Abstract
AIM
To identify whether chitinase 3-like 1 (CHI3L1) serves as a suitable biomarker for the prognosis of esophageal squamous cell carcinoma (ESCC) and to analyze this protein’s cellular source.
METHODS
An ELISA was conducted to detect the concentration of CHI3L1 in the serum of 150 ESCC patients diagnosed between January 2001 and February 2005. The prognostic relevance of CHI3L1 was evaluated by a Kaplan-Meier and Cox regression analysis. The immunohistochemistry was reanalyzed, and fluorescent staining was utilized to explore the cellular origins of CHI3L1. We stimulated monocyte-derived macrophages (MDMs) with either IL-6 or the supernatant of the ESCC cell line Eca-109 and later investigated the level of CHI3L1 by qPCR and ELISA.
RESULTS
The level of serum CHI3L1 was higher in older patients (≥ 60) than in patients under the age of 60 (P = 0.001). The patients with higher levels of CHI3L1 had a significantly shorter overall survival, whereas the traditional markers, carcinoembryonic antigen and squamous cell carcinoma antigen, were less effective (P > 0.05). A multivariate Cox analysis (P = 0.001) indicated that CHI3L1 was an independent prognostic factor for ESCC patients. Peritumoral macrophages in ESCC exhibited high levels of CHI3L1. Interleukin-6 (IL-6) and the supernatant of Eca-109 containing IL-6 stimulated MDMs to secrete CHI3L1. The serum concentration of CHI3L1 in the ESCC patients showed a weak correlation with the laboratory inflammatory parameters neutrophil (NEU, P = 0.045), neutrophil/lymphocyte rate (NLR, P = 0.016), and C-reactive protein (CRP, P < 0.001).
CONCLUSION
Our study first established a connection between the pretreated CHI3L1 and patients with ESCC, and the serum CHI3L1 was primarily secreted by ESCC-surrounded macrophages.
Keywords: Esophageal squamous cell carcinoma, Prognostic biomarker, Chitinase 3-like 1, Macrophage, Esophageal squamous cell carcinoma
Core tip: The current staging system is inadequate for predicting post-treated survival. Our study first established a connection between pretreated chitinase 3-like 1 (CHI3L1) and patients with esophageal squamous cell carcinoma (ESCC), and serum CHI3L1 was primarily secreted by ESCC-surrounded macrophages, suggesting that CHI3L1 was a simple and inexpensive prognostic factor. This simple, convenient serological testing allows for clinical application. In addition, in our study, the ESCC microenvironment, especially the secretion of IL - 6 by esophageal tumor cells, promotes the macrophage production of CHI3L1. These findings might help to identify high-risk patients for treatment decisions and to elucidate the mechanisms of ESCC.
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is one of the major histological subtypes of esophageal cancer. ESCC is the fourth most lethal cancer in China[1,2]. Ninety percent of the cases are squamous cell carcinomas[3,4]. With improvements in the diagnosis, staging system and treatment strategies, the overall 5-year survival rate of ESCC patients increased slightly. However, patients at the same stage undergoing similar treatment regimens often have notably different clinical outcomes, which suggests that the current staging system is inadequate for predicting survival. Several recent studies show that clinically used tumor markers, such as squamous cell carcinoma antigen (SCCA) and carcinoembryonic antigen (CEA), play important roles in tumorigenesis and the development of ESCC[5-7]. However, these antigens lack sufficient power and validity to be adopted for prognosis of cancer[8]. Certain studies have identified mRNA and protein biomarkers correlated with the prognosis of ESCC patients[9,10], but few biomarkers have sufficient evidence to be adopted for clinical use. Therefore, the identification of accurate biomarkers for ESCC is necessary to improve the clinical outcome of patients.
Chitinase 3-like 1 (CHI3L1) is a secreted glycoprotein that belongs to a group of mammalian proteins with an amino acid sequence that is similar to the 18-glycosyl hydrolase group of bacterial chitinases[11]. Various human cells, such as synovial, cartilage, endothelial, neutrophil and macrophage cells produce CHI3L1[12]. This protein is associated with the malignant behavior of several types of cancers, and elevated levels of CHI3L1 are notably correlated with a poor prognosis and short survival in ovarian cancer[13], breast cancer[14], lung cancer[15], hepatocellular carcinoma[16] and glioblastoma[17]. However, no information exists regarding whether serum CHI3L1 predicts ESCC patient survival.
Our previous study suggested that CHI3L1 may represent a diagnostic biomarker in ESCC patients[5]. In this study, we examined CHI3L1 expression in the serum from 150 ESCC patient by ELISA and later explored its cellular source. For the first time, we investigated the ability of CHI3L1 to predict ESCC patient survival, as well as the exact origin of CHI3L1 in ESCC. This signature represents a novel biomarker to predict ESCC patient survival more accurately and understand the mechanisms of the genesis and development of ESCC.
MATERIALS AND METHODS
Serum and tissue specimen
In this study, all the selected ESCC patients met the following inclusion criteria: Pathological examination confirmation of primary ESCC by the available biopsy samples at the Sun Yat-sen University Cancer Center (SYSUCC) and no anticancer treatments having been given previously. The absence of diseases, such as COPD and second primary carcinomas, was assessed by physical examination, clinical history, gastroscopy, colonoscopy and routine laboratory tests (including liver and renal function tests). Follow-up information was available for all of these patients.
The tumors were staged according to the 7th edition of the tumor-node-metastasis (TNM) classification for esophageal carcinoma (UICC, 2009).
Serum from 150 ESCC patients was obtained at the time of diagnosis before treatment from January 2001 to February 2005. The final confirmation date of the patients’ condition was in February 2010. Venous blood (3-5 mL) was clotted at room temperature, centrifuged at 3000 r/min for 10 min and stored at -80 °C until it was used. A total of 20 formalin-fixed and paraffin-embedded ESCC tumor specimens for immunochemistry and fluorescent staining were obtained from 2012 to 2013 as described before[8].
Prior to the use of these serum and tissues, informed consent was obtained from each participant. This experiment was approved by the Institute Research Ethics Committee of SYSUCC, Guangzhou.
Cell lines
The ESCC cell line Eca-109 (Chinese Academy of Sciences, Shanghai, China) was grown at 37 °C in 5% CO2 in RPMI 1640 (Invitrogen, United States) supplemented with 10% fetal bovine serum. The Eca-109 supernatant was collected after an incubation for 48 h. The cell line was obtained between 2012 and 2014.
