Abstract
Aim
To evaluate circulating plasma dipeptidyl peptidase-4 (DPP4) levels in non-obese Asian Indians with type 2 diabetes mellitus (T2DM), and to correlate these with metabolic profile and measures of anthropometry, skinfolds, abdominal adipose tissue depots, pancreatic volume, and liver span.
Methodology
Non-obese (body mass index (BMI) <25 kg/m2) patients with T2DM (cases, n=93), diagnosed within 1 year from recruitment, on metformin therapy and BMI-matched, and non-diabetic subjects (controls, n=40) were compared. Measurements of blood glucose, glycosylated hemoglobin, plasma insulin levels, lipid profile, hepatic transaminases and plasma DPP4 levels, and quantification of abdominal fat depots, pancreatic volume and liver span (MRI scan), were done.
Results
Significantly higher (p<0.001) circulating plasma DPP4 levels were observed in cases as compared to controls. Specifically, in patients with T2DM with non-alcoholic fatty liver disease (NAFLD) (n=48), the mean plasma DPP4 level (52.6±27.8 ng/mL) was significantly higher (p<0.05) as compared with those without NAFLD (n=43; 47±28.3 ng/mL). Significant positive correlation was observed for circulating plasma DPP4 levels with waist-to-hip ratio, total intra-abdominal adipose volume, and liver span. Fasting serum insulin, low-density lipoprotein cholesterol (LDL-C), triceps skinfolds, total intra-abdominal adipose tissue volume and presence of T2DM were significant determinants of circulating plasma DPP4 levels.
Conclusion
Non-obese Asian Indian patients with T2DM and on metformin therapy have significantly higher circulating plasma DPP4 levels as compared to non-obese non-diabetic controls, and these levels correlate with fasting insulin and LDL-C levels, upper limb subcutaneous adipose tissue, intra-abdominal adiposity and presence of diabetes.
Keywords: abdominal obesity, mri, Asian Indians, dp iv
Significance of this study.
What is already known about this subject?
The expression of dipeptidyl peptidase-4 (DPP4) has been shown to be dysregulated in obesity, type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease and cardiovascular diseases. However, it is unclear if increased circulating plasma DPP4 levels are associated with the development of obesity and diabetes.
Higher plasma DPP4 levels have been reported in obese as compared with non-obese individuals, but there are conflicting reports on circulating plasma DPP4 levels in subjects with T2DM of different ethnic groups.
What are the new findings?
This is the first study reporting elevated levels of circulating plasma DPP4 in young, non-obese Asian Indian patients with T2DM, on metformin therapy and diagnosed within 1 year from onset. Further, elevated circulating plasma DPP4 levels in patients with T2DM correlate with measures of anthropometry, skinfolds, intra-abdominal adipose tissue and hepatic fat.
We report fasting serum insulin, low-density lipoprotein cholesterol, triceps skinfolds, total intra-abdominal adipose tissue volume and presence of diabetes as significant determinants of circulating plasma DPP4 levels in non-obese Asian Indians with T2DM residing in North India.
How might these results change the focus of research or clinical practice?
This study has significant implications for DPP4 inhibitors therapy in non-obese Asian Indians with T2DM.
Introduction
Asian Indians have a body phenotype featured by excess body fat, higher truncal, subcutaneous and intra-abdominal adipose tissue, and lower muscle mass as compared with white Caucasians.1 ‘Non-obese’ Asian Indians (as defined by body mass index (BMI) < 25 kg/m2) also exhibit similar body composition features with biochemical features of insulin resistance. Further, associated with type 2 diabetes mellitus (T2DM) in ‘non-obese’ Asian Indians are excess liver span and increased pancreatic volume (surrogate markers for ectopic fat deposition in liver and pancreas, respectively), as reported previously by our group.2 Despite these reports, the pathogenesis of T2DM in such ‘non-obese’ Asian Indians remains poorly understood.
