Abstract
Background
A phase II study of methotrexate, etoposide, dexamethasone, and pegaspargase (MESA) sandwiched with radiotherapy for newly diagnosed, stage IE-IIE extranodal natural-killer/T-cell lymphoma, nasal-type (ENKTL) was conducted to explore its clinical efficacy and safety, as well as novel serum biomarkers upon anti-metabolic treatment.
Methods
Four cycles of MESA sandwiched with radiotherapy were administered. The primary end point was the overall response rate (ORR). Serum metabolomic profiles were assessed by liquid chromatography-mass spectrometry, with specific metabolites quantified by targeted metabolic analysis.
Findings
Forty patients were enrolled and the ORR was 92.1% (95%CI, 83.1%–100.0%). The 2-year progression-free survival (PFS) rate was 89.1% and overall survival (OS) rate was 92.0%. Grade 3/4 non-hematologic and hematologic toxicities were observed in 17 (42.5%) and 26 patients (65·0%) during chemotherapy, and in 9 (22.5%) and 0 (0.0%) patients during radiotherapy, respectively. Fifty-six significantly decreased and 59 increased metabolites were identified in ENKTL, as compared to healthy volunteers. A predictive principal components analysis model of asparaginase-associated metabolites, asparaginase-associated metabolic score (AspM), was established, including alanine, aspartate, glutamate, and succinic acid. Patients with high AspM score displayed superior survival and prognostic significance of AspM was validated in a historical cohort of early and advanced-stage ENKTL treated with asparaginase-based regimens. Multivariate analysis confirmed AspM as a prognostic score independent of PINK and PINK combined with Epstein-Barr virus DNA.
Interpretation
MESA sandwiched with radiotherapy is an effective and safe regimen for early-stage ENKTL. AspM score may be a promising prognostic index of serum metabolites in addition to clinical prognostic index in ENKTL.
Keywords: Extranodal natural-killer/T-cell lymphoma, nasal type; Asparaginase; Metabolomic profile; Prognosis
Highlights
-
•
MESA sandwiched with radiotherapy is an effective and safe regimen for early-stage ENKTL.
-
•
Asparaginase-associated metabolic score is a promising prognostic index of serum metabolites in ENKTL.
To explore regimen with more targeting effects and fewer toxicities, we conducted a trial of methotrexate, etoposide, dexamethasone, and pegaspargase (MESA) sandwiched with radiotherapy in newly diagnosed, stage IE-IIE extranodal natural-killer/T-cell lymphoma, nasal-type (ENKTL) and showed that MESA was highly effective and safe. Meanwhile, serum metabolomic profiles were studied to identify novel serum biomarkers. Asparaginase-associated metabolic score was established and could serve as a prognostic score independent of clinical prognostic index of ENKTL. Together, our study highlights the role of targeting metabolic aberrations in ENKTL and provides translational evidence of using serum metabolites in guiding risk stratification of this disease.
1. Introduction
Extranodal natural-killer/T-cell lymphoma, nasal-type (ENKTL) represents a distinct entity of extranodal non-Hodgkin lymphoma (NHL) and is prevalent in Asia. (Li et al., 2009) ENKTL is generally resistant to anthracycline-based chemotherapy and displays a highly aggressive behavior. (Tse and Kwong, 2013) Since nearly 75% of patients present with stage I or II disease within the nasal cavity and its adjacent sites, (Oshimi et al., 2005, Au et al., 2009) radiotherapy is routinely performed on early-stage ENKTL. (Yang et al., 2015) However, long-term survival for these patients treated with involved-field radiotherapy and conventional cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-like chemotherapy was not satisfactory, with overall response rate (ORR) and 5-year overall survival (OS) lower than 60% and 50%, respectively. (Yang et al., 2015, Kim et al., 2001, Cheung et al., 1998) Therefore, optimal chemotherapeutic regimen for early-stage ENKTL in combination with radiotherapy needs to be further investigated.
In the past decade, asparaginase-based regimens like dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE) and l-asparaginase, methotrexate, and dexamethasone (AspaMetDex) have significantly improved disease outcome. (Tse and Kwong, 2013) SMILE with sandwiched radiotherapy created a remarkable response rate reaching 90% in de novo ENKTL. (Kwong et al., 2012) Even in the refractory or relapsed setting, an ORR of 78% for AspaMetDex regimen was also achieved. (Jaccard et al., 2011) However, severe adverse events remain an obstacle in clinical practice. (Tse and Kwong, 2013, Kwong et al., 2012, Yamaguchi et al., 2011) Meanwhile, prognostic study was limited due to the rarity of the disease. International prognostic index (IPI), and newly established prognostic index of natural-killer lymphoma (PINK) and PINK in combination with peripheral blood Epstein-Barr virus (EBV) DNA (PINK-E) have been shown to predict disease outcome, especially in the era of anti-metabolic treatment. (Tse and Kwong, 2013, Kim et al., 2016) However, novel serum metabolic biomarkers have not been defined in ENKTL.
In the present study, a phase II trial of methotrexate, etoposide, dexamethasone, and pegaspargase (MESA) sandwiched with radiotherapy was conducted to evaluate its therapeutic efficacy and safety, in newly diagnosed, stage IE-IIE extranodal ENKTL (NCT02825147). Furthermore, serum biomarkers related to response to anti-metabolic treatment were assessed.
2. Patients and Methods
2.1. Eligibility Criteria
Inclusion criteria included: previously untreated ENKTL, nasal type, as defined by the World Health Organization classification; (Natkunam et al., 2008, Swerdlow et al., 2016) age 14 to 70 years old; Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2; stage IE-IIE; life expectancy > 6 months; informed consent was obtained from all subjects.
Exclusion criteria included: previous chemotherapy or bone marrow transplant; history of malignancy, excluding skin cancers or carcinomas in situ; uncontrollable cardio-cerebral vascular, coagulation, autoimmune, serious infectious disease (infection not progressing on therapy); left ventricular ejection fraction ≤ 50%; aminotransferase or aspartate aminotransferase levels > 3 upper limit of normal (ULN), alkaline phosphatase or bilirubin > 2.5 × ULN, creatinine > 1.5 × ULN; not able to comply to the protocol for mental or other unknown reasons; pregnancy or lactation; human immunodeficiency virus infection.
The study was approved by the Shanghai Rui Jin Hospital Review Board with informed consent obtained in accordance with the Declaration of Helsinki.
