Abstract
Humanin (HN) and Rattin (HNr), its homologous in the rat, are peptides with cytoprotective action in several cell types such as neurons, lymphocytes and testicular germ cells. Previously, we have shown that HNr is expressed in pituitary cells and that HN inhibited the apoptotic effect of TNF-α in both normal and tumor pituitary cells. The aim of the present study was to identify signaling pathways that mediate the antiapoptotic effect of HN in anterior pituitary cells from ovariectomized rats and in GH3 cells, a somatolactotrope cell line. We assessed the role of STAT3, JNK, Akt and MAPKs as well as proteins of the Bcl-2 family, previously implicated in the antiapoptotic effect of HN. We also evaluated the participation of NF-κB in the antiapoptotic action of HN. STAT3 inhibition reversed the inhibitory effect of HN on TNF-α-induced apoptosis in normal and pituitary tumor cells, indicating that STAT3 signaling pathway mediates the antiapoptotic effect of HN on pituitary cells. Inhibition of NF-κB pathway did not affect action of HN on normal anterior pituitary cells but blocked the cytoprotective effect of HN on TNF-α-induced apoptosis of GH3 cells, suggesting that the NF-κB pathway is involved in HN action in tumor pituitary cells. HN also induced NF-κB-p65 nuclear translocation in these cells. In pituitary tumor cells, JNK and MEK inhibitors also impaired HN cytoprotective action. In addition, HN increased Bcl-2 expression and decreased Bax mitochondrial translocation. Since HN expression in GH3 cells is higher than in normal pituitary cells, we may suggest that through multiple pathways HN could be involved in pituitary tumorigenesis.
Electronic supplementary material
The online version of this article (doi:10.1007/s12079-017-0388-4) contains supplementary material, which is available to authorized users.
Keywords: Humanin, Pituitary, Apoptosis, STAT3, NF-κB, Bcl-2 family proteins
Introduction
Humanin (HN) is a 24 aminoacid peptide encoded in 16S mitochondrial DNA. HN has been detected in many cell types such as endothelial cells (Bachar et al. 2010), germ cells (Moretti et al. 2010), and is also detectable in plasma (Widmer et al. 2013). Rattin, (HNr), a homologous peptide of HN in rat, is expressed in different tissues such as cerebral cortex, hippocampus, testis and heart, among others (Caricasole et al. 2002). We have demonstrated that HNr is expressed in anterior pituitary cells, especially in lactotropes and somatotropes in the normal anterior pituitary, and also in the tumoral somatolactotrope cell line, GH3 (Gottardo et al. 2014). The cytoprotective effects of HN and HNr have been described in different cell types including neurons (Zapala et al. 2010; Sponne et al. 2004), pancreatic β cells (Hoang et al. 2010), testicular germ cells, and Leydig cells (Lue et al. 2010). We have previously shown that HN exerts an antiapoptotic effect in anterior pituitary cells from both female and male rats as well as in GH3 cells (Gottardo et al. 2014).
HN is a secretory peptide that can bind to a membrane trimeric receptor composed of different subunits including the receptor for ciliary neurotrophic factor α (CNTFR-α), WSX-1, and glycoprotein 130 kDa (gp130), which has been involved in the neuroprotective action of HN (Hashimoto et al. 2009). Trimerization of the receptor induces activation of janus kinases (JAK1 and JAK2), which then subsequently activate signal transducer and activator of transcription 3 (STAT3) (Hashimoto et al. 2005). Dimerized STATs then translocate to the nucleus and regulate transcription. Other downstream signaling pathways, such as RAS/mitogen-activated protein kinase (MAPK), c-Jun N-terminal protein kinase (JNK) and phosphoinositide 3 kinase (PI3K)/Akt can also be activated in response to signaling through the CNTFR/WSX-1/gp130 complex (Kim et al. 2016). Another mechanism involved in HN action is activation of formylpeptide-receptor-like 1 (FPRL-1) which induces extracellular signal-regulated kinase (ERK 1/2) (Ying et al. 2004).
HN can also exert antiapoptotic action through an intrinsic mitochondrial mechanism by modulating expression and intracellular location of proteins of the Bcl-2 family. HN analogues decrease expression of proapoptotic protein Bax while simultaneously increasing expression of antiapoptotic protein Bcl-2 (Luciano et al. 2005). Intracellular HN can also interact with proapoptotic Bcl-2 family members such as Bax and Bid, interfering with their translocation to mitochondria and thereby inhibiting formation of the apoptosome and activation of caspase-3 (Guo et al. 2003; Zhai et al. 2005). The mitochondria-dependent intrinsic pathway was shown to be an indispensable signaling pathway for germ cell apoptosis across species after hormonal deprivation (Jia et al. 2013).
Since HN exerts antiapoptotic effect on rat anterior pituitary cells and GH3 cells, the aim of the present study was to evaluate mechanisms that mediate the cytoprotective action of HN in both normal and tumor pituitary cells. We assessed the role of STAT3 which has previously been involved in antiapoptotic effects of HN in other tissues. Also, considering that NF-κB and STAT3 cooperatively regulate antiapoptotic genes and that NF-κB and STAT3 physically interact and reciprocally modulate their transcriptional activity, we also evaluated the participation of NF-κB in the antiapoptotic action of HN. We also determined the participation of JNK, Akt and MAPKs as well as proteins of the Bcl-2 family in the cytoprotective effect of HN on pituitary tumor cells. Our results indicate that the antiapoptotic effect of HN was mediated by the STAT3 pathway in both normal and tumor pituitary cells. However, NF-κB pathway was involved in the antiapoptotic action of HN only in GH3 cells, but not in normal anterior pituitary cells. In pituitary tumor cells, JNK and MEK inhibitors also impaired HN cytoprotective action. In addition, HN increased Bcl-2 expression and decreased Bax mitochondrial translocation in GH3 cells.
Materials and methods
Drugs
All drugs and reagents were obtained from Sigma Aldrich. (St. Louis, MO) except for phenol red-free Dulbecco’s modified Eagle’s medium and supplements (D-MEM; GIBCO, Invitrogen, Carlsbad, CA), Fetal bovine serum (Natocor, Buenos Aires, Argentina), all terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) reagents (Roche Molecular Biochemicals, Mannheim, Germany), anti-rabbit IgG and anti-rabbit fluorescein-conjugated secondary antibody (Vector Laboratories Inc., Burlingame, CA), Humanin peptide (Genemed Synthesis, Inc., San Antonio, TX), JSI-214 (Sigma Aldrich), BAY 11–7082 (Enzo Life Sciences International, Plymouth Meeting, PA), SP600125 (Calbiochem, Nottingham, UK), Ro106–9920 (Tocris Bioscience, Minneapolis, MN), SB203580, LY294002 and PD98059 (Cell Signaling Technology Inc., Beverly, MA) and the materials indicated below.
Animals
Adult female Wistar rats (200-250 g) were kept in controlled conditions of light (12:12 h light-dark cycles) and temperature (20–25 °C). Rats were fed standard laboratory chow and water ad libitum and kept in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal protocols were previously approved by the Ethics Committee of the School of Medicine, University of Buenos Aires (Res. N° 2742/2013). The rats were ovariectomized (OVX) two weeks before experiments under ketamine (100 mg/Kg, i.p.) and xylazine (10 mg/Kg, i.p.) anesthesia and ketoprofen (5 mg/kg) analgesia. Anterior pituitary glands were removed within minutes after decapitation.
