Abstract
Parkinson's disease (PD) is one of the most prevalent neurodegenerative disease displaying negative impacts on both the health and social ability of patients and considerable economical costs. The classical anti-parkinsonian drugs based in dopaminergic replacement are the standard treatment, but several motor side effects emerge during long-term use. This mini-review presents the rationale to several efforts from pre-clinical and clinical studies using adenosine receptor antagonists as a non-dopaminergic therapy. As several studies have indicated that the monotherapy with adenosine receptor antagonists reaches limited efficacy, the usage as a co-adjuvant appeared to be a promising strategy. The formulation of multi-targeted drugs, using adenosine receptor antagonists and other neurotransmitter systems than the dopaminergic one as targets, have been receiving attention since Parkinson's disease presents a complex biological impact. While pharmacological approaches to cure or ameliorate the conditions of PD are the leading strategy in this area, emerging positive aspects have arisen from non-pharmacological approaches and adenosine function inhibition appears to improve both strategies.
Keywords: adenosine, A2AAR, dopaminergic system, neurodegeneration, Parkinson disease
General aspects of parkinson's disease
Parkinson's disease (PD) is the second most prevalent chronic neurodegenerative disease, affecting more than 1% of the elderly population, with diagnostic confirmation occurring when the loss of dopaminergic neurons in the striatum is close to 80% (de Rijk et al., 2000). PD is also diagnosed in people less than 40 years old, named early-onset PD (Crosiers et al., 2011). PD is associated with the formation of Lewy bodies and neurites (Braak et al., 2003), mainly composed of aggregated forms of α-synuclein (Spillantini et al., 1998). The loss of dopaminergic neurons causes a reduction in the release of dopamine, leading to motor symptoms such as bradykinesia, rigidity, imbalance and tremor (Jankovic, 2008). PD presents in sporadic and familial forms. The risk factors involved in the development of PD are both genetic and environmental (Mortimer et al., 2012; Noyce et al., 2012; Van der Mark et al., 2012; Pezzoli and Cereda, 2013). The familial form, with specific genetic targets, represents less than 10% of PD cases (Dawson and Dawson, 2010). The genetic aspects of the disease are linked to mutations in several genes related to a multitude of cellular mechanisms, such as protein aggregation, protein and membrane trafficking, lysosomal autophagy, immune response, synaptic function, endocytosis, inflammation, and metabolic pathways (Redenšek et al., 2017). The genes SNCA (PARK1), UCHL1 (PARK5), LRRK2 (PARK8), GIGYF2 (PARK11), OMI/HTRA2 (PARK13), VPS35 (PARK17), and EIF4G1 (PARK18) result in autosomal dominant PD, and PRKN (PARK2), DJ-1 (PARK7), ATP13A2 (PARK9), PLA2G6 (PARK14), FBX07 (PARK15), DNJC6 (PARK19), and SYNJ1 (PARK20) causes autosomal recessive PD (Lautier et al., 2008; Di Fonzo et al., 2009; Klein and Westenberger, 2012; Deng et al., 2015; Bartonikova et al., 2016; Miki et al., 2017; Scott et al., 2017). The gene contribution from other loci (PARK 3, 10, 12, and 16) is under investigation (Dawson and Dawson, 2010). However, a putative causative mutation in the gene that encodes the A1 adenosine receptor, located in the locus PARK16, has been related to susceptibility to PD (Jaberi et al., 2016). Among the environmental contributors to PD development are occupational exposure of pesticides, such as Rotenone and Paraquat, infection by Helicobacter and HCV, low body weight and sedentary lifestyle (McCarthy et al., 2004; Villar-Cheda et al., 2009; Golabi et al., 2017; Sharma and Lewis, 2017; Shen et al., 2017).
The relationship of adenosine and dopamine signaling
Adenosine affects dopaminergic signaling through receptor heteromer formations and shared intracellular pathways. Adenosine is a neuromodulator that acts through the A1 (A1AR) and A3 (A3AR) inhibitory adenosine receptors and A2A (A2AAR) and A2B (A2BAR) excitatory adenosine receptors (Ralevic and Burnstock, 1998). D1 (D1DR) and D2 (D2DR) dopamine receptors are found co-localized with A2AAR and A1AR, mGluR5 and NMDA (Hillion et al., 2002; Lee et al., 2002; Beggiato et al., 2016). The dopamine-adenosine receptor heteromers are constituted mainly of D1DR/A1AR and D2DR/A2AAR, displaying antagonistic properties. A1AR agonist decreases the binding potential of dopamine to D1DR, and reduces the D1DR-induced cAMP production, while A1AR antagonists activate D1DR increasing cAMP levels (Ferré et al., 1998). A3AR activation appears to have some influence on dopamine release and vesicular transport, while no functional impacts have been registered in dopamine receptors (Gołembiowska and Zylewska, 1998; Björklund et al., 2008; Shen et al., 2011).
The heteromerization of D2DR/A2AAR is one of the most studied receptors interaction. A2AAR agonists reduce the in vitro affinity of the D2DR agonist through an increase in D2DR Kd without affecting receptor density (Ferré et al., 1991). In vivo studies confirmed these findings since the administration of A2AAR antagonist increased the effects of the D2DR agonist in the rat striatum and basal ganglia, while the action of A2AAR agonists was opposite (Hillefors-Berglund et al., 1995; Strömberg et al., 2000). This heteromerization was confirmed through co-immunoprecipitation, fluorescence resonance energy, bioluminescence resonance energy transfer and ex vivo proximity ligation studies (Hillion et al., 2002; Canals et al., 2003; Trifilieff et al., 2011; Fernández-Dueñas et al., 2015). Studies with PET in the human brain showed the increased binding of a D2DR antagonist, after the administration of caffeine, a nonselective antagonist of adenosine receptors (Volkow et al., 2015).
The interaction between adenosinergic and dopaminergic receptors has been described as intramembrane, involving direct interaction between receptors, or the modulation of G-proteins and the consequent influence on cAMP-dependent proteins (Fuxe et al., 1998; Ferré et al., 2001; Hillion et al., 2002; Fredholm and Svenningsson, 2003). The administration of D2DR antagonists can reduce the cAMP production by A2AAR and the D2 agonist administration induces increase in cAMP levels by A2AAR (Vortherms and Watts, 2004; Botsakis et al., 2010). A2AAR stimulation, in vitro, causes the phosphorylation and activation of DARPP-32, which can be inhibited by D2DR activation (Nishi et al., 1997). A2AAR antagonists increase D2DR-dependent regulation of c-fos, which is more intense when dopaminergic neurodegeneration is presented (Pollack and Fink, 1995; Svenningsson et al., 1999). Compelling evidence for the impairment of D2DR/A2AAR oligomers in the striatum of rats was obtained in experimental Parkinsonism induced by 6-hydroxydopamine (6-OHDA) (Fernández-Dueñas et al., 2015). The ventral striopallidal GABA pathway appears to be a target of mGlu5R/D2DR/A2AAR interactions. The co-administration of A2AAR and mGlu5R agonist enhances GABA release compared with mGlu5R agonist alone, and this effect decreases with the administration of D2DR agonists (Díaz-Cabiale et al., 2002). In addition, D2DR/A2AAR controls NMDA-mediated excitation in neurons from the nucleus accumbens through a direct protein–protein interaction (Azdad et al., 2009).
Support for the A2AAR antagonism hypothesis from animal studies
The co-expression of D2DR/A2AAR receptors and their close functional and structural association in the striatopallidal GABAergic neurons reveals sites for therapeutic intervention and has received attention in the last three decades (Fink et al., 1992; Kase, 2001; Kelsey et al., 2009). The non-specific blockade of adenosine receptors by methylxanthines produces contralateral rotations in animals with dopaminergic lesions induced by 6-OHDA, since contralateral rotations have been related to an indirect stimulation of dopamine receptors in the lesioned area (Watanabe et al., 1981; Herrera-Marschitz et al., 1988).