2.2. Treatment and Dose Modifications
The doses and administration schedule of MESA were as follows: methotrexate 1 g/m2 given intravenously on day one, etoposide 100 mg given intravenously on day two to four, dexamethasone given 40 mg intravenously on day two to four, pegaspargase given 2500 U/m2 intramuscular on day five. The first 20% of the methotrexate were given over 1 h and the rest 80% of the doses were given over the next 23 h. Leucovorin was given 12 h after finishing methotrexate for rescue. The concentration of methotrexate was monitored every day from day two until it came to normal level. Chemotherapy was repeated every 21 days with a total of four cycles. Granulocyte-colony stimulating factor (G-CSF) prophylaxis was not planned in the study until absolute neutrophil count is < 1.0 × 109/L.
Radiotherapy was sandwiched 21 days after two cycles of MESA for the involved local focus. All patients were treated with a linear accelerator using 6-MV photons. Intensity-modulated radiotherapy planning was performed for all patients based on the planning CT. Inverse planning with 7–9 coplanar radiotherapy fields was constructed. The clinical target volume (CTV) of limited stage IE disease included the nasal cavity, bilateral frontal ethmoid sinuses, and the ipsilateral maxillary sinus. For extensive stage IE disease, CTV was extended to the involved organs and/or tissues. For stage IIE disease, CTV also included the affected cervical lymph node area. The planning target volume included the CTV with a five-mm margin. The radiotherapy prescription was 50 Gy in 25 fractions, once a day, and 5 fractions every week. Radiotherapy was postponed until the toxicity was reduced to grade 2 if one or more of the following adverse events were observed: grade 4 leukopenia or neutropenia, grade 4 thrombocytopenia, any grade 3 non-hematologic toxicities except for mucositis or dysphagia related to radiation, and/or ECOG performance status ≥ 3. MESA chemotherapy was restarted 28 days after radiotherapy.
2.3. Evaluation
Baseline evaluations were performed within 14 days of start of therapy. Pretreatment clinical investigations included complete blood cell count (CBC), serum biochemistry with lactate dehydrogenase and amylase, EBV-DNA, HIV, hepatitis B virus (HBV), electrocardiogram, echocardiography, coagulation panel including APTT, PT, and fibrinogen, bone marrow aspiration and trephine biopsy, nasopharyngeal endoscopy, positron emission tomography-computed tomography (PET-CT), magnetic resonance imaging (MRI) of the head and neck. The cases were staged according to the Ann Arbor staging system. PET-CT along with MRI was repeated after 2 cycles of MESA for interim evaluation, as well as at the end of treatment for final evaluation. MRI, as well as CT of the neck, thorax, abdomen, and pelvis was repeated every three months thereafter to monitor disease progression.
IPI (International Non-Hodgkin's Lymphoma Prognostic Factors Project, 1993), PINK (Kim et al., 2016), and PINK-E (Kim et al., 2016) were calculated, as previously described. Treatment response was assessed according to standardized response criteria for NHL. (Cheson et al., 2007) The primary endpoint was ORR, which was calculated as the proportion of patients having response at the end of treatment. The secondary endpoints were progression-free survival (PFS) and OS, as well as toxicity, which were evaluated according to the National Cancer Institute Common Terminology Criteria of Adverse Events, version 4.0.
2.4. Metabolomic Profiling of ENKTL
Metabolomic assay was performed on serum samples of 24 patients on the basis of patients' consents for additional blood sample collection, with no selection of patients, and 14 healthy volunteers (age and sex matched) as the normal controls. Informed consent was obtained from all subjects. Cell lysate (100 μl) was mixed with 400 μl methanol. After incubation for 15 min at 4 °C, the mixture was centrifuged at 15000 r.p.m. for 15 min and 200 μl supernatant was used for liquid chromatography (LC)-mass spectrometry (MS) (Ultimate 3000LC, Orbitrap Elite Thermo, San Jose, CA). The semi-quantitative analysis using multiple reactions monitoring approach was performed on a Hypergod C18 (100 × 4.6 mm × 3 μm) column. Detailed parameters of LC-MS were described in Table S1. Metabolic data were analyzed by SIEVE (Thermo) and SIMCA-P software (Umetrics AB, Umeå, Sweden).
2.5. Quantification of Targeted Amino Acids and Succinate
Serum samples of ENKTL patients were pretreated before targeted metabolic analysis with high-performance LC-MS/MS (Shimadzu LC20AD, Kyoto, Japan and API 3200MD TRAP, Framingham, MA, USA). For amino acids assay, serum samples (50 μl) were pretreated with protein precipitation solution (50 μl). The mixture was centrifuged at 4 °C, 13200 r.p.m. for 4 min. Supernatant (10 μl) was further mixed with binding buffer (50 μl) and derivative solution (20 μl) to derivate at room temperature for 15 min. For succinate assay, serum sample (100 μl) was premixed with acetonitrile (300 μl) and vortexed for 5 h. Detailed parameters of LC and MS analysis were described in Table S2. The standard curves were established to calculate the absolute concentrations.
2.6. Statistical Analysis
Previous studies showed that the ORR for SMILE with radiotherapy for untreated, localized nasal early-stage ENKTL was approximately 90% (Kwong et al., 2012) and radiotherapy of 50 Gy alone led to an ORR of about 78% (Cheung et al., 2002). If the true ORR for MESA is 90% or higher, a sample size of 40 will achieve about 80% power to show whether the lower bound of 95% confidence interval of the ORR will be no < 78% using a one-sided binomial test. The target significance level is 0.10 in sample size estimation.
χ2 and Fisher's exact tests were applied for non-ordinal categoric variables, and Wilcoxon's rank-sum test was applied for comparison of ordinal variables. Independent sample t-test or Mann-Whitney U test was applied, as appropriate, for numerical variables. Survival estimates were calculated using the Kaplan-Meier method and survival curves were compared by the log-rank test. Univariate analysis and multivariable hazard estimates were performed with Cox proportional hazards models. The contributions of the targeted metabolites were analyzed by principal component analysis (PCA). A two-sided P value of < 0.05 was considered significant. All analyses were performed using SPSS Statistics, version 20.0, software (SPSS, Chicago, IL, USA).
3. Results
3.1. Patient Characteristics
A total of 40 patients were enrolled in this study from September 2013 to October 2015, and referred as the training group in the establishment of asparaginase-associated prognostic system. The clinical characteristics of the patients were summarized in Table 1. The median age was 49 years. In terms of clinical prognostic index, 87.5% and 12.5% of the patients were categorized as low and low/intermediate risk by IPI, 45.0%, 50.0%, and 5.0% as low, intermediate, and high risk by PINK, 80.0% and 20.0% as low and intermediate risk by PINK-E. All the patients were cytoplasmic CD3 +, CD20 −, CD56 +, positive for cytotoxic molecules, and positive for EBV by fluorescence in situ hybridization. Thirty patients had Ki-67 > 50%.
Table 1.
