Abstract
Targeted therapy via imatinib appears to be a promising approach for chronic myeloid leukemia (CML) therapy. However, refractory and resistance to imatinib therapy has encouraged many investigators to get involved in development of new therapeutic agents such as Phorbol 12-myrestrat 13-acetate (PMA) for patients with CML. In that line, we attempted to investigate the chemosensitizing effect of PMA on the imatinib-resistant cells. Based on our western blot analyses, resistant K562 cells (K562R) showed high levels of FoxO3a and Bcl6 expressions which were not modulated by imatinib treatment. However, upon PMA treatment, the levels of both FoxO3a and Bcl6 were up-regulated among both the sensitive and the resistant cells and this treatment was associated with initiation of megakaryocytic differentiation of the cells. SiRNA-silencing of FoxO3a led to augmentation of megakaryocytic differentiation of the cells. Similarly, siRNA gene silencing of Bcl6 enhanced the differentiation and induced cell apoptosis among both types of cells. Regarding these results, it might be concluded that Bcl6 knockdown combined with PMA therapy could present a new therapeutical strategy for refractory CML patients to imatinib.
Keywords: Bcl6, Differentiation, FoxO3a, Imatinib, K562
Introduction
Many patients with chronic myeloid leukemia (CML) have a characteristic and specific chromosomal abnormality known as Philadelphia (Ph) chromosome that results from reciprocal translocation between the Abl gene on chromosome 9 and the Bcr gene on chromosome 22 in pluripotent hematopoietic stem cells (Nowell 1962; Rowley 1973). The resulting chimeric Bcr-Abl oncogene encodes p210Bcr-Abl protein with constitutive tyrosine kinase activity which is involved in growth factor independent cell proliferation, resistance to apoptosis and altered adhesion of cells in CML patients.
CML starts with an indolent chronic phase in which leukemia myeloid progenitors possess normal function and differentiation program accompanied by enhanced proliferation. By applying an effective therapeutic strategy in this phase, CML can be remedied for many years. Otherwise, CML progresses to irreversible blastic phase that is associated with accumulation of undifferentiated CML cells in bone marrow and peripheral blood which are invariably resistant to many therapeutic options (Druker et al. 2001; Klemm et al. 2009; Calabretta and Perrotti 2004; Quintas-Cardama and Cortes 2006). Among CML therapies, targeting Bcr-Abl protein by small molecule tyrosine kinase inhibitors (TKIs) such as imatinib has substantially improved CML prognosis (Deininger et al. 2005; Druker et al. 2006). However, despite the impressive imatinib clinical success the disease relapse appears to be an inevitable event in CML therapy due to its disability to eradicate CML stem cells and/or to the induced imatinib resistance (Hamilton et al. 2012; Corbin et al. 2011; Shah et al. 2002; Bhatia et al. 2003). The occurrence of imatinib resistance has motivated massive efforts for identification of new crucial targets besides of Bcr-Abl protein. In that respect, several signaling elements have been considered as critical factors responsible for resistance to imatinib (Naka et al. 2010).
Bcl6, as a functionally important survival factor in Bcr-Abl driven acute lymphoblastic leukaemia crisis, is induced following imatinib inhibition of Bcr-Abl (Duy et al. 2011). Bcl6 is a POZ/BTB transcription factor downstream of FoxO proteins which up-regulated in approximately one-third of the diffuse large cell lymphoma (DLCL) (Ye et al. 1993; Chang et al. 1996; Dalla-Favera et al. 1994). The products of Bcl6 targets genes implicated in proliferation, survival, cell growth, metabolism, differentiation and drug-resistance in various cell lines (Dent et al. 2002; Staudt et al. 1999; Seyfert et al. 1996). Based on previous findings, the unavoidable up-regulation of Bcl6 might limit the effectiveness of TKI therapy (Duy et al. 2011). In that line, it appears that modulation of Bcl6 activity via other therapeutic agents might constitute a new strategy for effective CML therapy.
Phorbol 12-myrestrat 13-acetate (PMA) as a potent activator of protein kinase C (PKC) induces megakaryocytic differentiation of K562 cells, a well established human CML cell line (Huberman and Callaham 1979). High affinity PMA binding to PKC leads, in turn, to activation of multiple signals implicated in megakaryocytic differentiation that is accompanied by changes in cell morphology, adhesive properties, cell growth arrest, polyploidization and increased expression of megakaryocytic cell surface markers (Hoffman and Huberman 1982; Murphy and Norton 1993). The occurrence of cell growth arrest following PMA treatment pertains to activation of the MEK/MAPK (ERK1/2) pathway, as well as induction of p21 and p27 proteins (Vrana et al. 1999; Hu et al. 2000). The positive outcome of PMA combination therapy of patients with refractory leukemia shows that PMA might reduce the myelosuppressive effects of the conventional cytotoxic chemotherapeutical drugs (Han et al. 1998a; Leszczyniecka et al. 2001; Han et al. 1998b). This fact might introduce alternative therapeutical strategies for imatinib-resistant patients pending further relevant research.
In that regard, this study was conducted to survey the involvement of Bcl6 in the PMA-induced megakaryocyte differentiation of sensitive and imatinib-resistant K562 cells. Our data demonstrated that PMA-induced Bcl6 expression might limit the PMA effectiveness via down-regulation of cyclinD2 and D3. Based on this conclusion, it is speculated that the combined treatment with Bcl6 siRNA and PMA might constitutive a new therapeutical strategy for refractory CML patients to imatinib.
Materials and methods
Materials
The cell culture plates were purchased from SPL life Science (South Korea). The culture medium (RPMI-1640), fetal bovine serum (FBS) and penicillin-streptomycin were purchased from Gibco BRL (Life Technology, Paisley, Scotland). Cell line was obtained from Pasteur Institute of Iran (Tehran, Iran).