Cell culture
A pool of anterior pituitary cells from 2 to 4 OVX rats were used for each culture. Anterior pituitary glands were washed several times with Dulbecco’s Modified Eagle’s Medium supplemented with 10 μl/ml MEM amino acids, 2 mM glutamine, 5.6 μg/ml amphotericin B, 100 U/ml penicillin, 100 μg/ml streptomycin (DMEM-S) and 3 mg/ml bovine serum albumin (BSA). Then, the glands were cut into small fragments. Sliced fragments were dispersed enzymatically by successive incubations in DMEM-S-BSA, containing 0.75% trypsin, 10% fetal bovine serum (FBS) previously treated with 0.025% dextran-0.25% charcoal (FBS-DCC) to remove steroids and 45 U/μl deoxyribonuclease type I (Invitrogen). Finally, cells were dispersed by extrusion through a Pasteur pipette in Krebs buffer without Ca2+ and Mg2+. Dispersed cells were washed and resuspended in DMEM-S with 10% FBS-DCC. Cell viability assessed by trypan blue exclusion was over 90%. Anterior pituitary cells or GH3 cells were seeded on cover slides in 24-well tissue culture plates (1 × 105 cells·ml−1·well−1) for TUNEL assay and immunocytochemistry or in 24-well tissue culture plates (3 × 105 cells·ml−1·well−1) for flow cytometry (FACS) analysis or in 6-well tissue culture plates (3 × 106 cells·ml−1·well−1) for Western Blot. Cells were cultured for 24 h in DMEM-S with 10% FBS-DCC and then incubated for 24 h in phenol red-free, serum-free DMEM-S supplemented with 0.1% BSA (DMEM-S-BSA 0.1%) with 17β-estradiol (E2, 10−9 M), for anterior pituitary cells from OVX rats, or without E2 for GH3 cells. Cells were preincubated with inhibitors of STAT3 (JSI-124, 1 μM), NF-κB (BAY-11 7082, 2.5 μM or Ro106–9920, 2.5 μM), JNK (SP600125, 5 μM), Akt (LY29402, 10 μM), MEK (PD98059, 10 μM) or p38 (SB203580, 10 μM) for 30 min, and then HN (0.5 μM) was added for 2 h and TNF-α (50 ng/ml) for a further 24 h in the same medium. Dose/response curves of the inhibitors used for the selection of non-cytotoxic doses are shown in Supplemental Fig. 1. In some experiments, GH3 cells were transiently transfected with a expression vector for the superrepressor IκB (ssIκB) kindly provided by Dr. M.A.Costas, Instituto Lanari, Universidad de Buenos Aires. The ssIκB expression vector (carrying the mutated IκB at Ser32 and Ser36 to prevent phosphorylation and proteolysis, keeping NF-κB inactive) was previously reported (Rubio et al. 2006; Alvarado et al. 2014). Cells were transiently transfected with a total of 1 μg of DNA for 16 h using Lipofectamine 2000 (Gibco) and then HN (0.5 μM) was added for 2 h and TNF-α (50 ng/ml) for a further 24 h. ssIκB reduced phospho-p65 NF-κB expression in GH3 cells (Supplemental Fig. 2).
Expression of Rattin (HNr) by flow cytometry and immunofluorescence
Cultured cells from anterior pituitary glands or GH3 cells were harvested with 0.025% trypsin-EDTA and washed in cold PBS. Cells were fixed with PFA 0.01% and then permeabilized with 0.1% saponin (MP Biomedicals, Inc., Solon, OH) for 10 min. Next, cells were incubated with anti-HNr antibody (1 μg/μl, Sigma Aldrich) in PBS for 1 h at 37 °C. Finally, cells were incubated with anti-rabbit antibody conjugated with FITC (1:50) in PBS for 1 h at 37 °C. The cells were washed, resuspended in PBS and analyzed by flow cytometry using a FACScan (Becton Dickinson). Data analysis was done with WinMDI 98 software. Cells were incubated with isotype control instead of primary antibody to determine the cut-off for HNr fluorescence.
The presence of HNr in anterior pituitary cells and GH3 cells was also evaluated by indirect immunofluorescent staining. Cells were incubated with anti-HNr antibody (Sigma, 1:100) for 1 h, washed, and incubated with anti-rabbit IgG antibody conjugated with fluorescein (1:50) for 1 h. Finally, slides were mounted with Vectashield (Vector Laboratories) containing 4,6-diamidino-2-phenylindoledihydrochloride (DAPI) for DNA staining and visualized in a fluorescent light microscope (Axiophot; Carl Zeiss, Jena, Germany). Control slides were incubated with control isotype instead of primary antibody.
Expression of NF-κB by immunofluorescence
To identify NF-κB/p65 in anterior pituitary cells and GH3 cells, cells were permeabilized with methanol for 15 min at −20 °C and incubated for 1 h with PBS and goat serum (10%). Then, cells were incubated for 1 h with anti-p-NF-κB/p65 antibody (Santa Cruz Biotechnology, 1:50), washed, and incubated for 1 h with anti-rabbit IgG fluorescein-conjugated antibody (Vector, 1:50). Finally, slides were mounted and visualized in a fluorescent light microscope. Control slides were incubated with control isotype instead of primary antibody. Images were captured using a fluorescence microscope. Phospho-p65 NF-κB immunoreactivity was quantified as pixels using Image J Software (NIH, USA) and shown as fluorescence intensity. Immunoreactive nuclei were counted on every image and the number of immunoreactive nuclei was averaged for each condition.
Microscopic detection of DNA fragmentation by TUNEL
Cultured cells were fixed with PFA 4% and permeabilized by microwave irradiation. DNA strand breaks were labelled with digoxigenin-deoxyuridine triphosphate using terminal deoxynucleotidyl transferase (0.18 U/μl) according to the manufacturer’s protocol. After incubation with 10% donkey serum in PBS for 90 min, cells were incubated for 1 h with fluorescein conjugated anti-digoxygenin antibody (1:10) to detect incorporation of nucleotides into the 3′-OH end of damaged DNA. Then, slides were mounted with mounting medium for fluorescence (Vectashield with DAPI) and visualized in a fluorescent light microscope. The percentage of apoptotic cells was calculated as [(TUNEL+)/total cells] × 100.
Western blot
Total proteins from GH3 cells were extracted in lysis buffer containing 250 mM NaCl, 5 mM MgCl2, 50 mM NaF, 1 mM dithiothreitol (DTT), 1% Igepal, 0.02% sodium azide, 0.1% sodium dodecyl sulphate (SDS) in 50 mM Tris–HCl pH 7.4 and protease inhibitor cocktail (1:100). Following centrifugation, the supernatant was used for immunoblot assay.
For isolation of mitochondria, cells were washed with PBS, harvested with 0.075% trypsin and centrifuged at 5000 g for 5 min. The pellet was resuspended in lysis buffer (10 mM Hepes pH 7.4, 250 mM Sucrose, 1 mM EGTA, 1 mM DTT and protease inhibitor cocktail) and centrifuged at 1500 g for 10 min. The supernatant was centrifuged at 10000 g for 10 min. The mitochondrial pellet was washed and resuspended in 10 μl of buffer solution. The purity of mitochondrial fraction is shown in Supplemental Fig. 3.
Protein concentration of each sample was determined by Bradford assay (BioRad Laboratories, Hercules, CA). Thirty μg of proteins were size-fractionated in 12% SDS–polyacrylamide gel, then electrotransferred to polyvinyl difluoride (PVDF) membranes. Blots were blocked for 90 min in 5% non-fat dry milk-TBS- 0.1% Tween 20 and incubated overnight with anti-Bax (1:20, BD Biosciences, San Jose, CA) or anti-Bcl-2 (1:30, Santa Cruz Biotechnology), and anti-β-actin (1:500, Santa Cruz Biotechnology) or Complex III subunit 2 antibody (1:1000 Anti-UQCRC2, Abcam, Cambridge, MA) in the same buffer at 4 °C. This was followed by 1 h incubation with the corresponding HRP-conjugated anti-mouse or anti-rabbit secondary antibody (1:1000, Millipore, Temecula, CA). Immunoreactivity was detected by enhanced chemiluminescence (Productos Bio-Lógicos, Buenos Aires, Argentina). Chemiluminescence was detected by chemiluminescence imaging system (G Box Chemi HR16, Syngene, Cambridge, U.K.) and bands were quantified using Gene Tools software (Syngene). Intensity data from Bax and Bcl-2 in total proteins were normalized with respect to the corresponding β-actin blot and expressed as relative increments compared to respective controls. Bax intensity data in mitochondrial fraction was normalized with respect to the corresponding complex III subunit 2 and expressed as the relative increment of the ratio of mitochondrial/total (mitochondrial + non mitochondrial fractions) compared to respective controls.