During the late 1990s and early 2000s, exciting results from animal models of Parkinsonism indicated that A2AAR antagonism improves motor activity by reducing the postsynaptic effects of dopamine depletion. Caffeine neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced lesion showed to be especially dependent on A2AAR from the striatal neurons, but not exclusively (Chen et al., 2001; Xu et al., 2016). The A2AAR antagonist KW6002 (Istradefylline) was shown to be powerful enough to increase locomotion activity and potentiate dopaminergic agonist motor effects in MPTP- and 6-OHDA-lesioned animals (Kanda et al., 1998, 2000; Grondin et al., 1999; Koga et al., 2000; Bibbiani et al., 2003). The anti-parkinsonian effects of KW6002 and similar drugs, such as KW17837, appear to be dose-dependent, effective in the postsynapse and beyond the direct effect on the dopaminergic system, and act on glutamatergic/gabaergic neurotransmission and monoamine oxidase activity (Bibbiani et al., 2003; Petzer et al., 2003; Tanganelli et al., 2004; Orru et al., 2011). MSX-3, a water-soluble precursor of the highly specific A2AAR antagonist MSX-2, which exhibits greater potency for A2AAR than KW6002, appeared to be a candidate of monotherapy since it alleviates the symptomatic parkinsonian locomotor deficiency in a genetic model of dopaminergic degeneration (Yang et al., 2007; Marcellino et al., 2010).
While some studies advocated that A2AAR antagonism, as a monotherapy, could reach a mildly lower or similar efficacy of L-DOPA treatment without inducing dyskinesia (Grondin et al., 1999; Pinna et al., 2007), the promisor effect of these drugs appeared to be when co-administrated with L-DOPA, simultaneously inhibiting A2AAR and activating D2DR. A2AAR-knockout animals demonstrated weak and transitory rotational sensitization and no sensitized grooming as a response to L-DOPA (Fredduzzi et al., 2002). The blockade of adenosine receptors by caffeine promoted additive or synergistic interactions with L-DOPA (Yu et al., 2006), whereas the co-administration of specific A2AAR antagonists, such as KW6002, ST1535, and L-DOPA, potentiated the anti-parkinsonian effect of L-DOPA without exacerbating dyskinesia (Kanda et al., 2000; Koga et al., 2000; Bibbiani et al., 2003; Matsuya et al., 2007; Tronci et al., 2007). However, some studies using several A2AAR antagonists, such as SCH4123-48, BIIB014 (Vipanedant), KW6002 and caffeine, when administered concomitantly and chronically with L-DOPA, failed to avoid dyskinesia (Jones et al., 2013).
The mechanism behind the effects of A2AAR antagonists alone or as co-adjuvant drugs appears to beyond actions on dopaminergic system (Fuxe et al., 2009; Maggio et al., 2009; Figure 1). The A2AAR exerts its neuronal activity in the striatum in a manner that is partially independent of D2Rs (Chen et al., 2001). Actually, KW6002 decreases the neuronal activity of the striatopallidal indirect pathway in the absence of D2R-mediated signaling (Aoyama et al., 2000). Dopaminergic neurodegeneration induced by transgenic mutant human α-synuclein is prevented in mice lacking the A2AAR reinforcing the potential of shared downstream pathways (Ferraro et al., 2012). However, the adenylate cyclase activity did not differ in a genetic model of PD, suggesting that coupling to G-proteins of dopaminergic and adenosinergic receptors should be a target (Botsakis et al., 2010). Regional differences appear in the anti-parkinsonian ability of A2AAR antagonism, since caffeine given at or before MPTP exposure blocks the nigral neurodegenerative process without restoring the striatal nerve terminal neurochemical features (Sonsalla et al., 2012). Motor sensitization developed in unilaterally 6-OHDA-lesioned rats submitted to L-DOPA has been associated with an overexpression of the GABA-synthesizing enzyme glutamic acid decarboxylase, dynorphin, and enkephalin mRNAs in the striatal efferent indirect pathway (Fink et al., 1992; Tronci et al., 2007). The impact of A2AAR antagonism over enkephalin content seems to promote motor recovery in D2DR-knockout animals, but did not promote changes in the preproenkephalin mRNA in a 6-OHDA model (Fink et al., 1992; Aoyama et al., 2000). The functional relation of D2DR/A2AAR in striatal medium spiny neurons appears to receive contributions of cholinergic signaling with consequences for the anti-tremor benefits of A2AAR antagonists (Simola et al., 2006; Tozzi et al., 2011; Salamone et al., 2013). The existence of A2AAR/mGlu5R heteromers and shared intracellular cascades steps, such as the stimulation of DARPP32 phosphorylation, increase in cAMP levels and elevated c-fos expression, provides clues to the possible contribution of glutamatergic and adenosinergic signaling to the beneficial effects of adenosine receptor antagonism (Nash and Brotchie, 2000; Kachroo et al., 2005). Effects resembling akinesia in 6-OHDA-lesioned rats were fully reversed by either a single treatment of an A2AAR antagonist or an mGlu5R antagonist at higher doses, or by a combined treatment with ineffective doses of each compound (Coccurello et al., 2004). Increased A2AAR mRNA levels, decreased DARPP-32 phosphorylation and increased phosphorylation of ERK1/2 appeared in 6-OHDA-lesioned rats that display L-DOPA motor sensitization (Tomiyama et al., 2004; Song et al., 2009). This altered downstream signaling pathway is recovered by CSC (8-(3-chlorostryryl) caffeine), an A2AAR antagonist (Song et al., 2009). Amelioration of motor response by A2AAR antagonism seems to be accompanied by the rescue of dopamine, dopamine metabolites, glutamate, and GABA striatal levels as well as the reversal of astroglial and microglial activation and antioxidant properties with beneficial outcomes on cognition (Aguiar et al., 2008; Gołembiowska et al., 2013; Uchida et al., 2014).
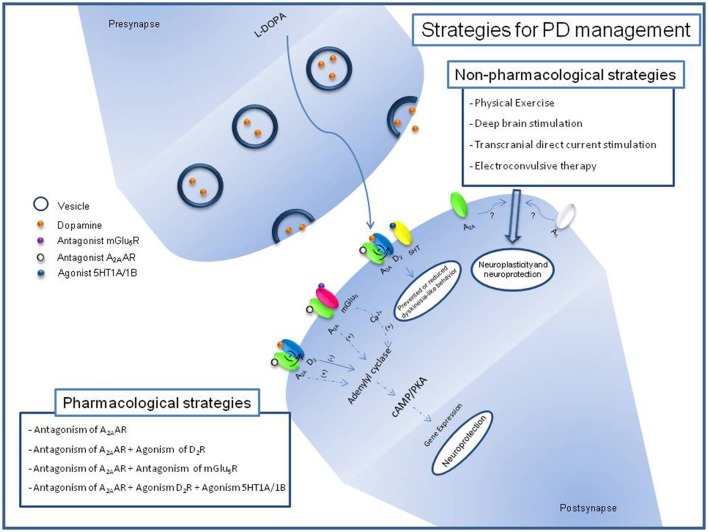
Figure 1.
Schematic description of pharmacological and non-pharmacological strategies for PD management and its relation with adenosinergic signaling. Block of A2AAR by antagonist induces reduction of positive effects over Adenylyl cyclase and negative effects over D2R signaling. Block of mGlu5R reduces its positive effects over Adenilyl cyclase through release of Ca2+. Recent studies with non-phamacological strategies for PD have been related it with adenosine receptors expression.
Prodrugs such as DP-L-A2AANT were designed to conjugate the beneficial effects against dopaminergic degeneration obtained by the combined action of dopamine and A2AAR antagonists in central nervous system (Dalpiaz et al., 2012). In addition to the potential dual action on adenosinergic and dopaminergic systems, the complimentary action on glutamatergic and adenosinergic systems appeared as prospective targets for dual anti-parkinsonian approaches. The combination of A2AAR antagonists and NR2B or mGlu5R antagonists has demonstrated attractive effects on motor activity with potential in the treatment of PD (Michel et al., 2014, 2015; Beggiato et al., 2016). A2AAR–CB1-D2DR-receptor-heteromer has been suggested as a component of motor alterations associated with dyskinesia and a possible target of multi-targeted drugs (Bonaventura et al., 2014; Pinna et al., 2014). The effects of caffeine-derived compounds over A2AAR and that of monoamine oxidase B have revealed that these proteins are targets for synergistic action with benefits on dopaminergic degeneration (Petzer and Petzer, 2015). Sulphanylphthalimides are also presented as a dual-targeted-direct compound acting in A1AR and monoamine oxidase B (Van der Walt et al., 2015). The association of L-dopa, serotonin 5-HT1A/1B receptor agonist and A2AAR antagonist also demonstrated a promissory strategy in 6-OHDA-lesioned rats exhibiting prevented or reduced dyskinetic-like behavior without impairing motor activity (Pinna et al., 2016).