Antibodies including anti-FoxO3a, anti-phosphorylated Akt, anti-cyclin D2, anti-cyclin D3 and Anti-phosphorylated Erk1/2 were obtained from Cell Signaling (MA. USA). Anti-p21 antibody was purchased from Bio-Source (Nivelles, Belgium). Anti-GPӀӀb antibody was from Ebioscience (San Diego, CA, USA). FoxO3a siRNA was purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
imatinib, anti-14-3-3ζ, anti-Bcl6 antibodies, control siRNA and Bcl6 siRNA were purchased from Santa Cruz Biotechnology (Canada). 14–3-3ζ inhibitor (R18 trifluoroacetate), Phorbol-12-myristate-13 acetate (PMA), 2, 3 diaminopyridine, MTT [3-(4, 5-dimethyl tiazol-2-yl)-2, 5-diphenyl tetrazolium bromide], propidiumiodode (PI), RNase (DNase free), phenylmethylsulphonyl fluoride (PMSF), leupeptin, pepstatin, aprotinin anti-Tubulin, mouse/rabbit horseradish peroxidase-conjugated secondary antibodies and sodium azide were obtained from Sigma Chem. Co. (Germany). Enhanced chemiluminescence detection systems (ECL) was purchased from Amersham-Pharmacia (Piscataway, NJ, USA). LipofectaminTM2000 was purchased from Invitrogen (UK).
Cell culture
The sensitive K562 (K562S) and imatinib-resistant K562 (K562R) cells were cultured in RPMI-1640 medium supplemented with FBS (10% v/v), streptomycine (100 μg/ml) and penicillin (100 U/ml). The cells were incubated under 5% CO2 humified atmosphere at 37 °C. To develop the K562R cell lines, the K562S cells were cultured in imatinib-containing medium. We increased the imatinib concentration in medium gradually from 0.1 μM to 2 μM. The cells were sub-cultured whenever the density reached 60–70% confluency. Cell numbers and viabilities were assessed using a hemocytometer and the abilities of the cells to exclude trypan blue.
Cell viability assay
The number of viable cells was estimated by trypan blue exclusion test. Cell number was assessed using a hemocytometer and the ability of the cells to exclude trypan blue. Cytotoxicity was also estimated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay. Viable cells with active mitochondria reduce the yellow tetrazolium salt giving dark blue water insoluble formazan crystals. Briefly, 1 × 104 cells were seeded per well in 96-well plates in triplicates 12 h prior to treatment. Cells were treated by different concentrations of imatinib or PMA separately and were incubated at 37 °C in 5% CO2 for 72 h. Then, the cells were treated with 10 μl MTT (5 mg/ml) solution for 4 h. After incubation, the medium was removed and 150 μl DMSO was added to each well. The formazan dye crystals were solubilized for 30 min, and the absorbance at 570 nm was measured with an ELISA reader (GENE5 Power waveXS2, Biothek, USA). Results were expressed as percentage of MTT reduction, assuming that the absorbance of the control cells was 100%.
Fluorescence microscopy evaluation of the apoptotic cells
The cells were seeded in 24-well plates and treated with PMA and Bcl6 siRNA for a time course of 48 h. Cells were washed with cold phosphate buffer saline and adjusted to a cell density of 1 × 106 using PBS. Apoptosis was determined morphologically after double staining the cells with acridine orange/ethidium bromide (AO/EtBr) using a fluorescence microscope (Zeiss, Germany). The AO/EtBr solution (1:1 v/v) was added to the cell suspension in a final concentration of 100 μg/ml. Using this procedure, cells can be discriminated as normal cells (uniformly stained green) and apoptotic cells which are stained orange on account of cell membrane destruction and the intercalation of ethidium bromide between the nucleotide bases of DNA. All examinations were done in triplicate, and the number of stained cells was calculated in 10 arbitrarily selected fields.
Western blot analysis
After treatment of K562S and K562R cells with imatinib (0.4 μM) or PMA (5 nM and 10 nM for K562R and K562S, respectively) for different times, the cells were harvested and lysed with lysis buffer containing 1% Triton X-100, 1% SDS, 10 mM Tris (pH 7.4), 100 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM sodium pyrophosphate, 2 mM Na3VO4, 1 mMNaF, 0.5% sodium deoxycholate, 10% glycerol, 1 mM phenylmethylsulphonyl fluoride (PMSF), 10 μg/ml leupeptin, 1 μg/ml pepstatin and 60 μg/ml aprotinin. After 30 min, the cell lysates were centrifuged at 14,000 rpm for 15 min at 4°C. The protein concentration was measured by the lowry’s protein assay (Lowry et al. 1951). Then 100 μg of each sample was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a PVDF membrane. Transfer of proteins was assessed by pre-stain ladder protein. Each membrane was blocked with 5% (w/v) non-fat dry milk in Tris-buffered salin pH 7.4, 0.1% Tween-20 and for an overnight at 4 °C. The blocked membranes were incubated with primary antibodies for 2 h at room temperature using antibody dilution as recommended by the manufacturer in Tris-buffered salin pH 7.4, 0.1% Tween-20. Following 1–2 h incubation with anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody, the proteins were detected by an enhanced chemiluminescence (ECL) detection system according to manufacturer’s instruction. For analysis of the western blotting data, densitometric analysis was performed using Image.J software and the densities were normalized with respect to β-tubulin as the internal control.
Cell cycle analysis by flow cytometer
To analyze the cell cycle profile, K562R cells were seeded into 24-well plates. After 24 h, cells were exposed to 0.4 μM imatinib or 5 nM PMA for 72 h. After treatment, cells were harvested and washed twice with cold PBS, fixed by 70% ethanol for at least 2 h at 4 °C and stained with 20 μg/ml PI containing 20 μg/ml RNase (DNase free) for 30 min at 37 °C. The stained cells were analyzed by a FACScan flow cytometer (Partec PAS, Munich, Germany).