Characteristics of the ENKTL patients and univariate analysis of predictors of progression-free survival (PFS) and overall survival (OS) in the validation group.
| Characteristics | Training group |
Validation group |
P valuea | P valueb | P value for PFS | P value for OS | |
|---|---|---|---|---|---|---|---|
| Stage I/II (n = 40) | Stage I/II (n = 39) | Stage III/IV (n = 24) | |||||
| Sex | |||||||
| Male | 33/40 (82.5%) | 33/39 (84.6%) | 16/24 (66.7%) | 1.000 | 0.223 | 0.760 | 0.936 |
| Female | 7/40 (17.5%) | 6/39 (15.4%) | 8/24 (33.3%) | ||||
| Age (years) | |||||||
| ≤ 60 | 34/40 (85.0%) | 32/39 (82.1%) | 17/24 (70.8%) | 0.770 | 0.208 | 0.096 | 0.111 |
| > 60 | 6/40 (15.0%) | 7/39 (17.9%) | 7/24 (29.2%) | ||||
| Performance status (ECOG) | |||||||
| 0–1 | 37/40 (92.5%) | 37/39 (94.9%) | 10/24 (41.7%) | 1.000 | < 0.001 | 0.002 | < 0.001 |
| 2 | 3/40 (7.5%) | 2/39 (5.1%) | 14/24 (58.3%) | ||||
| Lactic dehydrogenase | |||||||
| Normal | 25/40 (62.5%) | 22/39 (56.4%) | 10/24 (41.7%) | 0.650 | 0.126 | < 0.001 | 0.001 |
| Elevated | 15/40 (37.5%) | 17/39 (43.6%) | 14/24 (58.3%) | ||||
| B symptom | |||||||
| Yes | 24/40 (60.0%) | 24/39 (61.5%) | 19/24 (79.2%) | 1.000 | 0.170 | 0.757 | 0.977 |
| No | 16/40 (40.0%) | 15/39 (38.5%) | 5/24 (20.8%) | ||||
| Distant lymph node involvement | |||||||
| Yes | 18/40 (45.0%) | 17/39 (43.6%) | 14/24 (58.3%) | 1.000 | 0.439 | 0.627 | 0.406 |
| No | 22/40 (55.0%) | 22/39 (56.4%) | 10/24 (41.7%) | ||||
| Epstein-Barr Virus DNA (copies/ml) | |||||||
| ≥ 1000 | 9/40 (22.5%) | 12/39 (30.8%) | 12/24 (50.0%) | 0.453 | 0.030 | 0.003 | 0.001 |
| < 1000 | 31/40 (77.5%) | 27/39 (69.2%) | 12/24 (50.0%) | ||||
| International prognostic index (IPI) | |||||||
| Low risk (0–1) | 35/40 (87.5%) | 34/39 (87.2%) | 3/24 (12.5%) | 0.966 | < 0.001 | < 0.001 | < 0.001 |
| Low/intermediate risk (2) | 5/40 (12.5%) | 5/39 (12.8%) | 4/24 (16.7%) | ||||
| Intermediate/high risk (3) | 0/40 (0%) | 0/39 (0%) | 7/24 (29.2%) | ||||
| High risk (4–5) | 0/40 (0%) | 0/39 (0%) | 10/24 (41.7%) | ||||
| Prognostic index of natural-killer lymphoma (PINK) | |||||||
| Low risk (0) | 18/40 (45.0%) | 17/39 (43.6%) | 0/24 (0%) | 0.903 | < 0.001 | < 0.001 | < 0.001 |
| Intermediate risk (1) | 20/40 (50.0%) | 20/39 (51.3%) | 2/24 (8.3%) | ||||
| High risk (2–4) | 2/40 (5.0%) | 2/39 (5.1%) | 22/24 (91.7%) | ||||
| Prognostic index of natural-killer lymphoma- Epstein-Barr Virus (PINK-E) | |||||||
| Low risk (0–1) | 32/40 (80.0%) | 30/39 (76.9%) | 1/24 (4.2%) | 0.741 | < 0.001 | < 0.001 | < 0.001 |
| Intermediate risk (2) | 8/40 (20.0%) | 9/39 (23.1%) | 5/24 (20.8%) | ||||
| High risk (3–5) | 0/40 (0%) | 0/39 (0%) | 18/24 (75.0%) | ||||
| Treatment | |||||||
| MESA | 40/40 (100%) | 8/24 (33.3%) | < 0.001 | < 0.001 | |||
| CHOPE-L | 16/39 (41.0%) | 5/24 (20.8%) | |||||
| Hyper-CVAD-L | 13/39 (33.3%) | ||||||
| ESA | 10/39 (25.6%) | ||||||
| SMILE | 8/24 (33.3%) | ||||||
| GLIDE | 3/24 (12.5%) | ||||||
| Complete response | |||||||
| Yes | 34/38 (89.4%) | 32/39 (82.1%) | 11/24 (45.8%) | 0.517 | < 0.001 | < 0.001 | < 0.001 |
| No | 4/38 (10.5%) | 7/39 (17.9%) | 13/24 (54.2%) | ||||
| Asparagine-associated metabolic (AspM) score (μg/mL) | |||||||
| ≥ 209 | 12/24 (50.0%) | 17/39 (43.6%) | 13/24 (54.2%) | 0.796 | 1.000 | 0.044 | 0.048 |
| < 209 | 12/24 (50.0%) | 22/39 (53.8%) | 11/24 (45.8%) | ||||
ECOG, Eastern Cooperative Oncology Group.
CHOPE-L: cyclophosphamide, doxorubicin, vincristine, prednisone, etoposide, and pegaspargase.
Hyper-CVAD-L: cyclophosphamide, vincristine, doxorubicin,dexamethasone, and pegaspargase (course A); methotrexate and cytarabine (course B).
ESA: etoposide, dexamethasone, and pegaspargase.
SMILE: methotrexate, ifosfamide, dexamethasone, etoposide, and pegaspargase.
GLIDE: gemcitabine, pegaspargase, ifosfamide, dexamethasone, and etoposide.
P value indicated difference between training group and stage I/II validation group.
P value indicated difference between training group and stage III/IV validation group.
In this cohort, HBV surface antigen or HBV-DNA was all negative and no prophylactic anti-viral agent was given to patients with positive HBV surface/core antibody. However, serum HBV serology and HBV-DNA were monitored. No viral activation was observed during treatment and follow-up.
3.2. Dose Intensity
As shown in Fig. 1, eighty-five percent (35/40) of patients received all four cycles of MESA sandwiched with radiotherapy. Early withdrawal occurred in two cases. One patient experienced grade 3 pancreatitis, hyperbilirubinemia and AST/ALT elevation after cycle 1 and was changed to CHOPE (cyclophosphamide, doxorubicin, vincristine, prednisone and etoposide) followed by radiotherapy. Another patient experienced grade 3 neutropenia, refused chemotherapy after cycle 1, and switched to radiotherapy. Disease progression or death occurred in another three cases.