ELISA
Serum CHI3L1 levels were determined by a double-antibody sandwich ELISA according to the manufacturer’s instructions (R&D systems, United States). Briefly, 96-well microplates were coated with 100 μL/well of the capture antibody (rat anti-human CHI3L1, 2.0 μg/mL) overnight at room temperature. After blocking with 3% BSA for 1 h, 100 μL of the patients’ serum (1:100 diluted in blocking buffer) was added and incubated for 2 h at room temperature. Subsequently, 100 μL/well of the detection antibody (biotinylated goat anti-human CHI3L1, 200 ng/mL) was added and incubated for 2 h at room temperature. Next, 100 μL/well of Streptavidin-HRP (1:200) was added and incubated for 20 min at room temperature. Finally, the substrate (tetramethylbenzidine) solution was added, and the reaction was stopped with 2 mol/L H2SO4 and read at an OD of 450 nm. Each test included a standard control (CV < 12%).
CEA and SCCA assays
The level of CEA in the serum was assessed using an electrochemiluminescence immunoassay (ECLIA) kit (Roche, German) on a Roche Cobas8000 fully automatic electrochemistry luminescence immunity analyzer (Roche, Germany). The concentration of SCCA in the serum was detected using an ARCHITECT I2000SR immune analyze system (Abbott, United States). Each test included a standard control (CV < 5%).
Immunohistochemistry
IHC was performed in our previous study[8]. The degree of immunostaining was analyzed again by two independent observers.
Fluorescent staining of the paraffin-embedded sections
After rehydrating and blocking, the formalin-fixed, paraffin-embedded ESCC sections incubated with a rabbit polyclonal anti-CHI3L1 antibody (1:100, Bioss, China) and a mouse polyclonal anti-CD68 antibody (1:100, Zhongshan Golden Bridge, China) overnight at 4 °C. The slides were washed 3 times for 15 min each in PBST wash buffer and were then incubated with a goat anti-rabbit IgG secondary antibody (FITC) and a goat anti-mouse IgG secondary Antibody (PE) for 30 min at room temperature (1:500, Ebioscience, United States). After 3 washes, the tissue sections were incubated by diluted DAPI for 5 min at room temperature, mounted with an anti-fade mounting media and then visualized using a fluorescence microscope by two independent observers.
Cytokines
Cytokines in serum samples were measured with the BD CBA Th1/Th2/Th17 Cytokine Kit (BD Bioscience, United States). The kit was used for the simultaneous detection of interleukin-2 (IL-2), IL-4, IL-6, interferon-γ (IFN-γ) and IL-10 in a single sample. This array kit provides a mixture of capture beads with distinct fluorescence intensities are coated with capture antibodies specific for each cytokine. The operations were performed according to the manufacturer’s instructions. The individual cytokine concentrations were indicated by their fluorescent intensities. The concentrations of all the cytokines were reported in pg/mL.
Isolation and culturing of primary monocytes to obtain monocyte-derived macrophages
Human peripheral blood was collected from healthy donors, and monocytes from Ficoll-isolated PBMCs were separated from the lymphocytes by adherence to tissue culture-treated plates[18]. After 48 h of incubation, the non-adherent cells were removed via two washes with warm RPMI. The monocytes were differentiated into macrophages (MDMs) by culturing in RPMI medium supplemented with 10% fetal bovine serum for 15 d prior to stimulation. The MDMs were washed with phosphate-buffered saline (PBS), and the culture medium was replaced every 2 d. MDMs were stimulated for 24 h with IL-6 (1 μg/mL) or the Eca-109 cell line supernatant. After 24 h, the RNA was extracted for with the Trizol reagent (Invitrogen, United States), and the culture medium were collected for ELISA.
Real-time RT-PCR
Total RNA was extracted from the cell lines and frozen ESCC tissues using the Trizol reagent (Invitrogen, United States) according to the manufacturer’s instructions. Reverse transcription of the total RNA (2 μg) was derived using SuperScript II reverse transcriptase (BioRad, United States). The quantification of the target and reference (GAPDH) genes was performed in triplicate on a LightCycler® 480 II (Roche, Applied Science) using a SYBR green-based assay (BioRad, United States). The primers used in the real-time RT-PCR reaction were as follows: CHI3L1, NBCI RefSeq Database entry: NM_001276.2, forward 5’- GAGGATGGAACTTTGGGTCTC-3’ and reverse 5’- TCATTTCCTTGATTAGGGTGGT-3’ and GAPDH, NBCI RefSeq Database entry: NM_001256799.2, forward 5’-GACTCATGACCACAGTCCATGC-3’ and reverse 5’-AGAGGCAGGGATGATGTTCTG-3’. The results were expressed as mean ± SD.
White blood cell, neutrophil, lymphocyte, platelet counts and C-reactive protein assay
White blood cell (WBC), neutrophil (NEU), lymphocyte (LY) and platelet (PLT) were collected from the blood acquired during a routine examination, and the results were detected using a Sysmex XE5000 analyzer (Sysmex, Japan). The NLR was defined as the ratio of NEUs and LYs, and the PLR was defined as the ratio of PLTs and LYs. Serum C-reactive protein (CRP) was determined by a latex-enhanced homogeneous immunoassay on a Hitachi LAS008 analyzer (Hitachi, Japan).
Statistical analysis
The data were analyzed by SPSS standard version 16.0 (SPSS, Chicago, United States). The optimal cutoff level for CHI3L1 was determined as the value with the maximization of the Yuden index by the receiver operating characteristic (ROC) analysis, whereas the cutoff levels for the clinical markers, SCCA and CEA, were the upper limit of normal reference values, which were 5.0 ng/mL for CEA and 1.5 ng/mL for SCCA. The Kaplan-Meier method was used to estimate the overall survival (OS), and the multivariate analysis was performed using the Cox proportional hazards model. The χ2 test was used to analyze the relationship between CHI3L1 level and the clinicopathological characteristics. The associations of CHI3L1 with inflammatory indexes were analyzed by a Spearman correlation. All of the statistical tests were two-sided, and P < 0.05 was considered to be statistically significant.