Flow cytometry analyses of the cell surface markers
To measure megakaryocytic differentiation, we used flow cytometry analysis via evaluating the expression of CD41 (as a marker of megakaryocytic differentiation) among K562S cells independently treated with imatinib (0.4 μM) or PMA (10 nM). K562S cells (3 × 105 cells) were cultured in 12-well plates. After 12 h, cells were treated with 0.4 μM imatinib or 10 nM PMA for 72 h, then the cells were washed twice with cold PBS and resuspended in PBS containing 1% FBS and 0.1% sodium azide. The anti-human CD41a FITC monoclonal antibody (5 μl) was added to 100 μl of the cell suspension (3 × 105 cells), and incubated for 30 min at 4 °C. Then, the cells were washed with PBS and analyzed at least 104 cells by a flow cytometer (Partec PAS, Munich, Germany).
Inhibition of Bcl6 and FoxO3a expression by siRNA transfection
K562S cells were cultured in 24-well plates and transfected with 100 nM of FoxO3a siRNA (Cell Signaling Technology, # 63025, Lot: 1) and Bcl6 siRNA (Santa Cruz Biotechnology, sc-29,791, Lot #D2413) using Lipofectamin™2000 according to manufacturer’s instructions. Non-targeting siRNA (Santa Cruz Biotechnology, sc-37,007, Lot # G2913) was used as the control for non-sequence-specific effects of the transfected siRNA. The accuracy of gene knockdown was confirmed by western blot analysis.
Statistical analyses
Data are expressed as mean ± SD of three independent measurements and statistically analyzed using Student’s t test. Values of p < 0.05 were considered significant.
Results
Overexpression of Bcl6 in imatinib resistant cells
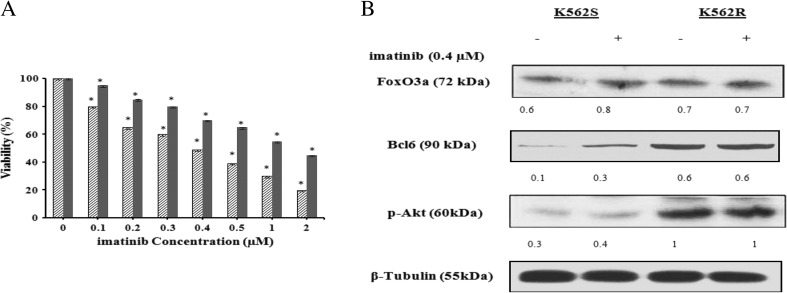
To develop imatinib-resistant K562 cells, treatment of the cells with imatinib was followed for about one year. After ten month of treatment, the concentration of the drug producing half maximal growth inhibition (IC50) of the so called resistant cells (K562R) was obtained to be around 1 μM (Fig. 1a) relative to that of the sensitive cells which was about 0.4 μM . Previous studies have characterized FoxO3a and Bcl6 proteins as the central factors implicated in induction of resistance to TKI treatment (Hurtz et al. 2011). Consistent with previous reports, our western blot analyses also revealed that FoxO3a and Bcl6 were overexpressed in the resistant relative to the sensitive K562 cells (Fig. 1b).
Fig. 1.
The effect of imatinib on K562R and K562S cells viabilities. (a) Relative cell viability was evaluated using MTT assay. The data are the means of three independent measurements ± SD (P ≤ 0.05). (b) Effects of imatinib on the expression of p-Akt, FoxO3a and Bcl6 in K562S and K562R cells. Cells were treated with imatinib (0.4 μM) for 72 h. The protein expression of p-Akt, FoxO3a and Bcl6 were determined in each lysate of the drug-treated cells by immunoblotting technique
Anti-cell growth and cell cycle effects of PMA in K562R cells
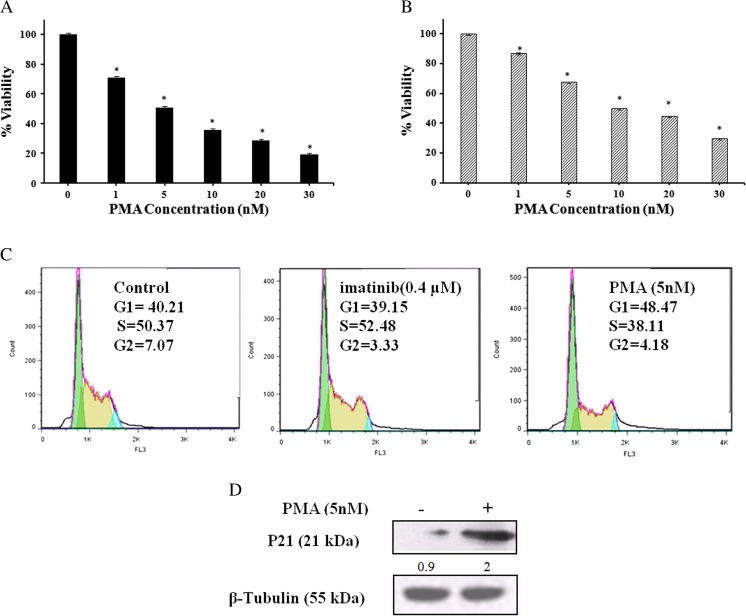
After 72 h exposure to 1–30 nM of PMA, the K562R cell proliferation was inhibited by 30–81% (Fig. 2a) while the extent of inhibition among the sensitive cells, under all identical experimental conditions was 10–70% (Fig. 2b). The half-inhibitory concentrations (IC50) were approximately 5 nM and 10 nM for K562R and K562S cells, respectively. In order to get a better understanding on the anti-proliferative activity of PMA, we examined the effects of PMA on cell cycle progression. Cell cycle distribution of K562R cells was analyzed by a flow cytometer after treating the cells with Imatinib (0.4 μM) and PMA (5 nM) for 72 h. Flow cytometry analyses of untreated cells indicated that almost 40.2, 50.4 and 7.1% of the K562R cells were distributed among G0/G1, S and G2/M-phases, respectively. As shown in Fig. 2c, the percent G1-phase distribution of K562R cells, after treatment with 5 nM PMA, increased to almost 48% at the expense of a decrease in the S or G2/M-phase population relative to control cells. According to Fig. 2c, the distribution of K562R cells remained almost unchanged under the influence of Imatinib. On the other hand, western blot analyses indicated that PMA potentiated the expression of p21 in K562R cells (Fig. 2d). This fact might account for the high cell population at G1-phase.
Fig. 2.
The effect(s) of PMA on K562R (a) and K562S (b) cell viabilities. K562R and K562S cells were treated with different concentrations of PMA for 72 h and cell viabilities were estimated by MTT assay. The data are the means of three independent measurements ± SD (P ≤ 0.05). (c, d) Evaluation of cell cycle distribution and expression of p21 among K562R cells upon imatinib and PMA treatments. (c) Cells were treated with imatinib (0.4 μM) or PMA (5 nM) for 72 h. In each phase, the percentage of cell population was determined by a flow cytometer. (d) After lysis of the harvested cells, equal protein amount of each sample was subjected to western blot analysis using specific antibody for p21 and the intensity of each band was estimated by densitometric analysis. Equal protein loading in each gel well was confirmed by the β-Tubulin content
Augmentation of the FoxO3a and Bcl6 expressions under the influence of PMA in K562R/K562S cells
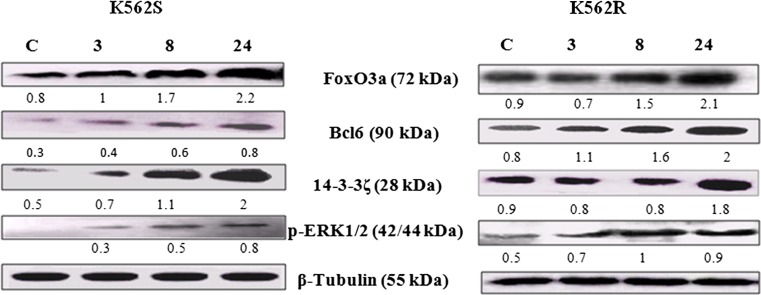
Considering the pivotal role of FoxO3a and Bcl6 factors in sensitivity and responsiveness of leukemia cells to therapeutic agents (O'Hare et al. 2012), we investigated the expression status of these factors under the influence of PMA in both imatinib resistant and sensitive K562 cells. Western blot analyses indicated that PMA affected, in a time dependent manner, the expression of FoxO3a and Bcl6 in K562R cells (Fig. 3). K562S/K562R cells respond to PMA via up regulation of FoxO3a and Bcl6. As shown in Figs. 3, 14–3-3ζ, as an important FoxO3a regulator (Tzivion et al. 2011), was also up-regulated under the influence of PMA in both K562R and K562S cells. Similarly, PMA brought about the overexpression of p-ERK1/2 in both cell types.
Fig. 3.
Evaluation of FoxO3a and Bcl6 protein expressions during megakaryocyte differentiation induced by PMA at different time intervals in K562S and K562R cells. K562S and K562R cells treated with10nM and 5 nM, respectively and the expressions of FoxO3a, Bcl6, 14–3-3ζ and p-ERK1/2 were determined at different time intervals by immunoblotting using monoclonal antibodies against the mentioned factors. The density of each band was estimated by densitometric analysis. Equal protein loading in each gel well was confirmed by the β-Tubulin content
FoxO3a role in PMA-induced megakaryocyte differentiation of K562S cells
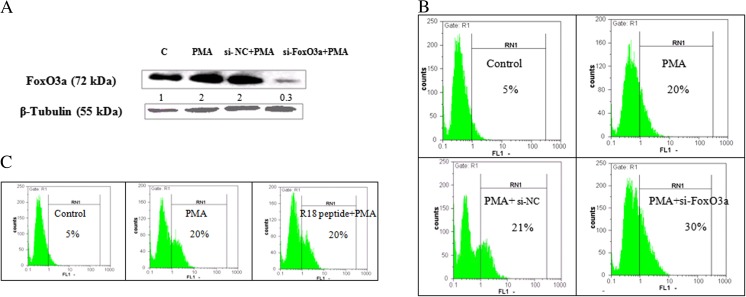
FoxO family members have various functions in the hematopoietic system. It has been well-established that FoxO3a is up-regulated and plays role during erythroid differentiation of leukemia cells (Marinkovic et al. 2007; Bakker et al. 2004). Regarding the up-regulation of FoxO3a under the influence of PMA (Fig. 3), we prompted to investigate whether FoxO3a has a role in PMA-induced megakaryocyte differentiation. In that line, K562S cells were first treated with 10 nM PMA followed by transfecting with scramble and FoxO3a siRNAs. Following these treatments, the expression level of FoxO3a decreased as it is evident in Fig. 4a. The effect of scramble siRNA (si-NC) on the expression of CD41 on the cell surface was evaluated among PMA-treated cells and there was no significant effect on the extent of differentiation relative to the PMA-treated cells (Fig. 4b). Similarly and as shown in Fig. 4b, down-regulation of FoxO3a by siRNA caused a 10% increase in CD41 content of K562S cells, confirming higher level of megakaryocytic differentiation of the cells. On the other hand, inhibition of 14–3-3 function, as the key regulator of FoxO3a, by R18 peptide in the presence of PMA did not induced variation in the CD41 cell surface content of the cells (Fig. 4c). Based on these data, it might be concluded that augmentation of FoxO3a level does not possess a key role in megakaryocyte differentiation of K562S cells under the influence of PMA.
Fig. 4.
Evaluation of the role of FoxO3a and 14–3-3 in PMA-induced megakaryocyte differentiation. (a) K562S cells were transfected with scramble siRNA (si-NC) and siRNA against FoxO3a. After 48 h of transfection, the expression of FoxO3a was measured by western blot analysis. (b)The percentage of CD41 was determined by a flow cytometer using FITC-conjugated mouse anti-human CD41 antibody. (c) K562S cells were treated with R18 peptide, the inhibitor of 14–3-3 protein, and after 48 h the percentage of CD41 marker was determined by a flow cytometer
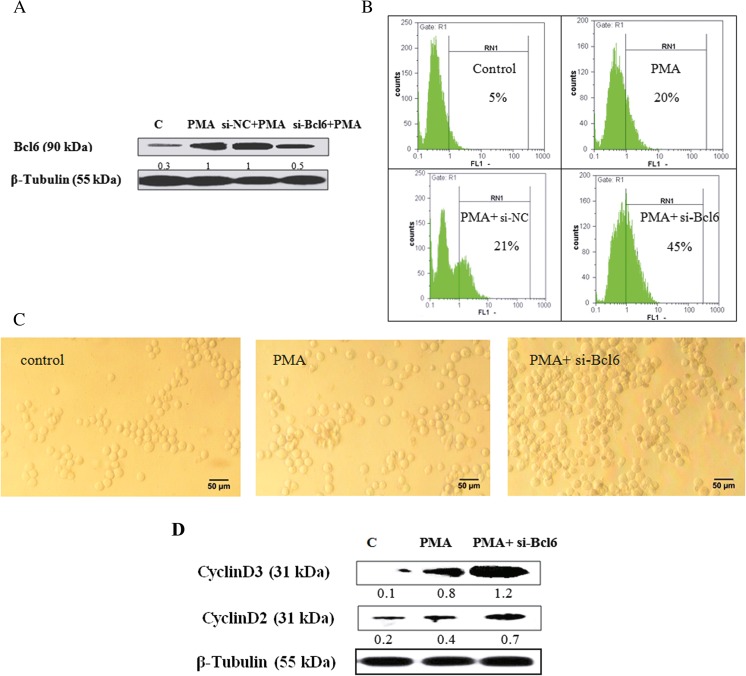
Attenuation of Bcl6 expression increases PMA-induced megakaryocyte differentiation of K562S cells
Bcl6 is an effective transcription factor downstream of FoxO3a factor. Given that Bcl6 expression was up-regulated in response to PMA-treatment of K562 cells (Fig. 3), the probable role of Bcl6 factor was evaluated in PMA-induced megakaryocyte differentiation. K562S cells, treated with 10 nM of PMA and then transfected with scramble and Bcl6 siRNAs, were evaluated for the extent of megakaryocytic differentiation using western blot analyses (Fig. 5a). In addition, after 48 h of transfection, the relative expression of cell surface antigen CD41 was evaluated by flow cytometery. As shown in Fig. 5b, down-regulation of Bcl6 by siRNA caused a 25% increase in CD41 content of K562S cells relative to PMA-treated cells. It is noteworthy that si-NC transfection had no significant effect on differentiation content among PMA-treated cells. Therefore, we only presented the results related to PMA- and PMA along with Bcl6 siRNA-treated cells in the following sections. Morphological changes due to Bcl6 down-regulation were also evaluated using an invert microscope. As evident from Fig. 5c, the K562S-transfected cells were morphologically bigger in size than the PMA-treated K562S cells. The occurrence of endomitosis during megakaryocyte differentiation depends on the expression of D-type cyclins which are critically important regulators of cell cycle G1/S progression (Sherr and Roberts 1995). In that respect, we examined the expression level of each of the cyclinDs. As depicted in Fig. 5d, western blot analyses clearly indicated that D2 and D3 cyclins were significantly overexpressed among the K562S-transfected cells.
Fig. 5.
Evaluation of functional role of Bcl6 during PMA-induced megakaryocyte differentiation. (a) K562S cells were transfected with scramble siRNA (si-NC) and siRNA against Bcl6. After 48 h of transfection, the expression of Bcl6 was measured by western blot analysis and then (b) the percentage of CD41 was determined by a flow cytometer using FITC-conjugated mouse anti-human CD41 antibody among K562S cells. (c) Morphological changes of transfected cells were observed by an invert microscope at ×20 magnifications (scale bar: 50 μm). (d) The protein expression of CyclinD2 and CyclinD3 among K562S cells were determined by immunoblotting using the relevant monoclonal antibodies and the density of each band was estimated by densitometry analysis. Equal protein loading in each gel well was confirmed by the β-Tubulin content
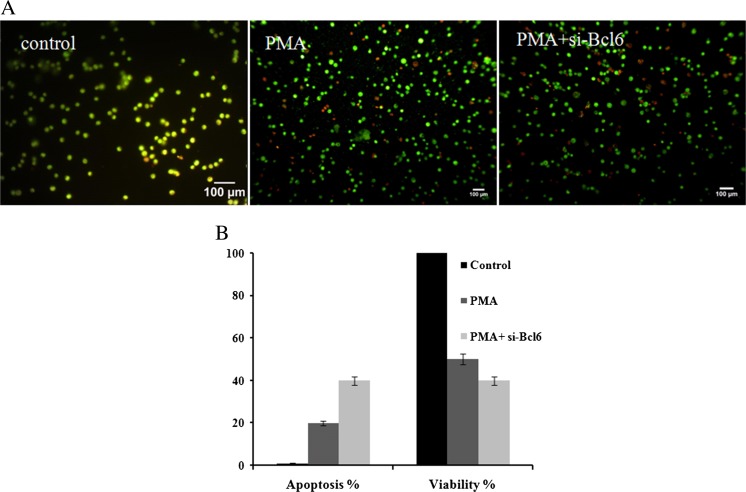
Modulation of cell viability and apoptosis by Bcl6 in PMA-treated K562S cells
Based on ethidium bromide and acridine orange double staining experiment, a significant population of cells was directed toward apoptosis following down-regulation of Bcl6. After 48 h, Bcl6-depleted cells experienced about 20% more apoptosis (Fig. 6a) and lost their viability by almost 10% compared to PMA-treated K562S cells (Fig. 6b). Regarding these data, it could be concluded that Bcl6 acts as a survival factor during PMA-induced megakaryocyte differentiation of K562S cells.
Fig. 6.
Modulation of the cell ʼ s apoptosis and viability by Bcl6 in PMA-treated K562S cells. (a) The percentage of apoptosis was determined by staining cells with EtBr/AO and photomicrographs of the cells were taken by a fluorescence microscope at ×10 magnifications (scale bar: 100 μm). (b) The proliferation of Bcl6-depleted cells was measured by the trypan blue exclusion test after transfection
Discussion
imatinib is the first successfull drug in the context of targeted therapy which created promising results in the treatment of CML patients. However, based on the extensive research on both CML biology and therapeutic strategies, pharmacological silencing of Bcr-Abl alone does not present an efficient strategy for CML therapy. The incidence of imatinib-resistance and insensitivity of CML stem cells to Bcr-Abl inhibitors, emphasize the essential need for other therapeutic approaches for treatment of CML patients (O'Hare et al. 2012).
Bcr-Abl oncoprotein promotes cytoplasmic retention of FoxO proteins as a result of PI3K/Akt activation which consequently inhibits the FoxO transcriptional activity (Skorski et al. 1995). The FoxO proteins are tumor suppressor factors that modulate the expression of cell cycle regulators, including p27KIP1, cyclin D and p130 (Burgering 2008). Inhibition of Bcr-Abl by TKIs has resulted in FoxO3a activation and the cell cycle arrest (Komatsu et al. 2003). Thus, induction of FoxO3a activity in leukemic cell lines might represent an effective strategy for induction of cell cycle arrest and apoptosis (Kikuchi et al. 2007). In that line, our western blot analyses showed that imatinib caused overexpression of FoxO3a in K562S cells associated with cell cycle arrest and apoptosis. Conversely, the expression level of FoxO3a was not modulated by imatinib treatment in K562R cells. FoxO3a has recently been identified as a main factor for the maintenance of leukemia-initiating cells (LICs) so that attenuation of FoxO3a activity has led to suppression of leukemia (Naka et al. 2010). In other words, it seems that FoxO3a is involved in induction of imatinib-resistance among K562R cells.
On the other hand, our results indicated that expression of FoxO3a was associated with high Bcl6 expression level among K562R cells (Fig. 1b). It has previously been shown that both FoxO3a and Bcl6 expression levels are tightly correlated in LICs during progression of chronic phase toward blastic crisis in CML (Hurtz et al. 2011). As presented in Fig. 1b, the expression of p-Akt, as a negative regulator of FoxO3a, was up-regulated among K562R cells (Dobson et al. 2011).
PMA as a potent modulator of cell differentiation among various cell lines has been successfully administrated to patients with refractory leukemia to all-trans retinoic acid, Ara-C and some other chemotherapeutic drugs (Han et al. 1998b). According to our results, PMA besides of inducing apoptosis stimulated megakaryocytic differentiation of K562R cells. Indeed, as presented in Fig. 2 (d, c), up-regulation of p21 and augmentation of G1 arrest following PMA treatment might be a prerequisite step for initiating megakaryocytic differentiation. Based on our western blot analyses (Fig. 5d), induction of FoxO3a activity by PMA was associated with up-regulation of cyclinD2 and D3 expressions. The occurrence of endomitosis is believed to depend on D-type cyclins which mediate polyploidy formation of megakaryocytes (Sherr and Roberts 1995). Wang et al. reported that suppression of cyclin D3 expression with antisense oligonucleotides significantly repressed megakaryocyte development of murine bone marrow cells. In addition, Zimmet et al. suggested a DNA replication regulatory role for cyclin D3 during endomitosis. Another study demonstrated that overexpression of D-type cyclin together with decreased cdc2 activity facilitated megakaryocytic differentiation of F-36p-mpl cells even without TPO treatment (Matsumura et al. 2000).
Our siRNA-based FoxO3a silencing experiment resulted in higher level of megakaryocytic differentiation of K562S cells. In that line, a recent in vivo study on megakaryopoiesis characterized the FoxO factors as negative regulators of murine megakaryocyte lineage specification during hematopoiesis (Cornejo et al. 2011). The fact that FoxO3a does not have binding site(s) in the cyclin D2 promoter (Fernandez de Mattos et al. 2004) guided us to evaluate the probable role of Bcl6 as an intermediate transcription factor which might mediate FoxO3a effect upon PMA treatment. Bcl6, as a transcription factor downstream of FoxO3a, is expressed in germinal center B cells but not in plasma cells. Based on gene deletion studies, Bcl6 plays an inhibitory role in differentiation of lymphocytes via repressing the genes accounted for B cell activation and terminal differentiation (Staudt et al. 1999). Conversely, a recent study has shown that Bcl6, as a master regulator, facilitates Follicular helper T (Tfh) cells differentiation and exerts multiple effects on Tfh biology (Hatzi et al. 2015). In CML cells, Bcl6 expression is repressed by Bcr-Abl signaling pathway and reactivated following TKI treatment (Pellicano and Holyoake 2011). Our results showed that PMA-induced accumulation of active FoxO3a was also associated with up-regulation of Bcl6 in K562S cells. Moreover, using siRNA-based gene silencing, we showed that expression of cyclin D2 and D3 genes were down-regulated by Bcl6 and so Bcl6 knockdown enhanced their expression. This situation might improve the ability of PMA to induced megakaryocytic differentiation among K562 sensitive and resistant cells. Indeed, our results describe a mechanism by which FoxO3a through up-regulation of Bcl6 exerts a repressory effect on the expression of cyclin D2 and D3.
In the present study, as presented in Fig. 6, Bcl6 gene-silencing also increased the growth inhibitory effects of PMA. In the absence of Bcl6 function, K562S cells tolerated more apoptosis by PMA. It seems that Bcl6 is part of a stress response mode by which K562S cells tolerate apoptosis. In this line, Duy et al. demonstrated that Bcl6 up-regulation allowed Philadelphia+ (Ph+) pre-B cell acute lymphoblastic leukemia (ALL) to survive TKI treatment. Moreover, it has been indicated that TKI-induced up-regulation of Bcl6 maintain the self-renewal capacity of CML-initiating cells and pharmacological inhibition of Bcl6, via retro-inverso Bcl6 peptide inhibitor, sensitizes them to TKIs (O'Hare et al. 2012). In fact, this report identified Bcl6 as a key effector molecule downstream of FoxO proteins accounting for the repression of p53/Arf signaling which eventually leads to protection and maintenance of LICs.
In conclusion, our study shows that Bcl6 knockdown leads to the enhancement of PMA-induced apoptosis and facilitates PMA-induced megakaryocytic differentiation in both K562S and K562R cells. Indeed, the present report describes a mechanism by which FoxO3a–mediated up-regulation of Bcl6 guides down-regulation of cyclin D2 and D3 expressions in K562S cells. Given that the pharmacological inhibition of Bcl6 induces LICs apoptosis, it seems that Bcl6 knockdown combined with PMA treatment could be an alternative effective therapeutical strategy for refractory CML patients to imatinib.
Acknowledgements
The authors appreciate the joint financial support of this investigation by the Research Council of University of Tehran and the University of Tehran Science and Technology Park.
Abbreviations
- CML
chronic myeloid leukemia
- PMA
phorbol 12-myrestrat 13-acetate
- Ph
Philadelphia
- TKIs
tyrosine kinase inhibitors
- DLCL
diffuse large cell lymphoma
- PKC
protein kinase C
- LICs
leukemia-initiating cells
- Tfh
facilitates Follicular helper T
- ALL
acute lymphoblastic leukemia
References
- Bakker WJ, Blázquez-Domingo M, Kolbus A, Besooyen J, Steinlein P, Beug H, Coffer PJ, Löwenberg B, von Lindern M, van Dijk TB. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J Cell Biol. 2004;164(2):175–184. doi: 10.1083/jcb.200307056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, Arber DA, Slovak ML, Forman SJ. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101(12):4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27(16):2258–2262. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103(11):4010–4022. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- Chang CC, Ye BH, Chaganti RS, Dalla-Favera R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci U S A. 1996;93(14):6947–6952. doi: 10.1073/pnas.93.14.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121(1):396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo MG, Mabialah V, Sykes SM, Khandan T, Lo Celso C, Lopez CK, Rivera-Munoz P, Rameau P, Tothova Z, Aster JC, DePinho RA, Scadden DT, Gilliland DG, Mercher T. Crosstalk between NOTCH and AKT signaling during murine megakaryocyte lineage specification. Blood. 2011;118(5):1264–1273. doi: 10.1182/blood-2011-01-328567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R, Ye BH, Lo Coco F, Gaidano G, Lista F, Knowles DM, Louie DC, Offit K, Chaganti RS. Identification of genetic lesions associated with diffuse large-cell lymphoma. Ann Oncol. 1994;5(Suppl 1):55–60. doi: 10.1093/annonc/5.suppl_1.S55. [DOI] [PubMed] [Google Scholar]
- Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105(7):2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- Dent AL, Vasanwala FH, Toney LM. Regulation of gene expression by the proto-oncogene BCL-6. Crit Rev Oncol Hematol. 2002;41(1):1–9. doi: 10.1016/S1040-8428(01)00164-0. [DOI] [PubMed] [Google Scholar]
- Dobson M, Ramakrishnan G, Ma S, Kaplun L, Balan V, Fridman R, Tzivion G. Bimodal regulation of FoxO3 by AKT and 14-3-3. Biochim Biophys Acta. 2011;1813(8):1453–1464. doi: 10.1016/j.bbamcr.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MWN, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA. Five-year follow-up of patients receiving Imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Duy C, Hurtz C, Shojaee S, Cerchietti L, Geng H, Swaminathan S, Klemm L, S-m K, Nahar R, Braig M, Park E, Y-m K, Hofmann W-K, Herzog S, Jumaa H, Koeffler HP, Yu JJ, Heisterkamp N, Graeber TG, Wu H, Ye BH, Melnick A, Müschen M. BCL6 enables Ph(+) acute lymphoblastic leukemia cells to survive BCR-ABL1 kinase inhibition. Nature. 2011;473(7347):384–388. doi: 10.1038/nature09883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez de Mattos S, Essafi A, Soeiro I, Pietersen AM, Birkenkamp KU, Edwards CS, Martino A, Nelson BH, Francis JM, Jones MC, Brosens JJ, Coffer PJ, Lam EW. FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol Cell Biol. 2004;24(22):10058–10071. doi: 10.1128/MCB.24.22.10058-10071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, Nicolini FE, Muller-Tidow C, Bhatia R, Brunton VG, Koschmieder S, Holyoake TL. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119(6):1501–1510. doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZT, Tong YK, He LM, Zhang Y, Sun JZ, Wang TY, Zhang H, Cui YL, Newmark HL, Conney AH, Chang RL. 12-O-Tetradecanoylphorbol-13-acetate (TPA)-induced increase in depressed white blood cell counts in patients treated with cytotoxic cancer chemotherapeutic drugs. Proc Natl Acad Sci U S A. 1998;95(9):5362–5365. doi: 10.1073/pnas.95.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZT, Zhu XX, Yang RY, Sun JZ, Tian GF, Liu XJ, Cao GS, Newmark HL, Conney AH, Chang RL. Effect of intravenous infusions of 12-O-tetradecanoylphorbol-13-acetate (TPA) in patients with myelocytic leukemia: preliminary studies on therapeutic efficacy and toxicity. Proc Natl Acad Sci U S A. 1998;95(9):5357–5361. doi: 10.1073/pnas.95.9.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. 2015;212(4):539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DR, Huberman E. The control of phospholipid methylation by phorbol diesters in differentiating human myeloid HL-60 leukemia cells. Carcinogenesis. 1982;3(8):875–880. doi: 10.1093/carcin/3.8.875. [DOI] [PubMed] [Google Scholar]
- Hu X, Moscinski LC, Valkov NI, Fisher AB, Hill BJ, Zuckerman KS. Prolonged activation of the mitogen-activated protein kinase pathway is required for macrophage-like differentiation of a human myeloid leukemic cell line. Cell Growth Differ. 2000;11(4):191–200. [PubMed] [Google Scholar]
- Huberman E, Callaham MF. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtz C, Hatzi K, Cerchietti L, Braig M, Park E, Kim YM, Herzog S, Ramezani-Rad P, Jumaa H, Muller MC, Hofmann WK, Hochhaus A, Ye BH, Agarwal A, Druker BJ, Shah NP, Melnick AM, Muschen M. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J Exp Med. 2011;208(11):2163–2174. doi: 10.1084/jem.20110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Nagai T, Kunitama M, Kirito K, Ozawa K, Komatsu N. Active FKHRL1 overcomes imatinib resistance in chronic myelogenous leukemia-derived cell lines via the production of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci. 2007;98(12):1949–1958. doi: 10.1111/j.1349-7006.2007.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm L, Duy C, Iacobucci I, Kuchen S, von Levetzow G, Feldhahn N, Henke N, Li Z, Hoffmann TK, Kim YM, Hofmann WK, Jumaa H, Groffen J, Heisterkamp N, Martinelli G, Lieber MR, Casellas R, Muschen M. The B cell mutator AID promotes B lymphoid blast crisis and drug resistance in chronic myeloid leukemia. Cancer Cell. 2009;16(3):232–245. doi: 10.1016/j.ccr.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Watanabe T, Uchida M, Mori M, Kirito K, Kikuchi S, Liu Q, Tauchi T, Miyazawa K, Endo H, Nagai T, Ozawa K. A member of Forkhead transcription factor FKHRL1 is a downstream effector of STI571-induced cell cycle arrest in BCR-ABL-expressing cells. J Biol Chem. 2003;278(8):6411–6419. doi: 10.1074/jbc.M211562200. [DOI] [PubMed] [Google Scholar]
- Leszczyniecka M, Roberts T, Dent P, Grant S, Fisher PB. Differentiation therapy of human cancer: basic science and clinical applications. Pharmacol Ther. 2001;90(2–3):105–156. doi: 10.1016/S0163-7258(01)00132-2. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117(8):2133–2144. doi: 10.1172/JCI31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura I, Tanaka H, Kawasaki A, Odajima J, Daino H, Hashimoto K, Wakao H, Nakajima K, Kato T, Miyazaki H, Kanakura Y. Increased D-type cyclin expression together with decreased cdc2 activity confers megakaryocytic differentiation of a human thrombopoietin-dependent hematopoietic cell line. J Biol Chem. 2000;275(8):5553–5559. doi: 10.1074/jbc.275.8.5553. [DOI] [PubMed] [Google Scholar]
- Murphy JJ, Norton JD. Phorbol ester induction of early response gene expression in lymphocytic leukemia and normal human B-cells. Leuk Res. 1993;17(8):657–662. doi: 10.1016/0145-2126(93)90070-2. [DOI] [PubMed] [Google Scholar]
- Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463(7281):676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- Nowell PC. The minute chromosome (Phl) in chronic granulocytic leukemia. Blutalkohol. 1962;8:65–66. doi: 10.1007/BF01630378. [DOI] [PubMed] [Google Scholar]
- O'Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer. 2012;12(8):513–526. doi: 10.1038/nrc3317. [DOI] [PubMed] [Google Scholar]
- Pellicano F, Holyoake TL. Assembling defenses against therapy-resistant leukemic stem cells: Bcl6 joins the ranks. J Exp Med. 2011;208(11):2155–2158. doi: 10.1084/jem.20112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintas-Cardama A, Cortes JE. Chronic myeloid leukemia: diagnosis and treatment. Mayo Clin Proc. 2006;81(7):973–988. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous Leukaemia identified by Quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Seyfert VL, Allman D, He Y, Staudt LM. Transcriptional repression by the proto-oncogene BCL-6. Oncogene. 1996;12(11):2331–2342. [PubMed] [Google Scholar]
- Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, Sawyers CL. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2(2):117–125. doi: 10.1016/S1535-6108(02)00096-X. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Skorski T, Kanakaraj P, Nieborowska-Skorska M, Ratajczak MZ, Wen SC, Zon G, Gewirtz AM, Perussia B, Calabretta B. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood. 1995;86(2):726–736. [PubMed] [Google Scholar]
- Staudt LM, Dent AL, Shaffer AL, Yu X. Regulation of lymphocyte cell fate decisions and lymphomagenesis by BCL-6. Int Rev Immunol. 1999;18(4):381–403. doi: 10.3109/08830189909088490. [DOI] [PubMed] [Google Scholar]
- Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813(11):1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Vrana JA, Kramer LB, Saunders AM, Zhang XF, Dent P, Povirk LF, Grant S. Inhibition of protein kinase C activator-mediated induction of p21CIP1 and p27KIP1 by deoxycytidine analogs in human leukemia cells: relationship to apoptosis and differentiation. Biochem Pharmacol. 1999;58(1):121–131. doi: 10.1016/S0006-2952(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Ye BH, Lista F, Lo Coco F, Knowles DM, Offit K, Chaganti RS, Dalla-Favera R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science (New York, NY) 1993;262(5134):747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]