Fig. 1.
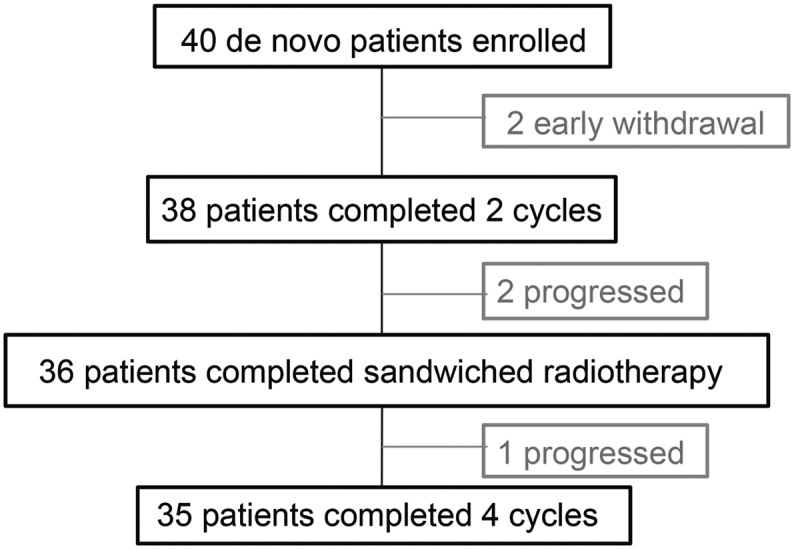
Flow chart of the study.
ENKTL: extranodal natural-killer/T-Cell lymphoma, nasal-type, MESA: methotrexate, etoposide, dexamethasone, and pegaspargase.
Forty patients received 148 cycles of protocol treatment, among which thirty-six cases received full radiotherapy dose. Five patients experienced chemotherapy dose reduction, among which two cases eliminated methotrexate for serious mucositis and skin infection, and three patients eliminated pegaspargase (one early withdrawal as mentioned above, two changed to methotrexate, etoposide and dexamethasone) for grade 3 pancreatitis. There was no treatment related death.
3.3. Response and Survival
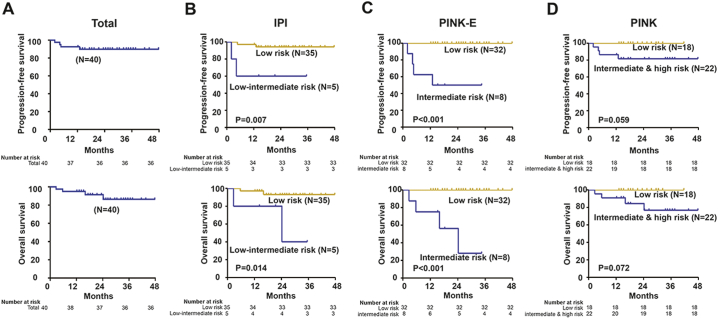
The two early-withdrawal patients achieved CR after treatment without disease progression or death. Among the remaining 38 patients, in the interim evaluation, the ORR was 92.1% (35/38, 95%CI, 83.1%–100.0%) with 71.1% (27/38, 95%CI, 57.9%–84.2%) achieving complete remission (CR), and in the final evaluation, the ORR was still 92.1% (35/38, 95%CI, 83.1%–100.0%) with 89.5% (34/38, 95%CI, 79.3%–99.7%) achieving CR. Three cases had disease progression during the treatment and another one case progressed 6 months after achieving CR. These four refractory/relapsed patients were defined as poor prognosis group, while the rest of the patients in remission were defined as good prognosis group in the following metabolomic study. Median follow-up of the patients was 24.5 months (range, 2.0 to 38.4 months). On an intent to treat analysis, the PFS and OS rate of the 40 patients was 89.1% and 92.0% at 24 months (Fig. 2A). IPI and PINK-E were both significantly associated with PFS and OS (Fig. 2B and C). PINK was of marginal prognostic significance (Fig. 2D).
Fig. 2.
Progression-free survival (PFS) and overall survival (OS) curves of patients with ENKTL treated by MESA.
(A) PFS and OS of the patients; (B) PFS and OS according to international prognostic index (IPI); (C) PFS and OS according to prognostic index of natural-killer lymphoma-Epstein-Barr Virus (PINK-E); (D) PFS and OS according to prognostic index of natural-killer lymphoma (PINK).
3.4. Toxicity
Hematologic and non-hematologic toxicities during treatment were summarized in Table 2. CBC was monitored weekly for all treatment cycles.
Table 2.
Toxicities of the major regimens in treating ENKTL.
| SMILE (ref. 8) Kwong et al. | SMILE (ref. 10) Yamaguchi M et al. | AspMetDex (ref. 9) Jaccard A et al. | RT-2/3DeVIC (ref. 26) Yamaguchi M et al. | CCRT-VIPD (ref. 27) Kim et al. | CCRT-VIPL (ref. 28) Kim et al. | MESA Xu et al. | |
|---|---|---|---|---|---|---|---|
| Patients | Newly diagnosed or refractory/relapsed, any stage | Newly diagnosed stage IV, relapsed/refractory | Relapsed/refractory, any stage | Newly diagnosed stage IE to IIE | Newly diagnosed stage IE to IIE | Newly diagnosed stage IE to IIE | Newly diagnosed stage IE to IIE |
| Number | 87 | 38 | 19 | 33 | 30 | 30 | 40 |
| Regimen | |||||||
| Methotrexate | 2000 mg/m2 day 1 | 2000 mg/m2 day 1 | 3000 mg/m2 day 1 | 1000 mg/m2 day 1 | |||
| Etoposide: | 100 mg/m2 days 2–4 | 100 mg/m2 days 2–4 | Level 1: 67 mg/m2 days 1–3 Level 2: 100 mg/m2 days 1–3 |
100 mg/m2 days 1–3 | 100 mg/m2 days 1–3 | 100 mg days 2–4 | |
| Dexamethasone | 40 mg days 2–4 | 40 mg days 2–4 | 40 mg days 1–4 | 40 mg days 1–3 | 40 mg days 1–4 | 40 mg days 1–3 | 40 mg days 2–4 |
| l-Asparaginase | 6000 U/m2 days 8, 10, 12, 14, 16, 18, and 20 | 6000 U/m2 days 8, 10, 12, 14, 16, 18, and 20 | 6000 U/m2 days 2, 4, 6, and 8 | 4000 U/m2 days 8, 10, 12, 14, 16, 18, and 20 | Pegaspargase 2500 U/m2 day 4 | ||
| Ifosfamide | 1500 mg/m2 days 2–4 | 1500 mg/m2 days 2–4 | Level 1: 1000 mg/m2 days 1–3 Level 2: 1500 mg/m2 days 1–3 |
1200 mg/m2 days 1–3 | 1200 mg/m2 days 1–3 | ||
| Carboplatin | Level 1:200 mg/m2 day 1 Level 2: 300 mg/m2 day 1 |
||||||