Support for the A2AAR antagonism hypothesis from clinical tests
The A2AAR biding sites and mRNA levels in PD patients with dyskinesia are increased in striatopallidal pathway neurons in relation to healthy patients (Martinez-Mir et al., 1991; Calon et al., 2004). These data, in association with the experimental benefits of A2AAR antagonists in dopaminergic degenerative diseases increased the enthusiasm regarding non-dopaminergic drug development. Table 1 updates the clinical trials assigned in the EUA and European Union using adenosine receptor antagonists. Istradefylline had long-term tolerability and safety, including as an adjuvant therapy to levodopa (Hauser et al., 2003; Stacy et al., 2008). In 2008, US Food and Drug Administration issued a non-approvable letter to the use of Istradefylline in humans based in the concern if the efficacy findings support clinical utility of Istradefylline in patients with PD. However, Kyowa Hakko Kirin has received approval for the use of Istradefylline as adjunctive therapy in Japan (Dungo and Deeks, 2013; Mizuno et al., 2013). After the additional data request, a 12-week randomized study to evaluate oral Istradefylline in subjects with moderate to severe PD ended with disappointing results, since Istradefylline did not change the off time per day (NCT01968031). However, a clinical trial is currently open (NCT02610231). Preladenant was evaluated as monotherapy to patients with early PD since it reduced the mean daily off time in a phase II study; however, no evidence has supported its efficacy in phase III studies (Hauser, 2011; Stocchi et al., 2017). BIIB014 and SCH900800 also failed to prove efficacy in clinical trials, while Tozadenant showed a mean daily off time reduction accompanied by adverse events of dyskinesia, nausea, and dizziness (Hauser et al., 2014). A safety and efficacy study of Tozadenant to treat end of dose wearing off in PD patients using L-DOPA is currently open (NCT02453386). Multiple epidemiological studies indicate that caffeine is able to prevent PD development (Ross et al., 2000; Ascherio et al., 2001). In a pilot study of caffeine for daytime sleepiness in PD, there was evident benefit on the motor manifestations of disease with no adverse effects (Postuma et al., 2012). Recently, a clinical trial has aimed to evaluate the efficacy of caffeine for motor and non-motor aspects of disease (NCT01738178). Nowadays, changing the dose and frequency of daily drug taking had no benefits in the use of adenosine receptor antagonists as a monotherapy or as an adjuvant of current Parkinsonism treatment.
Table 1.
A2AAR antagonists under clinical investigation for Parkinson's disease.
| Drug | Sponsor | Identifier number (year) | Parkinson's disease patient condition | Outcome measures (dose tested) | Phase | Status | Results |
|---|---|---|---|---|---|---|---|
| Istradefylline (KW6002) | Kyowa Hakko Kirin Co., Ltd |
NCT02610231*
(2015) |
Moderate to severe disease | Safety and tolerability (20 or 40 mg oral daily) |
III | Active – not recruiting | – |
|
NCT01968031*
(2013) 2013-002254-70** (2014) |
Moderate to severe disease | Efficacy and safety (20 or 40 mg daily) |
III | Completed | No change in the OFF time | ||
|
NCT00957203*
(2009) |
Advanced disease treated with levodopa | Long-term safety and efficacy (20 or 40 mg daily) |
III | Completed | |||
|
NCT00955526*
(2009) |
Levodopa-treated | Efficacy in reducing the mean total hours of awake time per day spent in the OFF state (20 or 40 mg daily) |
III | Completed | Reduction in daily OFF time | ||
|
NCT00456794*
(2007) |
Advanced disease treated with levodopa/carbidopa | Safety and efficacy compared with placebo in subjects with OFF-time (20 and 60 mg daily) | II | Completed | Significant reduction in OFF time, and was well tolerated as adjunctive treatment to levodopa | ||
|
NCT00456586*
(2007) |
Advanced disease treated with levodopa/carbidopa | Safety and efficacy compared with placebo in subjects with OFF phenomena (40 mg daily) | II | Completed | Istradefylline was safe, well toler-ated, and effective at improving end-of-dose wearing | ||
|
NCT00455507*
(2007) |
Advanced disease treated with levodopa | Efficacy for reducing the mean total hours of awake time per day spent in the OFF state(20 or 40 mg daily) | II | Completed | |||
| 2004-002844-93**
(2005) |
Motor response complications on levodopa therapy | Long-term tolerability and safety (20 or 40 mg daily) |
III | Completed | Istradefylline was well tolerated as adjunctive therapy to levodopa for subjects with Parkinson's disease | ||
|
NCT00250393*
(2005) |
Not specified | Change in Unified Parkinson's Disease Rating Scale (UPDRS) part-III (Motor examination) (40 mg daily) |
II | Completed | |||
|
NCT00203957*
(2005) |
Motor response complications on levodopa | Confirmation of long term tolerability and safety (20 or 40 mg daily) |
III | Completed | |||
|
NCT00199420*
(2005) |
Aadvanced disease treated with levodopa | Percentage of OFF time (10, 20 or 40 mg daily) |
III | Completed | |||
|
NCT00199407*
(2005) |
Advanced disease treated with levodopa | Efficacy for reducing the percentage of OFF time (20 mg daily) | III | Completed | |||
|
NCT00199394*
(2005) |
Advanced disease treated with levodopa | Percentage of awake time spent in the OFF state (40 mg daily) | III | Completed | |||
|
NCT00199381*
(2005) |
Patients who have recently completed one year of treatment with istradefylline | Long-term tolerability and safety (20 or 40 mg daily) |
III | Completed | The sponsor decided to terminate the study early (not for safety reasons) | ||
|
NCT00199368*
(2005) |
Patients with motor response complications on levodopa therapy. Who have completed prior istradefylline studies | Safety Study (20 or 40 mg daily) | III | Completed | |||
|
NCT00199355*
(2005) |
Advanced disease treated with levodopa /DCI. | OFF time (20 or 40 mg daily) | II | ||||
| NINDS |
NCT00006337*
(2000) |
Not specified | Effects on symptoms and dyskinesias | II | Completed | ||
| SCH900800 | Merck Sharp & Dohme Corp. |
NCT01500707*
(2011) |
Moderate to severe disease treated with levodopa | Pharmacokinetics of SCH 900800 (20 mg daily) |
I | Study withdrawn | - |
| Preladenant (SCH 420814) | Merck Sharp & Dohme Corp. |
NCT01294800*
(2011) |
Moderate to severe disease experiencing motor fluctuations and receiving levodopa | Efficacy on “off” time (2, 5, 10 mg twice/day) |
II | Completed | Change from baseline in mean “Off” time |
|
NCT01227265*
(2010) |
Moderate to severe disease | Efficacy and safety (2-5 mg twice/day) |
III | Completed | Not superior to placebo in reducing off time from baseline | ||
|
NCT01155479*
(2010) |
Early Parkinson's disease | Efficacy and safety (2,5, 10 mg twice/day) |
III | Completed | Change from baseline in motor impairments and disability | ||
| 2009-015161-31**
(2010) |
Moderate to severe disease | Efficacy and safety (2,5, 10 mg twice/day) |
III | Completed | |||
| 2009-015162-57**
(2010) |
Moderate to severe disease | Extension study (2,5, 10 mg twice/day) |
III | Study withdrawn | Lack of efficacy in the parent studies. | ||
|
NCT01155466*
(2010) |
Moderate to severe disease | Stability in levodopa dose (2, 5, 10 mg twice/day) |
III | Completed | No change from baseline in mean “Off” Time | ||
| 2009-013552-72**
(2010) |
Early Parkinson's disease | Dose-range-finding efficacy and safety (2, 5, or 10 mg twice/day) | III | Completed | No statistically significant or clinically meaningful difference vs. placebo |
||
|
NCT01215227*
(2010) |
Moderate to severe disease | Long-term safety and tolerability from patients of NCT01155466 and NCT01227265 (2, 5, 10 mg twice/day) | Terminated early due to the lack of efficacy in the parent studies NCT1155466 and NCT01227265 | ||||
|
NCT00845000*
(2009) |
Levodopa treated | Effects on the dyskinesia and antiparkinsonian actions of a levodopa infusion (10 or 100 mg daily) | I | Completed | |||
|
NCT00537017*
(2007) |
Moderate to severe disease | Long term safety (5 mg twice daily) |
II | Completed | Long-term preladenant treatment (5 mgtwice a day) was well tolerated and provided sustained OFF time reductions and ON time increases | ||
|
NCT00406029*
(2006) |
Not specified | Efficacy and safety when used together with a stable dose of L-dopa/dopa decarboxylase (1, 2, 5, and 10 mg twice a day) | II | Completed | Mean daily off time reduced (5 and 10 mg) | ||
| Tozadenant (SYN115) | Biotie Therapies Inc. |
NCT03051607*
2016-003961-25** (2017) |
Experiencing end of dose “Wearing-Off” | Safety and tolerability(120 mg oral twice daily) | III | Recruiting | - |
| 2014-005630-60 **
(2015) |
Levodopa-treated experiencing end-of-dose “Wearing-Off” | Efficacy and safety as adjunctive therapy to levodopa (60 mg oral daily) | III | Active | - | ||
| 2011-005054-59 **
(2013) |
Experiencing end of dose ”Wearing-Off” | Safety and efficacy as an adjunct to levodopa (60 mg oral daily) | II | Completed | |||
|
NCT01283594*
(2011) |
Motor fluctuations on levodopa | Safety and efficacy as an adjunct to levodopa(60, 120, 180, 240 mg twice/day) | II/III | Completed | Tozadenant (120 or 180 mg) was generally well tolerated and was effective at reducing off-time. | ||
| BIIB014 | Oxford BioMedica |
NCT00627588*
(2008) |
Early Parkinson's disease | Safety, efficacy and dose evaluation | I/II | Completed | |
| Caffeine | McGill University Health Center |
NCT01738178*
(2012) |
Not specified | Motor effects of caffeine persist (or even magnify) helps reduce dose of other PD meds and/or prevents their side effects (200 mg daily) | III | Completed | - |
| Ron Postuma |
NCT01190735*
(2010) |
Not specified | Optimal caffeine dose with maximal motor benefit and the least amount of undesirable adverse effects (100–200 mg twice/day) | II | Completed | ||
|
NCT00459420*
(2007) |
Not specified | Effect on sleepiness and motor symptoms (100–200 mg daily) | II/III | Completed | No significant benefit on excessive daytime sleepiness |
ClinicalTrials.gov
EU Clinical Trials Register.