Statistical analysis
Differences in the percentage of HNr-positive cells and the mean of HNr fluorescence intensity per cell (Gmean) between groups were analyzed by Student’s t test. The number of apoptotic cells evaluated by TUNEL in slides from three independent experiments was expressed as percentage of TUNEL positive cells ± 95% confidence limits (CL) of the total number of cells counted in each specific condition and analyzed by χ2 test. Phospho-p65 NF-κB fluorescence intensity data and Western blot data were expressed as mean ± SE and evaluated by two-way ANOVA, followed by Tukey’s test. Nuclear phospho-p65 NF-κB was expressed as percentage of immunoreactive nuclei ± 95% CL of the total number of cells counted in each specific condition and analyzed by χ2 test. p < 0.05 was considered significant. All experiments were performed at least three times.
Results
HNr expression in anterior pituitary cells from female rats was lower than in GH3 cells
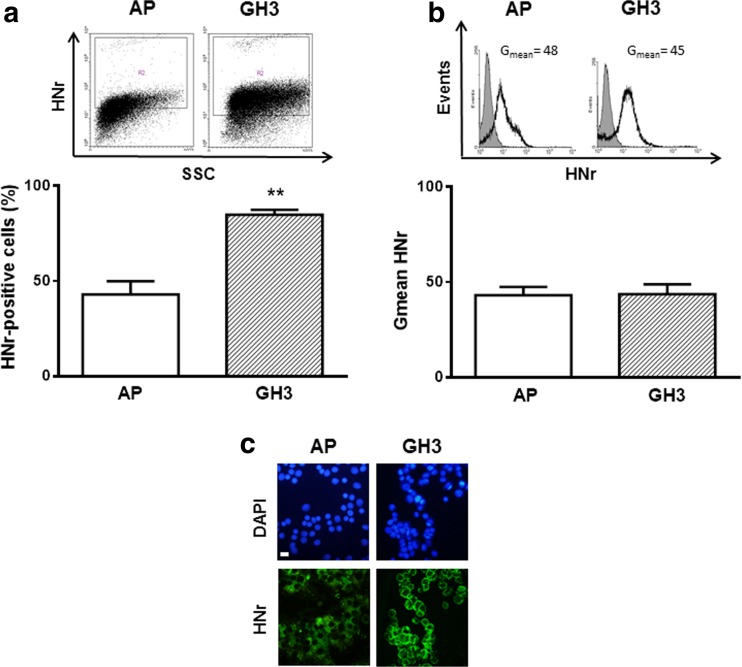
We previously reported that HNr is expressed in both anterior pituitary cells from female rats and in GH3 cells, a somatolactotrope tumor cell line (Gottardo et al. 2014). Since HN upregulation was suggested to be an important molecular event in tumor development (Cohen 2014), we compared HNr expression in normal and tumor pituitary cells. We determined HNr expression in cultured anterior pituitary cells from female rats and GH3 cells by FACS. The percentage of GH3 cells expressing HNr was higher than in normal anterior pituitary cells (Fig. 1), suggesting that HNr could play a role in tumor pituitary cell behavior.
Fig. 1.
HNr expression in anterior pituitary cells from female rats was lower than in GH3 cells. Cultured anterior pituitary (AP) cells from female rats or GH3 cells were incubated with anti-HNr antibody and analyzed by flow cytometry. Each column represents the mean ± SE (n = 3) of the percentage of HNr-positive cells (a), and the fluorescence intensity of HNr staining (Gmean) (b). * * p < 0.01. Student’s t test. The upper panels show representative dot plots and histograms of HNr expression. (c) Representative images of immunofluorescence for HNr in AP cells and GH3 cells. Scale bars: 10 μm
HN protected normal and tumor pituitary cells from TNF-α-induced apoptosis through activation of STAT3
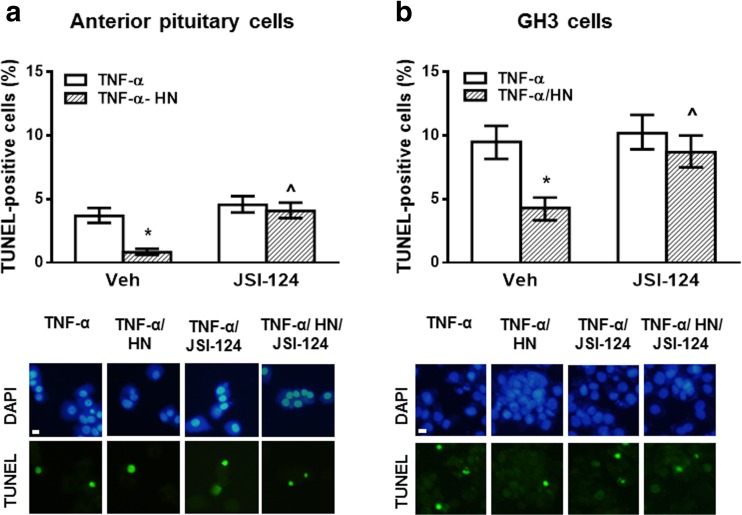
After binding to its specific receptor, HN was reported to exert its cytoprotective effect through activation of STAT3, JNK, and tyrosine kinases (Kim et al. 2016; Hashimoto et al. 2001). We previously showed that HN 0.5 μM, a concentration having no cytoprotective effect per se, inhibited the proapoptotic effect of TNF-α in anterior pituitary cells from ovariectomized (OVX) rats and GH3 cells (Gottardo et al. 2014). Since we reported that TNF-α induces apoptosis of anterior pituitary cells in an estrogen-dependent manner (Candolfi et al. 2002; Candolfi et al. 2005) but estrogens are not necessary to sensitize GH3 cells to TNF-α proapoptotic effect (Eijo et al. 2015), normal pituitary cells were incubated with 17β-estradiol (E2, 10−9 M) in all the following experiments. In order to study mechanisms involved in HN action in the pituitary, we investigated the effect of HN (0.5 μM) on TNF-α-induced apoptosis in anterior pituitary cells from OVX rats and GH3 cells incubated in absence or presence of a STAT3 inhibitor (JSI-124, 1 μM). The percentage of apoptotic cells was determined by TUNEL assay. As expected, HN reduced TNF-α-induced apoptosis in anterior pituitary cells (Fig. 2a) and GH3 cells (Fig. 2b). However, when the STAT3 pathway was inhibited, no antiapoptotic action of HN was observed either in anterior pituitary cells or in GH3 cells, suggesting that HN protects both normal and tumor pituitary cells from TNF-α-induced apoptosis through STAT3 activation.
Fig. 2.
