Abstract
The rapid clearance of malaria parasite DNA from circulation has widely been accepted as a fact without being systemically investigated. We assessed the persistence of parasite DNA in travelers treated for Plasmodium falciparum malaria in a malaria-free area.
Venous blood was collected at the time of admission and prospectively up to one year. DNA and RNA were extracted and analyzed using species-specific and gametocyte-specific real-time PCR as well as merozoite surface protein 2 (msp2)-PCR.
In 31 successfully treated individuals, asexual parasites were seen by microscopy until two days after treatment, whereas parasite DNA was detected by msp2- and species-specific PCR up to days 31 and 42, respectively. Statistical modelling predicted 26% (± 0·05 SE) species-specific PCR positivity until day 40 and estimated 48 days for all samples to become PCR negative. Gametocytes were detected by microscopy and PCR latest two days after treatment. CT values correlated well with microscopy-defined parasite densities before but not after treatment started.
These results reveal that PCR positivity can persist several weeks after treatment without evidence of viable sexual or asexual parasites, indicating that PCR may overestimate parasite prevalence after treatment.
Keywords: Malaria, Real-time PCR, msp2-PCR, Gametocyte, Traveler, Treatment
Highlights
-
•
PCR can remain positive several weeks after successful treatment of malaria with artemether-lumefantrine.
-
•
PCR-positivity could not be explained by circulating sub-microscopic asexual and sexual parasites.
-
•
The removal of dead parasites and their debris is not as rapid as it is believed.
The concept of rapid clearance of pathogens genetic material from circulation has long been accepted as a fact, with no systematic investigation to assess the actual duration of parasite-DNA persistence in the host, after pathogen is killed by immune response or medication. We observed that malaria parasite DNA remained detectable in blood, several weeks after infection is cleared, with no possible trace of viable parasite. Our finding highlights an important diagnostic matter not only in malaria but also in infectious diseases as whole; emphasizing that DNA-based detection method may underestimate drug efficiency in clinical management and trials.
1. Introduction
Microscopy has been the gold standard method for detection of malaria parasites for more than a century. Despite being a rather simple technique, sensitivity of this method depends on the experience of the microscopist as well as the quality of the microscope (Roth et al., 2016). Moreover, microscopy may fail to distinguish between Plasmodia species with similar morphology and often misses mixed infections (Di Santi et al., 2004). During the past decades, rapid diagnostic tests (RDTs) (Avila et al., 2002), immunoassays (Huong et al., 2002), and molecular methods have been increasingly used to detect malaria parasites. Several studies have compared the sensitivity and specificity of different techniques and showed that polymerase chain reaction (PCR) is the most sensitive method enabling more accurate identification of the parasite species especially in mixed infections. It also detects infections with parasite densities below the detection threshold of microscopy (sub-microscopic infections) (Barker et al., 1994, Lima et al., 2011, Okell et al., 2012, Tusting et al., 2014). Furthermore, real-time quantitative PCR (qPCR) has the potential to quantify the parasite density, is fast, and can be automated (Perandin et al., 2004). Despite the increasing number of studies reporting the advantages of PCR, the likelihood of PCR positive results due to amplification of DNA from dead parasites killed by treatment and/or host immune response has not been examined in depth. Assessments of post-treatment PCR positivity performed in malaria endemic areas are affected by the risk of re-infection as well as partial immunity in study populations, which may influence parasite clearance.
In this longitudinal study, we examined the duration of PCR positivity as well as the presence of gametocytes in travelers treated for Plasmodium falciparum malaria and followed up to 12 months in a malaria-free setting, using microscopy, species-specific qPCR, merozoite surface protein 2 (msp2)-genotyping PCR, and gametocyte-specific qPCR.
2. Methods
2.1. Study Population and Sample Collection
The study was performed on adults diagnosed with P. falciparum malaria (n = 36), enrolled in a malaria immunology study, at Karolinska University Hospital in Stockholm, Sweden. Patients with known HIV infection, planned visits to malaria endemic areas during the follow-up, or any other planned absence interfering with the sampling schedule, were not included in the study. Patients were treated according to the national guidelines for P. falciparum malaria with a full regimen of six doses of artemether-lumefantrine (AL) (20 mg/120 mg Riamet®), four tablets per dose at 0, 8, 24, 36, 48, and 60 h administered together with fatty meal or drink by a ward nurse as long as patients were hospitalized. Eight patients received one to three initial dose(s) of intravenous artesunate (2·4 mg/kg per dose) before a full course of AL due to hyperparasitaemia, of which three patients had also other signs of severe malaria (Supplementary Table 1).
Venous blood was collected in EDTA tubes at the time of admission, on consecutive days until discharge, after 10 days, as well as at one, three, six, and twelve months after treatment, according to a predefined protocol of an ongoing malaria immunology study. As a result of preliminary data on PCR positivity, Tempus™ Blood RNA tubes (Applied Biosystems) were added at each sampling occasion for gametocyte analysis, thus available only for a subset of patients (n = 12). The study was approved by the Ethical Review Board in Stockholm and informed consent was given by all participants.
2.2. Microscopy
Conventional light microscopy of Field's stained thin and thick smears was performed to detect and enumerate the parasites as proportion of infected erythrocytes expressed as percentage and as number of parasites per microliter (p/μl) of blood, assuming 5 × 106 erythrocytes per microliter whole blood.