Association of A2AAR antagonism and non-pharmacological approaches
Non-pharmacological approaches are strategies to combine, reinforce and complement the pharmacological options for the management and prevention of PD (Figure 1). Dance, treadmill and aquatic exercises feasibility to PD management have been evaluated in clinical trials with benefits to life quality, based in cognitive and motor features (Picelli et al., 2016; Carroll et al., 2017; Shanahan et al., 2017). Recently, it was demonstrated that treadmill exercises induce brain activation in PD (Maidan et al., 2017). These benefits have been reproduced in animal models of PD suggesting that physical exercise prevents the development of L-DOPA-induced dyskinesia and its association with hyperphosphorylation of DARPP-32, c-Fos expression and increased brain-derived neurotrophic factor (BDNF) levels (Gyárfás et al., 2010; Aguiar et al., 2013; Shin et al., 2017). Studies with wheel running rats revealed that A1AR and A2AAR expression is reduced in the striatum, reinforcing the idea that physical exercise is able to promote neuroplasticity and neuroprotection to brain regions related to motor control, probably through the reduction of antagonistic adenosine effects over dopamine signaling (Clark et al., 2014).
Deep Brain Stimulation (DBS) was approved by the FDA in 2002 as therapy for advanced PD (Suarez-Cedeno et al., 2017). From studies with animals, DBS appeared to have a neuroprotective effect against loss of dopaminergic neurons induced by classical dopaminergic neurotoxins (Maesawa et al., 2004). The use of A2AAR antagonism as an adjuvant of DBS in rodents suggests the potential to enhance the response in the treatment of parkinsonian symptoms, such as tremor (Collins-Praino et al., 2013). While clinical studies using transcranial direct current stimulation (tDCS) in PD suggest possible locomotor benefits, the biological mechanism is still under investigation (Benninger et al., 2011). In rodents, tDCS on the cerebral cortex promotes cognitive effects involving A1AR, although the adenosinergic participation in tDCS responses of PD has not been evaluated (Márquez-Ruiz et al., 2012). Electroconvulsive therapy (ECT) has been proposed to be efficient for both motor and non-motor symptoms in PD with psychological problems (Nishioka et al., 2014; Calderón-Fajardo et al., 2015). The proposed mechanism for ECT includes the enhancement of dopaminergic transmission in the striatum and an increase in the levels of levodopa by disrupting the blood–brain barrier (Kennedy et al., 2003). The purinergic system appears to be influenced by ECT, since the action, metabolism and release of nucleotide and nucleoside are altered under ECT, but no correlation with PD was identified until now (Gleiter et al., 1989; Busnello et al., 2008; Sadek et al., 2011). A combination of drugs and non-pharmacological therapies could warrant new investigations into the preclinical and clinical studies, with hope for the amelioration and affects in PD prevention, management and treatment.
Conclusions
This review highlights the need to intensify research into adenosine signaling in the development of PD therapies. The interaction between adenosine and dopamine signaling has been extensively studied and contributed to knowledge of the role of non-dopamingergic neurotransmitters in the PD. As cholinergic, glutamatergic, GABAergic, canabinergic and serotoninergic systems appear together with adenosinergic system in the myriad of pathways involved in the PD, appearing together with the possibility of improved results from dual or multi-targeted anti-parkisonism approaches opened a new area of drug development. In addition, the association of pharmacological and non-pharmacological approaches brings new perspectives for a more effective treatment of PD and improved of quality of life for PD patients.
Author contributions
LN, RdS, and CB equally contributed to the definition of the scope and to the writing of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
LN is a recipient of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/PROEX fellowship.
Glossary
Abbreviations
- A1AR
A1 adenosine receptor
- A2AAR
A2A adenosine receptor
- A2BAR
A2B adenosine receptor
- A3AR
A3 adenosine receptor
- BDNF
brain-derived neurotrophic factor
- DARPP-32
Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa
- D1DR
D1 dopamine receptor
- D2DR
D2 dopamine receptor
- PD
Parkinson's disease
- 6-OHDA
6-hydroxydopamine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Footnotes
Funding. RdS is a Research Career Awardees of the CNPq/Brazil (Proc: 301599/2016-5). CB is a Research Career Awardees of the CNPq/Brazil (Proc 305035/2015-0).
References
- Aguiar A. S., Jr., Moreira E. L., Hoeller A. A., Oliveira P. A., Córdova F. M., Glaser V., et al. (2013). Exercise attenuates levodopa-induced dyskinesia in 6-hydroxydopamine-lesioned mice. Neuroscience 243, 46–53. 10.1016/j.neuroscience.2013.03.039 [DOI] [PubMed] [Google Scholar]
- Aguiar L. M., Macêdo D. S., Vasconcelos S. M., Oliveira A. A., de Sousa F. C., Viana G. S. (2008). CSC, an adenosine A2A receptor antagonist and MAO B inhibitor, reverses behavior, monoamine neurotransmission, and amino acid alterations in the 6-OHDA-lesioned rats. Brain Res. 1191, 192–199. 10.1016/j.brainres.2007.11.051 [DOI] [PubMed] [Google Scholar]
- Aoyama S., Kase H., Borrelli E. (2000). Rescue of locomotor impairment in dopamine D2 receptor-deficient mice by an adenosine A2A receptor antagonist. J. Neurosci. 20, 5848–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A., Zhang S. M., Hernán M. A., Kawachi I., Colditz G. A., Speizer F. E., et al. (2001). Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann. Neurol. 50, 56–63. 10.1002/ana.1052 [DOI] [PubMed] [Google Scholar]
- Azdad K., Gall D., Woods A. S., Ledent C., Ferré S., Schiffmann S. N. (2009). Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology 34, 972–986. 10.1038/npp.2008.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartonikova T., Mensikova K., Mikulicova L., Vodicka R., Vrtel R., Godava M., et al. (2016). Familial atypical parkinsonism with rare variant in VPS35 and FBXO7 genes: a case report. Medicine (Baltimore) 95:e5398. 10.1097/MD.0000000000005398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggiato S., Tomasini M. C., Borelli A. C., Borroto-Escuela D. O., Fuxe K., Antonelli T., et al. (2016). Functional role of striatal A2A, D2, and mGlu5 receptor interactions in regulating striatopallidal GABA neuronal transmission. J. Neurochem. 138, 254–264. 10.1111/jnc.13652 [DOI] [PubMed] [Google Scholar]
- Benninger D. H., Lomarev M., Lopez G., Pal N., Luckenbaugh D. A., Hallett M. (2011). Transcranial direct current stimulation for the treatment of focal hand dystonia. Mov. Disord. 26, 1698–1702. 10.1002/mds.23691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbiani F., Oh J. D., Petzer J. P., Castagnoli N., Jr., Chen J. F., Schwarzschild M. A., et al. (2003). A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson's disease. Exp. Neurol. 184, 285–294. 10.1016/S0014-4886(03)00250-4 [DOI] [PubMed] [Google Scholar]
- Björklund O., Halldner-Henriksson L., Yang J., Eriksson T. M., Jacobson M. A., Daré E., et al. (2008). Decreased behavioral activation following caffeine, amphetamine and darkness in A3 adenosine receptor knock-out mice. Physiol. Behav. 95, 668–676. 10.1016/j.physbeh.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Bonaventura J., Rico A. J., Moreno E., Sierra S., Sánchez M., Luquin N., et al. (2014). L-DOPA-treatment in primates disrupts the expression of A2A adenosine-CB1 cannabinoid-D2 dopamine receptor heteromers in the caudate nucleus. Neuropharm. 79, 90–100. 10.1016/j.neuropharm.2013.10.036 [DOI] [PubMed] [Google Scholar]
