Abstract
Increased oxidative stress is implicated in the pathogenesis of experimental diabetic neuropathy, but translational evidence in recent-onset diabetes is scarce. We aimed to determine whether markers of systemic oxidative stress are associated with diabetic sensorimotor polyneuropathy (DSPN) in recent-onset diabetes. In this cross-sectional study, we measured serum concentrations of extracellular superoxide dismutase (SOD3), thiobarbituric acid reactive substances (TBARS), and reduced glutathione (GSH) in 107 type 1 and 215 type 2 diabetes patients from the German Diabetes Study baseline cohort and 37 glucose-tolerant individuals (controls). DSPN was defined by electrophysiological and clinical criteria (Toronto Consensus, 2011). SOD3 and GSH concentrations were lower in individuals with type 1 and type 2 diabetes compared with concentrations in controls (P<0.0001). In contrast, the TBARS concentration was higher in participants with type 1 diabetes and type 2 diabetes compared with levels in controls (P<0.0001). In addition, the SOD3 concentration was higher in participants with type 1 diabetes compared to concentrations in those with type 2 diabetes (P<0.0001). A low SOD3 concentration was associated with DSPN in individuals with type 1 diabetes (β=−0.306, P=0.002), type 2 diabetes (β=−0.164, P=0.017), and in both groups combined (β=−0.206, P=0.0003). Lower SOD3 concentrations were associated with decreased motor nerve conduction velocity (NCV) in men and, to a lesser degree, with reduced sensory NCV in women with diabetes. In conclusion, several biomarkers of oxidative stress are altered in recent-onset diabetes, with only a lower SOD3 concentration being linked to the presence of DSPN, suggesting a role for reduced extracellular antioxidative defense against superoxide in the early development of DSPN.
Introduction
Diabetic sensorimotor polyneuropathy (DSPN) is one of the most common and severe diabetes complications, associated with substantial morbidity, increased mortality and reduced quality of life.1 A decreased cellular protection through antioxidants can result in oxidative stress and cell death. Reactive oxygen species, including mitochondrial overproduction of superoxide, have been linked to the development of diabetic microvascular complications, including experimental neuropathy.2 Superoxide dismutases (SODs) are the primary antioxidant defense system, catalyzing dismutation of superoxide into O2 and H2O2.3 In mammals, there are cytosolic (SOD1), mitochondrial (SOD2) and extracellular (SOD3) isoforms that are all products of distinct genes.4 Reduced glutathione (GSH) is another important antioxidant, which functions, among other roles, as a co-substrate for glutathione peroxidase activity.5 Thiobarbituric acid reactive substances (TBARS) represent an indirect measure of early-stage lipid peroxidation triggered by oxidative stress.6, 7 A range of experimental studies indicate that oxidative stress is a major factor contributing to deficits in nerve conduction and nerve blood flow in diabetic neuropathy.7 In line with experimental evidence, we demonstrated previously that increased plasma superoxide generation is associated with the severity of DSPN8 and may predict the progression of nerve conduction slowing and cardiac autonomic dysfunction over six years in longer-term diabetes patients.9 In recently diagnosed type 2 diabetes, we found increased dermal expression of mitochondrial SOD2 in the lower limbs.10 In the present study, we aimed to determine whether markers of systemic oxidative stress and antioxidative defense are altered in relation to the presence of early DSPN in individuals with recently diagnosed type 1 or type 2 diabetes.
Materials and methods
Participants
Volunteers with type 1 diabetes (n=107) or type 2 diabetes (n=215) were consecutive participants from the prospective German Diabetes Study (GDS), which evaluates the long-term course of diabetes and its sequelae (ClinicalTrials.gov Identifier: NCT01055093). Inclusion criteria for entry into the GDS were type 1 diabetes or type 2 diabetes, known diabetes duration of ⩽1 year, and age ⩾18 years. Exclusion criteria were secondary diabetes, pregnancy, severe diseases such as cancer, psychiatric disorders, immunosuppressive therapy, limited cooperation ability or neuropathy from causes other than diabetes. The baseline cohort profile has been published elsewhere.11 Glucose-tolerant individuals were recruited as controls also at the German Diabetes Center (n=37; sex (male): 54.1% age: 40.4±2.5 years; BMI: 25.2±0.6; smokers: 13.5%). Inclusion criteria for this group were age ⩾18 years, while exclusion criteria corresponded to those defined in the persons with diabetes or an abnormal oral glucose tolerance test.
This cross-sectional study was approved by the local ethics committee (Heinrich Heine University, Düsseldorf). All participants provided a written informed consent according to the Declaration of Helsinki.