2.3. Species-specific qPCR and msp2-genotyping PCR
DNA was extracted from 400 μl blood using a magnetic bead separation method with Hamilton Chemagic Star Robot® (Bonadouz, Switzerland). Plasmodium species-specific qPCR was carried out targeting the 18S rRNA gene (Shokoples et al., 2009) on a QuantStudio™ 5 Real-Time PCR System (Applied Biosystems). A threshold cycle (CT) value over 40 was considered negative. Parasite densities estimated by species-specific qPCR were calculated computing the ΔΔCT.
Genotyping of P. falciparum msp2 gene was carried out using nested PCR followed by capillary electrophoresis (Liljander et al., 2009). All PCR analyses were repeated twice and sample identity was blinded during experiments. The detection limit, defined by serial dilutions of positive controls, was 0·12 and 5 parasite/μl of whole blood for species-specific and msp2-PCR, respectively.
2.4. Gametocyte Culture and Preparation of Positive Control
P. falciparum gametocyte culture was set up according to standard procedure (Carter et al., 1993), and aliquots were collected in Tempus™ Blood RNA tubes for further RNA extraction and cDNA synthetization.
2.5. RNA Extraction and cDNA Synthetization
RNA was extracted from 500 μl of samples (blood and culture) collected in Tempus Blood RNA tubes using Stabilized Blood-to-CT™ Nucleic Acid Preparation Kit for qPCR (ThermoFisher Scientific) following the manufacturer instructions. cDNA was synthesized by reverse transcriptase (RT)-PCR using Superscript® Vilo™ cDNA Synthesis Kit (Invitrogen). TaqMan® GAPDH Assay (Applied Biosystems) was carried out to verify the synthetization of cDNA.
2.6. Detection of Male and Female Gametocytes
Sex-specific real-time qPCR was performed on synthesized cDNA to detect both male and female P. falciparum gametocytes (modified from Schneider P et al.) (Schneider et al., 2015). Primer and probe sequences as well as PCR condition are presented in Table 1. Using positive controls with known gametocytaemia, a detection limit of 1·5 and 0·5 gametocyte/μl of blood was estimated for female and male gametocytes, respectively.
Table 1.
P. falciparum gametocyte sex-specific primers and probes.
| Name | Sequence | Sex |
|---|---|---|
| Pfs230p-FW | 5′-CCCAACTAATCGAAGGGATGAA-3′ | Male |
| Pfs230p-RV | 5′-TTTGTTGTTCGATTCCAGTTGGT-3′ | Male |
| Pfs230p-Probe | VIC-AAACGATCAAACCATCTCA-MGB | Male |
| Pfs25-FW | 5′-TAAAATAGATGGAAATCCCGTTTC-3′ | Female |
| Pfs25-RV | 5′-TACCGTTACCACAAGTTACATTCTTAC-3′ | Female |
| Pfs25-Probe | FAM-ATGTAATCTTGGATATGATATGG-MGB | Female |
Real-time PCR was carried out separately for male and female gametocytes targeting sex-specific transcripts (Schneider et al., 2015). PCR reactions were run in 25 μl total volume containing cDNA, 1 × TaqMan® Multiplex Master Mix (ThermoFisher Scientific), 200 nM of each primers, and 150 nM of probes (Life Technologies) using similar programme (an initial denaturation step at 95 °C for 20 s followed by 45 cycles of 95 °C for 1 s and 60 °C for 20 s) for both assays.
2.7. Validation of Real-time PCR Results
Although following a well-established protocol (Shokoples et al., 2009) for multiplex species-specific qPCR, a subset of samples were verified in singleplex reactions adopted to detect only P. falciparum in order to exclude probable inter-species cross-binding of primers. In addition, a randomly selected subset of blood samples (n = 20), blinded to previous results and sample identity, were re-analyzed (from DNA extraction to PCR) by a second researcher in our laboratory. In addition, the same subgroup of samples was analyzed by the Department of Clinical Microbiology at Karolinska University Hospital, serving as reference laboratory for malaria PCR in Sweden. DNA was extracted from whole blood using the Universal Pathogen protocol on MagNA Pure 96 system (Roche diagnostics). Real-time PCR was performed following a modified protocol of Shokoples et al. (2009) and Divis et al. (2010) on a QuanStudio™ 6 Flex Real-Time PCR System (Applied Biosystems).
2.8. Statistical Analyses
Statistical analyses were performed using R statistical software [v3.2.2; lme4, lmer, glmer, Cox proportional hazards model (Cox-PHZ), rms, simr, powersurvEpi].
The duration of microscopy PCR positivity over time [time-to-becoming negative] was analyzed using the Cox-PHZ; with time of follow-up treated as the main covariate for evaluating the proportion of positive samples (by microscopy and species-specific qPCR) as the outcome, with patient ID incorporated as a random effect [frailty function].
The correlations between parasite density defined by microscopy before treatment and the number of positive days by microscopy and PCRs were assessed using a linear regression model [lm]. Moreover, logistic regression [GLMM: glmer] was used to define the estimated proportion of positivity by PCR within 10 days intervals during follow-up, with patient study ID as a random effect in this model [frailty function]. The correlation between the species-specific qPCR-CT value and microscopy-defined parasite densities (log) in microscopy positive samples was analyzed using linear regression model [lm], following a log-normal distribution.
Mixed effects linear model [lmer] was used to evaluate the effect of multiple covariates (sex, age, patient origin, number of years out of endemic area, and duration of fever before treatment) on days of PCR-positivity as the outcome.
The influence of parasite density, age, sex, country of origin, days of fever, previous treatment, recrudescence, and parasite clone number on presence of gametocytaemia was evaluated using logistic regression [GLMM: glmer].
In all GLMM models, backward elimination was used for sequential removal of non-significant variables, to obtain the minimal statistically-significant model. Additional details of analyses are presented in Supplementary Material.