RESULTS
Patients’ characteristics
The clinicopathological and laboratory characteristics of 150 ESCC patients are summarized in Table 1. Among these patients, 113 (75.3%) were males, and 37 (24.7%) were females, exhibiting a median age of 60 (range 30-96). In total, there were 51 (34.0%) early stage (I-II) ESCC patients and 93 (62.0%) advanced stage (III-IV) patients in the cohort. Of the included patients, 73 patients underwent surgery. Of the 73 patients who underwent surgery, 3 patients received chemotherapy followed by surgical resection, 2 received radiation before surgery and the others did not undergo any additional treatment. Fifty-seven patients received chemotherapy administered concurrently with radiation therapy. Eleven subjects underwent systemic chemotherapy. A variety of chemotherapy regimens were employed, and most commonly, they were cisplatin and 5-Fluorouracil. The relationship between the CHI3L1 and clinicopathological characteristics of the ESCC patients is presented in Table 1. There were no differences of CHI3L1 level in regards to sex (P = 0.713), smoking behavior (P = 0.514), alcohol status (P = 0.984), tumor grade (P = 0.736) T classification (P = 0.886), N classification (P = 0.218), metastasis (P = 0.156), clinical stage (P = 0.712), SCCA level (P = 0.539) CEA level (P = 0.389) or treatment options (P = 0.138). However, the CHI3L1 level was associated with age (P = 0.001). The level of serum CHI3L1 was higher in older patients (≥ 60) than in patients under the age of 60.
Table 1.
Levels of chitinase 3-like 1 and clinical and laboratory characteristics of 150 esophageal squamous cell carcinoma patients
| Characteristics | No. of patients |
Levels of CHI3L1 |
||
| Low | High | P value | ||
| All | 150 | 97 | 53 | |
| Age | 0.009 | |||
| Median | 60 | |||
| Range | 30-96 | |||
| < 60 | 81 | 60 | 21 | |
| ≥ 60 | 69 | 37 | 32 | |
| Sex | 0.713 | |||
| Male | 113 | 74 | 39 | |
| Female | 37 | 23 | 14 | |
| Smoking status | 0.514 | |||
| Smoker | 99 | 66 | 33 | |
| Non-smoker | 49 | 30 | 19 | |
| Alcohol intake | 0.984 | |||
| Yes | 56 | 36 | 20 | |
| No | 90 | 58 | 32 | |
| Grade | 0.736 | |||
| Grade 1 | 24 | 16 | 8 | |
| Grade 2 | 57 | 36 | 21 | |
| Grade 3 | 41 | 29 | 12 | |
| T status | 0.886 | |||
| pT 1/2 | 31 | 21 | 10 | |
| pT 3/4 | 113 | 75 | 38 | |
| N status | 0.154 | |||
| pN 0 | 63 | 46 | 17 | |
| pN 1/2/3 | 81 | 50 | 31 | |
| M status | 0.156 | |||
| pM 0 | 112 | 78 | 34 | |
| pM 1 | 32 | 18 | 14 | |
| Stage | 0.712 | |||
| I–II | 51 | 35 | 16 | |
| III-IV | 93 | 61 | 32 | |
| SCCA | 0.539 | |||
| ≤ 1.5 | 101 | 67 | 34 | |
| > 1.5 | 49 | 30 | 19 | |
| CEA | 0.389 | |||
| ≤ 5.0 | 132 | 87 | 45 | |
| > 5.0 | 18 | 10 | 8 | |
| Treatment | 0.138 | |||
| Surgery | 73 | 49 | 24 | |
| Chemoradiotherapy | 57 | 37 | 20 | |
| Chemotherapy | 11 | 4 | 7 | |
CHI3L1: Chitinase 3-like 1; SCCA: Squamous cell carcinoma antigen; CEA: Carcinoembryonic antigen.
Prognostic value of CHI3L1 in ESCC
The median OS was 1.82 years for the entire cohort of patients with a five-year overall survival of 24.67%. The patient survival curves were constructed via the Kaplan-Meier method and were compared using the log-rank test. Kaplan-Meier estimates of the OS for patients with CHI3L1 and the levels of the clinically used ESCC markers, SCCA and CEA, are shown in Figure 1. The results showed that patients with elevated CHI3L1 levels were significantly associated with a shorter OS (P = 0.019; Figure 1A). Further stratification of the patient groups based on stage displayed that the correlation of elevated CHI3L1 levels and a shorter OS was statistically significant in Stage I-II patients with ESCC (P = 0.003; Figure 1B). However, in Stage III-IV, there was no significant association between elevated CHI3L1 levels and a shorter OS (P = 0.089; Figure 1C). The traditional ESCC markers, SCCA and CEA, were not significantly relevant to the OS (SCCA, All: P = 0.087, Figure 1D, Stage I-II: P = 0.756, Figure 1E, Stage III-IV: P = 0.003, Figure 1F; CEA, All: P = 0.847, Figure 1G, Stage I-II: P = 0.325, Figure 1H, Stage III-IV: P = 0.835, Figure 1I).
Figure 1.
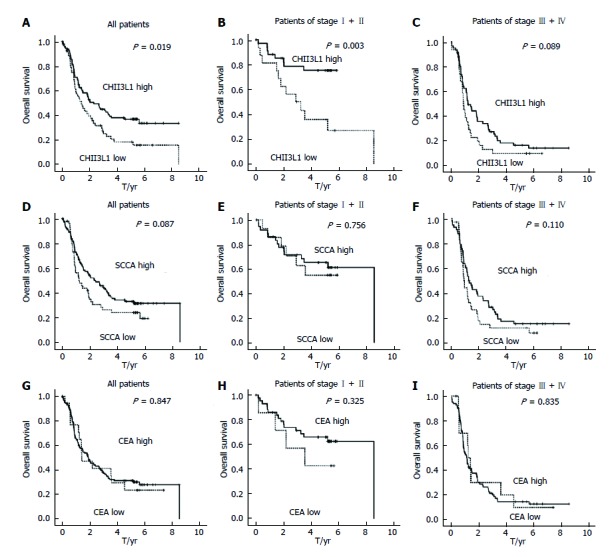
Kaplan-Meier survival curves of esophageal squamous cell carcinoma patients. A: Overall survival of patients with CHI3L1 in all ESCC patients; B: Overall survival of patients with CHI3L1 in patients of Stage I-II; C: Overall survival of patients with CHI3L1 in patients of Stage III-IV; D: Overall survival of patients with SCCA in all ESCC patients; E: Overall survival of patients with SCCA in patients of Stage I-II; F: Overall survival of patients with SCCA in patients of Stage III-IV; G: Overall survival of patients with CEA in all ESCC patients; H: Overall survival of patients with CEA in patients of Stage I-II; I: Overall survival of patients with patients of Stage III-IV. CHI3L1: Chitinase 3-like 1; ESCC: Esophageal squamous cell carcinoma; SCCA: Squamous cell carcinoma antigen.
Next, we examined the OS using the Cox proportional hazards model to determine whether the level of CHI3L1 serves as an independent predictor. A series of factors, including age, sex, alcohol intake, G grade, TNM stage, treatment options, SCCA, CEA, and CHI3L1 concentration, were entered into the univariate Cox regression analysis in Table 2 to assess their impact on the OS of ESCC patients. Sex, smoking status, TNM stage, treatment options, SCCA, and CHI3L1 were significant in the univariate analysis, and therefore, we further analyzed these using a multivariate analysis. The multivariate analysis model revealed that the predominant independent predictors of OS were the CHI3L1 level (HR, 1.004; 95%CI: 1.002-1.005; P < 0.001), sex (HR, 0.346; 95%CI: 0.154-0.776; P = 0.010), TNM stage (HR, 1.755; 95%CI: 1.288-2.390; P < 0.001) and SCCA concentration (HR, 1.056; 95%CI: 1.013-1.100; P = 0.010) as presented in Table 2. Separate analyses of the prognostic effect of CHI3L1 in the subgroup of patients treated with surgery also showed a significant effect for predicting the OS (P = 0.014, not shown).
Table 2.
Univariate and multivariate analysis of overall survival
| Variables |
Univariate analysis |
Multivariate analysis |
||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 0.989 | 0.970-1.009 | 0.271 | - | - | - |
| Sex | 0.489 | 0.293-0.815 | 0.006 | 0.346 | 0.154-0.776 | 0.010 |
| Smoke status | 0.745 | 0.595-0.932 | 0.010 | 1.071 | 0.761-1.507 | 0.694 |
| Alcohol intake | 0.817 | 0.546-1.223 | 0.327 | - | - | - |
| G grade | 1.131 | 0.836-1.530 | 0.533 | - | - | - |
| TNM stage | 2.001 | 1.564-2.560 | < 0.001 | 1.755 | 1.288-2.390 | < 0.001 |
| CHI3L1 | 1.002 | 1.001-1.004 | 0.011 | 1.004 | 1.002-1.005 | < 0.001 |
| SCCA | 1.065 | 1.028-1.104 | < 0.001 | 1.056 | 1.013-1.100 | 0.010 |
| CEA | 1.048 | 0.972-1.130 | 0.224 | - | - | - |
| Treatment | 1.402 | 1.218-1.613 | < 0.001 | 1.167 | 0.979-1.391 | 0.084 |
Variables: Age, ≥ 60 vs < 60; gender, female vs male; smoke, smoker vs non-smoker; alcohol intake, yes vs no; grade, grade 3 vs grade 2 vs grade 1; stage, IV vs III vs II vs treatment, surgery vs chemotherapy vs chemoradiotherapy; -: Represent “date not available”. CHI3L1: Chitinase 3-like 1; SCCA: Squamous cell carcinoma antigen; CEA: Carcinoembryonic antigen.
Tumor surrounded macrophages in ESCC show high expression levels of CHI3L1
In this experiment, we sought to identify the cells in the ESCC tissues secreting CHI3L1. In our previous study[8], we confirmed that CHI3L1 was expressed in 85% of ESCC tissues, among which 7/20 ESCC tissues had a strong stain, and 3/20 did not show any CHI3L1 expression. Additionally, the expression rate of CHI3L1 in the normal esophageal epithelium was only 10%. Through a reanalysis, we found that in 18/20 slices, the esophageal tumor surrounded cells had a high expression of CHI3L1 (Figure 2A). Fluorescence staining was performed to explore the origins of CHI3L1 in the surrounding areas of the tumor cells. As shown in Figure 2B, CD68 protein expression, indicated by the red stain, was found in the same areas as CHI3L1 expression, supporting that CHI3L1 was expressed by macrophages.
Figure 2.
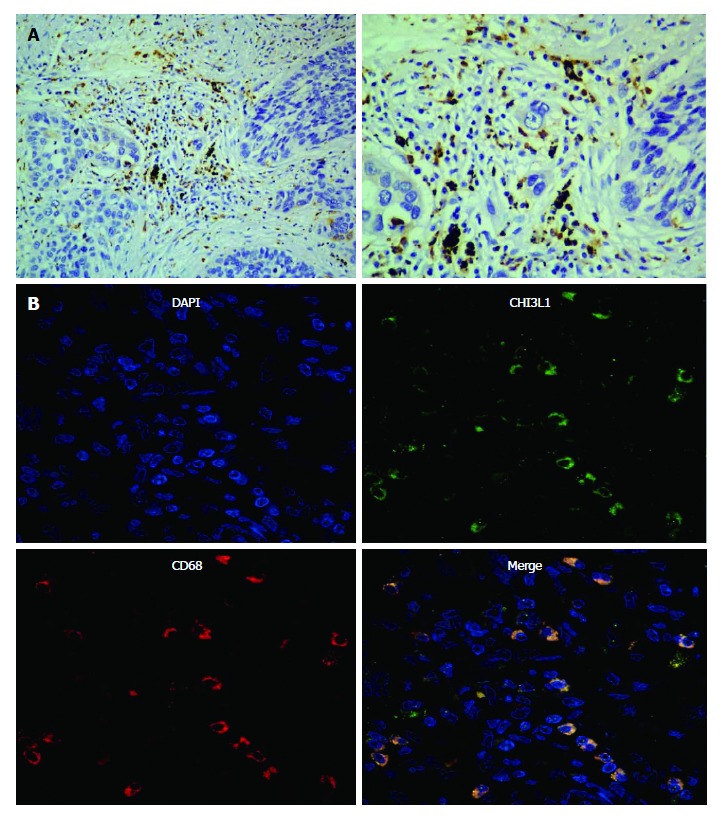
Representative micrographs of esophageal squamous cell carcinoma tissues after immunohistochemistry and immunofluorescence assay. A: Sections stained with a CHI3L1 specific antibody (left- × 200; right- × 40); B: Sections immunofluorescence stained with CHI3L1 (upper left), an antibody against the macrophage specific marker CD68 (upper right), a nuclear counterstain DAPI (lower left) and their merge (lower right). CHI3L1: Chitinase 3-like 1.
