Abstract
AIM
To evaluate the efficacy of thalidomide for treating troublesome cases of pediatric Crohn’s disease (CD) with tuberculosis infection.
METHODS
A retrospective study of clinical outcome among children treated with thalidomide was conducted. All patients had evidence of tuberculosis infection with a failure of anti-tuberculosis treatment for more than one year, and were subsequently diagnosed with CD. All the patients received thalidomide treatment with a starting dose of 1.2-2.5 mg/kg per day. Remission was defined as pediatric CD activity index less than or equal to 10.
RESULTS
Ten patients with CD were treated with thalidomide at an average age of 7.2 years and followed up for a median of 22.2 mo. Clinical remission rate was 60% after 9-12 mo of thalidomide treatment. One patient with no response had an interleukin-10 receptor alpha gene mutation. Erythrocyte sedimentation rate, C-reactive protein and platelet count showed a dramatic decrease; hemoglobin level and weight improved significantly after thalidomide treatment when compared with the baseline values.
CONCLUSION
Thalidomide is an effective and safe drug for remission of CD in pediatric patients who have been treated for tuberculosis.
Keywords: Inflammatory bowel disease, Intestinal tuberculosis, Anti- tubercular treatment, Thalidomide, Children
Core tip: Therapies for Crohn’s disease (CD) and intestinal tuberculosis are totally different, and anti-TNF alpha treatment may increase the risk of tuberculosis reactivation. That makes it still tough to treat patients with severe CD with concomitant tuberculosis, especially in high tuberculosis prevalence areas. In the current study, all patients had evidence of tuberculosis infection and diagnosed with CD. Thalidomide yielded a positive result for those special cases, and it could be an alternative drug after treatment of tuberculosis is completed.
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory disorder affecting an increasing number of patients each year around the world[1]. It is characterized by abdominal pain, diarrhea, bloody stool and other extra-intestinal manifestations, and impacts on growth in children and adolescents. Wang et al[2] reported a multicenter retrospective study in China which demonstrated that childhood-onset inflammatory bowel disease is an emerging disease with a 12-fold increase of incidence over the past decade. Intestinal tuberculosis (ITB) shares a close resemblance in clinical, endoscopic and histological manifestations with CD, making the differential diagnosis of these two diseases difficult[3]. At the same time, tuberculosis (TB) is and has always been a major public health problem worldwide, especially in the lower income countries[4]. With the changing epidemiology of TB and CD, it is not unusual to encounter the coexistence of these two diseases, especially in high TB endemic areas[5].
Managing CD with steroids, immunomodulatory therapy and biological agents in high TB endemic regions is challenging since those treatments are associated with an increased risk of tuberculosis reactivation for active or latent TB[6]. Considering a high incidence of TB in developing countries, empirical anti-tubercular treatment (ATT) is used to differentiate between ITB and CD[7]. However, the use of anti-TB medications may pose a risk of toxicity and cause unnecessary delay for management of CD patients.
In 2011, we reported in our pilot study our experience that three pediatric patients with CD concomitant with tuberculosis achieved clinical remission after six months of thalidomide treatment[8]. Similarly, a later case report presented an adult patient with CD and pulmonary TB who was steroid-dependent and unresponsive to infliximab, but the patient exhibited an excellent response to thalidomide treatment[9]. In the present study, we enrolled 10 pediatric-onset patients who had symptoms and evidence suggestive of CD and tuberculosis in attempt to evaluate the efficacy of thalidomide in clinical remission of these cases in a long-term follow-up.
MATERIALS AND METHODS
Patients
This is a tertiary medical center, retrospective study of pediatric CD patients (aged < 18 years) with tuberculosis treated with thalidomide at the Children’s Hospital of Fudan University from July 2009 to April 2016. Tuberculosis diagnosis was established based on at least one of the following criteria: (1) histological manifestation of acid fast bacilli (AFB) in intestinal tissues; (2) positive TB culture; and (3) positive tuberculin skin testing. All of the patients received a full course of anti-TB medications prior to thalidomide administration. The diagnosis of CD was based on endoscopic and clinical symptoms, which were defined as no improvement of clinical and endoscopic symptoms after anti-TB treatment for at least one year.