HN protected anterior pituitary cells and GH3 cells from TNF-α-induced apoptosis through STAT3 activation. (a) Anterior pituitary cells from OVX rats cultured with 17β-estradiol (E2, 10−9 M) or (b) GH3 cells were incubated with STAT3 inhibitor (JSI-124, 1 μM) for 30 min before adding HN (0.5 μM) for 2 h and TNF-α (50 ng/ml) for a further 24 h. Apoptosis was assessed by TUNEL. Each column represents the percentage ± CL of TUNEL-positive cells (n ≥ 1000 cell/group). * p < 0.05 vs respective control without HN, ^ p < 0.05 vs respective control without STAT3 inhibitor. χ2 test. The lower panels show representative images of TNF-α-induced apoptosis in anterior pituitary cells or GH3 cells incubated with HN in presence of STAT3 inhibitor. Scale bars: 10 μm
NF-κB pathway participated in cytoprotective action of HN in pituitary tumor cells but not in normal pituitary cells
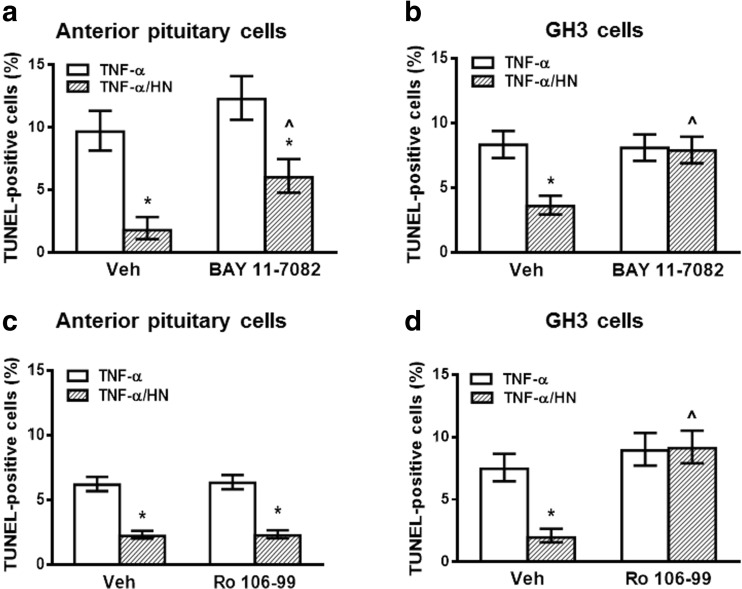
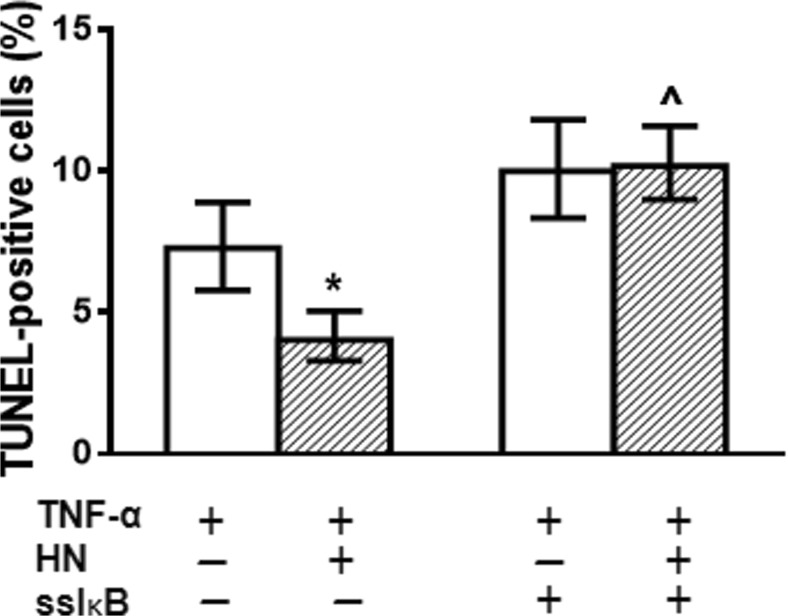
NF-κB is a pleiotropic transcription factor involved in the survival of normal and tumor cells (Vender et al. 2008; Karin and Ben-Neriah 2000; Hayden and Ghosh 2004). Thus, we aimed to evaluate the role of NF-κB pathway in the antiapoptotic action of HN in pituitary cells. We assessed the effect of HN on TNF-α-induced apoptosis of anterior pituitary cells from OVX rats and GH3 cells incubated in presence of BAY 11–7082 (BAY, 2.5 μM), an inhibitor of the NF-κB pathway. Although BAY inhibited TNF-α-induced expression of phospho-p65 NF-κB (Supplemental Fig. 4), inhibition of the NF-κB pathway did not affect the cytoprotective action of HN in anterior pituitary cells (Fig. 3a). In contrast, HN failed to protect GH3 cells from TNF-α-induced apoptosis when the NF-κB pathway was inhibited (Fig. 3b). Similarly, inhibition of NF-κB pathway with Ro 106–9920 (Ro, 2.5 μM) completely blocked the cytoprotective action of HN only in GH3 cells, but not in normal anterior pituitary cells (Fig. 3c, d). In order to confirm the functional role of NF-κB in the cytoprotective effect of HN, GH3 cells were transiently transfected with superrepressor IκB (ssIκB) that is not susceptible to phosphorylation and proteolysis upon TNF-α stimulation and hence, constitutively suppresses NF-κB activation (Rubio et al. 2006; Alvarado et al. 2014). Expression of ssIκB inhibited the antiapoptotic effect of HN on TNF-α-induced apoptosis (Fig. 4a).
Fig. 3.
NF-κB pathway participated in cytoprotective action of HN in GH3 cells, but not in normal pituitary cells. (a, c) Anterior pituitary cells from OVX rats cultured with 17β-estradiol (E2, 10−9 M) or (b, d) GH3 cells were incubated with NF-κB inhibitor (a, b) BAY 11–7082 (BAY, 2.5 μM) or vehicle, ethanol 0.05 ml/l) or (c, d) Ro 106–9920 (Ro, 2.5 μM or vehicle, DMSO 0.5 ml/l) for 30 min before adding HN (0.5 μM) for 2 h and TNF-α (50 ng/ml) for a further 24 h. Each column represents the percentage ± CL of TUNEL-positive cells (n ≥ 1000 cell/group). * p < 0.05 vs respective control without HN, ^ p < 0.05 vs respective control without NF-κB inhibitor. χ2 test
Fig. 4.
Inhibition of NF-κB pathway with superrepressor IκB impaired cytoprotective action of HN in GH3 cells. GH3 cells were transiently transfected with superrepressor IκB (ssIκB) for 16 h. Then, cells were incubated with HN (0.5 μM) for 2 h and TNF-α (50 ng/ml) for a further 24 h. Each column represents the percentage ± CL of TUNEL-positive cells (n ≥ 1000 cell/group). * p < 0.05 vs respective control without HN, ^ p < 0.05 vs respective control without ssIκB. χ2 test
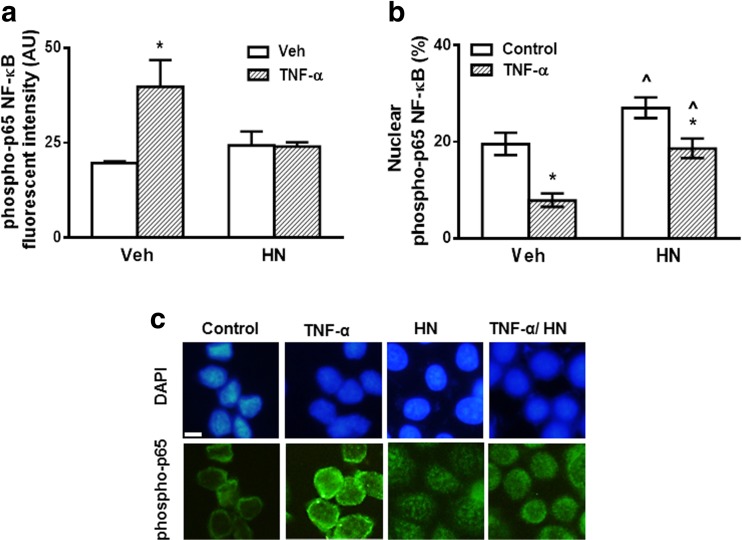
In the classical NF-κB pathway, NF-κB/p65 can translocate to the nucleus, thereby regulating gene transcription through binding to specific κB response elements (Hayden and Ghosh 2004; Hinz and Scheidereit 2014). To evaluate whether HN is involved in NF-κB nuclear translocation, we examined by immunocytochemistry the expression and location of phosphorylated p65 subunit of NF-κB in GH3 cells incubated in the presence of TNF-α and HN. TNF-α increased expression of phospho p65 NF-κB in GH3 cells (Fig. 5a, c). HN per se did not affect phospho-p65 NF-κB expression but blocked TNF-α-induced NF-κB upregulation. HN increased nuclear translocation of phospho-p65 NF-κB in both control and TNF-α treated cells (Fig. 5b). These results suggest that HN could protect GH3 cells from TNF-α-induced apoptosis through activation of NF-κB pathway by promoting nuclear translocation of phosphorylated p65 NF-κB.
Fig. 5.
HN promoted nuclear translocation of phosphorylated p65 NF-κB in GH3 cells. GH3 cells were incubated with HN (0.5 μM) for 2 h before adding TNF-α (50 ng/ml) for an additional 24 h and processed for detection of phosphorylated p65 subunit of NF-κB (phospho-p65) by immunofluorescent staining. (a) Each column represents fluorescence intensity expressed as arbitrary units (AU) ± SE. * p < 0.05 vs respective control without TNF-α, ^ p < 0.05 vs respective control without HN. ANOVA. (b) Each column represents the percentage of phospho-p65 NF-κB immunoreactive nuclei ± CL. * p < 0.05 vs respective control without TNF-α, ^ p < 0.05 vs respective control without HN. χ2 test. (c) Representative images of phospho-p65 immunostaining: Upper panels: DAPI; Lower panels: phospho-p65
HN protected GH3 cells from TNF-α-induced apoptosis through JNK and MEK activation
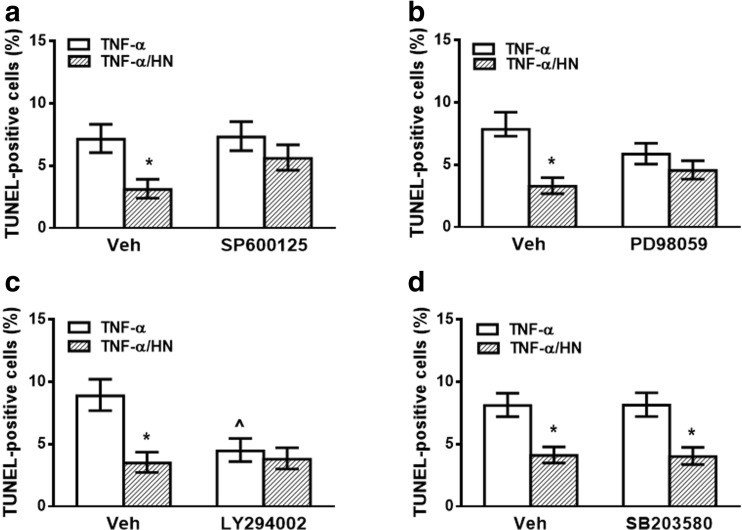
Since JNK, Akt, MEK, and p38 pathways were reported to mediate HN cytoprotective action in several cell types (Zapala et al. 2010; Hashimoto et al. 2001; Xu et al. 2008), we also evaluated whether these kinases are involved in antiapoptotic action of HN in GH3 cells. For this purpose, we determined the effect of HN on TNF-α-induced apoptosis in GH3 cells incubated in presence of specific inhibitors of JNK (SP600125, 5 μM), Akt (LY294002, 10 μM), MEK (PD98059, 10 μM) or p38 (SB203580, 10 μM). HN was unable to inhibit TNF-α-induced apoptosis of GH3 cells incubated with inhibitors of JNK (Fig. 6a) or MEK (Fig. 6b), suggesting that these pathways could be involved in the effect of HN on apoptosis in tumor cells. Akt inhibition per se reduced TNF-α-induced apoptosis but failed to modify the antiapoptotic effect of HN (Fig. 6c), suggesting that PI3K/Akt pathway is not involved in the cytoprotective action of HN on TNF-α-induced apoptosis in GH3 cells. Also, p38 inhibition did not affect the cytoprotective effect of HN in GH3 cells, suggesting that antiapoptotic action of HN is not dependent on the p38 pathway in these cells (Fig. 6d).
Fig. 6.
HN protected GH3 cells from TNF-α-induced apoptosis through JNK and MEK activation. GH3 cells were incubated with (a) JNK inhibitor (SP600125, 5 μM), (b) MEK inhibitor (PD98059, 10 μM), (c) Akt inhibitor (LY294002, 10 μM), (d) p38 inhibitor (SB203580, 10 μM) or vehicle (DMSO, 0.5 ml/l) for 30 min before adding HN (0.5 μM) for 2 h and TNF-α (50 ng/ml) for a further 24 h. Each column represents the percentage ± CL of TUNEL-positive cells (n ≥ 1000 cell/group). * p < 0.05 vs respective control without HN, ^ p < 0.05 vs respective control without inhibitor. χ2 test
The intrinsic pathway was involved in the antiapoptotic effect of HN in pituitary tumor cells
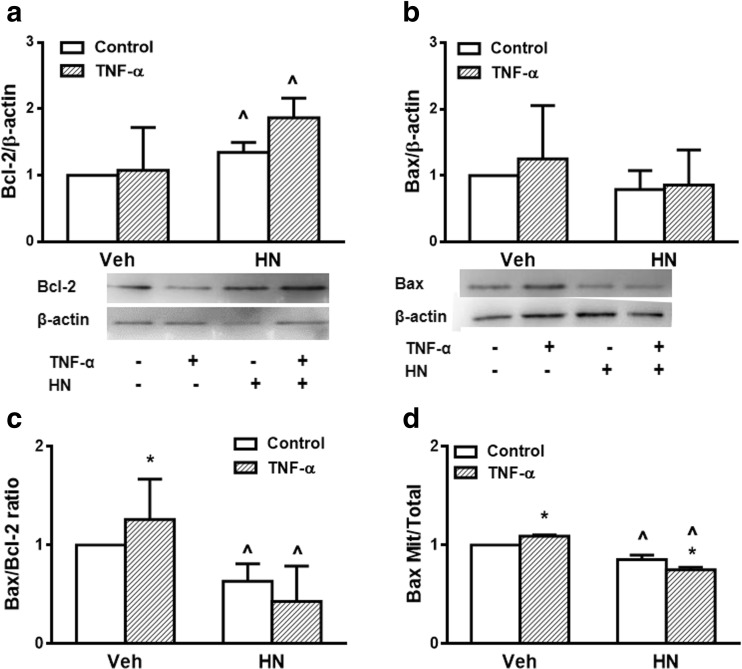
In order to investigate involvement of the intrinsic apoptotic pathway in the action of HN in the pituitary, we determined the effect of HN on expression of antiapoptotic protein Bcl-2 and proapoptotic protein Bax in GH3 cells incubated with or without TNF-α. As determined by Western blot, TNF-α did not significantly modify Bcl-2 or Bax expression (Fig. 7a, b). HN increased Bcl-2 expression in GH3 cells incubated either with or without TNF-α (Fig. 7a). Although HN did not modify Bax expression (Fig. 7b), it significantly decreased the Bax/Bcl-2 ratio in GH3 cells incubated in either presence or absence of TNF-α (Fig. 7c).
Fig. 7.
HN decreased Bax/Bcl-2 ratio and Bax translocation in GH3 cells. GH3 cells were incubated with HN (0.5 μ M) for 2 h before adding TNF-α (50 ng/ml) for a further 24 h. Total concentration of Bcl-2 (a) and Bax (b) were determined by Western blot. Densitometric data were normalized by the corresponding β-actin value and analyzed by ANOVA followed by Tukey’s test. (c) Bax/Bcl-2 ratio (d) Mitochondrial concentration of Bax was determined by Western blot. Densitometric data of mitochondrial Bax were normalized by the corresponding complex III value, expressed as Mitochondrial/Total (mitochondrial + non-mitochondrial) ratio and analyzed by ANOVA followed by Tukey’s test. Each column represents the mean ± SE from 3 independent experiments. * p < 0.05 vs respective control without TNF-α, ^ p < 0.05 respective control without HN
Since HN can interact with Bax in the cytoplasm and prevent its translocation to mitochondria (Guo et al. 2003), we evaluated Bax expression in isolated mitochondrial fractions of GH3 cells incubated with or without TNF-α. TNF-α stimulated Bax mitochondrial translocation, and HN inhibited both basal and TNF-α-induced Bax mitochondrial translocation (Fig. 7d), suggesting that blockage of Bax translocation from cytosol to mitochondria is one of the mechanisms involved in antiapoptotic action of HN.
Discussion
We have previously shown that HNr is present in the anterior pituitary gland where it exerts antiapoptotic action, suggesting that this peptide could be involved in tissue homeostasis of this gland. We also reported that HNr is expressed in the somatolactotrope GH3 cell line and protects these pituitary tumor cells from TNF-α-induced apoptosis (Gottardo et al. 2014).
One extracellular receptor of HN is the trimeric receptor complex composed of CNTFR, WSX-1 and gp130 which activates JAK2-STAT3 signaling pathway (Hashimoto et al. 2009). It was reported that HN activates gp130 and downstream signaling cascade of STAT3, inducing STAT3 phosphorylation (Hashimoto et al. 2005; Kim et al. 2016). The participation of this pathway in cytoprotective action of HN was demonstrated in several tissues. In the present study, we observed that HN effect on TNF-α-induced apoptosis in both normal and tumor pituitary cells was blocked by STAT3 inhibition, supporting the idea that STAT3 signaling pathway is an important mechanism for the antiapoptotic effect of HN on pituitary cells. STAT3 regulates a number of important mechanisms involved in tumorigenesis: it is involved in processes of tumor invasion and metastasis as well as evasion of the immune system (Grandis et al. 2000; Wang et al. 2004). STAT3 binding sites were found in promoters of genes whose products are related to cell cycle progression, apoptosis (c-fos, c-Myc, cyclin D1) (Bromberg et al. 1999), and angiogenesis (Vascular Endothelial Growth Factor) (Niu et al. 2002). Constitutive activation of STAT3 has been observed in cancer of several organs such as prostate, pancreas, brain, and ovary. In the anterior pituitary gland, STAT3 mediates gp130-controlled proliferation of corticotropes (Melmed 2015). Also, increased STAT3 expression was observed in human somatotrope adenomas (Zhou et al. 2015). Moreover, inhibition of STAT3 was reported to reduce GH3 cell proliferation and growth of xenografted GH3 cell tumors (Melmed 2015). Considering that HNr expression in GH3 cells is higher than in normal pituitary cells, it is possible to suggest that HN could be involved in constitutive activation of STAT3 in pituitary tumors, contributing to their growth and progression.
The NF-κB proteins are a family of transcription factors that not only mediate immune and inflammatory responses but were also shown to be involved in tumor development (Suh et al. 2002; Wang et al. 1999). Activation of NF-κB is associated with increased cell cycle progression, cell migration and invasion (Shishodia et al. 2005), and also with decreased cell apoptosis (Dutta et al. 2006). NF-κB is inactivated in the cytoplasm through its binding to IκB inhibitory proteins. Several stimuli promote dissociation of cytosolic inactive NF-κB/IκB complexes via IKK activation, which results in phosphorylation and degradation of IκB, followed by translocation of NF-κB p50:p65 dimers into the nucleus and binding to DNA. Specifically, NF-κB blocks apoptosis by stimulating expression of antiapoptotic genes, as well as suppressing apoptosis-inducing genes (Du et al. 2007). Interaction between STAT3 and NF-κB was postulated to play a crucial role in colon, gastric, and liver cancers (Fan and Yang 2013). NF-κB and STAT3 cooperatively regulate a number of target genes including antiapoptotic as well as cell cycle control genes. NF-κB was shown to interact physically with STAT3, facilitating NF-κB recruitment to STAT3 promoters and, reciprocally, STAT3 to those of NF-κB. In addition, increased constitutive activity of NF-κB stimulates the release of cytokines such as IL-6 which activate STAT3. In many tumor cell lines, including the somatolactotrope GH3 cell line, the NF-κB pathway is constitutively activated (Vender et al. 2008; Kojima et al. 2004; Nair et al. 2003). We previously reported that pharmacological inhibition of the NF-κB pathway with BAY 11–7082, a synthetic compound that irreversibly inhibits phosphorylation of IκB, thereby preventing its ubiquitination and subsequent proteasomal degradation (Gasparian et al. 2009), increased apoptosis in anterior pituitary cells and GH3 cells, suggesting that NF-κB acts as an important survival factor for these cells (Eijo et al. 2015). Our present data show that inhibition of the NF-κB pathway with either BAY or Ro, an inhibitor of TNF-α-induced IκB degradation and NF-κB activation (Swinney et al. 2002), did not affect the cytoprotective action of HN in normal anterior pituitary cells, suggesting that NF-κB does not participate in the antiapoptotic effect of HN in these cells. However, both BAY and Ro blocked the cytoprotective effect of HN on TNF-α-induced apoptosis of GH3 cells. Moreover, the superrepressor IκB, which prevents IκB phosphorylation and proteolysis, keeping NF-κB inactive (Rubio et al. 2006; Alvarado et al. 2014; Werbajh et al. 2000), inhibited the cytoprotective action of HN on TNF-α-induced apoptosis of GH3 cells, confirming that the NF-κB pathway is involved in antiapoptotic action of HN in pituitary tumor cells. Furthermore, HN induced NF-κB-p65 nuclear translocation in GH3 cells and reduced TNF-α-induced expression of NF-κB-p65, suggesting that HN could act by increasing nuclear translocation of NF-κB in pituitary tumor cells.
HN was also proposed to act through activation of kinases such as JNK, Akt, MEK and p38 (Hashimoto et al. 2005; Kim et al. 2016). In this study, we observed that JNK or MEK inhibitors decreased the antiapoptotic effect of HN on TNF-α-induced apoptosis of GH3 cells, suggesting that these kinases could be involved in this effect of HN. We previously reported that inhibition of JNK pathway increased apoptotic response to TNF-α in GH3 only when the NF-κB pathway was also blocked, suggesting that JNK pathway may be involved in survival of pituitary tumor cells (Eijo et al. 2015). Considering that TNF-α can activate JNK signaling, our results suggest that HN could protect GH3 cells from TNF-α-induced apoptosis through NF-κB-mediated JNK activation. Neither Akt nor p38 seem to be required for the cytoprotective action of HN on TNF-α-induced apoptosis of GH3 cells.
It has also been demonstrated that HN can modulate expression and intracellular location of Bcl-2 family proteins (Luciano et al. 2005; Guo et al. 2003). Our results show that HN did not modify Bax expression in pituitary tumor cells. However, HN increased expression of Bcl-2, resulting in a decrease in the Bax/Bcl-2 ratio, a good index of antiapoptotic cell behavior. HN was also reported to interact with proapoptotic Bcl-2 family members such as Bax and tBid, preventing their translocation to mitochondria (Luciano et al. 2005; Guo et al. 2003; Jia et al. 2013). We observed that TNF-α increased Bax translocation to mitochondria, and HN inhibited basal and TNF-α induced Bax translocation, suggesting that HN may interact with Bax in the cytoplasm as has been shown in other tissues (Guo et al. 2003; Jia et al. 2013).
Since HN was reported to be overexpressed in gastric cancer cells and considering that HN has antiapoptotic activity in these cells (Mottaghi-Dastjerdi et al. 2014), it was suggested that HN upregulation could be an important molecular event in tumorigenesis. In fact, GH3 cells seem to have higher expression of HNr than normal anterior pituitary cells, suggesting that HN expression is altered in tumor cells and could participate in the pathogenesis of pituitary tumors. Although HN was shown to have antitumor effect in some models (Eriksson et al. 2014; Lue et al. 2015) we have observed that HN increases the resistance of breast cancer cells to chemoteraphy (Gottardo et al. 2016), putting into question the validity of using HN analogs for cancer treatment.