Peripheral nerve function and neurological examination
Peripheral nerve function tests were performed as previously described.12 Motor nerve conduction velocity (MNCV) was measured in the median, ulnar and peroneal nerves, while sensory nerve conduction velocity (SNCV) was determined in the median, ulnar and sural nerves at a skin temperature of 33–34 °C using surface electrodes (Nicolet VikingQuest, Natus Medical, San Carlos, CA, USA). Quantitative sensory testing was evaluated by vibration perception threshold (VPT) at the second metacarpal bone and medial malleolus using the method of limits (Vibrameter, Somedic, Stockholm, Sweden) and by thermal detection thresholds (TDT) including warm and cold thresholds at the thenar eminence and dorsum of the foot using the method of limits (TSA-II NeuroSensory Analyzer, Medoc, Ramat Yishai, Israel).12 Neurological examination was performed using the Neuropathy Disability Score (NDS) and Neuropathy Symptom Score (NSS).13
Stages of DSPN were defined according to modified Toronto Consensus criteria.14 Subclinical DSPN (Stage 1a): NDS⩽2 points, NSS⩽2 points, and peroneal motor nerve conduction velocity (MNCV)<2.5th percentile and sural sensory nerve conduction velocity (SNCV) and/or sural sensory nerve amplitude (SNAP)<2.5th percentile; confirmed asymptomatic DSPN (Stage 1b): NDS⩾3 points and NSS⩽2 points and peroneal MNCV<2.5th percentile and sural SNCV<2.5th percentile and/or sural SNAP<2.5th percentile; confirmed symptomatic DSPN (Stage 2): NSS⩾3 points and peroneal MNCV<2.5th percentile and sural SNCV<2.5th percentile and/or sural SNAP<2.5th percentile.
Markers of oxidative stress
Extracellular superoxide dismutase (SOD3) concentration in serum samples was measured using the human Enzyme-linked Immunosorbent Assay kit for SOD3 (Hölzel Diagnostika, Germany) according to the manufacturer’s instructions. All samples were analyzed in duplicate. TBARS were assessed, as described before.15 In brief, the quantification of malonaldehyde in serum samples was performed using a TBARS assay (Sigma Aldrich, Germany) according to the manufacturer’s instructions. Serum concentration of reduced glutathione (GSH) was assessed by the enzymatic recycling method using the Sensolyte Glutathione assay kit (Anaspec, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
Categorical variables were compared using the χ2 test. Continuous data were analyzed using the Nonparametric Mann–Whitney U test. Categorical data were expressed as percentages of participants with 95% confidence intervals (CI) while continuous data were expressed as the mean ±s.e.m. Correlation and multiple linear regression analyses were performed to determine associations between two variables. Dependent variables were assessed for normality and transformed (natural logarithm or square root) where appropriate to obtain normal distribution. The full models were adjusted for sex, age, BMI and HbA1c. All statistical tests were performed two-sided. The level of significance was set at α=0.05. All analyses were performed using the SPSS v22.0 (Chicago, IL, USA).
Results
Anthropometric, demographic, clinical and functional data of the two diabetes groups are shown in Table 1. Participants with type 1 diabetes were younger, taller, and had lower BMI, lower diastolic blood pressure, and longer known diabetes duration than type 2 diabetes individuals (all P<0.05). There were no differences in neurophysiological function between both groups. DSPN was present in 21.5% of type 1 diabetes (subclinical: 17.8% confirmed asymptomatic: 0.9% confirmed symptomatic: 2.8%) individuals and 30.2% (16.3/7.0/6.9%) of those with type 2 diabetes.
Table 1. Anthropometric, demographic, clinical, and neurophysiological data of the individuals with diabetes studied.
| Type 1 diabetes (n=107) | Type 2 diabetes (n=215) | P-value | |
|---|---|---|---|
| Age (years) | 34.4±1.3 | 52.8±0.7 | <0.0001 |
| Sex (% male) | 57.0 | 66.0 | NS |
| BMI (kgm−2) | 24.6±0.4 | 31.7±0.4 | <0.0001 |
| Height (cm) | 175±1.0 | 172±0.6 | 0.014 |
| Heart rate (b.p.m.) | 79.3±1.4 | 80.8±0.9 | NS |
| Systolic BP (mmHg)a | 130±1.5 | 142±1.3 | NS |
| Diastolic BP (mmHg)a | 77.8±1.0 | 85.0±0.8 | 0.05 |
| Regular smokers (%) | 19.6 | 18.1 | NS |
| Diabetes duration (months) | 6.62±0.30 | 5.55±0.20 | 0.005 |
| HbA1c (mmolmol−1) | 50.6±1.5 | 47.4±0.7 | NS |
| HbA1c (%) | 6.78±0.14 | 6.48±0.06 | NS |
| Peroneal MNCV (m s−1)a | 46.4±0.4 | 44.4±0.3 | NS |
| Sural SNCV (ms−1)a | 45.5±0.5 | 43.9±0.4 | NS |
| Malleolar VPT (μm)a | 0.62±0.03 | 2.19±0.20 | NS |
| Warm TDT foot (°C)a | 38.2±0.3 | 40.4±0.3 | NS |
| Cold TDT foot (°C)a | 28.6±0.2 | 27.0±0.3 | NS |
| NSSa | 0.22±0.10 | 1.00±0.15 | NS |
| NDSa | 0.45±0.12 | 1.72±0.15 | NS |
| DSPN (%) | 21.5 | 30.2 | NS |
Abbreviations: DSPN, diabetic sensorimotor polyneuropathy; MNCV, motor nerve conduction velocity; NDS, Neuropathy Disability Score; NSS, Neuropathy Symptom Score; NS, not significant; SNCV, sensory nerve conduction velocity; TDT, thermal detection threshold; VPT, vibration perception threshold.