- Botsakis K., Pavlou O., Poulou P. D., Matsokis N., Angelatou F. (2010). Blockade of adenosine A2A receptors downregulates DARPP-32 but increases ERK1/2 activity in striatum of dopamine deficient “weaver” mouse. Neurochem. Int. 56, 245–249. 10.1016/j.neuint.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Braak H., Rüb U., Gai W. P., Del Tredici K. (2003). Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. (Vienna) 110, 517–536. 10.1007/s00702-002-0808-2 [DOI] [PubMed] [Google Scholar]
- Busnello J. V., Oses J. P., da Silva R. S., Feier G., Barichello T., Quevedo J., et al. (2008). Peripheral nucleotide hydrolysis in rats submitted to a model of electroconvulsive therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1829–1833. 10.1016/j.pnpbp.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Calderón-Fajardo H., Cervantes-Arriaga A., Llorens-Arenas R., Ramírez-Bermudez J., Ruiz-Chow Á., Rodríguez-Violante M. (2015). Electroconvulsive therapy in Parkinson's disease. Arq Neuropsiquiatr. 73, 856–860. 10.1590/0004-282X20150131 [DOI] [PubMed] [Google Scholar]
- Calon F., Dridi M., Hornykiewicz O., Bédard P. J., Rajput A. H., Di Paolo T. (2004). Increased adenosine A2A receptors in the brain of Parkinson's disease patients with dyskinesias. Brain 127(Pt 5), 1075–1084. 10.1093/brain/awh128 [DOI] [PubMed] [Google Scholar]
- Canals M., Marcellino D., Fanelli F., Ciruela F., de Benedetti P., Goldberg S. R., et al. (2003). Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 278, 46741–46749. 10.1074/jbc.M306451200 [DOI] [PubMed] [Google Scholar]
- Carroll L. M., Volpe D., Morris M. E., Saunders J., Clifford A. M. (2017). Aquatic exercise therapy for people with Parkinson disease: a randomized controlled trial. Arch. Phys. Med. Rehabil. 98, 631–638. 10.1016/j.apmr.2016.12.006 [DOI] [PubMed] [Google Scholar]
- Chen J. F., Xu K., Petzer J. P., Staal R., Xu Y. H., Beilstein M., et al. (2001). Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson's disease. J. Neurosci. 21:RC143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P. J., Ghasem P. R., Mika A., Day H. E., Herrera J. J., Greenwood B. N., et al. (2014). Wheel running alters patterns of uncontrollable stress-induced cfos mRNA expression in rat dorsal striatum direct and indirect pathways: a possible role for plasticity in adenosine receptors. Behav. Brain Res. 272, 252–263. 10.1016/j.bbr.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccurello R., Breysse N., Amalric M. (2004). Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing Parkinsonian deficits in rats. Neuropsychopharmacology 29, 1451–1461. 10.1038/sj.npp.1300444 [DOI] [PubMed] [Google Scholar]
- Collins-Praino L. E., Paul N. E., Ledgard F., Podurgiel S. J., Kovner R., Baqi Y., et al. (2013). Deep brain stimulation of the subthalamic nucleus reverses oral tremor in pharmacological models of parkinsonism: interaction with the effects of adenosine A2A antagonism. Eur. J. Neurosci. 38, 2183–2191. 10.1111/ejn.12212 [DOI] [PubMed] [Google Scholar]
- Crosiers D., Theuns J., Cras P., Van Broeckhoven C. (2011). Parkinson disease: insights in clinical, genetic and pathological features of monogenic disease subtypes. J. Chem. Neuroanat. 42, 131–141. 10.1016/j.jchemneu.2011.07.003 [DOI] [PubMed] [Google Scholar]
- Dalpiaz A., Cacciari B., Vicentini C. B., Bortolotti F., Spalluto G., Federico S., et al. (2012). A novel conjugated agent between dopamine and an A2A adenosine receptor antagonist as a potential anti-Parkinson multitarget approach. Mol. Pharm. 9, 591–604. 10.1021/mp200489d [DOI] [PubMed] [Google Scholar]
- Dawson T., Dawson V. L. (2010). The role of Parkin in Familial and Sporadic Parkinson's Disease. Mov. Disord. 25, S32–S39. 10.1002/mds.22798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Deng X., Yuan L., Song Z., Yang Z., Xiong W., et al. (2015). Genetic analysis of SNCA coding mutation in Chinese Han patients with Parkinson disease. Acta Neurol. Belg. 115, 267–271. 10.1007/s13760-014-0347-2 [DOI] [PubMed] [Google Scholar]
- de Rijk M. C., Launer L. J., Berger K., Breteler M. M., Dartigues J. F., Baldereschi M., et al. (2000). Prevalence of Parkinson's disease in Europe: a collaborative study of population-based cohorts. Neurology 54(11 Suppl. 5), S21–S23. 10.1212/WNL.54.11.21A [DOI] [PubMed] [Google Scholar]
- Díaz-Cabiale Z., Vivó M., Del Arco A., O'Connor W. T., Harte M. K., Müller C. E., et al. (2002). Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A2A and dopamine D2 receptors. Neurosci. Lett. 324, 154–158. 10.1016/S0304-3940(02)00179-9 [DOI] [PubMed] [Google Scholar]
- Di Fonzo A., Fabrizio E., Thomas A., Fincati E., Marconi R., Tinazzi M., et al. (2009). GIGYF2 mutations are not a frequent cause of familial Parkinson's disease. Parkinsonism Relat. Disord. 15, 703–705. 10.1016/j.parkreldis.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Dungo R., Deeks E. D. (2013). Istradefylline: first global approval. Drugs 73, 875–882. 10.1007/s40265-013-0066-7 [DOI] [PubMed] [Google Scholar]
- Fernández-Dueñas V., Taura J. J., Cottet M., Gómez-Soler M., López-Cano M., Ledent C., et al. (2015). Untangling dopamine-adenosine receptor-receptor assembly in experimental parkinsonism in rats. Dis. Model. Mech. 8, 57–63. 10.1242/dmm.018143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S., Popoli P., Giménez-Llort L., Rimondini R., Müller C. E., Strömberg I., et al. (2001). Adenosine/dopamine interaction: implications for the treatment of Parkinson's disease. Parkinsonism Relat. Disord. 7, 235–241. 10.1016/S1353-8020(00)00063-8 [DOI] [PubMed] [Google Scholar]
- Ferré S., Torvinen M., Antoniou K., Irenius E., Civelli O., Arenas E., et al. (1998). Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. J. Biol. Chem. 273, 4718–4724. 10.1074/jbc.273.8.4718 [DOI] [PubMed] [Google Scholar]
- Ferré S., von Euler G., Johansson B., Fredholm B. B., Fuxe K. (1991). Stimulation of high affinity adenosine A-2 receptors decreases the affinity of dopamine D-2 receptors in rat striatal membranes. Proc. Natl. Acad. Sci. U.S.A. 88, 7238–7241 10.1073/pnas.88.16.7238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L., Beggiato S., Tomasini M. C., Fuxe K., Antonelli T., Tanganelli S. (2012). A2A/D2 receptor heteromerization in a model of Parkinson's disease. Focus on striatal aminoacidergic signaling. Brain Res. 1476, 96–107. 10.1016/j.brainres.2012.01.032 [DOI] [PubMed] [Google Scholar]
- Fink J. S., Weaver D. R., Rivkees S. A., Peterfreund R. A., Pollack A. E., Adler E. M., et al. (1992). Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. Mol. Brain Res. 14, 186–195. 10.1016/0169-328X(92)90173-9 [DOI] [PubMed] [Google Scholar]
- Fredduzzi S., Moratalla R., Monopoli A., Cuellar B., Xu K., Ongini E., et al. (2002). Persistent behavioral sensitization to chronic L-DOPA requires A2A adenosine receptors. J. Neurosci. 22, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Svenningsson P. (2003). Adenosine-dopamine interactions: development of a concept and some comments on therapeutic possibilities. Neurology 61(11 Suppl. 6), S5–S9. 10.1212/01.WNL.0000095204.89871.FF [DOI] [PubMed] [Google Scholar]
- Fuxe K., Ferré S., Zoli M., Agnati L. F. (1998). Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res. Brain Res. Rev. 26, 258–273. 10.1016/S0165-0173(97)00049-0 [DOI] [PubMed] [Google Scholar]