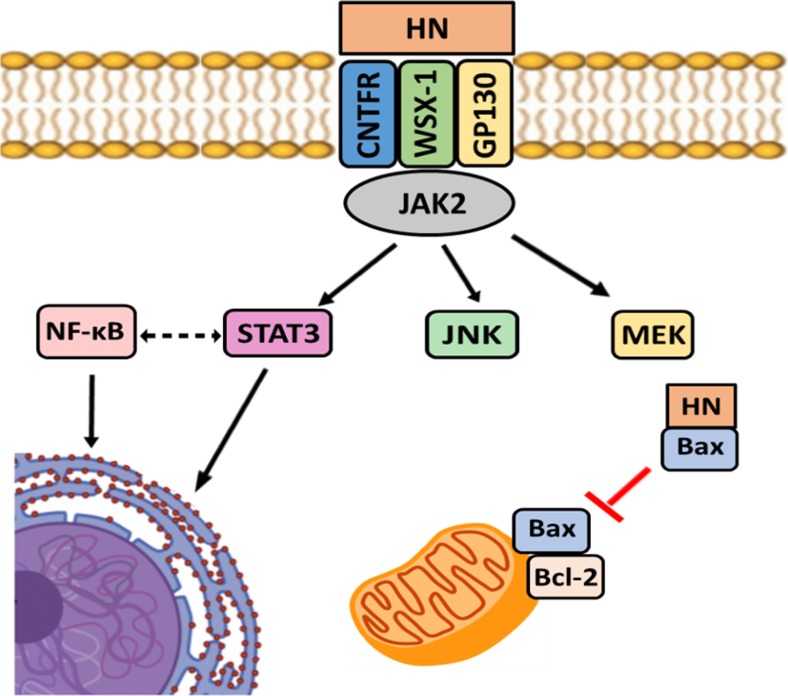
In conclusion, our results show that the mechanism of action by which HN inhibits TNF-α-induced apoptosis in normal and tumor anterior pituitary cells is complex, involving multiple pathways, including STAT3 activation (Fig. 8). It is noteworthy that NF-κB pathway is involved in antiapoptotic action of HN in pituitary tumor cells but not in normal pituitary cells, and thus this pathway could enhance survival of tumor cells. In pituitary tumor cells, HN also exerts antiapoptotic effect by increasing Bcl-2 expression and decreasing Bax translocation to mitochondria. These results could help to design therapies using inhibition of HN as a potential strategy for alternative treatment of pituitary tumors.
Fig. 8.
Schematic diagram of the Humanin-mediated signaling pathways in pituitary tumor cells
Electronic supplementary material
GH3 cells were incubated with (A) STAT3 inhibitor JSI-124, (B) NF-κB inhibitor Ro 106–9920, (C) JNK inhibitor SP600125, (D) Akt inhibitor LY294002, (E) MEK inhibitor PD98059, (F) p38 inhibitor SB203580 or the corresponding vehicle for 24 h. Each column represents the percentage ± CL of TUNEL-positive cells (n ≥ 1000 cell/group). * p < 0.05 vs respective control without inhibitor. χ2 test. (JPEG 57 kb)
(A) GH3 cells were transfected with ssIκB for 16 h then incubated with TNF-α (50 ng/ml) for 24 h and processed for detection of phosphorylated p65 subunit of NF-κB (phospho-p65) by immunofluorescent staining. Each column represents arbitrary units (AU) of phospho-p65 fluorescence intensity ± SE. Student’s t test. (B) Representative images of phospho-p65 NF-κB in GH3 cells transfected with ssIκB. Upper panels: DAPI; Lower panels: phospho-p65. Scale bars: 10 μm. (JPEG 29 kb)
Mitochondria were isolated from cultured GH3 cells as described in Material and Methods. Proteins from mitocondrial (Mit) and non-mitochondrial (Non-Mit) fractions were subjected to Western blot using anti-Complex III subunit 2 antibody. Immunoreactivity was detected by enhanced chemiluminescence. (JPEG 14 kb)
(A) Anterior pituitary cells from OVX rats cultured with 17β-estradiol (E2, 10−9 M) or (B) GH3 cells were incubated with NF-κB inhibitor BAY 11–7082 (BAY, 2.5 μM) or vehicle (ethanol 0.05 ml/l) for 30 min before adding TNF-α (50 ng/ml) for a further 24 h. Cells were processed for detection of phosphorylated p65 subunit of NF-κB (phospho-p65) by immunofluorescent staining. Each column represents the arbitrary units (AU) of phospho-p65 fluorescence intensity ± SE from 3 independent experiments. * p < 0.05 vs respective control without TNF-α, ^ p < 0.05 respective control without BAY. ANOVA (JPEG 34 kb)
Acknowledgements
We would like to thank Miss Mercedes Imsen for her kind help with animal care and handling. We thank Dr. Monica Costas for kindly providing the mutated inhibitor of NF-κB (ssIκB). This work was supported by the Consejo Nacional de Investigaciones Científicas y Tecnológicas, National Research Council (PIP 11420110100353 to M.C.; PIP 11220120100261 to A.S.), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2013-0310 to M.C.; PICT 2014-0334 to A.S.) and the University of Buenos Aires (20020130100020 to A.S.).
Abbreviations
- HN
Humanin
- HNr
Rattin
- STAT3
Signal transducer and activator of transcription 3
- NF-κB
nuclear factor-kappa B
- JNK
c-Jun N-terminal protein kinase
- MEK
Mitogen-activated protein/extracellular signal-regulated kinase kinase
- TNF-α
Tumor Necrosis Factor-alpha
- PI3K
Phosphoinositide 3-kinase
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12079-017-0388-4) contains supplementary material, which is available to authorized users.
References
- Alvarado CV, Rubio MF, Fernandez Larrosa PN, Panelo LC, Azurmendi PJ, Ruiz Grecco M, Martinez-Noel GA, Costas MA. The levels of RAC3 expression are up regulated by TNF in the inflammatory response. FEBS Open Bio. 2014;4:450–457. doi: 10.1016/j.fob.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Candolfi M, Zaldivar V, De Laurentiis A, Jaita G, Pisera D, Seilicovich A. TNF-alpha induces apoptosis of lactotropes from female rats. Endocrinology. 2002;143:3611–3617. doi: 10.1210/en.2002-220377. [DOI] [PubMed] [Google Scholar]
- Candolfi M, Jaita G, Zaldivar V, Zarate S, Ferrari L, Pisera D, Castro MG, Seilicovich A. Progesterone antagonizes the permissive action of estradiol on tumor necrosis factor-alpha-induced apoptosis of anterior pituitary cells. Endocrinology. 2005;146:736–743. doi: 10.1210/en.2004-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricasole A, Bruno V, Cappuccio I, Melchiorri D, Copani A, Nicoletti F. A novel rat gene encoding a Humanin-like peptide endowed with broad neuroprotective activity. FASEB J. 2002;16:1331–1333. doi: 10.1096/fj.02-0018fje. [DOI] [PubMed] [Google Scholar]
- Cohen P. New role for the mitochondrial peptide humanin: protective agent against chemotherapy-induced side effects. J Natl Cancer Inst. 2014;106:dju006. doi: 10.1093/jnci/dju006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Wei L, Murti A, Pfeffer SR, Fan M, Yang CH, Pfeffer LM. Non-conventional signal transduction by type 1 interferons: the NF-kappaB pathway. J Cell Biochem. 2007;102:1087–1094. doi: 10.1002/jcb.21535. [DOI] [PubMed] [Google Scholar]
- Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- Eijo G, Gottardo MF, Jaita G, Magri ML, Moreno Ayala M, Zarate S, Candolfi M, Pisera D, Seilicovich A. Lack of Oestrogenic inhibition of the nuclear factor-kappaB pathway in Somatolactotroph tumour cells. J Neuroendocrinol. 2015;27:692–701. doi: 10.1111/jne.12296. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Wickstrom M, Perup LS, Johnsen JI, Eksborg S, Kogner P, Savendahl L. Protective role of humanin on bortezomib-induced bone growth impairment in anticancer treatment. J Natl Cancer Inst. 2014;106:djt459. doi: 10.1093/jnci/djt459. [DOI] [PubMed] [Google Scholar]
- Fan YMR, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparian AV, Guryanova OA, Chebotaev DV, Shishkin AA, Yemelyanov AY, Budunova IV. Targeting transcription factor NFkappaB: comparative analysis of proteasome and IKK inhibitors. Cell Cycle. 2009;8:1559–1566. doi: 10.4161/cc.8.10.8415. [DOI] [PubMed] [Google Scholar]
- Gottardo MF, Jaita G, Magri ML, Zarate S, Moreno Ayala M, Ferraris J, Eijo G, Pisera D, Candolfi M, Seilicovich A. Antiapoptotic factor humanin is expressed in normal and tumoral pituitary cells and protects them from TNF-alpha-induced apoptosis. PLoS One. 2014;9:e111548. doi: 10.1371/journal.pone.0111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardo MF, Moreno Ayala A, Imsen M, Asad A, Pidre ML, Romanowski V, Candolfi M, Seilicovich A (2016) Humanin increases the resistance of breast cancer to chemotherapy. Medicina 76:201
- Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Ito Y, Niikura T, Shao Z, Hata M, Oyama F, Nishimoto I. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem Biophys Res Commun. 2001;283:460–468. doi: 10.1006/bbrc.2001.4765. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Suzuki H, Aiso S, Niikura T, Nishimoto I, Matsuoka M. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77:3092–3104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hinz M, Scheidereit C. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep. 2014;15:46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang PT, Park P, Cobb LJ, Paharkova-Vatchkova V, Hakimi M, Cohen P, Lee KW. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Lue YH, Swerdloff R, Lee KW, Cobb LJ, Cohen P, Wang C. The cytoprotective peptide humanin is induced and neutralizes Bax after pro-apoptotic stress in the rat testis. Andrology. 2013;1:651–659. doi: 10.1111/j.2047-2927.2013.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Guerrero N, Wassef G, Xiao J, Mehta HH, Cohen P, Yen K (2016) The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget 7:46899–46912 [DOI] [PMC free article] [PubMed]
- Kojima M, Morisaki T, Sasaki N, Nakano K, Mibu R, Tanaka M, Katano M. Increased nuclear factor-kB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004;24:675–681. [PubMed] [Google Scholar]
- Luciano F, Zhai D, Zhu X, Bailly-Maitre B, Ricci JE, Satterthwait AC, Reed JC. Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein BimEL. J Biol Chem. 2005;280:15825–15835. doi: 10.1074/jbc.M413062200. [DOI] [PubMed] [Google Scholar]
- Lue Y, Swerdloff R, Liu Q, Mehta H, Hikim AS, Lee KW, Jia Y, Hwang D, Cobb LJ, Cohen P, Wang C. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–357. doi: 10.1210/en.2009-0577. [DOI] [PubMed] [Google Scholar]
- Lue Y, Swerdloff R, Wan J, Xiao J, French S, Atienza V, Canela V, Bruhn KW, Stone B, Jia Y, Cohen P, Wang C. The potent Humanin analogue (HNG) protects germ cells and leucocytes while enhancing chemotherapy-induced suppression of cancer metastases in male mice. Endocrinology. 2015;156:4511–4521. doi: 10.1210/en.2015-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S. Pituitary tumors. Endocrinol Metab Clin N Am. 2015;44:1–9. doi: 10.1016/j.ecl.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti E, Giannerini V, Rossini L, Matsuoka M, Trabalzini L, Collodel G. Immunolocalization of humanin in human sperm and testis. Fertil Steril. 2010;94:2888–2890. doi: 10.1016/j.fertnstert.2010.04.075. [DOI] [PubMed] [Google Scholar]
- Mottaghi-Dastjerdi N, Soltany-Rezaee-Rad M, Sepehrizadeh Z, Roshandel G, Ebrahimifard F, Setayesh N. Genome expression analysis by suppression subtractive hybridization identified overexpression of Humanin, a target gene in gastric cancer chemoresistance. Daru. 2014;22:14. doi: 10.1186/2008-2231-22-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22:50–58. doi: 10.1038/sj.onc.1206043. [DOI] [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- Rubio MF, Werbajh S, Cafferata EG, Quaglino A, Colo GP, Nojek IM, Kordon EC, Nahmod VE, Costas MA. TNF-alpha enhances estrogen-induced cell proliferation of estrogen-dependent breast tumor cells through a complex containing nuclear factor-kappa B. Oncogene. 2006;25:1367–1377. doi: 10.1038/sj.onc.1209176. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Sponne I, Fifre A, Koziel V, Kriem B, Oster T, Pillot T. Humanin rescues cortical neurons from prion-peptide-induced apoptosis. Mol Cell Neurosci. 2004;25:95–102. doi: 10.1016/j.mcn.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gelinas C, Rabson AB. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;52:183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- Swinney DCXY, Scarafia LE, Lee I, Mak AY, Gan QF, Ramesha CS, Mulkins MA, Dunn J, So OY, Biegel T, Dinh M, Volkel P, Barnett J, Dalrymple SA, Lee S, Huber M. A small molecule ubiquitination inhibitor blocks NF-kappa B-dependent cytokine expression in cells and rats. J Biol Chem. 2002;277:23573–23581. doi: 10.1074/jbc.M200842200. [DOI] [PubMed] [Google Scholar]
- Vender JR, Laird MD, Dhandapani KM. Inhibition of NFkappaB reduces cellular viability in GH3 pituitary adenoma cells. Neurosurgery. 2008;62:1122–1127. doi: 10.1227/01.neu.0000325874.82999.75. [DOI] [PubMed] [Google Scholar]
- Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Werbajh S, Nojek I, Lanz R, Costas MA. RAC-3 is a NF-kappa B coactivator. FEBS Lett. 2000;485:195–199. doi: 10.1016/S0014-5793(00)02223-7. [DOI] [PubMed] [Google Scholar]
- Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Phys. 2013;304:H393–H397. doi: 10.1152/ajpcell.00347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chua CC, Gao J, Chua KW, Wang H, Hamdy RC, Chua BH. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res. 2008;1227:12–18. doi: 10.1016/j.brainres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying G, Iribarren P, Zhou Y, Gong W, Zhang N, Yu ZX, Le Y, Cui Y, Wang JM. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol. 2004;172:7078–7085. doi: 10.4049/jimmunol.172.11.7078. [DOI] [PubMed] [Google Scholar]
- Zapala B, Kaczynski L, Kiec-Wilk B, Staszel T, Knapp A, Thoresen GH, Wybranska I, Dembinska-Kiec A. Humanins, the neuroprotective and cytoprotective peptides with antiapoptotic and anti-inflammatory properties. Pharmacol Rep. 2010;62:767–777. doi: 10.1016/S1734-1140(10)70337-6. [DOI] [PubMed] [Google Scholar]
- Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies bid activity by blocking its activation of Bax and Bak. J Biol Chem. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- Zhou C, Jiao Y, Wang R, Ren SG, Wawrowsky K, Melmed S. STAT3 upregulation in pituitary somatotroph adenomas induces growth hormone hypersecretion. J Clin Invest. 2015;125:1692–1702. doi: 10.1172/JCI78173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GH3 cells were incubated with (A) STAT3 inhibitor JSI-124, (B) NF-κB inhibitor Ro 106–9920, (C) JNK inhibitor SP600125, (D) Akt inhibitor LY294002, (E) MEK inhibitor PD98059, (F) p38 inhibitor SB203580 or the corresponding vehicle for 24 h. Each column represents the percentage ± CL of TUNEL-positive cells (n ≥ 1000 cell/group). * p < 0.05 vs respective control without inhibitor. χ2 test. (JPEG 57 kb)
(A) GH3 cells were transfected with ssIκB for 16 h then incubated with TNF-α (50 ng/ml) for 24 h and processed for detection of phosphorylated p65 subunit of NF-κB (phospho-p65) by immunofluorescent staining. Each column represents arbitrary units (AU) of phospho-p65 fluorescence intensity ± SE. Student’s t test. (B) Representative images of phospho-p65 NF-κB in GH3 cells transfected with ssIκB. Upper panels: DAPI; Lower panels: phospho-p65. Scale bars: 10 μm. (JPEG 29 kb)
Mitochondria were isolated from cultured GH3 cells as described in Material and Methods. Proteins from mitocondrial (Mit) and non-mitochondrial (Non-Mit) fractions were subjected to Western blot using anti-Complex III subunit 2 antibody. Immunoreactivity was detected by enhanced chemiluminescence. (JPEG 14 kb)
(A) Anterior pituitary cells from OVX rats cultured with 17β-estradiol (E2, 10−9 M) or (B) GH3 cells were incubated with NF-κB inhibitor BAY 11–7082 (BAY, 2.5 μM) or vehicle (ethanol 0.05 ml/l) for 30 min before adding TNF-α (50 ng/ml) for a further 24 h. Cells were processed for detection of phosphorylated p65 subunit of NF-κB (phospho-p65) by immunofluorescent staining. Each column represents the arbitrary units (AU) of phospho-p65 fluorescence intensity ± SE from 3 independent experiments. * p < 0.05 vs respective control without TNF-α, ^ p < 0.05 respective control without BAY. ANOVA (JPEG 34 kb)