashi H. 1999. Identification of fish-pathogenic strains belonging to the genus Edwardsiella by sequence analysis of sodB. Fish Pathol 34:145–150. doi: 10.3147/jsfp.34.145. [DOI] [Google Scholar]
- 34.Matsuyama T, Kamaishi T, Ooseko N, Kurohara K, Iida T. 2005. Pathogenicity of motile and non-motile Edwardsiella tarda to some marine fish. Fish Pathol 40:133–135. doi: 10.3147/jsfp.40.133. [DOI] [Google Scholar]
- 35.Sakai T, Iida T, Osatomi K, Kanai K. 2007. Detection of type 1 fimbrial genes in fish pathogenic and non-pathogenic Edwardsiella tarda strains by PCR. Fish Pathol 42:115–117. doi: 10.3147/jsfp.42.115. [DOI] [Google Scholar]
- 36.Wang YM, Wang QY, Xiao JF, Liu Q, Wu HZ, Zhang YX. 2011. Genetic relationships of Edwardsiella strains isolated in China aquaculture revealed by rep-PCR genomic fingerprinting and investigation of Edwardsiella virulence genes. J Appl Microbiol 111:1337–1348. doi: 10.1111/j.1365-2672.2011.05166.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Takano T, Yasuike M, Sakai T, Matsuyama T, Sano M. 2013. Comparative genomics reveals that a fish pathogenic bacterium Edwardsiella tarda has acquired the locus of enterocyte effacement (LEE) through horizontal gene transfer. BMC Genomics 14:642. doi: 10.1186/1471-2164-14-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin MJ, Ware C, Quiniou SM, Steadman JM, Gaunt PS, Khoo LH, Soto E. 2014. Edwardsiella piscicida identified in the Southeastern USA by gyrB sequence, species-specific and repetitive sequence-mediated PCR. Dis Aquat Organ 108:23–35. doi: 10.3354/dao02687. [DOI] [PubMed] [Google Scholar]
- 39.Alcaide E, Herraiz S, Esteve C. 2006. Occurrence of Edwardsiella tarda in wild European eels Anguilla anguilla from Mediterranean Spain. Dis Aquat Organ 73:77–81. doi: 10.3354/dao073077. [DOI] [PubMed] [Google Scholar]
- 40.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 41.Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. 2010. A practical approach to RT-qPCR—publishing data that conform to the MIQE guidelines. Methods 50:S1–S5. doi: 10.1016/j.ymeth.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Griffin MJ, Reichley SR, Greenway TE, Quiniou SM, Ware C, Gao DX, Gaunt PS, Yanong RP, Pouder DB, Hawke JP, Soto E. 2016. Comparison of Edwardsiella ictaluri isolates from different hosts and geographic origins. J Fish Dis 39:947–969. doi: 10.1111/jfd.12431. [DOI] [PubMed] [Google Scholar]
- 43.Reichley SR, Waldbieser GC, Tekedar HC, Lawrence ML, Griffin MJ. 2016. Complete genome sequence of Edwardsiella piscicida isolate S11-285 recovered from channel catfish (Ictalurus punctatus) in Mississippi, USA. Genome Announc 4:e01259-16. doi: 10.1128/genomeA.01259-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichley SR, Waldbieser GC, Lawrence ML, Griffin MJ. 2015. Complete genome sequence of an Edwardsiella piscicida-like species recovered from tilapia in the United States. Genome Announc 3:e01004-15. doi: 10.1128/genomeA.01004-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichley SR, Waldbieser GC, Lawrence ML, Griffin MJ. 2017. Complete genome sequence of Edwardsiella hoshinae ATCC 35051. Genome Announc 5:e01605-16. doi: 10.1128/genomeA.01605-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichley SR, Waldbieser GC, Ucko M, Colorni A, Dubytska L, Thune RL, Lawrence ML, Griffin MJ. 2015. Complete genome sequence of an Edwardsiella piscicida-like species isolated from diseased grouper in Israel. Genome Announc 3:e00829-15. doi: 10.1128/genomeA.00829-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SB, Aoki T, Jung TS. 2012. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet Res 43:67. doi: 10.1186/1297-9716-43-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo PCY, Lau SKP, Teng JLL, Tse H, Yuen K-Y. 2008. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect 14:908–934. doi: 10.1111/j.1469-0691.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 49.McMeekin TA, Baranyi J, Bowman J, Dalgaard P, Kirk M, Ross T, Schmid S, Zwietering MH. 2006. Information systems in food safety management. Int J Food Microbiol 112:181–194. doi: 10.1016/j.ijfoodmicro.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 50.Janda JM, Abbott SL. 2002. Bacterial identification for publication: when is enough enough? J Clin Microbiol 40:1887–1891. doi: 10.1128/JCM.40.6.1887-1891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox GE, Wisotzkey JD, Jurtshuk P Jr. 1992. How close is close: 16s rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol 42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 52.Huang WM. 1996. Bacterial diversity based on type II DNA topoisomerase genes. Annu Rev Genet 30:79–107. doi: 10.1146/annurev.genet.30.1.79. [DOI] [PubMed] [Google Scholar]
- 53.Janda JM, Abbott SL. 2007. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol 45:2761–2764. doi: 10.1128/JCM.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turenne CY, Tschetter L, Wolfe J, Kabani A. 2001. Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. J Clin Microbiol 39:3637–3648. doi: 10.1128/JCM.39.10.3638-3648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nilsson RH, Ryberg M, Kristiansson E, Abarenkov K, Larsson KH, Koljalg U. 2006. Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PLoS One 1:e59. doi: 10.1371/journal.pone.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wragg P, Randall L, Whatmore AM. 2014. Comparison of Biolog GEN III MicroStation semi-automated bacterial identification system with matrix-assisted laser desorption ionization-time of flight mass spectrometry and 16S ribosomal RNA gene sequencing for the identification of bacteria of veterinary interest. J Microbiol Methods 105:16–21. doi: 10.1016/j.mimet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Clarridge JE., III 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17:840–862. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dauga C. 2002. Evolution of the gyrB gene and the molecular phylogeny of Enterobacteriaceae: a model molecule for molecular systematic studies. Int J Syst Evol Microbiol 52:531–547. doi: 10.1099/00207713-52-2-531. [DOI] [PubMed] [Google Scholar]
- 59.Versalovic J, Koeuth T, Lupski JR. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Versalovic J, Schneider M, DeBruijn FJ, Lupski JR. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol 5:25–40. [Google Scholar]
- 61.Meyer FP, Bullock GL. 1973. Edwardsiella tarda, a new pathogen of channel catfish (Ictalurus punctatus). Appl Microbiol 25:155–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fricke WF, Welch TJ, McDermott PF, Mammel MK, LeClerc JE, White DG, Cebula TA, Ravel J. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J Bacteriol 191:4750–4757. doi: 10.1128/JB.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welch TJ, Fricke WF, McDermott PF, White DG, Rosso M, Rasko DA, Mammal MK, Eppinger M, Rosovitz MJ, Wagner D, Rahalison L, LeClerc JE, Hinshaw JM, Lindler LE, Cebula TA, Carniel E, Ravel J. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. doi: 10.1371/journal.pone.0000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McIntosh D, Cunningham M, Ji B, Fekete FA, Parry EM, Clark SE, Zalinger ZB, Gilg IC, Danner R, Johnson KA, Beattie M, Ritchie R. 2008. Transferable, multiple antibiotic and mercury resistance in Atlantic Canadian isolates of Aeromonas salmonicida subsp. salmonicida is associated with carriage of an IncA/C plasmid similar to the Salmonella enterica plasmid pSN254. J Antimicrob Chemother 61:1221–1228. doi: 10.1093/jac/dkn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welch TJ, Evenhuis J, White DG, McDermott PF, Harbottle H, Miller RA, Griffin M, Wise D. 2009. IncA/C plasmid-mediated florfenicol resistance in the catfish pathogen Edwardsiella ictaluri. Antimicrob Agents Chemother 53:845–846. doi: 10.1128/AAC.01312-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LaFrentz BR, Welch TJ, Shoemaker CA, Drennan JD, Klesius PH. 2011. Modified live Edwardsiella ictaluri vaccine, AQUAVAC-ESC, lacks multidrug resistance plasmids. J Aquat Anim Health 23:195–199. doi: 10.1080/08997659.2011.642093. [DOI] [PubMed] [Google Scholar]
- 67.Purcell MK, Getchell RG, McClure CA, Garver KA. 2011. Quantitative polymerase chain reaction (PCR) for detection of aquatic animal pathogens in a diagnostic laboratory setting. J Aquat Anim Health 23:148–161. doi: 10.1080/08997659.2011.620217. [DOI] [PubMed] [Google Scholar]
- 68.Reichley SR, Ware C, Greenway TE, Wise DJ, Griffin MJ. 2015. Real-time polymerase chain reaction assays for the detection and quantification of Edwardsiella tarda, Edwardsiella piscicida, and Edwardsiella piscicida-like species in catfish tissues and pond water. J Vet Diagn Invest 27:130–139. doi: 10.1177/1040638714566672. [DOI] [PubMed] [Google Scholar]
- 69.Griffin MJ, Pote LM, Camus AC, Mauel MJ, Greenway TE, Wise DJ. 2009. Application of a real-time PCR assay for the detection of Henneguya ictaluri in commercial channel catfish ponds. Dis Aquat Organ 86:223–233. doi: 10.3354/dao02079. [DOI] [PubMed] [Google Scholar]
- 70.Griffin MJ, Mauel MJ, Greenway TE, Khoo LH, Wise DJ. 2011. A real-time polymerase chain reaction assay for quantification of Edwardsiella ictaluri in catfish pond water and genetic homogeneity of diagnostic case isolates from Mississippi. J Aquat Anim Health 23:178–188. doi: 10.1080/08997659.2011.637006. [DOI] [PubMed] [Google Scholar]
- 71.Griffin MJ, Goodwin AE, Merry GE, Liles MR, Williams MA, Ware C, Waldbieser GC. 2013. Rapid quantitative detection of Aeromonas hydrophila strains associated with disease outbreaks in catfish aquaculture. J Vet Diagn Invest 25:473–481. doi: 10.1177/1040638713494210. [DOI] [PubMed] [Google Scholar]
- 72.Singhal N, Kumar M, Kanaujia PK, Virdi JS. 2015. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dixon P, Davies P, Hollingworth W, Stoddart M, MacGowan A. 2015. A systematic review of matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry compared to routine microbiological methods for the time taken to identify microbial organisms from positive blood cultures. Eur J Clin Microbiol Infect Dis 34:863–876. doi: 10.1007/s10096-015-2322-0. [DOI] [PubMed] [Google Scholar]
- 74.Barbuddhe SB, Maier T, Schwarz G, Kostrzewa M, Hof H, Domann E, Chakraborty T, Hain T. 2008. Rapid identification and typing of Listeria species by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Appl Environ Microbiol 74:5402–5407. doi: 10.1128/AEM.02689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dieckmann R, Helmuth R, Erhard M, Malorny B. 2008. Rapid classification and identification of salmonellae at the species and subspecies levels by whole-cell matrix-assisted laser desorption ionization–time of flight mass spectrometry. Appl Environ Microbiol 74:7767–7778. doi: 10.1128/AEM.01402-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seibold E, Maier T, Kostrzewa M, Zeman E, Splettstoesser W. 2010. Identification of Francisella tularensis by whole-cell matrix-assisted laser desorption ionization–time of flight mass spectrometry: fast, reliable, robust, and cost-effective differentiation on species and subspecies levels. J Clin Microbiol 48:1061–1069. doi: 10.1128/JCM.01953-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reichley SR. 2017. Advancing our knowledge of the Edwardsiella. PhD dissertation. Mississippi State University, Mississippi State, MS. [Google Scholar]