3. Results
3.1. Study Population Characteristics
Thirty-six patients with P. falciparum malaria were enrolled in the study. The study participants, aged 21–70 years (median 39·5), were either of European (non-endemic) (n = 10) or African (endemic) origin (n = 26) living in Europe for 2–46 (median 15) years. Majority of patients were infected during visits to Sub-Saharan Africa. Patient characteristics including clinical presentations and treatment are presented in Supplementary Table 1.
Four patients had late treatment failure after three weeks and are reported separately. In addition, one patient had some uncertainty regarding slow response to the drug as well as incomplete DNA/RNA series, and was excluded from further analysis.
In patients with successful treatment (n = 31), availability of samples (slides, DNA and RNA) for the respective study participants at different time points are presented in Supplementary Table 2.
3.2. Detection of Parasites by Microscopy
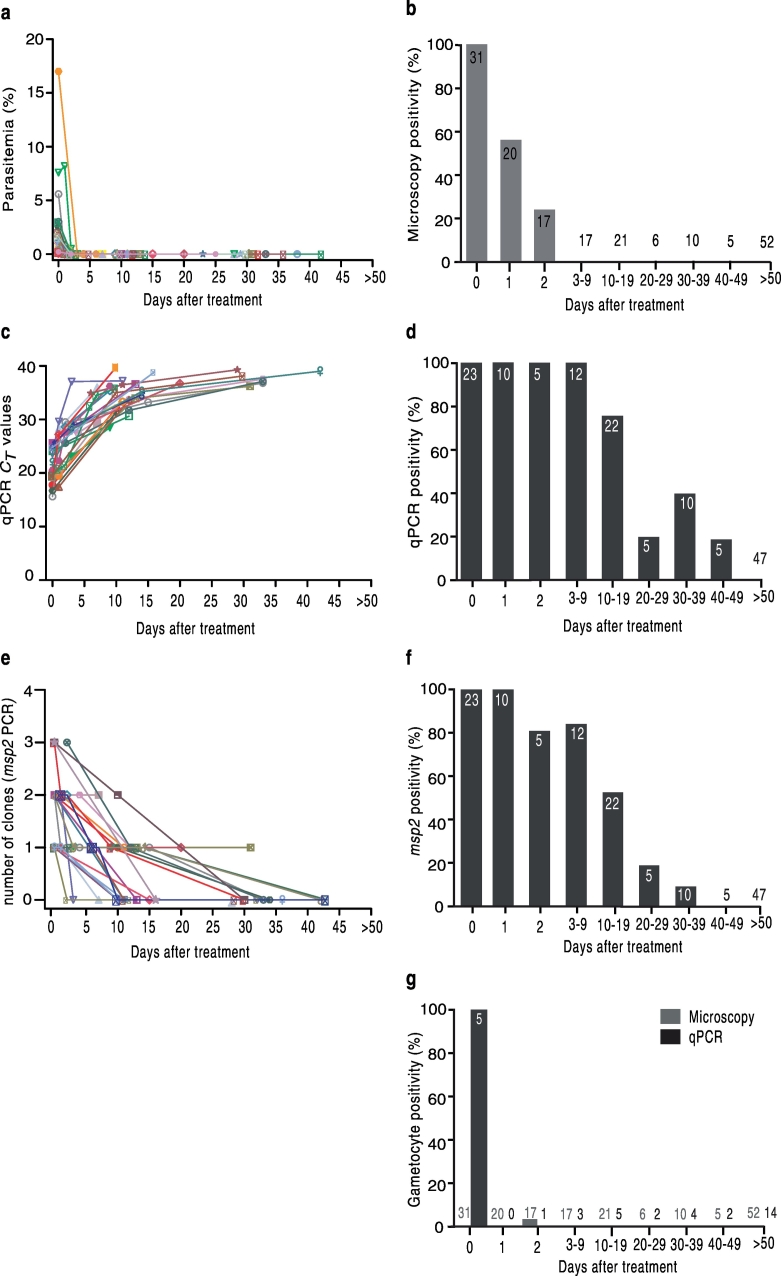
Microscopy-defined parasitemias were < 0·1–17% (median 0·95) at admission, with eight patients having parasitemias higher than 2% (corresponding to 100,000 p/μl of blood). Parasitemia declined rapidly after treatment initiation (Fig. 1a). Asexual parasites were detected by microscopy in 11/20 (55%) slides on day 1, and in 4/17 (23·5%) slides on day 2 after treatment (Fig. 1b). Gametocytes were detected by microscopy only in one patient on day 2 (Fig. 1g). On day 3 and onward, all slides were negative by microscopy (Fig. 1b, g).
Fig. 1.
Prevalence of P. falciparum parasite detected by microscopy and PCR in patients with successful treatment (n = 31).
a) Microscopy-defined parasitemias at different time points. Parasitemias declined rapidly during the first two days of treatment initiation. b) Proportion of microscopy positive individuals with available sample at different time-points. Asexual parasites were detected at the most two days after treatment. c) Species-specific qPCR-generated CT values (presented for all PCR positive samples) over time. qPCR remained positive up to 42 days after treatment. d) Proportion of individuals with species-specific qPCR positive samples over time. All samples collected during days 0–9 are positive by qPCR and thereafter in subsets of samples during different periods. e) Prevalence of infection and number of clones defined by msp2-genotyping PCR. The number of concurrent parasite clones decreased gradually over time. msp2-PCR remained positive the most until day 31 post-treatment. f) Proportion of individuals with positive msp2-PCR at different time points. g) Proportion of gametocyte positive samples by microscopy (gray bars) and gametocyte-specific qPCR (black bars). RNA samples were available for a total of 9 patients.