Treatment
Thalidomide (Changzhou Pharmaceutical Factory, Changzhou, China) was administered orally at a starting dose of 1.2-2.5 mg/kg per day. The decision to modify the dosage was made by the director of gastroenterology department according to the response and disease activity. To minimize adverse events, thalidomide was taken every evening. This study was approved by the Ethics Committee of Children’s Hospital, Fudan University. Written informed consent was obtained from either the parents or legal guardians of the patients after they were informed about possible adverse events. Contraception was controlled in the patients who were in reproduction age.
Outcome
A retrospective chart review of medical records was performed to collect baseline demographic and disease characteristics, results of clinical indices and adverse events during follow-up. Pediatric CD Activity Index (PCDAI)[10], as primary outcome, was used to evaluate the response to the treatment from the time of thalidomide initiation and at 9-12 mo thereafter. Each patient acted as his/her own historical control. Clinical remission was defined by PCDAI less than or equal to 10, significant response was a decrease in PCDAI at least 12.5 points from baseline[11]. The clinical indices of erythrocyte sedimentation rate (ESR; normal range 0-20 mm/h), C-reactive protein (CRP; normal range 0-8 mg/L), platelet count and hemoglobin were compared before and after treatment. Weight for age Z score by Chinese standardized growth curve was evaluated as a measurement of nutritional index.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL). Quantitative variables were presented as mean ± SD or median with range. Continuous variables were evaluated using paired Student’s t test with normal distribution and Wilcoxon test with non-normal distribution. Statistical significance level was set at two-sided P < 0.05.
RESULTS
Baseline characteristics
Ten pediatric patients treated with thalidomide were enrolled in the study. There were 6 females and 4 males, with an average age of 7.2 years at thalidomide treatment (range: 2-13.5 years). The mean disease duration before thalidomide therapy was 24 mo (range: 16-42 mo). The average length of follow-up was 22.2 mo (range: 9-44 mo) after the initiation of thalidomide. Clinical characteristics are summarized in Table 1.
Table 1.
Clinical characteristics of all cases treated with thalidomide
| Patient No. | Sex | Age at thalidomide treatment | Disease duration before thalidomide | Age at ATT treatment | Disease distribution | Extra-intestinal symptoms | TB status before ATT | ATT medications and duration | Thalidomide starting dose | Thalidomide final dose (duration) | Follow-up time (mo) | Response |
| 1 | M | 2 yr 8 mo | 26 mo | 1 yr 3 mo | Ileocolonic | Joint lesions | AFB (+), PPD (+) | HRZ, 6 m; | 2.5 mg/kg per day | - (34 m) | 38 mo | Remission |
| HRZEP, 6 mo | ||||||||||||
| 2 | F | 2 yr | 23 mo | 2 mo | Colon | Perianal abscess | AFB (+) | HRZ, 3 mo; | 2.5 mg/kg per day | - (7 m) | 10 mo | No response |
| HR, 15 mo | ||||||||||||
| 3 | F | 2 yr 4 mo | 28 mo | 7 mo | Colon | Perianal abscess | AFB (+) | HRZ, 9 mo; | 2 mg/kg per day | 0.33 mg/kg per day | 33 mo | Response |
| HREP, 27 mo | ||||||||||||
| 4 | F | 11 yr 7 mo | 42 mo | 8 yr 1 mo | Ileocolonic | Perianal skin tag | AFB (+) | HREZ, 12 mo | 1.8 mg/kg per day | 0.8 mg/kg per day | 15 mo | Response |
| 5 | M | 12 yr 6 mo | 18 mo | 11 yr 3 mo | Ileocolonic | Oral ulcers | TB culture (+), | HRS, 5 mo; | 2 mg/kg per day | 0.5 mg/kg per day | 36 mo | Remission |
| Spleen TB | HRE, 12 mo | |||||||||||
| 6 | M | 12 yr 5 mo | 20 mo | 11 yr 5 mo | Ileocolonic | Anal fistula | AFB (+) | HRZ, 12 mo | 1.2 mg/kg per day | 0.6 mg/kg per day | 12 mo | Remission |
| 7 | M | 8 yr 6 mo | 38 mo | 5 yr 4 mo | Ileal | Pleural and ascetic fluid | PPD (+) | HRE, 3 mo; | 2 mg/kg per day | - (44 m) | 44 mo | Remission |
| HRZ, 12 mo | ||||||||||||
| 8 | F | 3 yr 8 mo | 16 mo | 2 yr 4 mo | Ileocolonic | - | AFB (+) | HRZ, 14 mo; | 2 mg/kg per day | 1.6 mg/kg per day | 16 mo | Response |
| HRE, 12 mo | ||||||||||||
| 9 | F | 2 yr 4 mo | 16 mo | 1 yr | Ileocolonic | Anal fissure | AFB (+) | HRZ, 14 mo | 2.2 mg/kg per day | 1.8 mg/kg per day | 9 mo | Remission |
| 10 | F | 13 yr 6 mo | 17 mo | 12 yr 1 mo | Ileocolonic | Perianal skin tag | AFB (+) | HREZ, 3 mo; | 1.8 mg/kg per day | 1.3 mg/kg per day | 9 mo | Remission |
| HR, 15 mo |
ATT: Anti-tubercular treatment; H: Isoniazid; R: Rifampicin; Z: Pyrazinamide; E: Ethambutol; S: Streptomycin; P: Para-aminosalicylic acid.