| Cisplatin | 30 mg/m2 weekly during CCRT; 33 mg/m2 days 1–3 after CCRT | 30 mg/m2 weekly for 4 weeks during CCRT | |||||
| G-CSF prophylaxis | Yes | Yes | Yes | No | No | No | No |
| Anti-infection prophylaxis | Yes | Yes | Yes | NR | NR | NR | No |
| Hematological toxicity | |||||||
| Neutropenia | 66.7% (58 cases) | 100.0% (38 cases) | 42.1% (8 cases) | 90.9% (30 cases) | 46.7% (14 cases) | Leukopenia: 80.0% (24 cases) | 52.5% (21 cases) |
| Thrombocytopenia | 41.4% (36 cases) | 63.2% (24 cases) | 5.3% (1 case) | 18.2% (6 cases) | 23.3% (7 cases) | 13.3% (4 cases) | 7.5% (3 cases) |
| Anemia | NR | 50.0% (19 cases) | 21.1% (4 cases) | 24.2% (8 cases) | 26.7% (8 cases) | 10.0% (3 cases) | 12.5% (5 cases) |
| Non-hematological toxicity | |||||||
| Nausea | NR | 13.2% (5 cases) | NR | NR | 3.3% (1 case) | 10.0% (3 cases) | 10.0% (4 cases) |
| Vomiting | NR | 13.2% (5 cases) | NR | NR | 3.3% (1 case) | 0% | 10.0% (4 cases) |
| Diarrhea | NR | 10.5% (4 cases) | NR | NR | 0% | 0% | 0% |
| Hepatotoxicity | 6.9% (6 cases) | Hypoalbuminemia 15.8% (6 cases) Hyperbilirubinemia 10.5% (4 cases) AST elevation 31.6% (12 cases) ALT elevation 31.6% (12 cases) |
15.8% (3 cases) | NR | NR | Transaminase elevation: 10.0% (3 cases) | Hyperbilirubinemia: 10.0% (4 cases) AST/ALT elevation: 7.5% (3 cases) |
| Nephrotoxicity | 1.1% (1 case) | 5.3% (2 cases) | 0% | NR | NR | NR | 0% |
| Allergy | 1.1% (1 case) | 2 allergies, grading unknown | 5.3% (1 case) | NR | NR | NR | 0% |
| Infection | 31% (27 cases) for serious infection, grading unknown | 60.5% (23 cases) | 10.5% (2 cases) | Infection with grade 3 or 4 neutropenia: 12.1% (4 cases); Infection without neutropenia: 6.1% (2 cases) |
Two deaths were associated with infection | NR | 15.0% (6 cases) |
| Mucositis | NR | 13.2% (5 cases) | NR | Mucositis related to radiation: 36.4% (12 cases) | Stomatitis: 0% | Stomatitis during CCRT: 16.7% (5 cases) Stomatitis during chemotherapy: 21.4% (6 cases) |
Mucositis related to radiation:22.5% (9 cases) Mucositis related to chemotherapy: 10.0% (4 cases) |
| Amylase | NR | 18.4% (7 cases) | NR | NR | NR | NR | 7.5% (3 cases, all grade 3 pancreatitis) |
G-CSF, granulocyte-colony stimulating factor.
NR: not reported.
RT: radiotherapy.
CCRT: concurrent chemoradiotherapy.
During the chemotherapy, grade 3/4 hematologic toxicities were observed in 26 patients (65.0%), with grade 4 adverse events in 14 cases (35.0%). Grade 3/4 neutropenia were present in 21 cases (52.5%), who were given G-CSF support. Grade 3/4 thrombocytopenia did not occur. For non-hematologic toxicities, grade 3 events were observed in 16 cases (40.0%), and grade 4 events were observed in one case (2.5%), who experienced sepsis. During the radiotherapy, no grade 3/4 hematologic toxicities were observed, but 9 patients presented grade 3 mucositis (Table 2).
3.5. Asparaginase-Associated Metabolic (AspM) Score
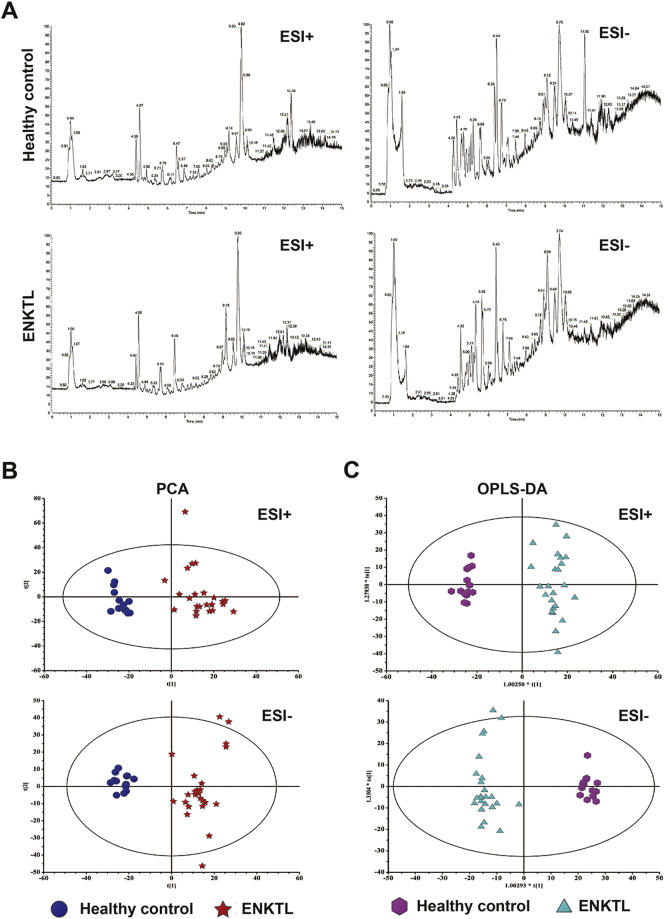
Considering that asparaginase and methotrexate are both anti-metabolic agents, we assessed serum metabolomic profile to screen potential metabolic biomarkers for risk stratification in ENKTL. Representative total ion chromatogram of ENKTL apparently differed from that of healthy volunteers (Supplemental Fig. 1A). PCA score plots were analyzed on 4120 features obtained after normalization, including 2081 spectral features for electrosprary ionization (ESI) + and 2039 features for ESI-, and showed a separating trend between ENKTL and healthy volunteers (Supplemental Fig. 1B). Orthogonal partial least-squares-discrimination analysis (OPLS-DA) models (Supplemental Fig. 1C), whose reliability was validated by response permutation test, revealed satisfactory modeling and predictive ability with one predictive component and one orthogonal components (R2X = 0.293, R2Y = 0.962, and Q2Y = 0.926) for ESI +, one predictive component and one orthogonal components (R2X = 0.248, R2Y = 0.971, and Q2Y = 0.916) for ESI −, respectively.