- Fuxe K., Marcellino D., Guidolin D., Woods A. S., Agnati L. (2009). Brain receptor mosaics and their intramembrane receptor-receptor interactions: molecular integration in transmission and novel targets for drug development. J. Acupunct. Meridian Stud. 2, 1–25. 10.1016/S2005-2901(09)60011-X [DOI] [PubMed] [Google Scholar]
- Gleiter C. H., Deckert J., Nutt D. J., Marangos P. J. (1989). Electroconvulsive shock (ECS) and the adenosine neuromodulatory system: effect of single and repeated ECS on the adenosine A1 and A2 receptors, adenylate cyclase, and the adenosine uptake site. J. Neurochem. 52, 641–646. 10.1111/j.1471-4159.1989.tb09168.x [DOI] [PubMed] [Google Scholar]
- Golabi P., Otgonsuren M., Sayiner M., Arsalla A., Gogoll T., Younossi Z. M. (2017). The Prevalence of Parkinson Disease Among Patients With Hepatitis C Infection. Ann. Hepatol. 16, 342–348. 10.5604/01.3001.0009.8588 [DOI] [PubMed] [Google Scholar]
- Gołembiowska K., Wardas J., Noworyta-Sokołowska K., Kaminska K., Górska A. (2013). Effects of adenosine receptor antagonists on the in vivo LPS-induced inflammation model of Parkinson's disease. Neurotox. Res. 24, 29–40. 10.1007/s12640-012-9372-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gołembiowska K., Zylewska A. (1998). N6-2-(4-aminophenyl)ethyladenosine (APNEA), a putative adenosine A3 receptor agonist, enhances methamphetamine-induced dopamine outflow in rat striatum. Pol. J. Pharmacol. 50, 299–305. [PubMed] [Google Scholar]
- Grondin R., Bédard P. J., Hadj T. A., Grégoire L., Mori A., Kase H. (1999). Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology 52, 1673–1677. 10.1212/WNL.52.8.1673 [DOI] [PubMed] [Google Scholar]
- Gyárfás T., Knuuttila J., Lindholm P., Rantamäki T., Castrén E. (2010). Regulation of brain-derived neurotrophic factor (BDNF) and cerebral dopamine neurotrophic factor (CDNF) by anti-parkinsonian drug therapy in vivo. Cell. Mol. Neurobiol. 30, 361–368. 10.1007/s10571-009-9458-3 [DOI] [PubMed] [Google Scholar]
- Hauser R. A. (2011). Future treatments for Parkinson's disease: surfing the PD pipeline. Int. J. Neurosci. 121, 53–62. 10.3109/00207454.2011.620195 [DOI] [PubMed] [Google Scholar]
- Hauser R. A., Hubble J. P., Truong D. D., Istradefylline US-001 Study Group (2003). Randomized trial of the adenosine A2A receptor antagonist istradefylline in advanced PD. Neurology 61, 297–303. 10.1212/01.WNL.0000081227.84197.0B [DOI] [PubMed] [Google Scholar]
- Hauser R. A., Olanow C. W., Kieburtz K. D., Pourcher E., Docu-Axelerad A., Lew M., et al. (2014). Tozadenant (SYN115) in patients with Parkinson's disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised Trial. Lancet Neurol. 13, 767–776. 10.1016/S1474-4422(14)70148-6 [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M., Casas M., Ungerstedt U. (1988). Caffeine produces contralateral rotation in rats with unilateral dopamine denervation: comparisons with apomorphine-induced responses. Psychopharmacology (Berl) 94, 38–45. 10.1007/BF00735878 [DOI] [PubMed] [Google Scholar]
- Hillefors-Berglund M., Liu Y., von Euler G. (1995). Persistent, specific and dose-dependent effects of toluene exposure on dopamine D2 agonist binding in the rat caudate-putamen. Toxicology 100, 185–194. 10.1016/0300-483X(95)03084-S [DOI] [PubMed] [Google Scholar]
- Hillion J., Canals M., Torvinen M., Casado V., Scott R., Terasmaa A., et al. (2002). Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 277, 18091–18097 10.1074/jbc.M107731200 [DOI] [PubMed] [Google Scholar]
- Jaberi E., Rohani M., Shahidi G. A., Nafissi S., Arefian E., Soleimani M., et al. (2016). Mutation in ADORA1 identified as likely cause of early-onset parkinsonism and cognitive dysfunction. Mov. Disord. 31, 1004–1011. 10.1002/mds.26627 [DOI] [PubMed] [Google Scholar]
- Jankovic J. (2008). Parkinson's disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatr. 79, 368–376. 10.1136/jnnp.2007.131045 [DOI] [PubMed] [Google Scholar]
- Jones N., Bleickardt C., Mullins D., Parker E., Hodgson R. (2013). A2A receptor antagonists do not induce dyskinesias in drug-naive or L-dopa sensitized rats. Brain Res. Bull. 98, 163–169. 10.1016/j.brainresbull.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Kachroo A., Orlando L. R., Grandy D. K., Chen J. F., Young A. B., Schwarzschild M. A. (2005). Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J. Neurosci. 25, 10414–10419. 10.1523/JNEUROSCI.3660-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Jackson M. J., Smith L. A., Pearce R. K., Nakamura J., Kase H., et al. (2000). Combined use of the adenosine A2A antagonist KW-6002 with L-DOPA or with selective D1 or D2 dopamine agonists increases antiparkinsonian activity but not dyskinesia in MPTP-treated monkeys. Exp. Neurol. 162, 321–327. 10.1006/exnr.2000.7350 [DOI] [PubMed] [Google Scholar]
- Kanda T., Tashiro T., Kuwana Y., Jenner P. (1998). Adenosine A2A receptors modify motor function in MPTP-treated common marmosets. Neuroreport. 9, 2857–2860. 10.1097/00001756-199808240-00032 [DOI] [PubMed] [Google Scholar]
- Kase H. (2001). New aspects of physiological and pathophysiological functions of adenosine A2A receptor in basal ganglia. Biosci. Biotechnol. Biochem. 65, 1447–1457. 10.1271/bbb.65.1447 [DOI] [PubMed] [Google Scholar]
- Kennedy P., Evans M. J., Berry C., Mullin J. (2003). Comparative analysis of goal achievement during rehabilitation for older and younger adults with spinal cord injury. Spinal Cord. 41, 44–52. 10.1038/sj.sc.3101386 [DOI] [PubMed] [Google Scholar]
- Kelsey J. E., Langelier N. A., Oriel B. S., Reedy C. (2009). The effects of systemic, intrastriatal, and intrapallidal injections of caffeine and systemic injections of A2A and A1 antagonists on forepaw stepping in the unilateral 6-OHDA-lesioned rat. Psychopharmacology (Berl) 201, 529–539. 10.1007/s00213-008-1319-0 [DOI] [PubMed] [Google Scholar]
- Klein C., Westenberger A. (2012). Genetics of Parkinson's disease. Cold Spring Harb. Perspect. Med. 2:a008888. 10.1101/cshperspect.a008888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K., Kurokawa M., Ochi M., Nakamura J., Kuwana Y. (2000). Adenosine A2A receptor antagonists KF17837 and KW-6002 potentiate rotation induced by dopaminergic drugs in hemi-Parkinsonian rats. Eur. J. Pharmacol. 408, 249–255. 10.1016/S0014-2999(00)00745-7 [DOI] [PubMed] [Google Scholar]
- Lautier C., Goldwurm S., Dürr A., Giovannone B., Tsiaras W. G., Pezzoli G., et al. (2008). Mutations in the GIGYF2 (TNRC15) gene at the PARK11 locus in familial Parkinson disease. Am. J. Hum. Genet. 82, 822–833. 10.1016/j.ajhg.2008.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. J., Xue S., Pei L., Vukusic B., Chéry N., Wang Y., et al. (2002). Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell 111, 219–230. 10.1016/S0092-8674(02)00962-5 [DOI] [PubMed] [Google Scholar]
- Maesawa S., Kaneoke Y., Kajita Y., Usui N., Misawa N., Nakayama A., et al. (2004). Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J. Neurosurg. 100, 679–687. 10.3171/jns.2004.100.4.0679 [DOI] [PubMed] [Google Scholar]
- Maggio R., Aloisi G., Silvano E., Rossi M., Millan M. J. (2009). Heterodimerization of dopamine receptors: new insights into functional and therapeutic significance. Parkinsonism Relat. Disord. 15, S2–7. 10.1016/S1353-8020(09)70826-0 [DOI] [PubMed] [Google Scholar]
- Maidan I., Rosenberg-Katz K., Jacob Y., Giladi N., Hausdorff J. M., Mirelman A. (2017). Disparate effects of training on brain activation in Parkinson disease. Neurology 89, 1804–1810. 10.1212/WNL.0000000000004576 [DOI] [PubMed] [Google Scholar]