Symbols of the same shape and color (graph a, c, and e) correspond to the same patients in three graphs. Numbers on the bars and over the x-axes (graph b, d, f, and g) represent the total number of individuals with available sample at that time point.
3.3. Detection of Parasite DNA by Species-specific qPCR
Species identification by microscopy and species-specific qPCR agreed 100% and confirmed mono-infections by P. falciparum in all patients. Once treatment was initiated, CT values increased rapidly until day 5 and thereafter raised gradually until the cycle 40 (threshold limit) (Fig. 1c). The species-specific qPCR was positive in all samples available between day 0 and 9 after treatment (Fig. 1d). Thereafter, PCR remained positive in 17/22 (77%) of patients with samples available during days 10–19, in 1/5 (20%) patients during days 20–29, in 4/10 (40%) patients during days 30–39, and in 1/5 (20%) patients during days 40–49. All samples thereafter until one year after treatment were negative by species-specific qPCR (Fig. 1d). The species-specific qPCR results were verified by re-analyzing subsets of samples (n = 20) in different settings (See Methods), confirming positive and negative PCR results in all samples by another investigator in the same laboratory, and only one discordant result of a 30 day sample with CT value of 37·6 that was negative by the reference laboratory (data not shown).
3.4. msp2-PCR positivity and number of clones over time.
The msp2-genotyping PCR was positive in all (n = 23) available samples collected on day 0, with 1–3 (median 2) P. falciparum clones per individual. The number of clones decreased over time after treatment within the respective individuals (Fig. 1e). The msp2-PCR assay was positive in all individuals on day 1 and remained positive in 4/5 (80%) on day 2, in 10/12 (83%) on days 3–9, in 12/22 (54·5%) on days 10–19, in 1/5 (20%) on days 20–29, and the longest in one individual until day 31 after treatment (Fig. 1f). All follow-up samples thereafter, up to twelve months were negative by msp2-PCR.
3.5. Detection of Gametocytes by Sex-specific qPCR
One hundred and eighty-five slides from 31 patients, at different time-points after treatment were screened by microscopy and gametocytes were seen without asexual parasites in one patient on day 2. RNA tubes were introduced later in the study (see Methods) and thus 55 RNA samples were available from 12 patients in total. Three of these patients are amongst treatment failure cases. Thirty-seven RNA samples were available from nine patients with successful treatment (Supplementary Table 2). Gametocytes were detected by PCR in all available samples collected at the time of admission (5/5) (Fig. 1g). No gametocyte was found at later time points (days 2–389) in any patient (32 RNA samples from nine participants).
3.6. Modelling of Kinetics of Species-specific qPCR-Generated CT Values over Time
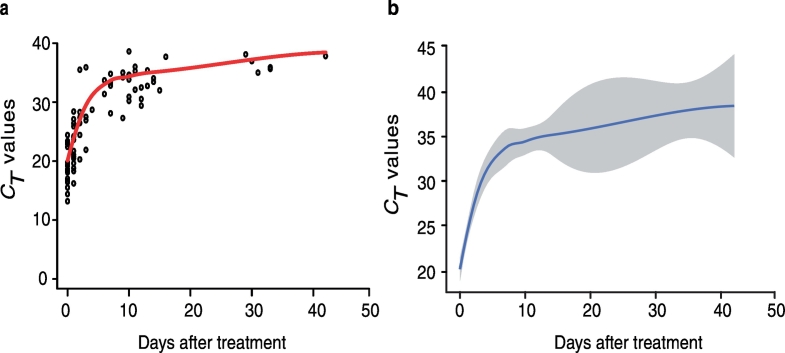
Mean CT value of available samples at different time points is presented in Fig. 2. Owing to long intervals between time points and incomplete sample series, it was not possible to determine the exact duration of PCR-positivity (i.e. the day that CT value reaches 40) in individual patient. Therefore, modelling of available data was performed to estimate the length of species-specific qPCR-positivity after treatment, which predicted 48 days for CT values to reach 40 [univariate Cox-PHZ with patient as random covariate, [HR = 0·75 (CI 95%: 0·69–0·79); p < 0·001]. In addition, a logistic regression model [glmer] for P. falciparum positivity with time modelled in 10 day strata, estimated that 56% (± 0·07% SE) of samples are positive by species-specific qPCR between days 20 and 29 and 26% (± 0·05% SE) remain positive during 30–39 days. Proportion of patients with positive microscopy and qPCR over time was also analyzed using Cox-PHZ, which showed that number of days for post-treatment qPCR-positivity are significantly higher than those estimated for microscopy [HR = 0·60 (CI 95%: 0·45–0·76), p < 0·001]. By microscopy, all samples (100%) are predicted to be negative by day 3 (Fig. 3).
Fig. 2.
Local regression fitting of CT value over time.
a) Empty circles represent individual samples at a certain time point and solid red line shows the overall uncertainty which is measured as how well the estimated curve fits the CT value. b) The solid blue line displays local weighted polynomial regression of CT value and shaded area shows the 95% confidence interval (CI) [using lm model, followed by loess function in ggplot2].
Fig. 3.
Predicted proportion of P. falciparum parasite positive samples by microscopy and species-specific qPCR, over time.
The shaded area shows 95% confidence interval (CI). Solid black line shows the model predicted probability of positive microscopy over time. The dotted line represents the model (Cox-HPZ) predicted probability of P. falciparum DNA being detected by species-specific qPCR.