Supplemental Fig. 1.
Metabolomic profile in patients with ENKTL. (A) Representative base peak intensity chromatograms of the ENKTL patients and the healthy volunteers detected by liquid chromatography-mass spectrometry under electrosprary ionization (ESI) + and ESI − mode. Different peaks were marked by arrows. (B) The score plots of the principal component analysis model and orthogonal projections to latent structures discriminant analysis model set up using data under ESI + and ESI − mode discriminated the ENKTL group (red plots) from the healthy volunteers (dark blue plots). (C) The orthogonal projections to latent structures discriminant analysis model set up using data under ESI + and ESI − mode discriminated the ENKTL group (light blue plots) from the healthy volunteers (purple plots).
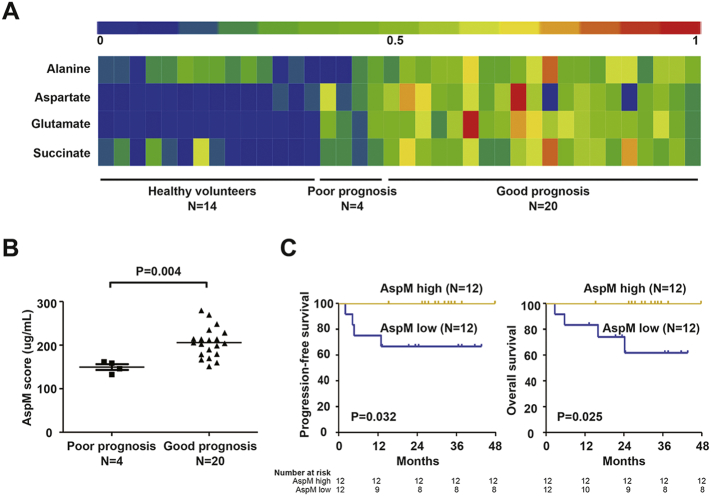
Significantly altered serum metabolites with the variable importance in projection threshold > 1 in the above OPLS-DA models were selected, showing 56 metabolites lower (abundance ratio < 1) and 59 higher (abundance ratio > 1) than healthy volunteers (P < 0.05). A total of 9 asparaginase-associated metabolites (alanine, aspartic acid, malic acid, ornithine, glutamate, glutamine, histidine, pantothenic acid, and succinic acid) were detected in the study to be differently expressed, as compared to healthy controls. Four of them (alanine, aspartate, glutamate, and succinic acid) formed a distinct asparaginase-associated metabolic signature and the expression of this panel was significantly increased in MESA-treated patients with good prognosis, compared with those with poor prognosis and healthy volunteers (Fig. 3A, software HemI 1.0.3.3(Deng et al., 2014)).
Fig. 3.
Asparaginase-associated metabolic (AspM) score in ENKTL.
(A) Heatmap showed four differentially expressed serum metabolites involved in asparaginase-associated metabolism in ENKTL with good and poor prognosis, as compared to healthy control. (B) Patients with good prognosis displayed higher AspM score than those with poor prognosis. (C) Progression-free survival and overall survival curves of patients according to the AspM score.
Targeted metabolic analysis was subsequently performed to quantify absolute serum concentration of these four metabolites in the training group. The patients with good prognosis showed significantly higher expression of these four metabolites than those with poor prognosis (P = 0.004, Fig. 3B). A predictive PCA model, AspM score, was fitted using the above four metabolites. The median of AspM based on the value from PCA model (Table 3) was 209 μg/ml in ENKTL. The patients with AspM score over the median were regarded as high AspM group, whereas those below the median were included in the low AspM group. The 2-year PFS and OS rate in high AspM group were 100% and 100%, as compared to 66.7% and 74.1% in low AspM group (P = 0.032 and P = 0.025, Fig. 3C).
Table 3.
A panel of 4 metabolites with prognostic value for ENKTL.
| Regression weighta | Median, range, μg/mL | |
|---|---|---|
| Alanine | 0.758 | 67.441 (27.439–201.343) |
| Aspartic acid | 0.212 | 14.002 (4.259–48.182) |
| Glutamate | 0.857 | 57.086 (18.656–133.300) |
| Succinic acid | 0.740 | 119.600 (15.000–306.800) |
AspM score of each sample is calculated according to followed equation: 0.758 ∗ alanine + 0.212 ∗ aspartic acid + 0.857 ∗ + 0.740 ∗ succinic acid.
The weight of the first principal component in PCA model.
3.6. Validation of AspM Score in a Historical Cohort of ENKTL
Prognostic significance of AspM score was further verified in a validation cohort of 63 de novo ENKTL patients from May 2011 to September 2016 receiving asparaginase-based treatment with available peripheral blood samples (Table 1). Thirty-nine patients had early-stage disease, while 24 patients were in advanced-stage. For the early-stage, male gender was predominant with the median age of 45. The distribution of patients according to IPI, PINK, and PINK-E were similar to the training group. For the advanced-stage group, significantly poor performance status and high EBV load were present, as compared to the training group (P < 0.001 and P = 0.030), leading to the higher proportion of high-risk patients in IPI, PINK and PINK-E (P all < 0.001).
The treatment in validation cohort were not unified, but all including pegaspargase. For the early-stage patients, chemotherapeutic regimens included CHOPE-L (cyclophosphamide, doxorubicin, vincristine, prednisone, etoposide, and pegaspargase, from 2011 to 2013), Hyper-CVAD-L (course A: cyclophosphamide, vincristine, doxorubicin, dexamethasone, and pegaspargase; course B: methotrexate and cytarabine, from 2011 to 2013), ESA (etoposide, dexamethasone, and pegaspargase, from 2015 to 2016). For the advanced-stage group, the regimens were CHOPE-L (from 2011 to 2013), SMILE (from 2014 to 2015), MESA (from 2015 to 2016), and GLIDE (gemcitabine, pegaspargase, ifosfamide, dexamethasone, and etoposide, 2016). The treatment response in the advanced-stage group was much lower than those of the early-stage group (P < 0.001). The median follow-up of early and advanced-stage patients was 26.5 and 11.7 months, respectively.