All patients have received ATT treatment for more than one year with the average age of 5.3 years (range: 0.2-12.0 years). Seven children were previously treated with isoniazide, rifampicin and pyrzinamide (HRZ). All patients had evidence of tuberculosis; positive AFB was observed in 8 cases and positive tuberculin skin testing in 2. One patient suffered from spleen TB, and cell culture of Mycobacterium was positive. The mean duration of ATT treatment was 18 mo (range: 12-36 mo).
In the present cases, 2 showed prominently colonic involvement, 7 had ileocolonic diseases and the remaining one had isolated ileal disease. Six patients presented with perianal disease, one suffered from joint involvement and one had oral ulcers.
Outcome
Disease activity: At baseline, 7 patients had moderate-to-severe disease activity (PCDAI > 30). There was a significant decrease in the PCDAI score from 37.3 ± 14.1 in the beginning to 16.0 ± 17.9 after 9-12 mo treatment (P < 0.05) (Figure 1). Clinical remission was achieved in 6 patients (60.0%) and there was a response in 3 cases (30.0%) at 9-12 mo after commencement of thalidomide treatment. For those 3 patients with follow-up duration longer than 36 mo, they were still in remission. Case 4 was unresponsive to infliximab prior to positive AFB, but was responsive to thalidomide. In case 2, the PCDAI increased from 52.5 to 60, indicating no response to thalidomide. Given early-onset symptoms and severe perianal abscess of the patient, we performed the whole exome sequencing and found a causative interleukin-10 receptor alpha (IL10 RA) mutation. One of the compound heterozygous variants was c.301C > T inherited from her father, and the other was c.537G > A from her mother. She has been treated with umbilical cord blood transplantation, which was described in our previous studies[12,13].
Figure 1.
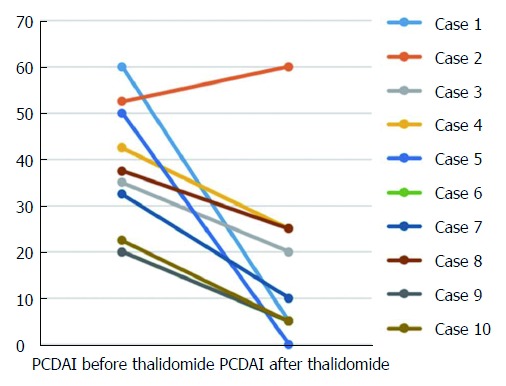
Changes in pediatric Crohn’s disease activity index before and after 9-12 mo of thalidomide treatment in 10 patients. There was a decrease of the pediatric CD activity index (PCDAI) scores in 9 patients (P < 0.05), 6 of them achieved clinical remission (PCDAI < 10). PCDAI: Pediatric Crohn’s disease activity index.
Given the lack of complete data, we only evaluated the response of some cases at different time points. Case 1 showed significant clinical response in the first one month (PCDAI decreased from 45 to 15), case 5 and case 7 attained clinical remission (PCDAI < 10) after 2 mo and one month of treatment, respectively. With regard to TB status, the positive AFB findings were turned to negative in case 3 and case 8 after 16 mo and 10 mo of treatment, respectively.