3.7. Duration of PCR Positivity and CT Kinetics in Relation to Microscopy-defined Parasite Densities
Parasite densities defined by microscopy before treatment were not correlated to days of positivity by microscopy [F = 2·7; (df = 1,5); p = 0·10], msp2-PCR [F = 0·94; (df = 1,11); p = 0·35], and species-specific qPCR [F = 0·58; (df = 1,12); p = 0·45]. Although, the qPCR applied here is not absolute-, rather relative quantitative method, a significant correlation was noted between CT values and microscopy-defined parasite densities before and/or on the day treatment started [linear regression, lm, F = 6·28; (df = 20); R2 = 0·24; p = 0·02]. This association was lost for microscopy positive samples collected one day after treatment and onwards [F = 1·80; (df = 10); R2 = 0·15; p = 0·20] (Supplementary Fig. 1). In PCR positive samples that were negative by microscopy (n = 39), CT values ranged between 23·1–39·8, which according to our serial dilution of positive samples with known parasitemia estimates parasite densities of 0·12 p/μl (CT 39·8) and 25,500 p/μl (CT 23·1).
3.8. Duration of PCR-positivity in Relation to Patient Characteristics
The number of post-treatment PCR-positive days was evaluated in relation to patient characteristics using linear mixed effects model [lmer]. Individuals receiving initial artesunate became qPCR negative in a significantly longer time as compared to those receiving only AL [χ21 = 4·79; p = 0·005], however, these patients also had high initial parasitemias. The potential interaction could not be tested due to sample size. Patients of malaria-endemic origin (n = 24) became qPCR negative significantly faster than those born in Europe (n = 7) [χ21 = 6·29; p < 0·001]. Nonetheless, 2/4 (50%) of patients with long qPCR-positivity (days 30–39) were of African origin. Furthermore, the number of years living out of endemic area did not significantly affect the clearance time of parasite DNA (defined by PCR positivity) after treatment [χ21 = 1·09; p = 0·38]. The other analyzed characteristics of patients (sex, age, and duration of fever before treatment) had no significant effect on days of PCR-positivity and were not included as covariates in the final model (p > 0·05).
3.9. Dynamics of PCR-positivity in Patients With Treatment Failure
In the four patients with late treatment failure, asexual parasites were seen by microscopy latest two days after treatment initiation of their first episode, while gametocytes were seen without asexual parasites in one individual until day 6. One of the patients was enrolled in the study at the time of recrudescence and thus had no DNA and RNA samples during the first infection. Gametocytes were detected by PCR, in all other three individuals, until day 3 post-treatment and remained detectable in one patient until day 13. The very last samples collected before recrudescence (day 13 in one and day 14 in two patients) were positive by both species-specific qPCR and msp2-PCR. Patients sought care again due to fever between 19 and 29 days after the first treatment. After recrudescence and second treatment initiation, asexual parasites and gametocytes were seen by microscopy latest on day 2 and 4, respectively. PCR detected gametocytes at the most on day 4 as well. Species-specific qPCR and msp2-PCR were positive until days 33 and 12, respectively. Additional details about these treatment failures including clinical presentation and management, parasite genotypes, drug resistance markers, and plasma drug concentrations are presented elsewhere (Sonden et al., 2017).
3.10. Gametocytaemia in Relation to Patient Characteristics and Recrudescence
Despite the low number of individuals with RNA samples, owing to the importance of gametocytes in all aspects of malaria, possible correlation between gametocytaemia and patient characteristics was tested. These analyses were performed on pooling data from 14 patients including all successfully treated individuals with available RNA samples (n = 9), patients with recrudescence (n = 4), and patient with microscopy detected gametocyte but missing RNA sample (n = 1). A significant association between the presence of gametocytes and recrudescence was noted (χ21 = 3·79; p = 0·049). No other significant associations were found between gametocytaemia and country of origin, age, days of fever, and number of parasite clone, or parasite density at enrolment [all p > 0.08]. The presence of gametocytes, at any time point, had no effect on the duration of PCR positivity after treatment (n = 15; χ21 = 1·68; p = 0·19).
4. Discussion
Many studies have compared methods to detect and quantify malaria parasites and have established advantages of PCR, especially for low-density infections and species identification (Hanscheid and Grobusch, 2002, Lima et al., 2011, Okell et al., 2012). In addition, PCR based-genotyping of P. falciparum parasites is universally used to distinguish new infections from recrudescence in order to monitor the effect of antimalarial drugs in clinical trials (WHO, 2015b). Here we demonstrate that in a malaria-free area with no risk of re-infection, PCR can remain positive up to six weeks after curative treatment. Moreover, using RNA-based analysis in a subset of individuals, we show that the DNA detected weeks after treatment does not seem to be originated from circulating sub-microscopic gametocytes.
We found that according to microscopy, parasites were rapidly cleared by AL treatment (in some cases AL + artesunate), with a significant decline to 55% already one day after treatment and dropped further down to 23·5% on day 2. On day 3 after treatment, all samples were negative by microscopy for presence of both asexual parasites and gametocytes. These results are in line with another study on travelers where according to microscopy, complete clearance of asexual parasites and gametocytes were achieved by day three and seven, respectively, after a six-dose AL regimen (Hatz et al., 2008).
Using species-specific qPCR, we detected P. falciparum DNA up to day 42 after treatment was started. Since the sampling intervals did not allow determining the exact duration of PCR-positivity, a statistical model was carried out which predicted; that positive PCR could last in 26% of the samples at 40 days; and that 48 days was needed for CT values to reach 40 (negative threshold).