Dipeptidyl peptidase- 4 (DPP4) is an exopeptidase glycoprotein of 110 kilodaltons released from differentiated adipocytes and ubiquitously expressed on the surface of a variety of cells and tissues such as pancreas, liver, spleen and adrenal glands.3 DPP4 exerts both paracrine and endocrine effects and is involved in cell signaling and insulin action. It selectively cleaves N-terminal dipeptides from cytokines, growth factors, neuropeptides and incretin hormones.4 The substrate for DPP4, namely glucagon-like peptide-1, is responsible for 60% of postprandial insulin secretion. It is inactivated by DPP4 within 20 minutes of release,5 leading to decreased plasma insulin levels resulting in elevation of blood glucose levels.6
The expression of DPP4 has been shown to be dysregulated in obesity,7 T2DM, non-alcoholic fatty liver disease (NAFLD)8 and cardiovascular diseases.9 Higher plasma DPP4 levels have been reported in obese as compared with lean Chinese men.10 Further, circulating plasma DPP4 levels are increased in Danish patients with T2DM,11 while decreased plasma DPP4 levels were reported in Irish Caucasian patients with T2DM.12 In one of the few studies on correlations of DPP4 expression with abdominal adipose tissue compartments, circulating DPP4 levels was positively correlated with BMI, subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) in German patients with wide ranges of BMI. Specifically, in lean subjects (mean BMI: 22±2 kg/m2) with impaired glucose tolerance, DPP4 expression was higher in VAT than SAT, and correlated positively with the amount of VAT, adipocyte size and adipose tissue inflammation. It has been opined that DPP4 may be a marker for visceral obesity, insulin resistance and the metabolic syndrome.13 Preliminary data also show that circulating DPP4 levels correlated with advanced glycosylation end (AGE) products and that AGEs significantly increased plasma DPP4 release from tubular cells and increased DPP4 expression in Japanese patients, thus suggesting their potential importance in the development of complications of diabetes as well.14
DPP4 inhibitors have been used successfully in the treatment of T2DM globally.15 In this context, research on DPP4 is particularly relevant for Asian Indians who have increasing prevalence of diabetes, generalized obesity and excess abdominal adiposity. Importantly, adipose tissue compartments, posterior abdominal SAT16 and deep abdominal SAT have been strongly linked to insulin resistance,17 especially in Asian Indians.18
Previous investigators did not specifically study circulating plasma DPP4 levels in non-obese patients (defined by BMI) with T2DM. Importantly, the relationships of circulating plasma DPP4 with various adipose tissue subcompartments-abdominal SAT (anterior, posterior and deep subcutaneous abdominal adipose tissue) and total intra-abdominal adipose tissue, (intraperitoneal and retroperitoneal adipose tissue, pancreatic volume and liver fat, which may have relevance to insulin resistance, have not been previously defined.
In view of the above gaps in knowledge and pursuant to possible association of circulating plasma DPP4 to intra-abdominal obesity in non-obese Asian Indians with T2DM, we conducted the present research. We hypothesized that ‘non-obese’ Asian Indians with T2DM may have increased circulating plasma DPP4 levels as compared with non-obese, normoglycemic subjects and aimed to show that such increased levels would correlate with waist-to-hip ratio, skinfolds, abdominal SAT, intra-abdominal adipose tissue (IAAT) volume, liver span (as surrogate measure of NAFLD,) pancreatic volume (surrogate marker for pancreatic, lipids) and fasting serum insulin levels.
Methodology
Ethical approval was obtained from the institutional review board and the study was conducted according to the Declaration of Helsinki 2013.19 The details of subject recruitment, inclusion and exclusion criteria and so on are mentioned in our previous study.2
Briefly, non-obese (BMI <25 kg/m2) patients with T2DM (cases: males n=83, females, n=10), diagnosed within one year from onset, and BMI-matched, normoglycaemic subjects (controls: males, n=24, females, n=16), aged between 18–40 years were recruited. In the first phase of the study, we recruited more of male T2DM subjects (n=83) as compared with females (n=10). This was because a higher number of male subjects fulfilled the recruitment criteria as compared to females.
None of the patients was on insulin therapy, sulfonylureas, DPP4 inhibitors, pioglitazone or any other antihyperglycemic treatment other than metformin. Those who were on metformin were continued on this drug and dose was modified as per clinical requirement. Peripheral skinfolds (biceps, triceps, thigh and calf skinfolds; sum of four skinfolds=total peripheral skinfolds) and truncal skinfolds (subscapular, suprailiac and abdominal skinfolds; sum of three skinfolds=total truncal skinfolds) were measured using a Lange skinfold calipers (Beta Technology, Santa Cruz, California, USA) as reported previously.20 Further, 1.5 Tesla MRI imaging was carried out using T1-weighted axial scans at lumbar vertebra 2 and 3 to estimate the volumes of total abdominal adipose tissue and abdominal adipose tissue compartments, viz SAT (anterior, posterior, superficial and deep subcutaneous) and total IAAT (intraperitoneal and retroperitoneal) with liver span (surrogate measure of NAFLD) and pancreatic volume, using previously published protocols.2 Pancreatic volume index was calculated as pancreatic volume (cm3)/body surface area (m2).21
Biochemical analysis
Fasting and postprandial blood samples were analyzed for plasma glucose levels. Serum lipid profile, hepatic transaminases and glycosylated hemoglobin were analyzed in fasting blood samples by methods described previously.2 Circulating plasma DPP4 and fasting serum insulin levels were measured by ELISA using commercial kits (USCN Life Science, Houston, Texas, USA). The intra-assay and inter-assay percentage coefficient variables for plasma DPP4 were 2.6 and 1.97, respectively. For serum insulin, the intra-assay and inter-assay percentage coefficient variables were 1.76 and 2.10, respectively.
Statistical analysis
Data were analyzed using STATA 11.0 (College Station, TX, USA). Continuous variables were presented as Mean±SD using Student’s t-test. As the data of cases and controls differed in age, the analysis of covariance (ANOCOVA) was used to adjust plasma DPP4 levels for age in cases and controls. The correlations of plasma DPP4 levels with measures of anthropometry, biochemical variables, and volumes of abdominal adipose tissue compartments, liver span and pancreatic volume were assessed using Pearson’s correlation. Stepwise multiple regression analysis was performed to derive predictor variables for plasma DPP4 levels in both cases and controls. The p value less than 0.05 was considered statistically significant.
Results
In this study, significant differences were observed for biceps and triceps skinfolds in non-obese patients with T2DM (cases) as compared with controls after adjustment for age and gender (table 1). Importantly, significantly higher (p<0.001) circulating plasma DPP4 levels were observed in cases as compared with controls (table 2). Significantly higher volumes of abdominal adipose tissue depots were observed in cases as compared with controls, after adjustment for age and gender as follows: total IAAT (49.7%; p=0.000), intraperitoneal adipose tissue (47.7%; p=0.000), retroperitoneal adipose tissue (70.7%; p=0.000) and liver span (10.8%; p=0.000). Specifically, no significant differences were observed between cases and controls for volumes of anterior subcutaneous, posterior subcutaneous, superficial and deep subcutaneous abdominal adipose tissue and total subcutaneous abdominal adipose tissue (figure 1A–F, table 3). Further, NAFLD was observed in 48 cases (51.6%; 36 cases had grade 1 and 12 cases had grade 2 NAFLD) and two controls (5%; grade 1 NAFLD). Specifically, in patients with T2DM with NAFLD (n=48), the mean circulating plasma DPP4 level (52.6±27.8 ng/mL) was significantly higher (p<0.05) as compared with patients with T2DM without NAFLD (n=43; mean circulating plasma DPP4 level; 47±28.3 ng/mL).
Table 1.
Anthropometric profile and skinfold measurements
| Unadjusted for age | Adjusted for age | p value | ||||
| Cases (n=93) | Controls (n=40) | p value | Cases (n=93) | Controls (n=40) | ||
| Body mass index (kg/m2) | 22.8±2.0 | 22.3±2.1 | 0.21 | 22.8±1.9 | 22.4±1.8 | 0.33 |
| Body surface area (cm3/m2) | 1.72±0.1 | 1.67±0.2 | 0.09 | 1.7±0.09 | 1.6±0.18 | 0.97 |
| Waist circumference (cms) | 85.7±6.8 | 82.8±7.7 | <0.01 | 85.8±4.8 | 84.5±5.6 | 0.02 |
| Hip circumference (cms) | 89.5±4.6 | 90.8±7.3 | 0.23 | 89.3±4.8 | 91.3±5.0 | 0.06 |
| Waist-to-hip ratio | 0.95±0.0 | 0.90±0.0 | <0.001 | 0.95±0.0 | 0.90±0.0 | <0.001 |
| Mid-arm circumference (cms) | 27.2±2.4 | 27.6±5.4 | 0.6 | 27.2±2.8 | 27.6±3.1 | 0.53 |
| Mid-thigh circumference (cms) | 49.7±4.2 | 48.7±4.8 | 0.24 | 49.7±3.8 | 48.7±4.4 | 0.26 |
| Biceps skinfolds (mms) | 8.4±4.1 | 13.6±9.8 | <0.01 | 8.3±5.7 | 13.8±6.3 | <0.01 |
| Triceps skinfolds (mms) | 15.6±5.3 | 18.9±7.7 | <0.01 | 15.5±0.3 | 19.3±6.3 | <0.01 |
| Thigh skinfolds (mms) | 23.6±5.7 | 28.7±10.1 | <0.01 | 23.5±6.6 | 28.9±7.5 | <0.01 |
| Calf skinfolds (mms) | 12.4±5.2 | 21.8±8.4 | <0.01 | 12.4±5.7 | 21.9±6.3 | <0.01 |
| Subscapular skinfolds (mms) | 22.2±5.8 | 21.8±8.5 | 0.79 | 21.9±6.7 | 22.3±6.9 | 0.8 |
| Suprailiac skinfolds (horizontal) (mms) | 17.8±4.9 | 20.7±5.6 | <0.01 | 17.7±4.8 | 20.8±5.0 | <0.01 |
| Suprailiac skinfolds (vertical) (mms) | 18.1±5.2 | 20.2±5.2 | <0.05 | 18.0±9.6 | 20.4±5.0 | <0.05 |
| Suprailiac skinfolds (average) (mms) | 17.9±4.8 | 19.9±5.6 | <0.05 | 17.8±9.6 | 20.1±5.0 | <0.05 |
| Abdominal skinfolds (vertical) (mms) | 23.9±5.3 | 24.1±7.8 | 0.87 | 23.8±5.7 | 24.6±8.1 | 0.61 |
| Abdominal skinfolds (horizontal) (mms) | 24.8±5.4 | 24.1±7.6 | 0.60 | 24.8±5.7 | 24.6±8.1 | 0.91 |
| Abdominal skinfolds (average) (mms) | 24.2±5.3 | 23.8±7.9 | 0.71 | 24.0±5.7 | 24.7±8.1 | 0.66 |
| Total peripheral skinfolds (mms) | 60.1±17.3 | 78.0±35.2 | <0.01 | 59.8±24 | 78.7±23.9 | <0.001 |
| Total truncal skinfolds (mms) | 106±21.8 | 102.0±29.6 | 0.32 | 106.7±24 | 101.9±25.2 | 0.32 |
Values are presented as Mean±SD; p<0.05, statistically significant.
Table 2.
Biochemical profile
| Biochemical variables | Unadjusted for age | p value | Adjusted for age | p value | ||
| Cases (n=93) | Controls (n=40) | Cases (n=93) | Controls (n=40) | |||
| Fasting blood glucose (mg/dL) |
147.8±51. 1 | 89.4±2.6 | <0.000 | 148.1±12.4 | 88.8±32 | 0.92 |
| Postprandial blood glucose (mg/dL) | 222.6±82.9 | 89.36±13.7 | <0.01 | 222.9±70.5 | 79.8±12.6 | <0.01 |
| Fasting serum insulin (mIU/dL) |
9.5±0.6 | 5.5±0.4 | <0.001 | 10.0±9.5 | 6.8±8.1 | <0.05 |
| Postprandial serum insulin (mIU/mL) | 22.3±3.0 | 8.0±0.5 | <0.001 | 21.5±44.6 | 13.0±28.6 | 0.07 (NS) |
| Glycosylated hemoglobin (%) | 9.00±2.5 | 5.2±0.37 | <0.01 | 9.0±2 | 5.1±2 | <0.001 |
| Total cholesterol (mg/dL) | 175.4±41.5 | 152.4±29.1 | <0.01 | 175.4±38.4 | 152.6±42.2 | 0.34 |
| Serum triglycerides (mg/dL) | 170.1±99.4 | 97.15±45.8 | <0.01 | 170.0±87.3 | 97.4±88.2 | <0.001 |
| High-density lipoprotein cholesterol (mg/dL) | 40.7±8.9 | 42.4±8.3 | 0.31 | 40.8±8.6 | 42.4±8.1 | <0.05 |
| Low-density lipoprotein cholesterol (mg/dL) | 110.3±31.2 | 96.81±25.3 | <0.05 | 110.2±29.7 | 98.0±29.6 | <0.001 |
| Very low-density lipoprotein cholesterol (mg/dL) | 33.5±19.3 | 19.47±9.1 | <0.01 | 19.5±16.3 | 33.5±17.0 | <0.001 |
| Aspartate aminotransferase (U/L) | 57.81±21.7 | 45.60±18.1 | <0.01 | 57.9±20.1 | 47.1±21.4 | 0.17 |
| Alanine aminotransferase (U/L) | 27.89±12.8 | 23.68±9.1 | <0.05 | 27.8±11.5 | 24.6±11.9 | <0.001 |
| Serum dipeptidyl peptidase-4 (ng/mL) |
52.0±3.0 | 20.1±2.3 | <0.001 | 50.2±9.6 | 23.5±6.3 | <0.001 |
Values are presented as Mean±SD; p<0.05, statistically significant.
NS, not significant.
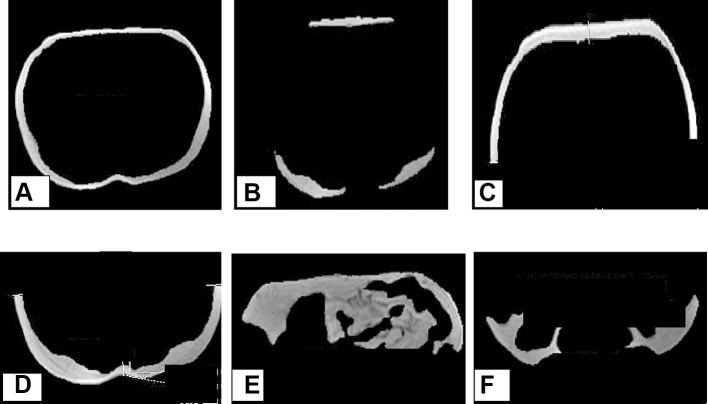
Figure 1.
Abdominal adipose tissue depots and pancreatic volume in a 29-year-old non-obese (BMI: 21.9 kg/m2) patient with type 2 diabetes, images obtained using MRI (1.5 Tesla) and quantified by ROI analysis using GE Advantage Workstation Volume Viewer softwares. (A) Superficial subcutaneous abdominal adipose tissue, (B) Deep subcutaneous abdominal adipose tissue, (C) Anterior subcutaneous abdominal adipose tissue, (D) Posterior subcutaneous abdominal adipose tissue, (E) Intraperitoneal adipose tissue and (F) Retroperitoneal adipose tissue.
Table 3.
Abdominal fat depots, pancreatic volume and liver span measured by MRI (1.5 Tesla)
| Variables measured by MRI (1.5 Tesla) | Unadjusted for age | Adjusted for age | ||||
| Cases (n=93) | Controls (n=40) | p value | Cases (n=93) | Controls (n=40) | p value | |
| Anterior subcutaneous adipose tissue volume (cm3) | 49.0±21.7 | 46.7±20.1 | 0.56 | 48.3±19.9 | 48.4±20.7 | 0.98 |
| Posterior subcutaneous adipose tissue volume (cm3) | 55.8±20.6 | 57.0±19.4 | 0.75 | 55.2±19.9 | 58.0±20.1 | 0.41 |
| Superficial subcutaneous adipose tissue volume (cm3) | 80.9±28.7 | 82.4±30.8 | 0.77 | 79.8±28.5 | 84.8±28.9 | 0.35 |
| Deep subcutaneous adipose tissue volume (cm3) | 22.3±10.5 | 19.6±8.3 | 0.15 | 22.1±9.5 | 20.3±9.4 | 0.35 |
| Total subcutaneous adipose tissue volume (cm3) | 103.3±35.3 | 102.1±38.0 | 0.86 | 101.9±35.1 | 105.1±35.2 | 0.64 |
| Retroperitoneal adipose tissue volume (cm3) | 33.5±14.5 | 19.6±9.0 | <0.001 | 33.4±12.3 | 19.9±13.2 | <0.001 |
| Intraperitoneal adipose tissue volume (cm3) | 69.3±29.5 | 48.0±22.0 | <0.001 | 62.2±27.5 | 44.4±27.7 | <0.001 |
| Total intra-abdominal adipose tissue volume (cm3) | 102.8±39.8 | 68.5±30.5 | <0.001 | 69.1±37.0 | 49.3±37.8 | <0.001 |
| Liver span (mm) | 165±16.5 | 149.0±19.1 | <0.001 | 165.1±17.1 | 149.0±17.6 | <0.001 |
| Pancreatic volume (cm3) | 67.4±24.9 | 53.2±20.8 | <0.01 | 67.1±23.7 | 53.9±23.9 | <0.01 |
| Pancreatic volume index (cm3) | 38.7±13.8 | 31.9±11.1 | <0.01 | 38.6±12.3 | 32.2±13.2 | <0.05 |
Values are presented as Mean±SD; p<0.05, statistically significant.
Pearson’s correlation analysis revealed significant positive correlation in cases for circulating plasma DPP4 with waist-to-hip ratio (online supplementary figure 1) and MRI quantified volumes of total IAAT (p<0.001) (figure 2) and liver span p <0.05 (p<0.001) (online supplementary figure 2). No significant correlation was observed between plasma DPP4 levels in T2DM patients with NAFLD. Further, no significant correlation was observed for pancreatic volume and pancreatic volume index, anterior subcutaneous, posterior subcutaneous, superficial deep SAT compartments and total abdominal fat. For biochemical variables, significant positive correlation was observed for circulating plasma DPP4 levels with fasting insulin in cases but not in controls (online supplementary figure 3). Importantly, circulating plasma DPP4 levels correlated negatively and significantly (p<0.05) with high-density lipoprotein cholesterol (HDL-C) in cases but not in controls (table 4 and online supplementary figure 4).
Figure 2.
Showing significant positive correlation of circulating plasma DPP4 levels with total intra-abdominal adipose tissue volume in patients with T2DM (A) and lack of significant positive correlation of circulating plasma DPP4 with total intra-abdominal adipose tissue volume in non-obese, non-diabetic subjects (B). DDP4, plasma dipeptidyl peptidase-4; T2DM, type 2 diabetes mellitus.
Table 4.
Correlation of plasma DPP4 levels with waist-to-hip ratio, biochemical variables, total abdominal fat volume and Liver span in non-obese patients with T2DM (Cases: n = 93) and non obese, non diabetic subjects (Controls: n = 40)
| Cases (n=93) Pearson’s correlation coefficient |
Controls (n=40) Pearson’s correlation coefficient |
|||
| r | p value | r | p value | |
| Waist-to-hip ratio | 0.32 | 0.04 | 0.21 | 0.2 |
| Fasting serum insulin (ng/dL) | 0.31 | 0.04 | 0.09 | 0.39 |
| High density lipoprotein cholesterol (mmol/L) | −0.48 | 0.02 | 0.10 | 0.53 |
| Total abdominal fat volume (cm3) | 0.24 | 0.04 | 0.10 | 0.75 |
| Total intra-abdominal fat volume (cm3) | 0.19 | 0.04 | 0.04 | 0.78 |
| Liver span (mm) | 0.24 | 0.02 | 0.10 | 0.98 |
p<0.05: statistically significant.
DDP4, plasma dipeptidyl peptidase-4; T2DM, type 2 diabetes mellitus.
bmjdrc-2017-000393supp001.docx (47.7KB, docx)
On stepwise multiple linear regression analysis, variables such as fasting serum insulin, low-density lipoprotein cholesterol (LDL-C), triceps skinfolds, total IAAT volume and presence of T2DM were derived as significant determinants of circulating plasma DPP4 levels (table 5).
Table 5.
Stepwise multiple linear regression analysis for determinants of circulating plasma DPP4 levels
| Predictor variables | Beta coefficient (95% CI) | p value |
| Presence of diabetes | 7.1 (5.0 to 9.2) | 0.001 |
| Fasting serum insulin | 0.23 (0.09 to 0.37) | 0.04 |
| Triceps skinfold thickness | 0.25 (0.11 to 0.42) | 0.02 |
| Total intra-abdominal fat volume (cm3) | 0.40 (0.20 to 0.63) | 0.001 |
| Low-density lipoprotein cholesterol | 0.80 (0.14 to 0.92) | 0.04 |
Adjusted r2 value: 0.57, p<0.05: statistically significant.
DDP4, plasma dipeptidyl peptidase-4.
Discussion
Although circulating plasma DPP4 levels in T2DM have been researched, data in non-obese patients with T2DM are few. This is the first study showing elevated levels of circulating plasma DPP4 levels in young, non-obese Asian Indian patients with T2DM. Further, for the first time, correlations of circulating plasma DPP4 with anthropometric measures (including detailed skinfold thickness measurements), volumes of abdominal adipose tissue compartments, pancreatic volume and liver span have been carried out.
Specifically, circulating plasma DPP4 levels correlated significantly and positively with waist-to-hip ratio and IAAT volume in T2DM cases as compared with controls, even after adjustment for age and gender. Further, on stepwise multiple linear regression analysis, total intra-abdominal fat volume appeared a significant predictor (β coefficient: 0.40, p<0.05) of circulating plasma DPP4 levels. Similarly, a recent CT-based study on elderly Japanese men with T2DM showed significant positive correlation between circulating DPP4 levels and intra-abdominal fat area, indicating that circulating DPP4 levels were significantly associated with intra-abdominal visceral fat in Japanese patients with T2DM.22 Of note, as compared with white Caucasians, Asian Indians have higher magnitude of abdominal adiposity and larger adipocyte size23 correlating with increased release of non-esterified free fatty acids.24 It is reported that DPP4 levels correlate with adipocyte size and metabolic syndrome.10 Whether increased intra-abdominal obesity and larger adipocytes in non-obese Asian Indians lead to concomitant increase in DPP4 levels needs to be researched.
We used liver span quantified by MRI as a measure of hepatic fat, and also graded fatty liver. Importantly, nearly 52% of patients with T2DM in this study had NAFLD and elevated DPP4 levels. DPP4 is highly expressed in the liver tissues and its activity has been correlated with hepatic steatosis and grading of NAFLD in North Americans25 and Japanese subjects.26 It is important to note that as compared with white Caucasians, hepatic triglyceride accumulation is significantly higher in Asian Indians and is associated with higher magnitude of insulin resistance.27 In this context, our observations being reported for the first time in Asian Indians achieve clinical importance. Further studies in the context of plasma DPP4 levels and liver steatosis in Asian Indians are needed, using more specific measures of quantification of liver fat (eg, proton magnetic resonance spectroscopy).
In our study, patients were non-obese by definition of BMI, hence circulating plasma DPP4 levels may not be similarly increased as seen in studies done on obese patients. Second, patients with T2DM in our study were diagnosed within one year prior to recruitment and were already on lifestyle management and metformin therapy. Whether such management could alter levels of circulating plasma DPP4 levels and its relationship with abdominal adipose tissue compartments is not known. In this context, it is important to note that in Korean patients with T2DM on metformin therapy or dual combination therapy (metformin with thiazolidinedione), plasma DPP4 levels were lower as compared to patients with T2DM on other antihyperglycemic drugs. These observations were attributed to the suppressive effects of metformin and thiazolidinedione drugs on the in vivo release of the plasma isoforms of DPP4.28 29
Importantly, circulating plasma DPP4 levels have been reported to increase when HbA1c increases above 9.0%,30 similar to patients in our study (HbA1c levels ranged between 9.0% and 11% (75–97 mmol/mol)). We also show significant positive correlations of fasting insulin with high circulating plasma DPP4 levels. It is unclear if high levels of circulating plasma DPP4 as seen in patients with marked hyperglycemia is a primary or secondary effect. It is possible that gene expression for plasma DPP4 is upregulated on exposure to elevated blood glucose levels.31 In this context it is important to note that circulating plasma DPP4 levels are significantly upregulated by serum insulin levels32 and tumor necrosis factor alpha33, especially in patients with T2DM with non-alcoholic steatohepatitis.34 Importantly, circulating plasma DPP4 levels in peripheral blood cells improved after better glucose control in drug-naive T2DM patients with T2DM, indicating the possible role of DPP4 in insulin signaling pathway.33
Of further importance, we observed significant negative correlation of circulating plasma DPP4 levels with HDL-C, despite lack of significant differences in mean values of HDL-C between the two groups. In a study on middle-aged Chinese individuals, HDL-C levels have been reported to correlate with plasma DPP4 levels only in subjects with diabetes and not in normoglycemic subjects.26 Similar observations have been reported in another study on Chinese individuals.10 Further in a study on non-obese, elderly Japanese subjects, HDL-C was shown to correlate independently with plasma DPP4 levels and showed significant dose–response relationship.14 This interesting relationship should be investigated. Finally, independent correlations of LDL-C and circulating DPP4 levels need to be researched in view of high predisposition of Asian Indians for development of coronary heart disease.
The clinical significance of high circulating plasma DPP4 levels in ‘non-obese’ Asian Indians with diabetes is not clear, but some reported effects could be relevant. Specifically, subclinical inflammation may be increased by plasma DPP4 due to induction of obesity-induced inflammation in IAAT.35 Further, deterioration of insulin resistance and adiposity may be induced by high DPP4 levels.36 These effects of high DPP4 levels could be particularly detrimental to Asian Indians who already have higher magnitude of subclinical inflammation and abdominal adiposity as compared with other races,37 thus escalating cardiovascular risk.38 Whether high levels of circulating plasma DPP4 levels have any influence on therapy with DPP4 inhibitors remains to be researched. Finally, in view of high load of diabetes-related complications, the relationship of AGE products and circulating DPP4 levels in Asian Indians needs further research.39
In summary, non-obese Asian Indian patients with T2DM on metformin have significantly higher circulating plasma DPP4 level as compared with non-obese, non-diabetic controls, which correlate with fasting insulin and LDL-C levels, triceps skinfolds, and intra-abdominal adiposity, and presence of diabetes. However, this is a cross-sectional study and detailed studies on DPP4 expression in intra-abdominal adipocytes need to be conducted to validate our observations.
Acknowledgments
The authors thank the participants of this study. Mr Gokulraj Prabhakaran, Mahajan Imaging Centre, New Delhi, India, is acknowledged for technical support in MRI imaging.
Footnotes
Contributors: AM conceived the study, reviewed and edited the manuscript. SA conducted the study and wrote the manuscript. SPB performed laboratory analysis of samples. SG contributed to the discussion and reviewed the manuscript. RMP analysed, interpreted the data and contributed to discussion. HM offered MRI services for the study. AM is the guarantor for this manuscript.
Funding: A partial financial support towards imaging and laboratory investigation charges was received from Merck Limited, Mumbai, India (formerly E Merck). The Centre of Nutrition & Metabolic Research (C-NET), New Delhi, India, supported staff salaries, costs for MRI imaging and DPP4 analysis in this study.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Institutional ethics committee of Fortis C-DOC Centre of Excellence for Diabetes, Metabolic Diseases and Endocrinology, Chirag Enclave, Nehru Place, New Delhi.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. Int J Obes 2011;35:167–87. 10.1038/ijo.2010.135 [DOI] [PubMed] [Google Scholar]
- 2. Misra A, Anoop S, Gulati S, et al. Body fat patterning, hepatic fat and pancreatic volume of non-obese Asian Indians with type 2 diabetes in North India: a case-control study. PLoS One 2015;10:e0140447 10.1371/journal.pone.0140447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mentzel S, Dijkman HB, Van Son JP, et al. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem 1996;44:445–61. 10.1177/44.5.8627002 [DOI] [PubMed] [Google Scholar]
- 4. Rohrborn D, Wronkowitz N, Eckel J. DPP4 in diabetes. Front Immunol 2015;27:6–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deacon CF, Knudsen LB, Madsen K, et al. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia 1998;41:271–8. 10.1007/s001250050903 [DOI] [PubMed] [Google Scholar]
- 6. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–705. 10.1016/S0140-6736(06)69705-5 [DOI] [PubMed] [Google Scholar]
- 7. Stengel A, Goebel-Stengel M, Teuffel P, et al. Obese patients have higher circulating protein levels of dipeptidyl peptidase IV. Peptides 2014;61:75–82. 10.1016/j.peptides.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 8. Williams KH, Vieira De Ribeiro AJ, Prakoso E, et al. Circulating dipeptidyl peptidase-4 activity correlates with measures of hepatocyte apoptosis and fibrosis in non-alcoholic fatty liver disease in type 2 diabetes mellitus and obesity: a dual cohort cross-sectional study. J Diabetes 2015;7:809–19. 10.1111/1753-0407.12237 [DOI] [PubMed] [Google Scholar]
- 9. Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis 2013;226:305–14. 10.1016/j.atherosclerosis.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 10. Lamers D, Famulla S, Wronkowitz N, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 2011;60:1917–25. 10.2337/db10-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryskjaer J, Deacon CF, Carr RD, et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol 2006;155:485–93. 10.1530/eje.1.02221 [DOI] [PubMed] [Google Scholar]
- 12. McKillop AM, Duffy NA, Lindsay JR, et al. Decreased dipeptidyl peptidase-IV activity and glucagon-like peptide-1(7-36)amide degradation in type 2 diabetic subjects. Diabetes Res Clin Pract 2008;79:79–85. 10.1016/j.diabres.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 13. Sell H, Bluher M, Kloting N, et al. Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care 2013;36:4083–90. 10.2337/dc13-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tahara N, Yamagishi S, Takeuchi M, et al. Serum levels of advanced glycation end products (AGEs) are independently correlated with circulating levels of dipeptidyl peptidase-4 (DPP-4) in humans. Clin Biochem 2013;46:300–3. 10.1016/j.clinbiochem.2012.11.023 [DOI] [PubMed] [Google Scholar]
- 15. Son JW, Kim S. Dipeptidyl peptidase 4 inhibitors and the risk of cardiovascular disease in patients with type 2 diabetes: a tale of three studies. Diabetes Metab J 2015;39:373–83. 10.4093/dmj.2015.39.5.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Misra A, Garg A, Abate N, et al. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res 1997;5:93–9. 10.1002/j.1550-8528.1997.tb00648.x [DOI] [PubMed] [Google Scholar]
- 17. Marinou K, Hodson L, Vasan SK, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 2014;37:821–9. 10.2337/dc13-1353 [DOI] [PubMed] [Google Scholar]
- 18. Anand SS, Tarnopolsky MA, Rashid S, et al. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the molecular study of health and risk in ethnic groups (mol-SHARE). PLoS One 2011;6:e22112 10.1371/journal.pone.0022112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 20. Dudeja V, Misra A, Pandey RM, et al. BMI does not accurately predict overweight in Asian Indians in northern India. Br J Nutr 2001;86:105–12. 10.1079/BJN2001382 [DOI] [PubMed] [Google Scholar]
- 21. Goda K, Sasaki E, Nagata K, et al. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol 2001;38:145–9. 10.1007/s005920170012 [DOI] [PubMed] [Google Scholar]
- 22. Tanaka S, Kanazawa I, Notsu M, et al. Visceral fat obesity increases serum DPP-4 levels in men with type 2 diabetes mellitus. Diabetes Res Clin Pract 2016;116:1–6. 10.1016/j.diabres.2016.04.027 [DOI] [PubMed] [Google Scholar]
- 23. Chandalia M, Lin P, Seenivasan T, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One 2007;2:e812 10.1371/journal.pone.0000812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abate N, Garg A, Peshock RM, et al. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest 1995;96:88–98. 10.1172/JCI118083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balaban YH, Korkusuz P, Simsek H, et al. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol 2007;6:242–50. [PubMed] [Google Scholar]
- 26. Miyazaki M, Kato M, Tanaka K, et al. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep 2012;5:729–33. 10.3892/mmr.2011.707 [DOI] [PubMed] [Google Scholar]
- 27. Petersen KF, Dufour S, Feng J, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A 2006;103:18273–7. 10.1073/pnas.0608537103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee SA, Kim YR, Yang EJ, et al. CD26/DPP4 levels in peripheral blood and T Cells in patients with Type 2 diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism 2013;98:2553–61. 10.1210/jc.2012-4288 [DOI] [PubMed] [Google Scholar]
- 29. Lenhard JM, Croom DK, Minnick DT. Reduced serum dipeptidyl peptidase-IV after metformin and pioglitazone treatments. Biochem Biophys Res Commun 2004;324:92–7. 10.1016/j.bbrc.2004.09.021 [DOI] [PubMed] [Google Scholar]
- 30. Green BD, Irwin N, Duffy NA, et al. Inhibition of dipeptidyl peptidase-IV activity by metformin enhances the antidiabetic effects of glucagon-like peptide-1. Eur J Pharmacol 2006;547:192–9. 10.1016/j.ejphar.2006.07.043 [DOI] [PubMed] [Google Scholar]
- 31. Mannucci E, Pala L, Ciani S, et al. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia 2005;48:1168–72. 10.1007/s00125-005-1749-8 [DOI] [PubMed] [Google Scholar]
- 32. Pala L, Mannucci E, Pezzatini A, et al. Dipeptidyl peptidase-IV expression and activity in human glomerular endothelial cells. Biochem Biophys Res Commun 2003;310:28–31. 10.1016/j.bbrc.2003.08.111 [DOI] [PubMed] [Google Scholar]
- 33. Wu L, Gong Q, Na R, et al. Dipeptidyl peptidase 4 concentration influenced by serum insulin levels rather than arterial stiffness index in type 2 diabetics. Int J Clin Exp Med 2015;8:6236–41. [PMC free article] [PubMed] [Google Scholar]
- 34. Firneisz G, Varga T, Lengyel G, et al. Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: a novel liver disease biomarker. PLoS One 2010;5:e12226 10.1371/journal.pone.0012226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhong J, Rao X, Deiuliis J, et al. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 2013;62:149–57. 10.2337/db12-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aso Y, Terasawa T, Kato K, et al. The serum level of soluble CD26/dipeptidyl peptidase 4 increases in response to acute hyperglycemia after an oral glucose load in healthy subjects: association with high-molecular weight adiponectin and hepatic enzymes. Transl Res 2013;162:309–16. 10.1016/j.trsl.2013.07.011 [DOI] [PubMed] [Google Scholar]
- 37. Chandalia M, Cabo-Chan AV, Devaraj S, et al. Elevated plasma high-sensitivity C-reactive protein concentrations in Asian Indians living in the United States. J Clin Endocrinol Metab 2003;88:3773–6. 10.1210/jc.2003-030301 [DOI] [PubMed] [Google Scholar]
- 38. Shrivastava U, Misra A, Mohan V, et al. Obesity, diabetes and cardiovascular diseases in India: public health challenges. Curr Diabetes Rev 2017;13:65–80. 10.2174/1573399812666160805153328 [DOI] [PubMed] [Google Scholar]
- 39. Gupta R, Misra A. Epidemiology of microvascular complications of diabetes in South Asians and comparison with other ethnicities. J Diabetes 2016;8:470–82. 10.1111/1753-0407.12378 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2017-000393supp001.docx (47.7KB, docx)