Overall, the laboratory assessment showed significant drop after thalidomide therapy in ESR (baseline: 26.1 mm/h ± 14.5 mm/h; follow-up: 11.5 mm/h ± 12.0 mm/h) (normal range: 0-20 mm/h) (P = 0.037) (Figure 2A), CRP (baseline: 64.0 mg/L ± 46.5 mg/L; follow-up: 25.1 mg/L ± 47.8 mg/L) (normal range: 0-8 mg/L) (P = 0.004) (Figure 2B) and platelet (baseline: 471.1 × 109/L ± 178.3 × 109/L; follow-up: 328.4 × 109/L ± 163.8 × 109/L) (P = 0.002) (Figure 2D), with a marked improvement in hemoglobin level (baseline: 111.0 g/L ± 13.4 g/L; follow-up: 119.5 g/L ± 19.7 g/L) (normal range: 110-160 g/L) (P = 0.105) (Figure 2C). Weight values at 9-12 mo after starting the treatment showed a dramatic increase when compared with the baselines (21.03 ± 13.4 vs 27.8 ± 19.3) (P < 0.05). The changes of weight for age Z score are shown in Figure 3.
Figure 2.
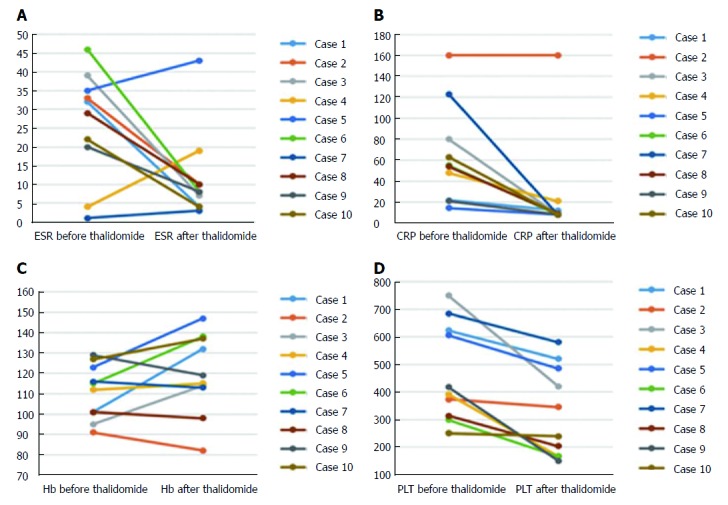
Changes of laboratory indices before and after 9-12 mo treatment in 10 Crohn’s disease patients with tuberculosis. A: Erythrocyte sedimentation rate (ESR); B: C-reactive protein (CRP); C: Hemoglobin (Hb); D: Platelet (PLT). It showed significant reductions in ESR, CRP and platelet levels (P < 0.05); and an increasing trend in Hb levels.
Figure 3.
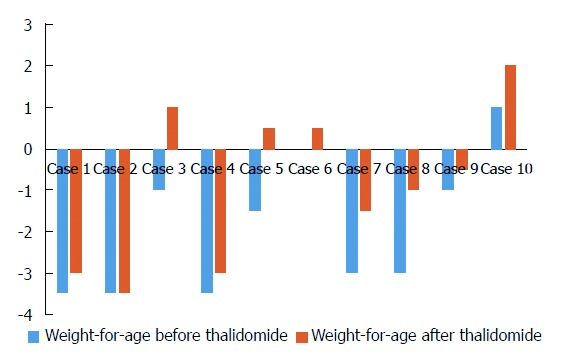
Changes in weight for age Z score before and after 9-12 mo treatment in 10 cases treated with thalidomide.
Dose escalation: The majority of patients (70%) started on an initial dose of 2 mg/kg per day or more. During follow-up, four cases had thalidomide dose increased (to a maximum of 3 mg/kg per day). And the details of dose changes in each individual patient are listed in Table 2. Doses were successfully decreased for all cases after responding to thalidomide. Two cases discontinued thalidomide administration during follow-up due to clinical remission, and one patient with IL10 RA deficiency stopped the treatment due to no response (Table 1). The median cumulative dose for all patients until the end of follow-up time was 16.0 g. For case 5, the cumulative dose was over 28 g. He discontinued thalidomide after 22 mo of treatment, but the symptom of oral ulcers relapsed. However, the patient later opted to restart thalidomide and responded quickly. He was in remission with a minimum dose of 0.5 mg/kg per day. Among all cases, 4 completely discontinued anti-tuberculous drugs and then took thalidomide, the other 6 patients continued anti-tuberculous medications. In terms of the treatment for CD, 6 cases were treated with 5-ASA as well as thalidomide, one patient received nutritional treatment.
Table 2.