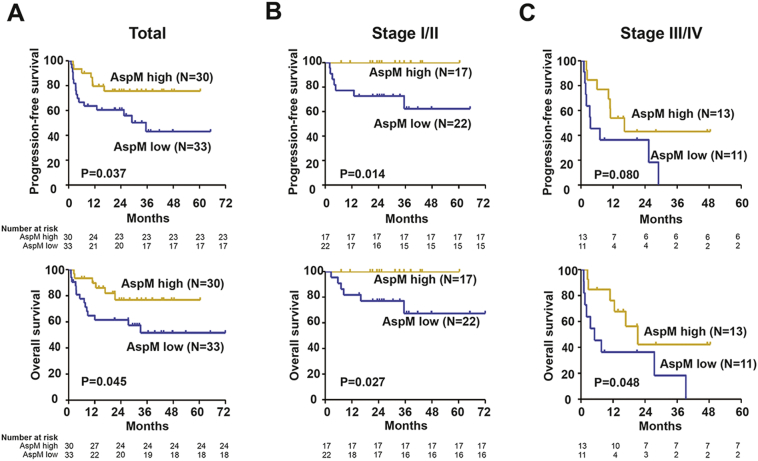
The distribution of AspM score (cut-off value: 209 μg/ml) was not varied between the training and validation sets. Patients with low AspM suffered from remarkably short survival time than those with high AspM expression. The 2-year PFS and OS for the validation group with high AspM score were 75.6% and 77.0%, as compared to 60.5% and 63.3% for those with low AspM score (P = 0.037 and P = 0.045, Fig. 4A).
Fig. 4.
Progression-free survival (PFS) and overall survival (OS) curves of the validation cohort of ENKTL.
(A) PFS and OS according to asparaginase-associated metabolic (AspM) score in all the patients; (B) PFS and OS according to the AspM score in early-stage patients; (C) PFS and OS according to the AspM score in advanced-stage patients.
Within validation set, AspM displayed prognostic capability in both early-stage (P = 0.014 and P = 0.027, Fig. 4B) and advanced-stage patients (P = 0.080 and P = 0.048, Fig. 4C). In univariate analysis, IPI, PINK, PINK-E (P all < 0.001) and AspM score (P = 0.044 and P = 0.048) were significant prognostic factors for both PFS and OS, as well as performance status, LDH, EBV-DNA, and treatment response (Table 1). In multivariate analysis, when PINK or PINK-E was controlled, AspM score was an independent prognostic factor for PFS and OS (Table 4).
Table 4.
Multivariate analysis of predictors of progression-free survival (PFS) and overall survival (OS) in patients with ENKTL (validation group) controlled by international prognostic index (IPI), prognostic index of natural-killer lymphoma (PINK), and prognostic index of natural-killer lymphoma- Epstein-Barr Virus (PINK-E).
| Variable | RR | PFS |
P value | RR | OS |
P value |
|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||
| IPI | ||||||
| 0-1/2/3/4-5 | 2.454 | 1.705–3.531 | < 0.001 | 2.641 | 1.793–3.890 | < 0.001 |
| AspM score | ||||||
| High vs low | 0.455 | 0.185–1.116 | 0.086 | 0.453 | 0.174–1.182 | 0.106 |
| PINK | ||||||
| 0/1/2–4 | 6.757 | 2.895–15.769 | < 0.001 | 7.214 | 2.910–17.885 | < 0.001 |
| AspM score | ||||||
| High vs low | 0.243 | 0.096–0.616 | 0.003 | 0.241 | 0.091–0.644 | 0.004 |
| PINK-E | ||||||
| 0–1/2/3–5 | 3.946 | 2.284–6.818 | < 0.001 | 4.294 | 2.396–7.693 | < 0.001 |
| AspM score | ||||||
| High vs low | 0.265 | 0.106–0.661 | 0.004 | 0.252 | 0.096–0.659 | 0.005 |
4. Discussion
MESA was derived from SMILE and AspaMetDex regimen, aiming to maintain the efficacy and minimize the toxicity in early-stage ENKTL. Etopside was kept as an important agent to treat hemophagocytosis that frequently occurs in ENKTL (Tse and Kwong, 2013). Comparing with l-asparaginase, pegaspargase depletes asparagine more efficiently by achieving rapid peak levels, with significant reduction of hypersensitive reactions and dose frequencies (Fu and Sakamoto, 2007, Shrivastava et al., 2016). Methotrexate (at a dose of 2–3 g/m2) in combination with etoposide, dexamethasone, and pegaspargase has been recently reported on refractory or de novo ENKTL patients (Ding et al., 2015, Liang et al., 2016). With regards to the severe toxicity of high-dose methotrexate (reviewed in Table 2) and the dose-independent anti-ENKTL activity of methotrexate in vitro (Kim et al., 2014b) here we reduced the dose of methotrexate to 1 g/m2, as suggested in HyperCVAD/MA regimen (Cortes et al., 1995) and evaluated the efficacy and toxicity of MESA as a first-line treatment on early-stage ENKTL. With similar clinical characteristics as previous studies of SMILE (Jiang et al., 2012, Wang et al., 2013) comparable response rate and survival time were observed in MESA-treated patients. Of note, severe toxicity was less common. As for the hematological toxicity, due to the dose reduction of methotrexate, the grade 3/4 toxicity has been remarkably decreased, in the setting of no infection for prophylaxis or G-CSF support. Recent prospective studies on limited-stage ENKTL (Jiang et al., 2012, Wang et al., 2013, Yamaguchi et al., 2009, Kim et al., 2009, Kim et al., 2014a) showed ORR ranging from 81% to 96%, and our study is 92.1%. MESA showed higher hematological and non-hematological toxicities than L-asparaginase, vincristine, and prednisone sandwiched with radiotherapy, but still comparable with other studies. To our knowledge, this is one of the largest phase II studies of asparaginase-based chemotherapy in de novo ENKTL that indicated low-dose methotrexate could be considered in early-stage patients.
Aberrant metabolism reprogramming plays an important role in cancer progression. Although asparaginase and methotrexate are the most commonly used anti-metabolite agents, metabolomic study was rarely performed in ENKTL. Asparaginase selectively hydrolyzes the extra-cellular amino acid L-asparagine into l-aspartate and ammonia. Most of the ENKTL cells are lacking asparagine synthetase, unable to carry out primary asparagine synthesis, and sensitive to asparaginase-induced cell death. However, high level of cellular asparagine synthetase expression, which allows intrinsic asparagine production and protein synthesis, is considered major cause of resistance to asparaginase-based therapy (Shrivastava et al., 2016). Alanine, aspartic acid, glutamate, and succinic acid attribute to asparagine synthesis, as depicted in KEGG (Kyoto Encyclopedia of Genes and Genomes) alanine, aspartate and glutamate metabolism pathway (hsa00250), and were found to be highly expressed in patients with good response to asparaginase, not only in early-stage, but also in advanced-stage ENKTL. Otherwise, patients with lower expression of the four metabolites displayed poor response and inferior survival, which might be associated with overexpression of cellular asparagine synthetase in these patients. For healthy people, the lower levels of alanine, aspartic acid, glutamate and succinic acid are considered as the outcome of normal cellular asparagine synthesis. Attainable from peripheral blood metabolites and independent from PINK and PINK-E, AspM score may be a simple and useful prognostic tool in ENKTL. However, longer follow-up time and larger sample size should be accumulated, particularly in prospective clinical trials of asparaginase-based regimens. In conclusion, MESA sandwiched with radiotherapy is an effective and safe treatment for newly diagnosed, stage IE-IIE ENKTL, highlighting the role of targeting metabolic aberrations in this disease.