Supernatant of the ESCC cell line Eca-109 and IL-6 stimulate Monocyte-derived macrophages to secret CHI3L1
To explore the role of the tumor microenvironment in the secretion of CHI3L1 by macrophages, we used the supernatant of the ESCC cell line Eca-109 to culture the MDMs. Compared with the controls, the stimulation group had a higher level of CHI3L1 expression at both the mRNA level (Figure 3A left, 2.186 ± 1.719 vs 0.1982 ± 0.2918, P = 0.0378) and the protein level (Figure 3A right, 59.31 ± 28.31 vs 27.28 ± 16.89, P = 0.0337). In order to access which component in the Eca-109 cell supernatant works, we detected the cytokine and CHI3L1 levels in the Eca-109 cell supernatant. As shown in Figure 3B, the IL-6 concentration was the highest (119.21 pg/mL), whereas IL-4, IL-2, IL-10, IFN-γ and TNF-α were rarely detected (< 2.5 pg/mL). To imitate the cell media, we used 2 ng/mL of IL-6 to stimulate healthy MDMs. The results showed that the IL-6 group had a higher level of CHI3L1 expression at both the mRNA level (Figure 3C left, 3.841 ± 3.637 vs 1.465 ± 1.813, P = 0.0493) and the protein level (Figure 3C right, 160.9 ± 187.8 vs 116.8 ± 194.7, P = 0.0174).
Figure 3.
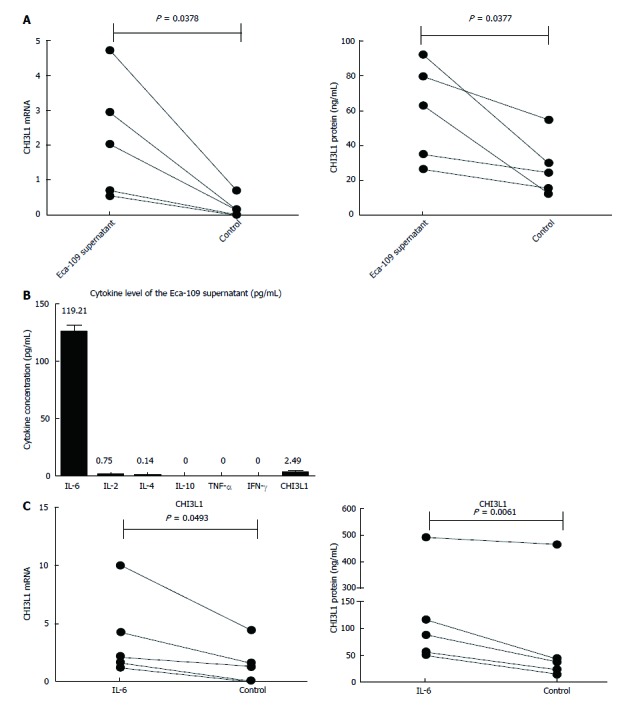
Expression level of chitinase 3-like 1 in monocyte-derived macrophages after stimulation of either supernatant of esophageal squamous cell carcinoma cell line Eca-109 or interleukin-6. A: Expression of CHI3L1 mRNA and protein in monocyte-derived macrophages (upper, mRNA; lower, protein) after stimulation of Eca-109 cell line supernatant was analyzed by real-time PCR and ELISA, respectively; B: Cytokine levels of Eca-109 supernatant were measured with BD CBA Th1/Th2/Th17 Cytokine Kit; C: Level of CHI3L1 mRNA (left) and protein (right) in monocyte-derived macrophages after stimulation of interleukin-6 (IL-6) was analyzed by real-time PCR and ELISA, respectively. Error bars represent standard deviations (SD) calculated from three parallel experiments. CHI3L1: Chitinase 3-like 1.
Correlation of CHI3L1 and laboratory inflammatory parameters
Macrophages are the central cell type that directs host inflammatory and immune processes, and inflammation plays a vital role in tumorigenesis. Thus, we further investigated the correlation of CHI3L1 and laboratory inflammatory parameters. The serum concentration of CHI3L1 in the ESCC patients showed a weak correlation with NEUs (R2 = 0.027, P = 0.045), NLR (R2 = 0.039, P = 0.016), and CRP (R2 = 0.093, P < 0.001) but no correlation with WBCs, LYs, PLTs, and PLR when measured in the serum of the same patients (Figure 4).
Figure 4.
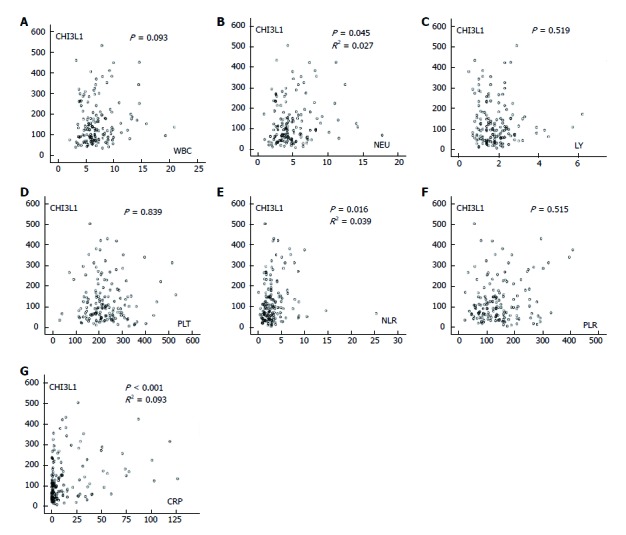
Associations of chitinase 3-like 1 with inflammatory indexes were analyzed by Spearman correlation. A: The associations of chitinase 3-like 1 (CHI3L1) with white blood counts (WBC); B: The associations of CHI3L1 with neutrophil counts (NEU); C: The associations of CHI3L1 with lymphocyte counts (LY); D: The associations of CHI3L1 with platelet counts (PLT); E: The associations of CHI3L1 with NLR (NEU/LY); F: The associations of CHI3L1 with PLR (PLT/LY); G: The associations of CHI3L1 with C-reactive protein (CRP).
DISCUSSION
To the best of our knowledge, this is the first report that demonstrates serum CHI3L1 could serve as a prognostic predictor for esophageal cancer. ESCC is the eighth most common tumor in the world. Over the past 20 years, with the development of translational medicine and clinical practice, esophageal cancer treatments have made substantial progress. However, the 5-year survival rate of esophageal cancer still varies between 15% and 25%. Thus, an effective biomarker is urgently needed to both screen early ESCC and accurately predict its prognosis.
In our previous study, we observed that CHI3L1 exhibited a higher expression level in esophageal squamous cell carcinoma and served as an ideal tumor marker applied in ESCC screening[8]. In this study, we further explored this protein’s prognostic value in ESCC patients. Serum CHI3L1 concentrations were significantly associated with a poor clinical outcome. This was confirmed by Kaplan-Meier survival curves, showing that patients with serum CHI3L1 levels above the cutoff value showed a significantly reduced OS compared to patients with levels below the cutoff value. Moreover, compared to the advanced stages, a stronger correlation between elevated CHI3L1 and short survivals was found at the early stages. Moreover, CHI3L1 performed better than the conventional parameters CEA and SCCA in predicting prognosis. Concordantly, the Cox proportional hazards regressions model analysis illustrated that the level of serum CHI3L1 was an independent prognostic factor, similar to the TMN staging system. Different from the Kaplan-Meier analysis, in the Cox proportional hazards regression model, SCCA was defined as an independent prognostic factor. The statistical approaches may account for some of the differences in error management. Many investigators report similar findings in other types of tumors. Thom et al[15] quantified circulating CHI3L1 in the serum of non-small cell lung cancer and found it was an independent predictor of prognosis. This protein’s serum level was elevated in patients with poor prognoses. In studies by Zhu et al[16], elevated serum CHI3L1 levels also predicted poor prognosis in hepatocellular carcinoma serum. Hogdall EV[19] concluded that high plasma CHI3L1 levels in patients with ovarian cancer stage III is related to a shorter survival. Thus, we speculate that serum CHI3L1 might also serve as a prognostic biomarker for ESCC.
Since elevated serum CHI3L1 is found in patients with different types of solid tumors[20,21], the manner in which serum CHI3L1 is released and regulated is essential for the understanding of tumors. Several studies mention the origin of CHI3L1. It is reported that the human osteosarcoma cell line MG-63[22] and the glioblastoma cell line U87 produces CHI3L1, as does the pluripotent myeloid leukemia cell lines HL-60, THP-1 and U937 when they are differentiated into a macrophage-like cell types[23-25]. The location of CHI3L1 expression was explored in several in vivo settings. Studies by Roslind A showed the expression of CHI3L1 in malignant HNSCC (squamous cell carcinoma of the head and neck) cells[26]. Junker et al[27] concluded that the predominant source of elevated serum CHI3L1 in SCLC is peritumoral macrophages. These studies suggest that CHI3L1 in human tumors is expressed either by cancer cells or stromal cells, such as macrophages, perhaps depending on the cancer type. Appealingly, no observations concerning cell specific expression in ESCC have previously been published. Here, we find pronounced CHI3L1 expression in tumor-surrounded macrophages at the tumor stroma, suggesting that these cells are responsible for the high serum CHI3L1 levels in ESCC patients.
Our observation that ESCC macrophages express CHI3L1 fits well into current knowledge and theories. Serum concentrations of CHI3L1 are often elevated in patients with diseases characterized by extensive inflammation, the development of fibrosis and remodeling of the extra cellular matrix, such as inflammatory bowel disease[28], active rheumatoid arthritis[29], giant cell arteritis[30], severe bacterial infections[31,32] and development of fibrosis[33,34]. In all the above situations, macrophages are involved. Simultaneously, CHI3L1 expression in macrophages has been confirmed in several in vivo settings[24,30,35]. All these phenomena suggest the rationality of the secretion of CHI3L1 by ESCC-associated macrophages. Moreover, CHI3L1 levels were significantly correlated with CRP levels, a systemic marker of inflammation. These findings are consistent with previous studies that reported significant correlations between CHI3L1 levels and CRP[36]. Additionally, the levels of other inflammation markers, such as neutrophil counts (NEU) and the neutrophil/ lymphocyte ratio (NLR), positively correlated with CHI3L1. Therefore, it is possible that CHI3L1 may be involved in the formation and development of tumors by promoting ESCC-related inflammation.
We further explored the release mechanisms of CHI3L1 by peritumoral macrophages. This study shows that CHI3L1 concentrations increase in monocyte-derived macrophages in response to IL-6 stimulation, indicating that a certain increase in IL-6 concentration, as seen in esophageal cancer cell, is needed to stimulate the release of CHI3L1. These findings are consistent with previous studies reporting significant correlations between CHI3L1 levels and IL-6[36,37]. Matsumoto T showed that CHI3L1 was correlated positively with IL-6, and this association seems to be determined by IL-6[36]. Johansen et al[38] indicated that CHI3L1 and IL-6 correlate positively in low concentrations of IL-6. Anders R. Nielsen reported that following the IL-6 infusion, the plasma level of CHI3L1 increased from 30 to 57 ng/mL (P < 0.05) at 24 h and returned to normal values after 48 h[39]. Therefore, we propose that esophageal cancer cells have the ability to produce IL-6, which promotes the secretion of CHI3L1 by ESCC-associated macrophages.
In conclusion, our study first showed that the level of serum CHI3L1 in patients with ESCC provides a reference mark for evaluating prognosis. This simple, convenient serological testing enables clinical application. In addition, the ESCC microenvironment, in our study, especially the secretion of IL-6 by esophageal tumor cells, promotes the macrophage production of CHI3L1. This provides some guidance for elucidating the pathogenesis of ESCC. Further studies are needed to determine how CHI3L1, produced by ESCC-associated macrophages, influences the development of ESCC. Besides, which type of macrophage, M1 or M2, is responsible for the secretion of CHI3L1 remains to be further explored.
Our study first established a connection between pretreated CHI3L1 and patients with ESCC, and the serum CHI3L1 was primarily secreted by ESCC surrounded macrophages, suggesting that CHI3L1 was a simple, non-invasive and inexpensive prognostic factor. These finding may help to identify high-risk patients for treatment decisions and to understand the mechanisms of ESCC.
ARTICLE HIGHLIGHTS
Research background
Esophageal squamous cell carcinoma (ESCC) is one of the most aggressive and lethal human malignancies. With improvements in the diagnosis, staging system and treatment strategies, the overall 5-year survival rate of ESCC patients yielded a slight increase. However, patients at the same stage undergoing similar treatment regimens often have quite different clinical outcomes, which suggests that the current staging system is inadequate for predicting survival. Therefore, the identification of accurate biomarkers for ESCC is necessary to improve the clinical outcome of patients.
Research motivation
Elevated serum chitinase 3-like 1 (CHI3L1) is notably correlated with a poor prognosis and short survival in many malignant tumors. However, no observation concerning the role of CHI3L1 in ESCC prognosis has been reported. In addition, previous studies suggest that CHI3L1 in human tumors are expressed either by cancer cells or stromal cells, such as macrophages. However, no observations concerning the cell specific expression in ESCC were published previously.
Research objectives
To identify whether CHI3L1 serves as a suitable biomarker for the prognosis of ESCC and to analyze this protein’s cellular source.
Research methods
ELISA was conducted to detect the concentration of CHI3L1 in serum of 150 ESCC patients. Immunohistochemistry (IHC) was reanalyzed and fluorescent staining utilized to explore the cellular origins of CHI3L1. Cytokines in serum samples were measured with BD CBA Th1/Th2/Th17 Cytokine Kit. We stimulated the monocyte-derived macrophages (MDMs) with either interleukin-6 (IL-6) or the supernatant of ESCC cell line Eca-109 and then investigated the level of CHI3L1 by qPCR and ELISA.
Research results
This study finds that the level of serum CHI3L1 in patients with ESCC provides a reference mark for evaluating prognosis. In addition, the ESCC microenvironment, in our study, especially the secretion of IL-6 by esophageal tumor cells promotes the macrophage production of CHI3L1.
Research conclusions
This study first established a connection between pretreated CHI3L1 and patients with ESCC, and the serum CHI3L1 was primarily secreted by ESCC surrounded macrophages, suggesting that CHI3L1 was a simple, non-invasive and inexpensive prognostic factor. These finding may help to identify high-risk patients for treatment decisions and to understand the mechanisms of ESCC.
Research perspectives
Further studies are needed to determine how CHI3L1, produced by ESCC-associated macrophages, influences the development of ESCC. As CHI3L1 is reported to be associated with the malignant behavior, especially angiogenesis. We will seek whether it plays a role on the development of ESCC through promoting angiogenesis. And which type of macrophage, M1 or M2, is responsible for the secretion of CHI3L1 remains to be further explored.
ACKNOWLEDGMENTS
We would like to thank all of the study participants for agreeing to participate in medical research. We are grateful to Mr. Christopher Lavender, the International Communication Coordinator of the Office of International Collaboration and Public Relations in the Sun Yat-Sen University Cancer Center, for his assistance in the language editing process.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Sun Yat-sen University Cancer Center.
Conflict-of-interest statement: The authors declare no potential conflicts of interest.
Data sharing statement: No additional data are available.
Peer-review started: June 30, 2017
First decision: August 15, 2017
Article in press: September 26, 2017
P- Reviewer: Eshraghian A, Guo Y S- Editor: Ma YJ L- Editor: A E- Editor: Lu YJ
Contributor Information
Shan Xing, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Guangdong Province, China; Department of Clinical Laboratory, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China.
Xin Zheng, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Guangdong Province, China; Department of Clinical Laboratory, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China.
Tao Zeng, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Guangdong Province, China; Department of Clinical Laboratory, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China.
Mu-Sheng Zeng, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Guangdong Province, China.
Qian Zhong, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Guangdong Province, China.
Yue-Song Cao, Department of Biotechnology, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China.
Kai-Lu Pan, Department of Clinical Laboratory, Guangdong Medical University, Dongguan 523808, Guangdong Province, China.
Chu Wei, Department of Clinical Laboratory, Guangdong Medical University, Dongguan 523808, Guangdong Province, China.
Fan Hou, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Guangdong Province, China; Department of Clinical Laboratory, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China.
Wan-Li Liu, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Guangdong Province, China; Department of Clinical Laboratory, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong Province, China. liuwl@sysucc.org.cn.
References
- 1.Yang L, Parkin DM, Ferlay J, Li L, Chen Y. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev. 2005;14:243–250. [PubMed] [Google Scholar]
- 2.Su H, Hu N, Yang HH, Wang C, Takikita M, Wang QH, Giffen C, Clifford R, Hewitt SM, Shou JZ, et al. Global gene expression profiling and validation in esophageal squamous cell carcinoma and its association with clinical phenotypes. Clin Cancer Res. 2011;17:2955–2966. doi: 10.1158/1078-0432.CCR-10-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 5.Zheng X, Xing S, Liu XM, Liu W, Liu D, Chi PD, Chen H, Dai SQ, Zhong Q, Zeng MS, et al. Establishment of using serum YKL-40 and SCCA in combination for the diagnosis of patients with esophageal squamous cell carcinoma. BMC Cancer. 2014;14:490. doi: 10.1186/1471-2407-14-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen GQ, Tian H, Yue WM, Li L, Li SH, Qi L, Gao C, Si LB, Lu M. NCOA5 low expression correlates with survival in esophageal squamous cell carcinoma. Med Oncol. 2014;31:376. doi: 10.1007/s12032-014-0376-y. [DOI] [PubMed] [Google Scholar]
- 7.Cao HH, Zhang SY, Shen JH, Wu ZY, Wu JY, Wang SH, Li EM, Xu LY. A three-protein signature and clinical outcome in esophageal squamous cell carcinoma. Oncotarget. 2015;6:5435–5448. doi: 10.18632/oncotarget.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Zhu Z, Liu Y, Jin X, Xu Z, Yu Q, Li K. Diagnostic value of multiple tumor markers for patients with esophageal carcinoma. PLoS One. 2015;10:e0116951. doi: 10.1371/journal.pone.0116951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji L, Cao XF, Wang HM, Li YS, Zhu B, Xiao J, Wang D. Expression level of beta-catenin is associated with prognosis of esophageal carcinoma. World J Gastroenterol. 2007;13:2622–2625. doi: 10.3748/wjg.v13.i18.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong M, Chan KW, Bao JY, Wong KY, Chen JN, Kwan PS, Tang KH, Fu L, Qin YR, Lok S, et al. Rab25 is a tumor suppressor gene with antiangiogenic and anti-invasive activities in esophageal squamous cell carcinoma. Cancer Res. 2012;72:6024–6035. doi: 10.1158/0008-5472.CAN-12-1269. [DOI] [PubMed] [Google Scholar]
- 11.Bleau G, Massicotte F, Merlen Y, Boisvert C. Mammalian chitinase-like proteins. EXS. 1999;87:211–221. doi: 10.1007/978-3-0348-8757-1_15. [DOI] [PubMed] [Google Scholar]
- 12.Johansen JS, Jensen HS, Price PA. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993;32:949–955. doi: 10.1093/rheumatology/32.11.949. [DOI] [PubMed] [Google Scholar]