- Marcellino D., Lindqvist E., Schneider M., Müller C. E., Fuxe K., Olson L., et al. (2010). Chronic A2A antagonist treatment alleviates parkinsonian locomotor deficiency in MitoPark mice. Neurobiol. Dis. 40, 460–466. 10.1016/j.nbd.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Márquez-Ruiz J., Leal-Campanario R., Sánchez-Campusano R., Molaee-Ardekani B., Wendling F., Miranda P. C., et al. (2012). Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc. Natl. Acad. Sci. U.S.A. 109, 6710–6715. 10.1073/pnas.1121147109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Mir M. I., Probst A., Palacios J. M., Adenosine A. (1991). Receptors: selective localization in the human basal ganglia and alterations with disease. Neuroscience 42, 697–706. 10.1016/0306-4522(91)90038-P [DOI] [PubMed] [Google Scholar]
- Matsuya T., Takuma K., Sato K., Asai M., Murakami Y., Miyoshi S., et al. (2007). Synergistic effects of adenosine A2A antagonist and L-DOPA on rotational behaviors in 6-hydroxydopamine-induced hemi-Parkinsonian mouse model. Pharmacol Sci. 103, 329–332. 10.1254/jphs.SCZ070058 [DOI] [PubMed] [Google Scholar]
- McCarthy S., Somayajulu M., Sikorska M., Borowy-Borowski H., Pandey S. (2004). Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol. Appl. Pharmacol. 201, 21–31. 10.1016/j.taap.2004.04.019 [DOI] [PubMed] [Google Scholar]
- Michel A., Downey P., Nicolas J. M., Scheller D. (2014). Unprecedented therapeutic potential with a combination of A2A/NR2B receptor antagonists as observed in the 6-OHDA lesioned rat model of Parkinson's disease. PLoS ONE 9:e114086. 10.1371/journal.pone.0114086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A., Downey P., Van Damme X., De Wolf C., Schwarting R., Scheller D. (2015). Behavioural Assessment of the A2a/NR2B combination in the unilateral 6-OHDA-lesioned rat model: a new method to examine the therapeutic potential of non-dopaminergic drugs. PLoS ONE 10:e0135949. 10.1371/journal.pone.0135949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y., Tanji K., Mori F., Kakita A., Takahashi H., Wakabayashi K. (2017). PLA2G6 accumulates in Lewy bodies in PARK14 and idiopathic Parkinson's disease. Neurosci. Lett. 645, 40–45. 10.1016/j.neulet.2017.02.027 [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Kondo T., Japanese Istradefylline Study Group (2013). Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson's disease. Mov. Disord. 28, 1138–1141. 10.1002/mds.25418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer J. A., Borenstein A. R., Nelson L. M. (2012). Associations of welding and manganese exposure with Parkinson disease: review and meta-analysis. Neurology 79, 1174–1180. 10.1212/WNL.0b013e3182698ced [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash J. E., Brotchie J. M. (2000). A common signaling pathway for striatal NMDA and adenosine A2a receptors: implications for the treatment of Parkinson's disease. J. Neurosci. 20, 7782–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A., Snyder G. L., Greengard P. (1997). Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J. Neurosci. 17, 8147–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K., Tanaka R., Shimura H., Hirano K., Hatano T., Miyakawa K., et al. (2014). Quantitative evaluation of electroconvulsive therapy for Parkinson's disease with refractory psychiatric symptoms. J. Neural. Transm. (Vienna) 121, 1405–1410. 10.1007/s00702-014-1212-4 [DOI] [PubMed] [Google Scholar]
- Noyce A. J., Bestwick J. P., Silveira-Moriyama L., Hawkes C. H., Giovannoni G., Lees A. J., et al. (2012). Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann. Neurol. 72, 893–901. 10.1002/ana.23687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M., Bakešová J., Brugarolas M., Quiroz C., Beaumont V., Goldberg S. R., et al. (2011). Striatal pre-and postsynaptic profile of adenosine A2A receptor antagonists. PLoS ONE 6:e16088 10.1371/journal.pone.0016088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzer J. P., Petzer A. (2015). Caffeine as a lead compound for the design of therapeutic agents for the treatment of Parkinson's disease. Curr. Med. Chem. 22, 975–988. 10.2174/0929867322666141215160015 [DOI] [PubMed] [Google Scholar]
- Petzer J. P., Steyn S., Castagnoli K. P., Chen J. F., Schwarzschild M. A., Van der Schyf C. J., et al. (2003). Inhibition of monoamine oxidase B by selective adenosine A2A receptor antagonists. Bioorg. Med. Chem. 11, 1299–1310. 10.1016/S0968-0896(02)00648-X [DOI] [PubMed] [Google Scholar]
- Pezzoli G., Cereda E. (2013). Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 80, 2035–2041. 10.1212/WNL.0b013e318294b3c8 [DOI] [PubMed] [Google Scholar]
- Picelli A., Varalta V., Melotti C., Zatezalo V., Fonte C., Amato S., et al. (2016). Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson's disease: a pilot, single-blind, randomized controlled trial. Funct. Neurol. 31, 25–31. 10.11138/FNeur/2016.31.1.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna A., Bonaventura J., Farré D., Sánchez M., Simola N., Mallol J., et al. (2014). L-DOPA disrupts adenosine A2A-cannabinoid CB(1)-dopamine D(2) receptor heteromer cross-talk in the striatum of hemiparkinsonian rats: biochemical and behavioral studies. Exp. Neurol. 253, 180–191. 10.1016/j.expneurol.2013.12.021 [DOI] [PubMed] [Google Scholar]
- Pinna A., Ko W. K., Costa G., Tronci E., Fidalgo C., Simola N., et al. (2016). Antidyskinetic effect of A2A and 5HT1A/1B receptor ligands in two animal models of Parkinson's disease. Mov. Disord. 31, 501–511. 10.1002/mds.26475 [DOI] [PubMed] [Google Scholar]
- Pinna A., Pontis S., Borsini F., Morelli M. (2007). Adenosine A2A receptor antagonists improve deficits in initiation of movement and sensory motor integration in the unilateral 6-hydroxydopamine rat model of Parkinson's disease. Synapse 61, 606–614. 10.1002/syn.20410 [DOI] [PubMed] [Google Scholar]
- Pollack A. E., Fink J. S. (1995). Adenosine antagonists potentiate D2 dopamine-dependent activation of Fos in the striatopallidal pathway. Neuroscience 68, 721–728. 10.1016/0306-4522(95)00168-I [DOI] [PubMed] [Google Scholar]
- Postuma R. B., Lang A. E., Munhoz R. P., Charland K., Pelletier A., Moscovich M., et al. (2012). Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology 79, 651–658. 10.1212/WNL.0b013e318263570d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V., Burnstock G. (1998). Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492. [PubMed] [Google Scholar]
- Redenšek S., Trošt M., DolŽan V. (2017). Genetic determinants of parkinson's disease: can they help to stratify the patients based on the underlying molecular defect? Front. Aging Neurosci. 9:20. 10.3389/fnagi.2017.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. W., Abbott R. D., Petrovitch H., Morens D. M., Grandinetti A., Tung K. H., et al. (2000). Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283, 2674–2679. 10.1001/jama.283.20.2674 [DOI] [PubMed] [Google Scholar]
- Sadek A. R., Knight G. E., Burnstock G. (2011). Electroconvulsive therapy: a novel hypothesis for the involvement of purinergic signalling. Purinergic Signal. 7, 447–452. 10.1007/s11302-011-9242-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J. D., Collins-Praino L. E., Pardo M., Podurgiel S. J., Baqi Y., Müller C. E., et al. (2013). Conditional neural knockout of the adenosine A2A receptor and pharmacological A2A antagonism reduce pilocarpine-induced tremulous jaw movements: studies with a mouse model of parkinsonian tremor. Eur. Neuropsychopharmacol. 23, 972–977. 10.1016/j.euroneuro.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Scott L., Dawson V. L., Dawson T. M. (2017). Trumping neurodegeneration: targeting common pathways regulated by autosomal recessive Parkinson's disease genes. Exp. Neurol. 298(Pt B), 191–201. 10.1016/j.expneurol.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan J., Morris M. E., Bhriain O. N., Volpe D., Lynch T., Clifford A. M. (2017). Dancing for Parkinson Disease: a randomized trial of irish set dancing compared with usual care. Arch. Phys. Med. Rehabil. 98, 1744–1751. 10.1016/j.apmr.2017.02.017 [DOI] [PubMed] [Google Scholar]