The following are the supplementary data related to this article.
Supplementary tables
Contributors
P-PX collected and analyzed data, and wrote the article. JX performed the experiment, analyzed the data and wrote the article. XZ and H-JZ collected clinical data. C-FW was responsible for pathology review. GC and J-YC were responsible for the plan of patients' radiotherapy. H-YH was responsible for statistical review. SC supervised the study. W-LZ designed and supervised the study, and wrote the article.
Declaration of Interests
All authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was supported, in part, by research funding from the National Natural Science Foundation of China (81325003, 81520108003, 81670716 and 81201863), the Shanghai Commission of Science and Technology (14430723400, 14140903100 and 16JC1405800), Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152206 and 20152208), Clinical Research Plan of SHDC (16CR2017A), Multi-center clinical research project by Shanghai Jiao Tong University school of medicine (DLY201601), SMC-Chen Xing Scholars Program, Chang Jiang Scholars Program, Innovation Fund Projects of Shanghai Jiao Tong University (BXJ201607), Collaborative Innovation Center of Systems Biomedicine and the Samuel Waxman Cancer Research Foundation.
References
- Au W.Y., Weisenburger D.D., Intragumtornchai T. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- Cheson B.D., Pfistner B., Juweid M.E. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Cheung M.M., Chan J.K., Lau W.H. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J. Clin. Oncol. 1998;16:70–77. doi: 10.1200/JCO.1998.16.1.70. [DOI] [PubMed] [Google Scholar]
- Cheung M.M., Chan J.K., Lau W.H. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int. J. Radiat. Oncol. Biol. Phys. 2002;54:182–190. doi: 10.1016/s0360-3016(02)02916-4. [DOI] [PubMed] [Google Scholar]
- Cortes J., O'Brien S.M., Pierce S. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995;86:2091–2097. [PubMed] [Google Scholar]
- Deng W., Wang Y., Liu Z. HemI: a toolkit for illustrating heatmaps. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Chang J., Liu L.G. High-dose methotrexate, etoposide, dexamethasone and pegaspargase (MEDA) combination chemotherapy is effective for advanced and relapsed/refractory extranodal natural killer/T cell lymphoma: a retrospective study. Int. J. Hematol. 2015;102:181–187. doi: 10.1007/s12185-015-1809-x. [DOI] [PubMed] [Google Scholar]
- Fu C.H., Sakamoto K.M. PEG-asparaginase. Expert. Opin. Pharmacother. 2007;8:1977–1984. doi: 10.1517/14656566.8.12.1977. [DOI] [PubMed] [Google Scholar]
- Jaccard A., Gachard N., Marin B. Efficacy of l-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- Jiang M., Zhang H., Jiang Y. Phase 2 trial of “sandwich” l-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012;118:3294–3301. doi: 10.1002/cncr.26629. [DOI] [PubMed] [Google Scholar]
- Kim W.S., Song S.Y., Ahn Y.C. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann. Oncol. 2001;12:349–352. doi: 10.1023/a:1011144911781. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Kim K., Kim B.S. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: consortium for improving survival of lymphoma study. J. Clin. Oncol. 2009;27:6027–6032. doi: 10.1200/JCO.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- Kim T.M., Kim S., Ahn Y.O. Anti-cancer activity of gemcitabine against natural killer cell leukemia/lymphoma. Leuk. Lymphoma. 2014;55:940–943. doi: 10.3109/10428194.2013.813505. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Yang D.H., Kim J.S. Concurrent chemoradiotherapy followed by l-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann. Hematol. 2014;93:1895–1901. doi: 10.1007/s00277-014-2137-6. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Yoon D.H., Jaccard A. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. The Lancet. Oncology. 2016;17:389–400. doi: 10.1016/S1470-2045(15)00533-1. [DOI] [PubMed] [Google Scholar]
- Kwong Y.L., Kim W.S., Lim S.T. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- Li Y.X., Liu Q.F., Fang H. Variable clinical presentations of nasal and Waldeyer ring natural killer/T-cell lymphoma. Clin. Cancer Res. 2009;15:2905–2912. doi: 10.1158/1078-0432.CCR-08-2914. [DOI] [PubMed] [Google Scholar]
- Liang R., Gao G.X., Chen J.P. A phase 2 study of methotrexate, etoposide, dexamethasone, and pegaspargase chemotherapy for newly diagnosed, relapsed, or refractory extranodal natural killer/T-cell lymphoma, nasal type: a multicenter trial in Northwest China. Hematol. Oncol. 2016 doi: 10.1002/hon.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkunam Y., Farinha P., Hsi E.D. LMO2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy with and without rituximab. J. Clin. Oncol. 2008;26:447–454. doi: 10.1200/JCO.2007.13.0690. [DOI] [PubMed] [Google Scholar]
- Oshimi K., Kawa K., Nakamura S. NK-cell neoplasms in Japan. Hematology. 2005;10:237–245. doi: 10.1080/10245330400026162. [DOI] [PubMed] [Google Scholar]
- Shrivastava A., Khan A.A., Khurshid M. Recent developments in l-asparaginase discovery and its potential as anticancer agent. Crit. Rev. Oncol. Hematol. 2016;100:1–10. doi: 10.1016/j.critrevonc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Swerdlow S.H., Campo E., Pileri S.A. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Non-Hodgkin's Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin's lymphoma. N.Engl. J. Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- Tse E., Kwong Y.L. How I treat NK/T-cell lymphomas. Blood. 2013;121:4997–5005. doi: 10.1182/blood-2013-01-453233. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang Z.H., Chen X.Q. First-line combination of gemcitabine, oxaliplatin, and l-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119:348–355. doi: 10.1002/cncr.27752. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tobinai K., Oguchi M. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J. Clin. Oncol. 2009;27:5594–5600. doi: 10.1200/JCO.2009.23.8295. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Kwong Y.L., Kim W.S. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J. Clin. Oncol. 2011;29:4410–4416. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhu Y., Cao J.Z. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood. 2015;126:1424–1432. doi: 10.1182/blood-2015-04-639336. (quiz 1517) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables