ABSTRACT
The effectiveness of antimicrobial binding resins present in blood culture (BC) bottles in removing meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam is unknown. We assessed the time to detection (TTD) and growth of 2 Pseudomonas aeruginosa isolates in the presence of clinically meaningful concentrations of these antibiotics. Bactec Plus Aerobic/F and BacT/Alert FA Plus BC bottles were inoculated with one of two isolates (1 meropenem susceptible and 1 resistant), followed by fresh whole blood containing the peak, midpoint, or trough plasma concentrations for meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam. Matching bottles were loaded into their respective detection instruments and a standard incubator at 37°C, with TTD and CFU being monitored for up to 72 h. Bacterial growth was observed for 11/48 (22.9%), 22/48 (45.8%), and 47/48 (97.9%) of all BC bottles inoculated with the peak, midpoint, and trough concentrations, respectively (P ≤ 0.001). When P. aeruginosa was isolated, the TTD was typically <26 h, and no differences between Bactec and BacT/Alert bottles were observed. In both systems, meropenem was removed to a greater degree than were ceftolozane and ceftazidime; however, concentrations for all antibiotics remained above the MIC for the susceptible organisms at 12 h. BC bottles containing antibiotic binding resins may not sufficiently inactivate achievable concentrations of meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam. The consistent identification of both P. aeruginosa isolates was observed only in the presence of antibiotic trough concentrations. To minimize false-negative BC results for patients already receiving these antibiotics, cultures should be collected just prior to the next dose, when antibiotic concentrations are lowest.
KEYWORDS: Pseudomonas aeruginosa, bacteremia, antimicrobial stewardship, carbapenem, cephalosporin
INTRODUCTION
Bloodstream infections (BSIs) are associated with major morbidity and mortality worldwide (1). Rapid identification of a culprit pathogen and prompt appropriate antimicrobial administration are essential for reducing mortality rates (2). Regardless of the source, a positive blood culture (BC) currently remains the gold standard for establishing this diagnosis; moreover, several clinical and laboratory variables determine the detection of pathogens in BC bottles (3). Notably, an important limitation involves the delay in, or lack of, recovery of bloodstream pathogens when BCs are obtained from patients already receiving antibiotics (4, 5). To overcome this, BC media contain proprietary antimicrobial binding resins that reduce antibiotic concentrations to prevent artificial sterilization of the sample (6–11). In vitro and clinical studies have suggested variability in performances for different combinations of microorganisms, antibiotics, and binding systems (6–12). For example, Zadroga and colleagues (12) observed the recovery of Gram-negative bacteria in Becton Dickinson Bactec Plus BC bottles when piperacillin-tazobactam was administered prior to collection, while cultures from the same patients using bioMérieux BacT/Alert FAN bottles demonstrated residual piperacillin concentrations above the MIC, with no recovery.
While the reliable detection of Pseudomonas aeruginosa by BC detection systems is a top priority for treatment optimization and effective antimicrobial stewardship (13–16), no studies have evaluated these BC bottle systems for meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam. These potent broad-spectrum β-lactams are frequently administered in high doses and with prolonged infusions to optimize their activity (17); therefore, there is an increased likelihood that the time to detection (TTD) will be delayed or that samples will be sterilized. We aimed to investigate the effect of the in vitro neutralization of clinically achievable concentrations of meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam on TTDs and growth curves of P. aeruginosa in two widely used BC bottle systems, the Bactec Plus Aerobic Plus/F, and BacT/Alert FA Plus Aerobic bottles. Additionally, we sought to propose a BC collection time during the dosing interval when bacteria could still be recovered from these systems, thereby minimizing the risk of artificial sterilization and false-negative results.
RESULTS
Baseline colony counts and whole-blood antibiotic concentrations.
The starting inoculum for all studies was 15.6 ± 5.7 CFU/bottle (range, 7 to 30 CFU/bottle). Inoculums per bottle were similar between P. aeruginosa ATCC 27853 and P. aeruginosa 147 (P = 0.2) as well as between experiments with meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam (P = 0.27). Observed whole-blood concentrations for meropenem were in agreement with target concentrations (Table 1). Observed whole-blood concentrations for ceftolozane and ceftazidime were slightly higher than the targeted concentrations but within clinically observed concentration ranges (18, 19).
TABLE 1.
Observed meropenem, ceftolozane, and ceftazidime concentrations in spiked whole-blood stock solutions before inoculation of BC bottlesa
| Antibiotic | Mean observed peak concn (μg/ml) ± SD (whole-blood target concn [μg/ml]) |
Mean observed midpoint concn (μg/ml) ± SD (whole-blood target concn [μg/ml]) |
Mean observed trough concn (μg/ml) ± SD (whole-blood target concn [μg/ml]) |
|||
|---|---|---|---|---|---|---|
| Bactec | BacT/Alert | Bactec | BacT/Alert | Bactec | BacT/Alert | |
| Meropenem | 22.1 ± 9.5 (20) | 20.5 ± 1.6 (20) | 10.5 ± 4.8 (10) | 10.8 ± 0.5 (10) | 2.9 ± 1.8 (2.5) | 2.7 ± 0.2 (2.5) |
| Ceftolozane | 113.9 ± 57.3 (75) | 88.0 ± 0.6 (75) | 32.4 ± 5.0 (25) | 30.2 ± 5.7 (25) | 5.0 ± 0.3 (4) | 5.3 ± 0.5 (4) |
| Ceftazidime | 66.0 ± 3.3 (45) | 64.3 ± 0.1 (45) | 22.6 ± 1.2 (12.5) | 22.8 ± 1.9 (12.5) | 10.5 ± 3.5 (5) | 9.3 ± 0.5 (5) |
Data are presented as means ± standard deviations for duplicate observed concentrations (whole-blood target concentrations). The target whole-blood concentration was calculated as one-half of the target plasma concentration assuming 50% hematocrit of blood from healthy volunteers.
P. aeruginosa growth.
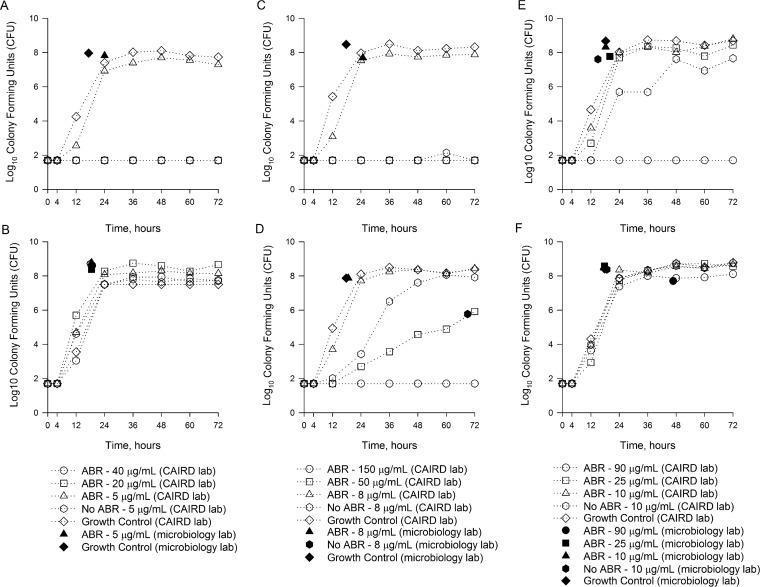
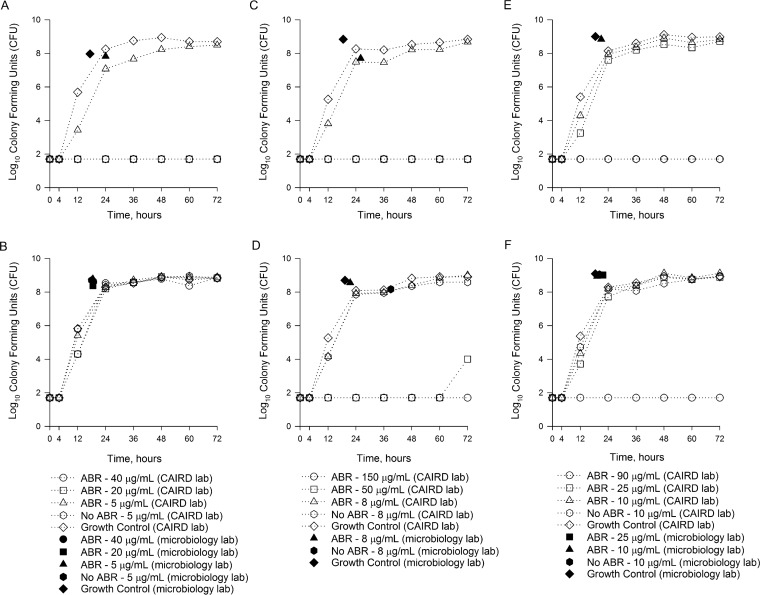
Growth curves over 72 h are presented in Fig. 1 and 2 for Bactec and BacT/Alert BC bottles, respectively. Controls (no antibiotic present) grew to 2.4 × 108 CFU/ml and 7.4 × 108 CFU/ml in Bactec and BacT/Alert BC bottle studies, respectively.
FIG 1.
Growth curves over 72 h for P. aeruginosa isolates in Bactec Plus Aerobic/F and Bactec Standard/10 Aerobic/F bottles inoculated with meropenem versus P. aeruginosa ATCC 27853 (A), meropenem versus P. aeruginosa 147 (B), ceftolozane-tazobactam versus P. aeruginosa ATCC 27853 (C), ceftolozane-tazobactam versus P. aeruginosa 147 (D), ceftazidime-avibactam versus P. aeruginosa ATCC 27853 (E), and ceftazidime-avibactam versus P. aeruginosa 147 (F). ABA, blood culture bottles containing antibiotic binding agents.
FIG 2.
Growth curves over 72 h for P. aeruginosa isolates in BacT/Alert FA Plus Aerobic and BacT/Alert SA Standard Aerobic bottles inoculated with meropenem versus P. aeruginosa ATCC 27853 (A), meropenem versus P. aeruginosa 147 (B), ceftolozane-tazobactam versus P. aeruginosa ATCC 27853 (C), ceftolozane-tazobactam versus P. aeruginosa 147 (D), ceftazidime-avibactam versus P. aeruginosa ATCC 27853 (E), and ceftazidime-avibactam versus P. aeruginosa 147 (F). The corresponding keys for each antibiotic are located below the specific antibiotic graphs. ABA, blood culture bottles containing antibiotic binding agents.
Combining the results for all BC bottles containing resins, P. aeruginosa growth was observed in 11/48 (22.9%), 22/48 (45.8%), and 47/48 (97.9%) bottles inoculated with peak, midpoint, and trough concentrations, respectively (P ≤ 0.001). For Bactec Plus Aerobic Plus/F studies, growth was observed in 7/24 (29.2%), 12/24 (50.0%), and 24/24 (100.0%) BC bottles inoculated with peak, midpoint, and trough concentrations, respectively (P ≤ 0.001). For BacT/Alert FA Plus studies, growth was observed in 4/24 (16.7%), 10/24 (41.7%), and 23/24 (95.8%) BC bottles inoculated with peak, midpoint, and trough concentrations, respectively (P ≤ 0.001). No statistically significant differences in growth were observed between Bactec and BacT/Alert systems in the presence of peak (P = 0.492), midpoint (P = 0.772), and trough (P = 1.0) concentrations.
Both P. aeruginosa ATCC 27853 and P. aeruginosa 147 grew in all Bactec Plus Aerobic Plus/F and BacT/Alert FA Plus bottles in the presence of meropenem trough concentrations; however, peak and midpoint concentrations inhibited the growth of P. aeruginosa ATCC 27853 (Fig. 1A and 2A), while P. aeruginosa 147 grew in the presence of all meropenem concentrations (Fig. 1B and 2B). Both P. aeruginosa isolates grew in all Bactec Plus Aerobic Plus/F and BacT/Alert FA Plus bottles in the presence of ceftolozane-tazobactam trough concentrations (Fig. 1C and D and 2C and D). However, neither isolate grew in any BC bottles containing ceftolozane-tazobactam peak or midpoint concentrations (Fig. 1C and D and 2C and D). For ceftazidime-avibactam, both isolates grew in the presence of trough and midpoint concentrations in Bactec Plus Aerobic Plus/F bottles, while peak concentrations inhibited the growth of P. aeruginosa ATCC 27853 (Fig. 1E and F). In BacT/Alert FA Plus bottles, both isolates grew in the presence of ceftazidime-avibactam trough concentrations (Fig. 2E and F). Peak concentrations inhibited both isolates, while midpoint ceftazidime-avibactam concentrations inhibited the growth of only P. aeruginosa ATCC 27853. Five individual runs were continued to 120 h, including 2 Bactec (1 each with ceftolozane-tazobactam and ceftazidime-avibactam) and 3 bioMérieux (1 with meropenem and 2 with ceftolozane-tazobactam) studies, with no new positive growth being identified beyond 72 h (data not shown).
During experiments with Bactec and BacT/Alert Standard BC bottles (no antibiotic binding resins), growth was observed for the less susceptible isolate, P. aeruginosa 147, in the presence of trough concentrations of all antibiotics (Fig. 1B, D, and F and 2B, D, and F). This was not unexpected given that the diluted trough concentrations for all antibiotics were lower than the MICs. For P. aeruginosa ATCC 27853, however, growth was inhibited in both bottles containing meropenem and ceftazidime-avibactam trough concentrations (Fig. 1A and E and 2A and E). This was in contrast to results with these antibiotics in the bottles containing binding resins. Notably, growth of P. aeruginosa ATCC 27853 was observed in Bactec Standard BC bottles containing ceftolozane-tazobactam trough concentrations (FIG 2C).
Time to detection.
A comparison of TTDs between Bactec and BacT/Alert antibiotic binding agent BC bottles is provided in Table 2. For control experiments, the TTD was 17.4 ± 1.2 h in Bactec BC bottles versus 18.4 ± 1.0 h in BacT/Alert BC bottles (P = 0.037). When P. aeruginosa was detected in antibiotic-containing bottles, the TTD was typically within 26 h of inoculation. No significant differences in TTDs were observed between Bactec and BacT/Alert BC bottles except in the case of studies with ceftazidime-avibactam peak concentrations (TTD of 46.8 ± 1.8 for P. aeruginosa 147 in Bactec bottles versus no growth in BacT/Alert bottles).
TABLE 2.
Comparative summary of TTD observations using microbiology laboratory detection instruments for BC bottles containing antimicrobial binding agentsa
| Antibiotic | Concn category | Mean TTD (h) ± SD |
P valueb | |||
|---|---|---|---|---|---|---|
| Bactec Plus Aerobic Plus/F |
BacT/Alert FA Plus Aerobic |
|||||
| P. aeruginosa ATCC 27853 | P. aeruginosa 147 | P. aeruginosa ATCC 27853 | P. aeruginosa 147 | |||
| Meropenem | Peak | NG | 17.8 ± 0.4 | NG | 18.8 ± 0.4 | 0.130 |
| Midpoint | NG | 18 ± 0 | NG | 18.5 ± 0.7 | 0.419 | |
| Trough | 18.6 ± 0.5 | 18 ± 0 | 24 ± 1.4 | 18.5 ± 0.7 | 0.122 | |
| Ceftolozane-tazobactam | Peak | NG | NG | NG | NG | ND |
| Midpoint | NG | NG | NG | NG | ND | |
| Trough | 24.8 ± 0.4 | 18.6 ± 0.9 | 26c | 21.5 ± 0.7 | 0.622 | |
| Ceftazidime-avibactam | Peak | NG | 46.8 ± 1.8 | NG | NG | ND |
| Midpoint | 20c | 17.8 ± 0.4 | NG | 21.8 ± 0.4 | 0.061 | |
| Trough | 18.3 ± 1.1 | 17.5 ± 0 | 21 ± 2.8 | 19.3 ± 0.4 | 0.077 | |
Data are presented as mean TTDs (hours) ± standard deviations for duplicates. NG, no growth detected; ND, not done.
P values represent comparisons between Bactec and BacT/Alert bottles at specific antibiotic concentrations for all P. aeruginosa cultures that grew.
Only a single TTD was reported due to a lack of growth in a duplicate experiment.
Antibiotic concentrations.
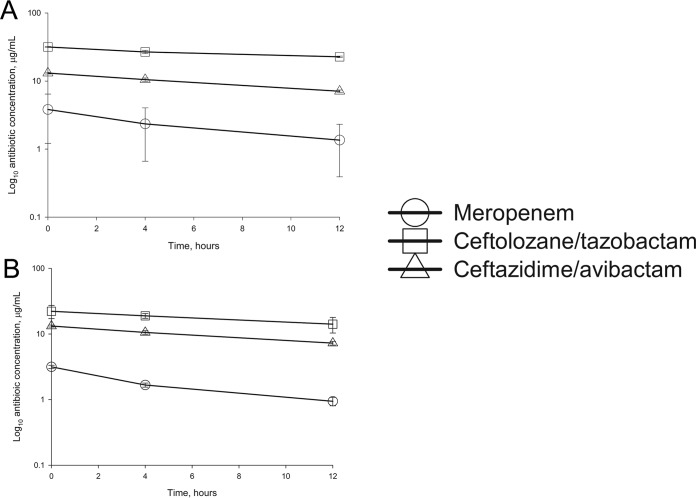
The reductions of meropenem, ceftolozane, and ceftazidime concentrations over the first 12 h from BC bottles spiked with peak concentrations are presented in Fig. 3. This decline suggests first-order elimination in both Bactec and BacT/Alert bottles (i.e., the concentration of the drug decreases at a rate that is proportional to the amount of drug remaining). Notably, beginning with peak levels, concentrations at 12 h remained above the MIC susceptibility breakpoints for all agents. The percent reductions in drug concentrations within 10 min, and at 4 and 12 h, are listed in Table 3. Both Bactec and BacT/Alert bottles removed meropenem with the highest percentage, followed by ceftazidime and, finally, ceftolozane. Percent reductions for meropenem and ceftazidime were similar between systems, while BacT/Alert bottles reduced the concentrations of ceftolozane to a greater degree than did Bactec bottles.
FIG 3.
Meropenem, ceftolozane, and ceftazidime concentrations over the initial 12 h in Bactec Plus Aerobic/F (A) and BacT/Alert FA Plus Aerobic (B) blood culture bottles spiked with peak concentrations of the antibiotics.
TABLE 3.
Percent reduction in meropenem, ceftolozane, and ceftazidime concentrations over 12 h in Bactec Plus Aerobic/F and BacT/Alert FA Plus Aerobic blood culture bottles inoculated with peak antibiotic concentrations
| Antimicrobial agent (target concn at 0 h in bottle [μg/ml]) | Mean % reduction of observed concn ± SDa |
|||||
|---|---|---|---|---|---|---|
| 0–10 min |
4 h |
12 h |
||||
| Bactec | BacT/Alert | Bactec | BacT/Alert | Bactec | BacT/Alert | |
| Meropenem (10) | 61.7 ± 26.2 | 68.6 ± 3.0 | 76.5 ± 16.9 | 83.3 ± 1.2 | 86.4 ± 9.6 | 90.6 ± 1.4 |
| Ceftolozane (37.5) | 15.5 ± 1.3 | 40.8 ± 13.4 | 28.5 ± 4.0 | 49.5 ± 4.2 | 39.4 ± 1.5 | 62.3 ± 10 |
| Ceftazidime (22.5) | 41.6 ± 1.6 | 44.3 ± 7.9 | 53.6 ± 2.7 | 57.0 ± 7.5 | 68.3 ± 1.2 | 69.9 ± 6.3 |
Data are presented as mean percent reductions ± standard deviations of observed concentrations in samples obtained at the specified time points relative to the target concentration in peak-concentration bottles at 0 h. The target peak concentration in the BC bottles is the plasma concentration diluted 4-fold by the volume in each bottle.
DISCUSSION
Identification of bacterial pathogens from BC bottles remains the gold standard for the diagnosis of invasive BSI and clinical monitoring of pathogen clearance after the start of antibiotic treatment (3). During the initial diagnosis of bloodstream infections, it is recommended that cultures ideally be collected prior to the administration of empirical antibiotics. However, this is not always feasible, and previous studies observed prior antibiotic therapy for 50% to 82% of patients in ward and intensive care units, respectively (12). Furthermore, when monitoring the clearance of blood cultures while a patient is on antibiotics, it is not possible to obtain a sample without treatment having already been administered. In either of these clinical scenarios, residual antibiotic concentrations in BC bottles may interrupt pathogen growth and prevent detection, resulting in falsely negative cultures. As a result, various proprietary antibiotic binding resins are included in BC bottles to reduce antibiotic concentrations and allow pathogen growth.
In this study, we tested the abilities of two popular BC bottle systems (Bactec Plus Aerobic/F and BacT/Alert FA Plus Aerobic) to identify P. aeruginosa after exposure to clinically meaningful concentrations of meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam. Although the antibiotic binding resins reduced antibiotic concentrations in the bottles, inhibition of P. aeruginosa was observed in the presence of peak and midpoint concentrations. P. aeruginosa was detected in 22.9% and 45.8% of bottles containing peak and midpoint concentrations, respectively. The less susceptible isolate was more likely to grow in bottles containing these higher concentrations, but detection varied by antibiotic (Fig. 1 and 2). Only trough concentrations consistently allowed the growth of the test organisms, with detection in 97.9% of bottles and a TTD of typically <26 h. Notably, there were no differences in the detection of P. aeruginosa between the Bactec Plus and BacT/Alert FA Plus bottles, both of which contain antibiotic binding resins. These observations have significant implications for the timing and interpretation of blood cultures when patients receive these antibiotics.
Meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam were selected for this study because they are potent, broad-spectrum antibiotics typically reserved for multidrug-resistant Gram-negative infections, including those caused by P. aeruginosa. The empirical use of these agents for a BSI will be strongly scrutinized by antimicrobial stewardship teams, with recommendations for discontinuation promptly after a report of a negative blood culture when no other source is identified (14, 15, 16). Alternatively, if the antibiotic is used as directed therapy, the time to clearance of infection and potential development of resistance will be monitored. As a result, the implications are that falsely negative blood cultures could lead to the early discontinuation of required antibiotic therapy and suboptimal outcomes.
Although BC bottle studies with meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam have not been extensively performed, similar studies with other beta-lactam antibiotics are available for comparison. Zadroga and colleagues (12) determined the level of recovery of bacteria in clinical blood cultures considering the timing of administration of prior antibiotics. Recovery was reduced in the presence of prior antimicrobial administration, specifically in BacT/Alert FA FAN bottles (which contain activated charcoal instead of the adsorbent polymeric beads in the FA Plus bottles studied here) compared with Bactec Plus bottles. When those investigators focused on patients receiving piperacillin, residual concentrations above the MIC were observed in BacT/Alert bottles sampled near the peak or midpoint but not in those sampled near the trough. Lovern and colleagues (11) performed an in vitro assessment of the same blood culture bottles used in our study in the presence of various antimicrobials, including cefepime and ceftriaxone. When peak concentrations of cefepime and ceftriaxone were simulated, none of the five bottles demonstrated recovery of Escherichia coli or P. aeruginosa in either system. Those data agree with our observations suggesting that peak and potentially midpoint concentrations of beta-lactams may not be adequately reduced, thereby causing in vitro sterilization in the bottles. In contrast, a previous study of meropenem peak concentrations reported the recovery of a susceptible P. aeruginosa isolate (meropenem MIC, 0.094 μg/ml) in BacT/Alert FA Plus bottles (9). However, those investigators utilized banked blood instead of fresh whole blood and a larger bacterial inoculum (up to 100 CFU/bottle), which may contribute to the different observations.
Trough concentrations in Bactec and BacT/Alert Standard BC bottles, which contain no binding resins, were included as negative controls. Based on predicted antibiotic concentrations in the bottles after dilution, which is 5-fold for these bottles, compared with 4-fold for the BC bottles containing binding resins, trough concentrations should have led to the inhibition of susceptible strain ATCC 27853 and the growth of the less susceptible P. aeruginosa isolate 147. As a result of these dilutions, unbound trough concentrations should be lower than the MIC for P. aeruginosa 147 yet still higher than the MIC for ATCC 27853. In fact, we observed these exact results, with the exception of the ceftolozane experiments, which resulted in organism growth for ATCC 27853. We speculate that the actual ceftolozane trough concentration was just below the MIC for this organism during these experiments, thereby preventing inhibition. When the results for ATCC 27853 are compared between Standard BC bottles and those containing binding resins, it should be apparent that the binding resins, combined with dilution of the media, result in binding that is sufficient to prevent the artificial sterilization of the bottles.
By measuring the concentrations of meropenem, ceftolozane (active component), and ceftazidime (active component) in the bottles over 12 h, it was apparent that the resins in both systems reduce drug concentrations (Fig. 3), as noted above. However, beginning with peak concentrations, residual levels remained above the MICs for susceptible organisms. Within the first 10 min of inoculation, concentrations of meropenem and ceftazidime were reduced by ∼60 to 70% and ∼40 to 45% in both systems, respectively (Table 3). Ceftolozane concentrations in BacT/Alert bottles were also decreased by ∼40%; however, reductions of only 15% were found for the Bactec bottles. We did not test the removal of the beta-lactamase inhibitors tazobactam and avibactam, but these drugs do not themselves display activity against Gram-negative bacteria.
There are some limitations of the present study. This was an ex vivo (using fresh human blood and simulated concentrations)/in vitro experiment and did not use actual clinical samples from patients. Such a study would be challenging given the sparse use of these restricted antibiotics and the low rate of BC positivity. Nonetheless, the natural human variability in plasma concentrations would provide more robust data on recovery during sampling near the trough. Second, only 2 P. aeruginosa isolates were included. We selected isolates with a wide range of MICs (very susceptible to less susceptible) for each drug, albeit they were clinically relevant since resistance to ceftolozane-tazobactam and ceftazidime-avibactam among P. aeruginosa isolates is still uncommon (20, 21). More susceptible Gram-negative organisms, such as Enterobacteriaceae, may be more prone to false-negative results since the MICs for these organisms are typically lower than those for P. aeruginosa. We simulated the most aggressive dosing of these antibiotics typically administered in clinical practice; notably, the doses in the meropenem and ceftolozane-tazobactam regimens used are higher than currently approved doses. Lower doses of these compounds may result in improved recovery. Nonetheless, sampling at the trough should still retain the same detection as that observed here. Finally, although we stopped our experiments at 72 h, a few of our studies were continued to 120 h, without any changes in results. This is consistent with data from other studies indicating that most pathogens are detected prior to 72 h (22–24).
In conclusion, clinically achievable peak and midpoint concentrations of meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam may overwhelm the neutralization ability of antibiotic binding resins within the Bactec Plus and BacT/Alert FA Plus BC bottles and lead to artificial sterilization. However, consistent identification of both P. aeruginosa isolates was observed in the presence of trough concentrations. To minimize false-negative BC results for patients already receiving these antibiotics, cultures should be collected just prior to the next dose, when concentrations are lowest.
MATERIALS AND METHODS
Study design.
This was an ex vivo/in vitro study conducted at the Center for Anti-Infective Research and Development (CAIRD) (Hartford Hospital, Hartford, CT) and the clinical microbiology laboratories of Hartford Hospital (i.e., Bactec experiments) and Manchester Memorial Hospital (Manchester, CT) (i.e., BacT/Alert experiments).
Bacteria.
Two P. aeruginosa strains were selected based on their meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam MICs determined in triplicate by using broth microdilution (25). P. aeruginosa ATCC 27853 was susceptible to all antibiotics. P. aeruginosa 147, a clinical isolate originating from a previous susceptibility surveillance study (20), was meropenem resistant but susceptible to ceftolozane-tazobactam and ceftazidime-avibactam with MICs that were at the current FDA susceptibility breakpoints (Table 4). All bacteria were frozen in skim milk at −80°C until needed. Isolates were subcultured twice on solid medium (Trypticase soy agar plates containing 5% blood) from frozen stocks and incubated at 37°C overnight before experiments were performed.
TABLE 4.
Modal MICs and categorical interpretation for meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam against the 2 P. aeruginosa isolates used in the study
| P. aeruginosa isolate | Antibiotic MIC (μg/ml) (interpretation)a |
||
|---|---|---|---|
| Meropenem | Ceftolozane-tazobactam | Ceftazidime-avibactam | |
| ATCC 27853 | 0.5 (S) | 0.5/4 (S) | 2/4 (S) |
| 147 | 8 (R) | 4/4 (S) | 8/4 (S) |
The breakpoints for meropenem are 2, 4, and 8 μg/ml for susceptible (S), intermediate, and resistant (R) strains, respectively; the breakpoints for ceftolozane-tazobactam are 4, 8, and 16 μg/ml for susceptible, intermediate, and resistant strains, respectively (ceftolozane component); and the breakpoints for ceftazidime-avibactam are 8 and 16 μg/ml for susceptible and resistant strains, respectively (ceftazidime component).
BC bottle media.
Bactec Plus Aerobic/F (Becton Dickinson and Company, Franklin Lakes, NJ) and BacT/Alert FA Plus Aerobic (bioMérieux Inc., Durham, NC) bottles, both of which contain proprietary antibiotic binding resins, were purchased from manufacturers. Bactec Standard/10 Aerobic/F (Becton Dickinson and Company, Franklin Lakes, NJ) and BacT/Alert SA Standard Aerobic (bioMérieux Inc., Durham, NC) bottles, free of any antibiotic binding resins, were included to serve as a negative control, as residual antibiotic concentrations in these bottles should inhibit P. aeruginosa growth above the MIC.
Antibiotics.
Commercially available formulations of meropenem (Fresenius Kabi USA Inc., Lake Zurich, IL), ceftolozane-tazobactam (Zerbaxa; Merck & Co. Inc., Kenilworth, NJ), and ceftazidime-avibactam (Avycaz; Allergan Inc., Jersey City, NJ) were purchased from Cardinal Health (Dublin, OH). Vials were reconstituted according to the manufacturers' instructions for clinical use.
BC bottle preparation and sampling.
Fresh whole-blood samples were collected from two healthy adult volunteers on the day of each experiment. Pooled whole blood was inoculated with meropenem, ceftolozane-tazobactam, or ceftazidime-avibactam to obtain average peak, midpoint, or trough concentrations for the most aggressive dosing regimens used clinically (Table 5) (26–28). For ceftolozane-tazobactam and ceftazidime-avibactam, targeted antibiotic concentrations were based on the β-lactam component only. Antibiotics were added to whole blood in consideration of a normal hematocrit to simulate plasma concentrations (29).
TABLE 5.
Target mean plasma concentrations for meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam dosing regimens
| Antibiotic | Dosing regimena | Concn (μg/ml) |
||
|---|---|---|---|---|
| Peak | Midpoint | Trough | ||
| Meropenemb | 2 g q8h (3-h infusion) | 40 | 20 | 5 |
| Ceftolozane-tazobactamc | 3 g (2 g ceftolozane–1 g tazobactam) q8h (1-h infusion) | 150 | 50 | 8 |
| Ceftazidime-avibactamd | 2.5 g (2 g ceftazidime–0.5 g avibactam) q8h (2-h infusion) | 90 | 25 | 10 |
q8h, every 8 h.
Meropenem pharmacokinetics were reported previously (24).
Ceftolozane-tazobactam pharmacokinetics were reported previously (25); target concentrations were based on the ceftolozane component only.
Ceftazidime-avibactam pharmacokinetics were reported previously (26); target concentrations were based on the ceftazidime component only.
Each isolate/antibiotic combination was prepared by using a set of five BC bottles from each manufacturer (3 Bactec Plus Aerobic/F and 2 Bactec Standard/10 Aerobic/F or 3 BacT/Alert FA Plus Aerobic and 2 BacT/Alert SA Standard Aerobic bottles). Inoculums were prepared from bacteria suspended to a McFarland density standard of 1 in sterile 0.9% sodium chloride, followed by serial dilution with the target of 7 to 30 CFU in a volume of 0.5 ml; this volume was inoculated into each BC bottle with the aim of achieving the lowest number of CFU identifiable in these systems (11). Immediately following bacterial inoculation, 10 ml of whole blood containing the targeted antibiotic concentrations was added to the three BC bottles containing antibiotic binding resins. The two standard bottles containing no antibiotic binding resins received whole blood with no antibiotics added (growth control) or whole blood inoculated with trough concentrations only (negative control). Replicate BC bottles were prepared so that one set of five bottles was incubated at 37°C at CAIRD, while the other set was loaded into the Becton Dickinson Bactec automatic blood culture system or the bioMérieux BacT/Alert-3D automated system at the respective clinical microbiology laboratories within 2 h of preparation. All CAIRD bottles were incubated for 72 h. BC bottles in the clinical microbiology laboratories were incubated for up to 72 h or until the system alerted the technician to positive growth. At least one run from each antibiotic was extended to 120 h.
Each BC bottle incubated at CAIRD was sampled for CFU counts and antibiotic concentrations within 10 min of predefined time points (0, 4, 12, 24, 36, 48, 60, and 72 h after inoculation). The growth control bottles were sampled for antibiotic concentrations only at 0 h. Samples used to assess antibiotic concentrations in the BC bottles were promptly frozen at −80°C until assayed. CFU were determined by using standard plating and manual colony counts via serial dilutions. The lower limit of detection was 1.7 log10 CFU/ml. Replicate BC bottles loaded into the clinical microbiology laboratory systems were sampled at 0 h as described above and then within 6 h of a positive alert; the TTD was recorded. BC bottles that remained negative at 72 h were subcultured to confirm the lack of growth. All experiments were conducted in duplicate on separate weeks.
Data analyses.
The TTD in positive BC bottles was normalized to the time of BC bottle inoculation. BC bottles that remained negative at 72 h were reported as having no growth. The mean CFU per milliliter at each time point for the duplicate BC bottles were calculated and plotted on growth curves for visualization. A final categorization of growth was based on agreement observed at 72 h for at least 75% of the identically prepared BCs.
Meropenem, ceftolozane, and ceftazidime concentrations in whole blood and BC bottles were determined at CAIRD by using validated high-performance liquid chromatography assays. The individual inhibition kinetics of meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam within the BC bottles was estimated separately for Bactec Plus Aerobic Plus/F and BacT/Alert FA Plus Aerobic BC bottles using the meropenem, ceftolozane, and ceftazidime log-transformed concentrations at 0, 4, and 12 h.
Statistical analyses were conducted with Sigma Plot version 13 (Systat Inc., San Jose, CA). Comparisons between categorical variables were evaluated with a chi-square or Fisher's exact test. Analysis of variance was used to compare the mean CFU per bottle inoculum and TTD between systems, isolates, and antibiotics. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank Lee Steere, Marguerite Monogue, Sean Stainton, Elizabeth Cyr, Kimelyn Greenwood, Raquel L. Diaz, Christina Sutherland, and Debora Santini for their assistance during the study.
This work was supported by a 2016 Society of Infectious Disease Pharmacists (SIDP)/bioMérieux antimicrobial stewardship and microbial diagnostics grant.
REFERENCES
- 1.Goto M, Al-Hasan MN. 2013. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 2.Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton R, D'Inzeo T, Fadda G, Cauda R, Spanu T. 2007. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 51:1987–1994. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubourg G, Raoult D. 2016. Emerging methodologies for pathogen identification in positive blood culture testing. Expert Rev Mol Diagn 16:97–111. doi: 10.1586/14737159.2016.1112274. [DOI] [PubMed] [Google Scholar]
- 4.Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. 2016. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol 7:697. doi: 10.3389/fmicb.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell JJ, Hujer AM, Sampath R, Bonomo RA. 2015. Salvage microbiology: opportunities and challenges in the detection of bacterial pathogens following initiation of antimicrobial treatment. Expert Rev Mol Diagn 15:349–360. doi: 10.1586/14737159.2015.989216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flayhart D, Borek AP, Wakefield T, Dick J, Carroll KC. 2007. Comparison of BACTEC PLUS blood culture media to BacT/Alert FA blood culture media for detection of bacterial pathogens in samples containing therapeutic levels of antibiotics. J Clin Microbiol 453:816–821. doi: 10.1128/JCM.02064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaargaren J, van Boven CP, Voorn GP. 1998. Effectiveness of resins in neutralizing antibiotic activities in Bactec plus Aerobic/F culture medium. J Clin Microbiol 36:3731–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viganò EF, Vasconi E, Agrappi C, Clerici P, Melloni P. 2004. Use of simulated blood cultures for antibiotic effect on time to detection of the two blood culture systems BacT/ALERT and BACTEC 9240. New Microbiol 27:235–248. [PubMed] [Google Scholar]