- 13.Zou L, He X, Zhang JW. The efficacy of YKL-40 and CA125 as biomarkers for epithelial ovarian cancer. Braz J Med Biol Res. 2010;43:1232–1238. doi: 10.1590/s0100-879x2010007500133. [DOI] [PubMed] [Google Scholar]
- 14.Shao R, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. Breast cancer expression of YKL-40 correlates with tumour grade, poor differentiation, and other cancer markers. Br J Cancer. 2011;105:1203–1209. doi: 10.1038/bjc.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thöm I, Andritzky B, Schuch G, Burkholder I, Edler L, Johansen JS, Bokemeyer C, Schumacher U, Laack E. Elevated pretreatment serum concentration of YKL-40-An independent prognostic biomarker for poor survival in patients with metastatic nonsmall cell lung cancer. Cancer. 2010;116:4114–4121. doi: 10.1002/cncr.25196. [DOI] [PubMed] [Google Scholar]
- 16.Zhu CB, Chen LL, Tian JJ, Su L, Wang C, Gai ZT, Du WJ, Ma GL. Elevated serum YKL-40 level predicts poor prognosis in hepatocellular carcinoma after surgery. Ann Surg Oncol. 2012;19:817–825. doi: 10.1245/s10434-011-2026-3. [DOI] [PubMed] [Google Scholar]
- 17.Horbinski C, Wang G, Wiley CA. YKL-40 is directly produced by tumor cells and is inversely linked to EGFR in glioblastomas. Int J Clin Exp Pathol. 2010;3:226–237. [PMC free article] [PubMed] [Google Scholar]
- 18.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Høgdall EV, Johansen JS, Kjaer SK, Price PA, Christensen L, Blaakaer J, Bock JE, Glud E, Høgdall CK. High plasma YKL-40 level in patients with ovarian cancer stage III is related to shorter survival. Oncol Rep. 2003;10:1535–1538. [PubMed] [Google Scholar]
- 20.Tanwar MK, Gilbert MR, Holland EC. Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res. 2002;62:4364–4368. [PubMed] [Google Scholar]
- 21.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–4434. [PubMed] [Google Scholar]
- 22.Johansen JS, Williamson MK, Rice JS, Price PA. Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res. 1992;7:501–512. doi: 10.1002/jbmr.5650070506. [DOI] [PubMed] [Google Scholar]
- 23.Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, Andreesen R, Krause SW. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem. 2003;278:44058–44067. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick RB, Emery JG, Connor JR, Dodds R, Lysko PG, Rosenberg M. Induction and expression of human cartilage glycoprotein 39 in rheumatoid inflammatory and peripheral blood monocyte-derived macrophages. Exp Cell Res. 1997;237:46–54. doi: 10.1006/excr.1997.3764. [DOI] [PubMed] [Google Scholar]
- 25.Verhoeckx KC, Bijlsma S, de Groene EM, Witkamp RF, van der Greef J, Rodenburg RJ. A combination of proteomics, principal component analysis and transcriptomics is a powerful tool for the identification of biomarkers for macrophage maturation in the U937 cell line. Proteomics. 2004;4:1014–1028. doi: 10.1002/pmic.200300669. [DOI] [PubMed] [Google Scholar]
- 26.Roslind A, Johansen JS, Christensen IJ, Kiss K, Balslev E, Nielsen DL, Bentzen J, Price PA, Andersen E. High serum levels of YKL-40 in patients with squamous cell carcinoma of the head and neck are associated with short survival. Int J Cancer. 2008;122:857–863. doi: 10.1002/ijc.23152. [DOI] [PubMed] [Google Scholar]
- 27.Junker N, Johansen JS, Andersen CB, Kristjansen PE. Expression of YKL-40 by peritumoral macrophages in human small cell lung cancer. Lung Cancer. 2005;48:223–231. doi: 10.1016/j.lungcan.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Vind I, Johansen JS, Price PA, Munkholm P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol. 2003;38:599–605. doi: 10.1080/00365520310000537. [DOI] [PubMed] [Google Scholar]
- 29.Volck B, Johansen JS, Stoltenberg M, Garbarsch C, Price PA, Ostergaard M, Ostergaard K, Løvgreen-Nielsen P, Sonne-Holm S, Lorenzen I. Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthritis Cartilage. 2001;9:203–214. doi: 10.1053/joca.2000.0377. [DOI] [PubMed] [Google Scholar]
- 30.Johansen JS, Baslund B, Garbarsch C, Hansen M, Stoltenberg M, Lorenzen I, Price PA. YKL-40 in giant cells and macrophages from patients with giant cell arteritis. Arthritis Rheum. 1999;42:2624–2630. doi: 10.1002/1529-0131(199912)42:12<2624::AID-ANR17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Kronborg G, Ostergaard C, Weis N, Nielsen H, Obel N, Pedersen SS, Price PA, Johansen JS. Serum level of YKL-40 is elevated in patients with Streptococcus pneumoniae bacteremia and is associated with the outcome of the disease. Scand J Infect Dis. 2002;34:323–326. doi: 10.1080/00365540110080233. [DOI] [PubMed] [Google Scholar]
- 32.Nordenbaek C, Johansen JS, Junker P, Borregaard N, Sørensen O, Price PA. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J Infect Dis. 1999;180:1722–1726. doi: 10.1086/315050. [DOI] [PubMed] [Google Scholar]
- 33.Johansen JS, Christoffersen P, Møller S, Price PA, Henriksen JH, Garbarsch C, Bendtsen F. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 34.Nøjgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U; EMALD Group. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39:179–186. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 35.Boot RG, van Achterberg TA, van Aken BE, Renkema GH, Jacobs MJ, Aerts JM, de Vries CJ. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999;19:687–694. doi: 10.1161/01.atv.19.3.687. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto T, Tsurumoto T. Serum YKL-40 levels in rheumatoid arthritis: correlations between clinical and laborarory parameters. Clin Exp Rheumatol. 2001;19:655–660. [PubMed] [Google Scholar]
- 37.Hempen M, Kopp HP, Elhenicky M, Höbaus C, Brix JM, Koppensteiner R, Schernthaner G, Schernthaner GH. YKL-40 is elevated in morbidly obese patients and declines after weight loss. Obes Surg. 2009;19:1557–1563. doi: 10.1007/s11695-009-9917-4. [DOI] [PubMed] [Google Scholar]
- 38.Johansen JS, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK, Bruunsgaard H. High serum YKL-40 level in a cohort of octogenarians is associated with increased risk of all-cause mortality. Clin Exp Immunol. 2008;151:260–266. doi: 10.1111/j.1365-2249.2007.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen AR, Plomgaard P, Krabbe KS, Johansen JS, Pedersen BK. IL-6, but not TNF-α, increases plasma YKL-40 in human subjects. Cytokine. 2011;55:152–155. doi: 10.1016/j.cyto.2011.03.014. [DOI] [PubMed] [Google Scholar]