Dose changes at different time points after thalidomide treatment
| Patient No. | Dose (mg/kg per day) at different follow-up time (mo) |
| 1 | 2.5 (baseline) - 2.5 (1 mo) - 1.2 (12 mo) - discontinue (34 mo) |
| 2 | 2.5 (baseline) - discontinue (7 mo) |
| 3 | 2 (baseline) - 1.1 (12 mo) - 0.7(18 mo) - 0.4 (21 mo) - 0.33 (33 mo) |
| 4 | 1.8 (baseline) - 3 (5 mo) - 1 (12 mo) - 0.8 (15 mo) |
| 5 | 2 (baseline) - 0.5 (2 mo) - 1.8(9 mo) - discontinue (22 mo) - 0.5 (36 mo) |
| 6 | 1.2 (baseline) - 0.6 (12 mo) |
| 7 | 2 (baseline) - 1.8 (1 mo) - 1.1 (10 mo) – stop (44 mo) |
| 8 | 2 (baseline) – 2.4 (10 mo) - 1.6 (16 mo) |
| 9 | 2.2 (baseline) -1.4 (5 mo) - 1.8 (9 mo) |
| 10 | 1.8 (baseline) -1.3 (9 mo) |
No significant relations were seen between the disease duration, disease location or age of diagnosis and remission or response to thalidomide treatment.
Adverse events
One patient (case 5) was complained of drowsiness and one (case 1) had dryness in eyes, the symptoms were relieved without reducing dose, or discontinuation. One patient (case 8) developed dryness in eyes and knee pain, and recovered after discontinuation of thalidomide. Subsequent thalidomide was readminstered without any adverse effects. None of these patients presented with symptoms and signs of sensory impairment during the follow-up period.
DISCUSSION
This study presents an experience with thalidomide for CD patients who have been treated for tuberculosis at a young age. And 60% of patients in our study achieved clinical remission after 9-12 mo of treatment and the measured parameters were significantly improved in most patients.
Previous studies have well described the efficacy of thalidomide in adult-onset CD patients[14-17]. Facchini et al[18] first reported five pediatric CD patients who were administered with thalidomide as refractory cases or the last medical resort before surgical intervention, 4 of them were in remission after 19-24 mo of treatment. In a long-term retrospective study, remission was achieved with thalidomide in 17 of 19 children and adolescent patients with CD and 80% of patients suspended steroids successfully[19]. In a later retrospective series of 12 children with severe refractory CD who failed to respond to infliximab and adalimumab, a 83.3% clinical remission rate and a rate of 71.4% complete fistula closure after thalidomide treatment as a rescue therapy were achieved[20]. In our study, one case (case 4) also failed to respond to infliximab, but responded to thalidomide therapy. Recently, the first multicenter, double-blind randomized clinical trial provided more information concerning the efficacy and safety of thalidomide on active pediatric CD despite immunosuppressive treatment. In that study, 31 of 49 (63.3%) children achieved clinical remission and 65.3% achieved a 75% response rate after 1.5 to 2.5 mg/kg per day thalidomide treatment[21]. The 60% remission and 30% response rates in our study are comparable with those studies.
One of the most important features in the current study is that all patients had laboratory findings consistent with tuberculosis infection. Of note, we found evidence of AFB in 80% of patients, which indicated tuberculosis infection. Thus, it is reasonable that they were first treated with anti-TB medications, however, the treatment failed despite administration for more than one year. Given that CD and ITB have marked overlap in clinical, endoscopic and histologic features, CD diagnosis was then established due to the failure of ATT therapy, and the majority of our cases had perianal diseases which were more common in CD than ITB. While it is undeniable that the two conditions could coexist in countries with a high TB prevalence, one hypothesis suggests that Mycobacterium avium subspecies might be a cause of CD[22]. In 2011, a successful use of thalidomide under such circumstances in patients with CD and also tuberculosis infection was also reported[9]. In fact, thalidomide has been shown to be effective as an adjuvant treatment for intractable intracranial tuberculosis and central nervous system tuberculosis infection that did not respond to standard medical and surgical therapy[23-25]. It has been postulated that the mechanism of action of thalidomide is associated with inhibiting tumor necrosis factor-alpha secretion. It could also co-stimulate T lymphocytes and have a greater effect on CD8+ than CD4+ T cells since CD8+ T cells have a protective immunological effect in Mycobacterium tuberculosis infection[26]. Therefore, due to its positive role in tuberculosis infection, the application of thalidomide seems to be able to avoid the contraindication for the use of infliximab in patients with latent or active TB and reduce the damage from delaying treatment of CD.