- 78.Vandepitte J, Lemmens P, De Swert L. 1983. Human Edwardsiellosis traced to ornamental fish. J Clin Microbiol 17:165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Javier S. 2012. Edwardsiellosis, an emerging zoonosis of aquatic animals. Biohelikon Immun Dis 1:1–2. [Google Scholar]
- 80.Haenen O, Evans J, Berthe F. 2013. Bacterial infections from aquatic species: potential for and prevention of contact zoonoses. Rev Sci Tech 32:497–507. doi: 10.20506/rst.32.2.2245. [DOI] [PubMed] [Google Scholar]
- 81.Gilman R, Madasamy M, Gan E, Mariappan M, Davis C, Kyser K. 1971. Edwardsiella tarda in jungle diarrhoea and a possible association with Entamoeba histolytica. Southeast Asian J Trop Med Public Health 2:186–189. [PubMed] [Google Scholar]
- 82.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY. [Google Scholar]
- 86.Reichley SR, Waldbieser GC, Tekedar HC, Lawrence ML, Griffin MJ. 2015. Complete genome sequence of Edwardsiella tarda isolate FL95-01, recovered from channel catfish. Genome Announc 3:e00682-15. doi: 10.1128/genomeA.00682-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams ML, Gillaspy AF, Dyer DW, Thune RL, Waldbieser GC, Schuster SC, Gipson J, Zaitshik J, Landry C, Banes MM, Lawrence ML. 2012. Genome sequence of Edwardsiella ictaluri 93-146, a strain associated with a natural channel catfish outbreak of enteric septicemia of catfish. J Bacteriol 194:740–741. doi: 10.1128/JB.06522-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chou L, Griffin MJ, Fraites T, Ware C, Ferguson H, Keirstead N, Brake J, Wiles J, Hawke JP, Kearney MT, Getchell RG, Gaunt P, Soto E. 2014. Phenotypic and genotypic heterogeneity among Streptococcus iniae isolates recovered from cultured and wild fish in North America, Central America and the Caribbean islands. J Aquat Anim Health 26:263–271. doi: 10.1080/08997659.2014.945048. [DOI] [PubMed] [Google Scholar]
- 89.Sakai T, Yuasa K, Sano M, Iida T. 2009. Identification of Edwardsiella ictaluri and E. tarda by species-specific polymerase chain reaction targeted to the upstream region of the fimbrial gene. J Aquat Anim Health 21:124–132. doi: 10.1577/H08-061.1. [DOI] [PubMed] [Google Scholar]
- 90.Besemer J, Borodovsky M. 1999. Heuristic approach to deriving models for gene finding. Nucleic Acids Res 27:3911–3920. doi: 10.1093/nar/27.19.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu W, Lomsadze A, Borodovsky M. 2010. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res 38:e132. doi: 10.1093/nar/gkq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salzberg SL, Delcher AL, Kasif S, White O. 1998. Microbial gene identification using interpolated Markov models. Nucleic Acids Res 26:544–548. doi: 10.1093/nar/26.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res 27:4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Darling ACE, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Evol Microbiol 46:1088–1092. [DOI] [PubMed] [Google Scholar]
- 96.Bilodeau AL, Waldbieser GC, Terhune JS, Wise DJ, Wolters WR. 2003. A real-time polymerase chain reaction assay of the bacterium Edwardsiella ictaluri in channel catfish. J Aquat Anim Health 15:80–86. doi:. [DOI] [Google Scholar]
- 97.Shao J, Yuan J, Shen Y, Hu R, Gu Z. 2016. First isolation and characterization of Edwardsiella tarda from diseased Asian swamp eel, Monopterus albus (Zuiew). Aquac Res 47:3684–3688. doi: 10.1111/are.12791. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.