- 9.Mitteregger D, Barousch W, Nehr M, Kundi M, Zeitlinger M, Makristathis A, Hirschl AM. 2013. Neutralization of antimicrobial substances in new BacT/Alert FA and FN Plus blood culture bottles. J Clin Microbiol 515:1534–1540. doi: 10.1128/JCM.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller NS, Rogan D, Orr BL, Whitney D. 2011. Comparison of BD Bactec Plus blood culture media to VersaTREK Redox blood culture media for detection of bacterial pathogens in simulated adult blood cultures containing therapeutic concentrations of antibiotics. J Clin Microbiol 494:1624–1627. doi: 10.1128/JCM.01958-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovern D, Katzin B, Johnson K, Broadwell D, Miller E, Gates A, Deol P, Doing K, van Belkum A, Marshall C, Mathias E, Dunne WM Jr. 2016. Antimicrobial binding and growth kinetics in BacT/ALERT FA Plus and BACTEC Aerobic/F Plus blood culture media. Eur J Clin Microbiol Infect Dis 35:2033–2036. doi: 10.1007/s10096-016-2759-9. [DOI] [PubMed] [Google Scholar]
- 12.Zadroga R, Williams DN, Gottschall R, Hanson K, Nordberg V, Deike M, Kuskowski M, Carlson L, Nicolau DP, Sutherland C, Hansen GT. 2013. Comparison of 2 blood culture media shows significant differences in bacterial recovery for patients on antimicrobial therapy. Clin Infect Dis 56:790–797. doi: 10.1093/cid/cis1021. [DOI] [PubMed] [Google Scholar]
- 13.Funk DJ, Kumar A. 2011. Antimicrobial therapy for life-threatening infections: speed is life. Crit Care Clin 27:53–76. doi: 10.1016/j.ccc.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:1197–1202. doi: 10.1093/cid/ciw217. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. 2017. Surviving Sepsis campaign. International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 16.Hughes J, Huo X, Falk L, Hurford A, Lan K, Coburn B, Morris A, Wu J. 2017. Benefits and unintended consequences of antimicrobial de-escalation: implications for stewardship programs. PLoS One 12:e0171218. doi: 10.1371/journal.pone.0171218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grupper M, Kuti JL, Nicolau DP. 2016. Continuous and prolonged intravenous β-lactam dosing: implications for the clinical laboratory. Clin Microbiol Rev 29:759–772. doi: 10.1128/CMR.00022-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monogue ML, Pettit RS, Muhlebach M, Cies JJ, Nicolau DP, Kuti JL. 2016. Population pharmacokinetics and safety of ceftolozane-tazobactam in adult cystic fibrosis patients admitted with acute pulmonary exacerbation. Antimicrob Agents Chemother 21:6578–6584. doi: 10.1128/AAC.01566-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loo AS, Neely M, Anderson EJ, Ghossein C, McLaughlin MM, Scheetz MH. 2013. Pharmacodynamic target attainment for various ceftazidime dosing schemes in high-flux hemodialysis. Antimicrob Agents Chemother 57:5854–5859. doi: 10.1128/AAC.00474-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland CA, Nicolau DP. 2015. Susceptibility profile of ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from US hospitals. Clin Ther 37:1564–1571. doi: 10.1016/j.clinthera.2015.05.501. [DOI] [PubMed] [Google Scholar]
- 21.Flamm RK, Nichols WW, Sader HS, Farrell DJ, Jones RN. 2016. In vitro activity of ceftazidime/avibactam against Gram-negative pathogens isolated from pneumonia in hospitalized patients, including ventilated patients. Int J Antimicrob Agents 47:235–242. doi: 10.1016/j.ijantimicag.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Reisner BS, Woods GL. 1999. Times to detection of bacteria and yeasts in BACTEC 9240 blood culture bottles. J Clin Microbiol 37:2024–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durmaz G, Us T, Aydinli A, Kiremitci A, Kiraz N, Akgün Y. 2003. Optimum detection times for bacteria and yeast species with the BACTEC 9120 aerobic blood culture system: evaluation for a 5-year period in a Turkish university hospital. J Clin Microbiol 41:819–821. doi: 10.1128/JCM.41.2.819-821.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourbeau PP, Pohlman JK. 2001. Three days of incubation may be sufficient for routine blood cultures with BacT/Alert FAN blood culture bottles. J Clin Microbiol 39:2079–2082. doi: 10.1128/JCM.39.6.2079-2082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. CLSI document M100-S24 U. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Crandon JC, Ariano RE, Zelenitsky SA, Nicasio AM, Kuti JL, Nicolau DP. 2011. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med 37:632–638. doi: 10.1007/s00134-010-2105-0. [DOI] [PubMed] [Google Scholar]
- 27.Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 56:3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merdjan H, Rangaraju M, Tarral A. 2015. Safety and pharmacokinetics of single and multiple ascending doses of avibactam alone and in combination with ceftazidime in healthy male volunteers: results of two randomized placebo-controlled studies. Clin Drug Invest 35:307–317. doi: 10.1007/s40261-015-0283-9. [DOI] [PubMed] [Google Scholar]
- 29.Grupper M, Kuti JL, Nicolau DP. 2017. In vitro blood culture bottle inoculation of whole blood with clinically relevant antibiotic concentrations: a word of caution. Eur J Clin Microbiol Infect Dis 36:917–919. doi: 10.1007/s10096-016-2874-7. [DOI] [PubMed] [Google Scholar]