We additionally performed a PCR targeting msp2-gene, which is widely used as a genetic marker to characterise P. falciparum populations and to distinguish reinfection from recrudescence in drug efficacy studies. The msp2-PCR also remained positive in a subset of samples between days 20–39 after treatment, with a decreasing number of concurrent genotypes over time suggesting gradual clearance of the different infecting parasite clones. Detection of parasites with the same genotype longer than 28 days after treatment in drug trials following the 28-days protocol (WHO, 2015a), may consequently be misinterpreted as a treatment failure. Whether application of more sensitive methods such as ultrasensitive PCR (Hofmann et al., 2015, Imwong et al., 2014) would result in even longer duration of PCR-positivity post treatment, remains to be addressed in future studies.
In line with our findings, other studies on travelers have reported similar persistence of PCR positivity after curative treatment (Dakic et al., 2014, Phuong et al., 2015). A duration of positive microscopy and real-time qPCR up to five and 28 days, respectively, was observed in Serbia (Dakic et al., 2014). In addition, a study from Canada reports real-time PCR positivity up to 19 days after microscopy turned negative in successfully treated patients, and suggested the slow clearance of parasite DNA from the bloodstream, pointing out the risk of false positive PCR after treatment (Phuong et al., 2015). Moreover, PCR positivity up to 42 days post-treatment has been observed in endemic areas where neither recrudescence nor reinfection were considered as underlying cause for positive PCR, with no further explanation or investigations into the actual cause of the long PCR positivity (Aydin-Schmidt et al., 2013). All together, these reports suggest that occasional PCR positivity detected weeks after successful treatment might not be an unlikely event in malaria infections.
The higher sensitivity of PCR compared to microscopy in detection of both sexual and asexual parasites as well as prolonged survival of gametocytes after treatment (Bousema et al., 2010) could be possible reasons for significantly longer post-treatment PCR positivity as compared to microscopy. A Limit of Detection (LoD) of 5–100 p/μl is estimated for microscopy performed by microscopists with different levels of expertise using microscopes with various qualities (Roth et al., 2016). Here, species-specific qPCR generated CT values down to 23·1 in microscopy negative samples during follow up. Although, the applied qPCR method in this study is not absolute quantitative, low CT values reflecting parasite densities well above LoD of microscopy, cannot merely mirror sub-microscopic infections. Furthermore, using RNA-based analysis in a subset of samples, no gametocytes were detected already three days after successful treatment. These observations weaken the possibility of circulating sub-microscopic viable asexual and/or sexual parasites as underlying causes for persistent positive PCR in this study, and suggest the dead/severely-damaged parasites and/or their debris as a possible source of DNA detected by PCR weeks after curative treatment. Although a study in rodent malaria suggested that DNA from parasites killed by drug or immune response are rapidly removed from circulation (Jarra and Snounou, 1998), the clearance speed of malaria parasite DNA in humans has not been assessed. Here we estimated that it could take up to 48 days for drug-killed parasites and/or their DNA to be entirely removed from circulation or descend lower than PCR detection limit.
Duration of PCR positivity was correlated neither with the initial (asexual) parasite densities nor with the initial presence of gametocytes (defined by microscopy and PCR). Contrary to expectations, patients receiving initial intravenous artesunate, remained qPCR-positive significantly longer as compared to those treated with AL only. This effect could be owing to the sudden release of sequestered parasites killed by artesunate, into circulation, which may require longer time to clean up.
Patients of African origin cleared the infection faster as compared to those born and grown up in Europe, suggesting that previous exposure and partial immunity may speed up removal of parasite debris from circulation. However, number of years living out of endemic areas did not significantly affect the length of PCR-positivity. In addition, individuals with long PCR positivity (days 30–39) were also of endemic origins. Regardless of differences in earlier exposure between non- and semi-immune patients, inter-individual variation in PCR-positivity duration might partly result from diversity in the proportion of antibody classes/sub-classes, which in turn may contribute to evading the ingestion by host immune cells as well as stabilisation of parasite DNA in the form of immune complexes and thus delaying their clearance from circulation (Schifferli and Taylor, 1989).
A recent review on post-treatment gametocytaemia has reported that treatment with AL does not potentially promote the development of gametocytes and rather rapidly clears the pre-existing gametocytaemias (Group, 2016). In line with this, we observed that gametocytes were no longer detected by microscopy or PCR later than day 2 post AL treatment. It has also been shown that regardless of anti-malarial medicine, individuals experiencing treatment failure by day 28 were significantly more probable to be gametocytaemic at any time point during follow up (Group, 2016). In concordant with this, a significant correlation between gametocytaemia and recrudescence was observed in our study. However, in this small subset of patients, we did not find that gametocytaemia correlated with various host- and parasite-related factors such as age, country of origin, fever, as well as initial parasite density, observed in other studies (Group, 2016). This discrepancy could partly be due to the smaller size of our study population as well as variation in previous exposure and consequently different levels of immunity in one-time infected travelers and immigrants living years in malaria-free areas as compared to individuals living in malaria endemic areas.
Interestingly, persistence of positive PCR long after curative treatment has also been reported in sleeping sickness (Deborggraeve et al., 2011) and brucellosis (Marei et al., 2011), suggesting that slow clearance of pathogen debris is not a rare phenomenon. All together, these findings highlight the shortcoming of PCR to distinguish active infections from dead pathogens or their debris. Importantly, our results emphasize that real-time qPCR might underestimate drug efficiency in clinical management and trials, overestimate after-treatment parasite prevalence in epidemiological studies, and possibly misinterpret the results of other interventions. Nonetheless, our findings do not deny the advantage of real-time qPCR for diagnosis and quantification of initial parasite load before treatment, as in challenge studies of controlled human malaria infections (Kamau et al., 2014). It rather points out that the choice of detection method(s), e.g. microscopy or PCR, should be selected according to the purpose of analysis. Novel RNA-based methods, which allow for more accurate detection of viable parasites of both sexual and asexual forms (Tadesse et al., 2017), could be considered as the alternative approach in diagnosis of malaria parasite in clinical practice as well as in all area of malaria research. However, such advanced methods are less feasible and more costly to be routinely performed in all study settings.
Regardless of the underlying reason for vast inter-individual variation in clearance-time of parasite debris after treatment, this report underlines; an important diagnostic matter essentially in infectious diseases and particularly in malaria; the need for a detection tool as sensitive as PCR and as accurate as microscopy; and the necessity for further studies to uncover the actual cause of slow clearance of parasite debris.
Contributors
MVH was the main organizer of experiments and data analysis; carried out majority of experiments; compiled the data and drafted the manuscript. SNE cultured gametocytes; performed statistical analyses and modelling; contributed with result presentation and manuscript revision. VY was involved in organizing the follow-up and collection of clinical data. CS performed microscopy analysis. KS was involved in patient inclusion and follow up. HR and MK repeated the DNA extraction and real-time PCR for a subset of samples. MA designed the gametocyte-specific PCR; contributed with experiments, result preparation and manuscript revision. AF was the principle supervisor of the study; responsible for clinical supervision and treatment of the patients; critically revised the manuscript. All authors reviewed and approved the final report.
Declaration of Interests
We declare no competing interests.
Funding
This study was funded by; The Swedish Research Council (521-2012-3311 and 348-2013-6573) and Stockholm County Council (20130207), grants to Anna Färnert; and by The Swedish Society for Medical Research (SSMF), Stiftelsen Sigurd och Elsa Goljes Minne, and Karolinska Institutet Research Foundation, grants to Muhammad Asghar. The funders had no role in study design, data analysis, manuscript preparation or the decision for publication.
Acknowledgments
Acknowledgments
We are grateful to the patients for their invaluable participation in the study. We also wish to thank the clinicians and laboratory staff for assistance with patient recruitment, Ingrid Andrén for sample collection as well as Nicholas Duffin and Ulf Hammar for valuable comments on the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.10.003.
Appendix A. Supplementary data
Supplementary material
References
- Avila P.E., Kirchgatter K., Brunialti K.C., Oliveira A.M., Siciliano R.F., Di Santi S.M. Evaluation of a rapid dipstick test, Malar-Check, for the diagnosis of Plasmodium falciparum malaria in Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2002;44:293–296. doi: 10.1590/s0036-46652002000500012. [DOI] [PubMed] [Google Scholar]
- Aydin-Schmidt B., Mubi M., Morris U., Petzold M., Ngasala B.E., Premji Z., Bjorkman A., Martensson A. Usefulness of Plasmodium falciparum-specific rapid diagnostic tests for assessment of parasite clearance and detection of recurrent infections after artemisinin-based combination therapy. Malar. J. 2013;12:349. doi: 10.1186/1475-2875-12-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R.H., Jr., Banchongaksorn T., Courval J.M., Suwonkerd W., Rimwungtragoon K., Wirth D.F. Plasmodium falciparum and P. vivax: factors affecting sensitivity and specificity of PCR-based diagnosis of malaria. Exp. Parasitol. 1994;79:41–49. doi: 10.1006/expr.1994.1057. [DOI] [PubMed] [Google Scholar]
- Bousema T., Okell L., Shekalaghe S., Griffin J.T., Omar S., Sawa P., Sutherland C., Sauerwein R., Ghani A.C., Drakeley C. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Ranford-Cartwright L., Alano P. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol. Biol. 1993;21:67–88. doi: 10.1385/0-89603-239-6:67. [DOI] [PubMed] [Google Scholar]
- Dakic Z., Ivovic V., Pavlovic M., Lavadinovic L., Markovic M., Djurkovic-Djakovic O. Clinical significance of molecular methods in the diagnosis of imported malaria in returning travelers in Serbia. Int. J. Infect. Dis. 2014;29:24–30. doi: 10.1016/j.ijid.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Deborggraeve S., Lejon V., Ekangu R.A., Mumba Ngoyi D., Pati Pyana P., Ilunga M., Mulunda J.P., Buscher P. Diagnostic accuracy of PCR in gambiense sleeping sickness diagnosis, staging and post-treatment follow-up: a 2-year longitudinal study. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santi S.M., Kirchgatter K., Brunialti K.C., Oliveira A.M., Ferreira S.R., Boulos M. PCR — based diagnosis to evaluate the performance of malaria reference centers. Rev. Inst. Med. Trop. Sao Paulo. 2004;46:183–187. doi: 10.1590/s0036-46652004000400002. [DOI] [PubMed] [Google Scholar]
- Divis P.C., Shokoples S.E., Singh B., Yanow S.K. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar. J. 2010;9:344. doi: 10.1186/1475-2875-9-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, W.G.S Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med. 2016;14:79. doi: 10.1186/s12916-016-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanscheid T., Grobusch M.P. How useful is PCR in the diagnosis of malaria? Trends Parasitol. 2002;18:395–398. doi: 10.1016/s1471-4922(02)02348-6. [DOI] [PubMed] [Google Scholar]
- Hatz C., Soto J., Nothdurft H.D., Zoller T., Weitzel T., Loutan L., Bricaire F., Gay F., Burchard G.D., Andriano K. Treatment of acute uncomplicated falciparum malaria with artemether-lumefantrine in nonimmune populations: a safety, efficacy, and pharmacokinetic study. Am. J. Trop. Med. Hyg. 2008;78:241–247. [PubMed] [Google Scholar]
- Hofmann N., Mwingira F., Shekalaghe S., Robinson L.J., Mueller I., Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huong N.M., Davis T.M., Hewitt S., Huong N.V., Uyen T.T., Nhan D.H., Cong le D. Comparison of three antigen detection methods for diagnosis and therapeutic monitoring of malaria: a field study from southern Vietnam. Tropical Med. Int. Health. 2002;7:304–308. doi: 10.1046/j.1365-3156.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Imwong M., Hanchana S., Malleret B., Renia L., Day N.P., Dondorp A., Nosten F., Snounou G., White N.J. High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J. Clin. Microbiol. 2014;52:3303–3309. doi: 10.1128/JCM.01057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarra W., Snounou G. Only viable parasites are detected by PCR following clearance of rodent malarial infections by drug treatment or immune responses. Infect. Immun. 1998;66:3783–3787. doi: 10.1128/iai.66.8.3783-3787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau E., Alemayehu S., Feghali K.C., Komisar J., Regules J., Cowden J., Ockenhouse C.F. Measurement of parasitological data by quantitative real-time PCR from controlled human malaria infection trials at the Walter Reed Army Institute of Research. Malar. J. 2014;13:288. doi: 10.1186/1475-2875-13-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljander A., Wiklund L., Falk N., Kweku M., Martensson A., Felger I., Farnert A. Optimization and validation of multi-coloured capillary electrophoresis for genotyping of Plasmodium falciparum merozoite surface proteins (msp1 and 2) Malar. J. 2009;8:78. doi: 10.1186/1475-2875-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima G.F., Levi J.E., Geraldi M.P., Sanchez M.C., Segurado A.A., Hristov A.D., Inoue J., Costa-Nascimento Mde J., Di Santi S.M. Malaria diagnosis from pooled blood samples: comparative analysis of real-time PCR, nested PCR and immunoassay as a platform for the molecular and serological diagnosis of malaria on a large-scale. Mem. Inst. Oswaldo Cruz. 2011;106:691–700. doi: 10.1590/s0074-02762011000600008. [DOI] [PubMed] [Google Scholar]
- Marei A., Boghdadi G., Abdel-Hamed N., Hessin R., Abdoel T., Smits H., Fathey F. Laboratory diagnosis of human brucellosis in Egypt and persistence of the pathogen following treatment. J. Infect. Dev. Ctries. 2011;5:786–791. doi: 10.3855/jidc.1538. [DOI] [PubMed] [Google Scholar]
- Okell L.C., Bousema T., Griffin J.T., Ouedraogo A.L., Ghani A.C., Drakeley C.J. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat. Commun. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perandin F., Manca N., Calderaro A., Piccolo G., Galati L., Ricci L., Medici M.C., Arcangeletti M.C., Snounou G., Dettori G. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J. Clin. Microbiol. 2004;42:1214–1219. doi: 10.1128/JCM.42.3.1214-1219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong M., Lau R., Ralevski F., Boggild A.K. Survival analysis of diagnostic assays in Plasmodium falciparum malaria. Malar. J. 2015;14:350. doi: 10.1186/s12936-015-0882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J.M., Korevaar D.A., Leeflang M.M., Mens P.F. Molecular malaria diagnostics: a systematic review and meta-analysis. Crit. Rev. Clin. Lab. Sci. 2016;53:87–105. doi: 10.3109/10408363.2015.1084991. [DOI] [PubMed] [Google Scholar]
- Schifferli J.A., Taylor R.P. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989;35:993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- Schneider P., Reece S.E., van Schaijk B.C., Bousema T., Lanke K.H., Meaden C.S., Gadalla A., Ranford-Cartwright L.C., Babiker H.A. Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR. Mol. Biochem. Parasitol. 2015;199:29–33. doi: 10.1016/j.molbiopara.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Shokoples S.E., Ndao M., Kowalewska-Grochowska K., Yanow S.K. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J. Clin. Microbiol. 2009;47:975–980. doi: 10.1128/JCM.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonden K., Wyss K., Jovel I., Vieira da Silva A., Pohanka A., Asghar M., Homann M.V., Gustafsson L.L., Hellgren U., Farnert A. High rate of treatment failures in nonimmune travelers treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria in Sweden: retrospective comparative analysis of effectiveness and case series. Clin. Infect. Dis. 2017;64:199–206. doi: 10.1093/cid/ciw710. [DOI] [PubMed] [Google Scholar]
- Tadesse F.G., Lanke K., Nebie I., Schildkraut J.A., Goncalves B.P., Tiono A.B., Sauerwein R., Drakeley C., Bousema T., Rijpma S.R. Molecular markers for sensitive detection of Plasmodium falciparum asexual stage parasites and their application in a malaria clinical trial. Am. J. Trop. Med. Hyg. 2017;97:188–198. doi: 10.4269/ajtmh.16-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusting L.S., Bousema T., Smith D.L., Drakeley C. Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Adv. Parasitol. 2014;84:151–208. doi: 10.1016/B978-0-12-800099-1.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Third edition. 2015. Guidelines for the Treatment of Malaria.http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf (Accessed April 2015) [Google Scholar]
- World Health Organization Methods and Techniques for Clinical Trials on Antimalarial Drug Efficacy: Genotyping to Identify Parasite Populations 2008. 2015. http://www.who.int/malaria/publications/atoz/9789241596305/en/ (Accessed 29 June 2015)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material