As a sedative and antiemetic agent during pregnancy in the 1950s, thalidomide was withdrawn from the market due to potential teratogenicity. The most commonly encountered adverse effect of thalidomide treatment is the peripheral neuropathy which impedes its long-term use. Our experience showed that drowsiness was the most common adverse reaction to thalidomide. The relationship between peripheral neuropathy and cumulative dose has been investigated in a previous study which showed that 25% of patients complained of peripheral neuropathy and all received cumulative doses over 28 g[19]. In our cases, only one reached high cumulative dose during the follow-up period. The low-dose administration may be one of the reasons for no obvious adverse events, or it could be due to the short-term follow-up in our study. However, it is noteworthy that one case with a cumulative dose over 28 g experienced disease relapse after thalidomide discontinuation and recovered soon after restarting thalidomide at a very low daily dose, being indicative of thalidomide-dependency. Clearly, this still needs further investigation with similar cases.
Our study had some limitations. There was no control group to compare the effect of other medications because the natural course of CD can achieve spontaneous remission and exacerbation. We selected the self-control study to reduce the bias. Although our study is the largest reported series treated with thalidomide in pediatric patients with CD who has been treated for tuberculosis, its retrospective design may create several limitations. A prospective study should be carried out in the future. We also did not perform Mycobacterium culture and electromyography because no patient presented with symptoms and signs of sensory impairment.
In conclusion, thalidomide is an effective and safe drug in inducing clinical remission and improving laboratory parameters for pediatric patients with CD who have been treated for tuberculosis infection. It can be used as an alternative drug after treatment of TB is completed. A controlled, long-term trial of thalidomide in patients with those conditions awaits further investigation.
COMMENTS
Background
Since Crohn’s disease (CD) and tuberculosis (TB) could be present in the same patient, especially in high tuberculosis endemic areas. However, biological agents or immunosuppressive drugs, as effective medications for CD, may increase the risk of tuberculosis reactivation. Therefore, it is urgent to develop a suitable therapy for those troublesome cases of CD with tuberculosis infection.
Research frontiers
Anti-TNF alpha treatment may increase the risk of tuberculosis reactivation. This makes it difficult to treat severe inflammatory bowel disease patients in high endemic area of tuberculosis. Thalidomide is reported to be effective in pediatric CD, and it is also safe for treatment of intracranial tuberculosis as an adjuvant therapy.
Innovations and breakthroughs
The authors describe a small series of 10 pediatric patients with evidence of tuberculosis. Following at least one year of anti-tubercular therapy, patients started on thalidomide due to persistence of gastrointestinal symptoms and endoscopic abnormalities consistent with CD. Clinical remission rate reached 60% after 9-12 mo of thalidomide treatment. Importantly, no exacerbation of TB was reported during a mean follow-up period of 22.2 mo. The results of the study may be relevant for clinicians dealing with CD in countries with high prevalence of tuberculosis.
Applications
This is a retrospective case series of 10 pediatric CD patients complicated with tuberculosis from one institution. In TB prevalent region where CD is also prevalent, the potential benefit using thalidomide provides a reasonable option.
Terminology
Anti-tubercular treatment refers to the use of response to the therapy to differentiate between tuberculosis and CD. PCDAI is the Pediatric CD Activity Index, a scale to assess the severity of the disease.
Peer-review
This study presented the retrospective experience with thalidomide to treat children with CD who had laboratory evidence of infection of Mycobacterium tuberculosis. The patient numbers included in this study are small. However, it provides useful information for the management of pediatric CD.
ACKNOWLEDGMENTS
We would like to express the gratitude to all the patients and their families for their participation and support of this study.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Medical Science and Technology Planning Project of Zhejiang Province, China, No. 2015KYB430 (to Hong Y).
Institutional review board statement: The study was approved by the ethics committee of Children’s Hospital of Fudan University, Shanghai, China.
Informed consent statement: All the patients and/or their legal guardians provided written consent forms for the study.
Conflict-of-interest statement: All the authors listed here declare no conflicts of interest related to this study.
Data sharing statement: The dataset is available from the corresponding author at yhuang815@163.com.