- Sharma J. C., Lewis A. (2017). Weight in Parkinson's Disease: phenotypical significance. Int. Rev. Neurobiol. 134, 891–919. 10.1016/bs.irn.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Shen H., Luo Y., Yu S. J., Wang Y. (2011). Enhanced neurodegeneration after a high dose of methamphetamine in adenosine A3 receptor null mutant mice. Neuroscience 194, 170–180. 10.1016/j.neuroscience.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Yang H., Wu Y., Zhang D., Jiang H. (2017). Association of Helicobacter pylori infection with Parkinson's diseases: a meta-analysis. Helicobacter 22:e12398. 10.1111/hel.12398 [DOI] [PubMed] [Google Scholar]
- Shin H. K., Lee S. W., Choi B. T. (2017). Modulation of neurogenesis via neurotrophic factors in acupuncture treatments for neurological diseases. Biochem Pharmacol. 141, 132–142. 10.1016/j.bcp.2017.04.029 [DOI] [PubMed] [Google Scholar]
- Simola N., Fenu S., Baraldi P. G., Tabrizi M. A., Morelli M. (2006). Dopamine and adenosine receptor interaction as basis for the treatment of Parkinson's disease. J. Neurol. Sci. 248, 48–52. 10.1016/j.jns.2006.05.038 [DOI] [PubMed] [Google Scholar]
- Song L., Kong M., Ma Y., Ba M., Liu Z. (2009). Inhibitory effect of 8-(3-chlorostryryl) caffeine on levodopa-induced motor fluctuation is associated with intracellular signaling pathway in 6-OHDA-lesioned rats. Brain Res. 1276, 171–179. 10.1016/j.brainres.2009.04.028 [DOI] [PubMed] [Google Scholar]
- Sonsalla P. K., Wong L. Y., Harris S. L., Richardson J. R., Khobahy I., Li W., et al. (2012). Delayed caffeine treatment prevents nigral dopamine neuron loss in a progressive rat model of Parkinson's disease. Exp. Neurol. 234, 482–487. 10.1016/j.expneurol.2012.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M., Goedert M. (1998). Alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473. 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy M., Silver D., Mendis T., Sutton J., Mori A., Chaikin P., et al. (2008). A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology 70, 2233–2240. 10.1212/01.wnl.0000313834.22171.17 [DOI] [PubMed] [Google Scholar]
- Stocchi F., Rascol O., Hauser R. A., Huyck S., Tzontcheva A., Capece R., et al. (2017). Randomized trial of preladenant, given as monotherapy, in patients with early Parkinson disease. Neurology 88, 2198–2206. 10.1212/WNL.0000000000004003 [DOI] [PubMed] [Google Scholar]
- Strömberg I., Popoli P., Müller C. E., Ferré S., Fuxe K. (2000). Electrophysiological and behavioural evidence for an antagonistic modulatory role of adenosine A2A receptors in dopamine D2 receptor regulation in the rat dopamine-denervated striatum. Eur. J. Neurosci. 12, 4033–4037. 10.1046/j.1460-9568.2000.00288.x [DOI] [PubMed] [Google Scholar]
- Suarez-Cedeno G., Suescun J., Schiess M. C. (2017). Earlier Intervention with Deep Brain Stimulation for Parkinson's Disease. Parkinsons. Dis. 2017:9358153. 10.1155/2017/9358153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P., Fourreau L., Bloch B., Fredholm B. B., Gonon F., Le Moine C. (1999). Opposite tonic modulation of dopamine and adenosine on c-fos gene expression in striatopallidal neurons. Neuroscience 89, 827–837. 10.1016/S0306-4522(98)00403-5 [DOI] [PubMed] [Google Scholar]
- Tanganelli S., Sandager Nielsen K., Ferraro L., Antonelli T., Scheel-Krüger J. (2004). Striatal plasticity at the network level. Focus on adenosine A2A and D2 interactions in models of Parkinson's Disease. Parkinsonism Relat. Disord. 10, 273–280. 10.1016/j.parkreldis.2004.02.015 [DOI] [PubMed] [Google Scholar]
- Tomiyama M., Kimura T., Maeda T., Tanaka H., Kannari K., Baba M. (2004). Upregulation of striatal adenosine A2A receptor mRNA in 6-hydroxydopamine-lesioned rats intermittently treated with L-DOPA. Synapse 52, 218–222. 10.1002/syn.20011 [DOI] [PubMed] [Google Scholar]
- Tozzi A., de Iure A., Di Filippo M., Tantucci M., Costa C., Borsini F., et al. (2011). The distinct role of medium spiny neurons and cholinergic interneurons in the D2/A2A receptor interaction in the striatum: implications for Parkinson's disease. J. Neurosci. 31, 1850–1862. 10.1523/JNEUROSCI.4082-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P., Rives M. L., Urizar E., Piskorowski R. A., Vishwasrao H. D., Castrillon J., et al. (2011). Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 51, 111–118. 10.2144/000113719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronci E., Simola N., Borsini F., Schintu N., Frau L., Carminati P., et al. (2007). Characterization of the antiparkinsonian effects of the new adenosine A2A receptor antagonist ST1535: acute and subchronic studies in rats. Eur. J. Pharmacol. 566, 94–102 10.1016/j.ejphar.2007.03.021 [DOI] [PubMed] [Google Scholar]
- Uchida S., Kadowaki-Horita T., Kanda T. (2014). Effects of the adenosine A2A receptor antagonist on cognitive dysfunction in Parkinson's disease. Int. Rev. Neurobiol. 119, 169–189. 10.1016/B978-0-12-801022-8.00008-8 [DOI] [PubMed] [Google Scholar]
- Van der Mark M., Brouwer M., Kromhout H., Nijssen P., Huss A., Vermeulen R. (2012). Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ. Health Perspect. 120, 340–347. 10.1289/ehp.1103881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Walt M. M., Terre'Blanche G., Petzer A., Petzer J. P. (2015). The adenosine receptor affinities and monoamine oxidase B inhibitory properties of sulfanylphthalimide analogues. Bioorg. Chem. 59, 117–123. 10.1016/j.bioorg.2015.02.005 [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B., Sousa-Ribeiro D., Rodriguez-Pallares J., Rodriguez-Perez A. I., Guerra M. J., Labandeira-Garcia J. L. (2009). Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson's disease. J. Cereb. Blood Flow Metab. 29, 230–234. 10.1038/jcbfm.2008.127 [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Logan J., Alexoff D., Fowler J. S., Thanos P. K., et al. (2015). Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl. Psychiatry 5, e549. 10.1038/tp.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortherms T. A., Watts V. J. (2004). Sensitization of neuronal A2A adenosine receptors after persistent D2 dopamine receptor activation. J. Pharmacol. Exp. Ther. 308, 221–227. 10.1124/jpet.103.057083 [DOI] [PubMed] [Google Scholar]
- Watanabe H., Ikeda M., Watanabe K. (1981). Properties of rotational behaviour produced by methylxanthine derivatives in mice with unilateral striatal 6-hydroxydopamine-induced lesions. J. Pharmacobiodyn. 4, 301–307. 10.1248/bpb1978.4.301 [DOI] [PubMed] [Google Scholar]
- Xu K., Di Luca D. G., Orrú M., Xu Y., Chen J. F., Schwarzschild M. A. (2016). Neuroprotection by caffeine in the MPTP model of parkinson's disease and its dependence on adenosine A2A receptors. Neuroscience. 322, 129–137. 10.1016/j.neuroscience.2016.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Soohoo D., Soelaiman S., Kalla R., Zablocki J., Chu N., et al. (2007). Characterization of the potency, selectivity, and pharmacokinetic profile for six adenosine A2A receptor antagonists. Naunyn Schmiedebergs. Arch. Pharmacol. 375, 133–144. 10.1007/s00210-007-0135-0 [DOI] [PubMed] [Google Scholar]
- Yu L., Schwarzschild M. A., Chen J. F. (2006). Cross-sensitization between caffeine- and L-dopa-induced behaviors in hemiparkinsonian mice. Neurosci. Lett. 393, 31–35. 10.1016/j.neulet.2005.09.036 [DOI] [PubMed] [Google Scholar]