Peer-review started: July 26, 2017
First decision: August 10, 2017
Article in press: September 13, 2017
P- Reviewer: Tommasini A, Zhang L, Lakatos PL S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Huang Y
Contributor Information
Lin Wang, Department of Gastroenterology, Pediatric Inflammatory Bowel Disease Research Center, Children’s Hospital of Fudan University, Shanghai 201102, China.
Yan Hong, Department of Gastroenterology, Pediatric Inflammatory Bowel Disease Research Center, Children’s Hospital of Fudan University, Shanghai 201102, China; Department of Pediatrics, Taizhou Women’s and Children’s Hospital of Wenzhou Medical University, Taizhou 318000, Zhejiang Province, China.
Jie Wu, Department of Gastroenterology, Pediatric Inflammatory Bowel Disease Research Center, Children’s Hospital of Fudan University, Shanghai 201102, China.
Ying-Kit Leung, Department of Gastroenterology, Pediatric Inflammatory Bowel Disease Research Center, Children’s Hospital of Fudan University, Shanghai 201102, China.
Ying Huang, Department of Gastroenterology, Pediatric Inflammatory Bowel Disease Research Center, Children’s Hospital of Fudan University, Shanghai 201102, China. yhuang815@163.com.
References
- 1.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 2.Wang XQ, Zhang Y, Xu CD, Jiang LR, Huang Y, Du HM, Wang XJ. Inflammatory bowel disease in Chinese children: a multicenter analysis over a decade from Shanghai. Inflamm Bowel Dis. 2013;19:423–428. doi: 10.1097/MIB.0b013e318286f9f2. [DOI] [PubMed] [Google Scholar]
- 3.Almadi MA, Ghosh S, Aljebreen AM. Differentiating intestinal tuberculosis from Crohn’s disease: a diagnostic challenge. Am J Gastroenterol. 2009;104:1003–1012. doi: 10.1038/ajg.2008.162. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global tuberculosis report 2015. Available from: http://www.who.int/tb /publications/global_report/en/.
- 5.Onal IK, Kekilli M, Tanoglu A, Erdal H, Ibis M, Arhan M. Tuberculosis and Crohn’s Disease Revisited. J Coll Physicians Surg Pak. 2015;25:443–448. [PubMed] [Google Scholar]
- 6.Epstein D, Watermeyer G, Kirsch R. Review article: the diagnosis and management of Crohn’s disease in populations with high-risk rates for tuberculosis. Aliment Pharmacol Ther. 2007;25:1373–1388. doi: 10.1111/j.1365-2036.2007.03332.x. [DOI] [PubMed] [Google Scholar]
- 7.Jayanthi V, Robinson RJ, Malathi S, Rani B, Balambal R, Chari S, Taghuram K, Madanagopalan N, Mayberry JF. Does Crohn’s disease need differentiation from tuberculosis? J Gastroenterol Hepatol. 1996;11:183–186. doi: 10.1111/j.1440-1746.1996.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 8.Zheng CF, Xu JH, Huang Y, Leung YK. Treatment of pediatric refractory Crohn’s disease with thalidomide. World J Gastroenterol. 2011;17:1286–1291. doi: 10.3748/wjg.v17.i10.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leite MR, Santos SS, Lyra AC, Mota J, Santana GO. Thalidomide induces mucosal healing in Crohn’s disease: case report. World J Gastroenterol. 2011;17:5028–5031. doi: 10.3748/wjg.v17.i45.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, Griffiths AM, Katz AJ, Grand RJ, Boyle JT. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. [PubMed] [Google Scholar]
- 11.Griffiths AM, Otley AR, Hyams J, Quiros AR, Grand RJ, Bousvaros A, Feagan BG, Ferry GR. A review of activity indices and end points for clinical trials in children with Crohn’s disease. Inflamm Bowel Dis. 2005;11:185–196. doi: 10.1097/00054725-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Ye ZQ, Zhou Y, Huang Y, Wang Y, Lu J, Tang Z, Miao S, Dong K, Jiang Z. Phenotype and management of infantile-onset inflammatory bowel disease: Experience from a Tertiary Care Center in China. Inflamm Bowel Dis. 2017;23:2154–2164. doi: 10.1097/MIB.0000000000001269. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z, Peng K, Li X, Zhao R, You J, Cheng X, Wang Z, Wang Y, Wu B, Wang H, et al. Mutations in Interleukin-10 Receptor and Clinical Phenotypes in Patients with Very Early Onset Inflammatory Bowel Disease: A Chinese VEO-IBD Collaboration Group Survey. Inflamm Bowel Dis. 2017;23:578–590. doi: 10.1097/MIB.0000000000001058. [DOI] [PubMed] [Google Scholar]
- 14.Sabate JM, Villarejo J, Lemann M, Bonnet J, Allez M, Modigliani R. An open-label study of thalidomide for maintenance therapy in responders to infliximab in chronically active and fistulizing refractory Crohn’s disease. Aliment Pharmacol Ther. 2002;16:1117–1124. doi: 10.1046/j.1365-2036.2002.01273.x. [DOI] [PubMed] [Google Scholar]
- 15.Vasiliauskas EA, Kam LY, Abreu-Martin MT, Hassard PV, Papadakis KA, Yang H, Zeldis JB, Targan SR. An open-label pilot study of low-dose thalidomide in chronically active, steroid-dependent Crohn’s disease. Gastroenterology. 1999;117:1278–1287. doi: 10.1016/s0016-5085(99)70277-5. [DOI] [PubMed] [Google Scholar]
- 16.Plamondon S, Ng SC, Kamm MA. Thalidomide in luminal and fistulizing Crohn’s disease resistant to standard therapies. Aliment Pharmacol Ther. 2007;25:557–567. doi: 10.1111/j.1365-2036.2006.03239.x. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenpreis ED, Kane SV, Cohen LB, Cohen RD, Hanauer SB. Thalidomide therapy for patients with refractory Crohn’s disease: an open-label trial. Gastroenterology. 1999;117:1271–1277. doi: 10.1016/s0016-5085(99)70276-3. [DOI] [PubMed] [Google Scholar]
- 18.Facchini S, Candusso M, Martelossi S, Liubich M, Panfili E, Ventura A. Efficacy of long-term treatment with thalidomide in children and young adults with Crohn disease: preliminary results. J Pediatr Gastroenterol Nutr. 2001;32:178–181. doi: 10.1097/00005176-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Lazzerini M, Martelossi S, Marchetti F, Scabar A, Bradaschia F, Ronfani L, Ventura A. Efficacy and safety of thalidomide in children and young adults with intractable inflammatory bowel disease: long-term results. Aliment Pharmacol Ther. 2007;25:419–427. doi: 10.1111/j.1365-2036.2006.03211.x. [DOI] [PubMed] [Google Scholar]
- 20.Felipez LM, Gokhale R, Tierney MP, Kirschner BS. Thalidomide use and outcomes in pediatric patients with Crohn disease refractory to infliximab and adalimumab. J Pediatr Gastroenterol Nutr. 2012;54:28–33. doi: 10.1097/MPG.0b013e318228349e. [DOI] [PubMed] [Google Scholar]
- 21.Lazzerini M, Martelossi S, Magazzù G, Pellegrino S, Lucanto MC, Barabino A, Calvi A, Arrigo S, Lionetti P, Lorusso M, et al. Effect of thalidomide on clinical remission in children and adolescents with refractory Crohn disease: a randomized clinical trial. JAMA. 2013;310:2164–2173. doi: 10.1001/jama.2013.280777. [DOI] [PubMed] [Google Scholar]
- 22.Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet. 2004;364:1039–1044. doi: 10.1016/S0140-6736(04)17058-X. [DOI] [PubMed] [Google Scholar]
- 23.Schoeman JF, Fieggen G, Seller N, Mendelson M, Hartzenberg B. Intractable intracranial tuberculous infection responsive to thalidomide: report of four cases. J Child Neurol. 2006;21:301–308. doi: 10.1177/08830738060210040801. [DOI] [PubMed] [Google Scholar]
- 24.Buonsenso D, Serranti D, Valentini P. Management of central nervous system tuberculosis in children: light and shade. Eur Rev Med Pharmacol Sci. 2010;14:845–853. [PubMed] [Google Scholar]
- 25.Schoeman JF, Andronikou S, Stefan DC, Freeman N, van Toorn R. Tuberculous meningitis-related optic neuritis: recovery of vision with thalidomide in 4 consecutive cases. J Child Neurol. 2010;25:822–828. doi: 10.1177/0883073809350507. [DOI] [PubMed] [Google Scholar]
- 26.Fu LM, Fu-Liu CS. Thalidomide and tuberculosis. Int J Tuberc Lung Dis. 2002;6:569–572. [PubMed] [Google Scholar]