Data are expressed as the mean ±s.e.m. except for sex, regular smokers and DSPN variables, which are expressed as %. Mann–Whitney-U-test was used where appropriate to calculate differences between groups. P<0.05 was considered significant.
Group comparisons were adjusted for age, sex, BMI, smoking, known diabetes duration, and HbA1c.
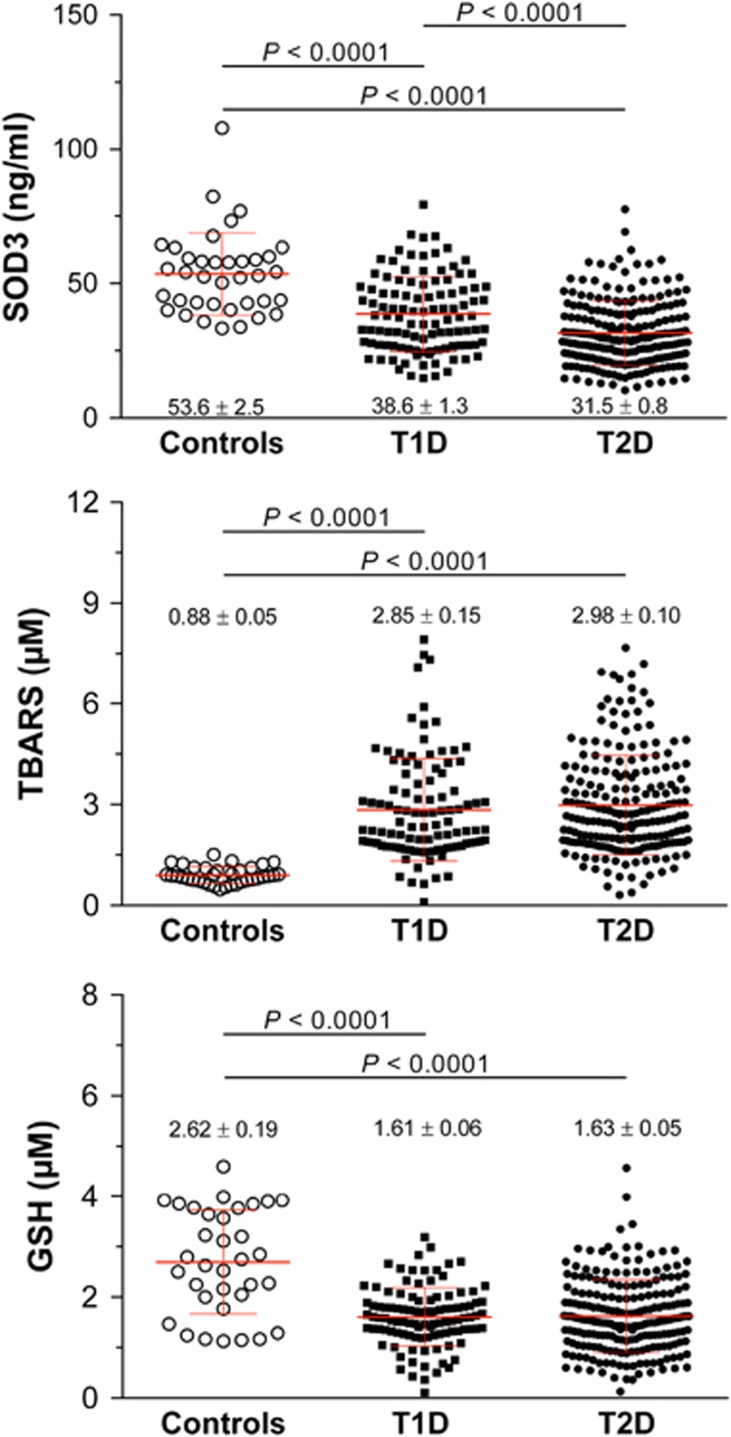
The mean concentrations of SOD3, TBARS and GSH are also shown in Figure 1. After adjustment for age, sex, BMI and HbA1c, SOD3 concentration was reduced in both type 1 (38.6±1.3 ng ml−1) and type 2 diabetes (31.5±0.8 ng ml−1) patients compared with controls (53.6±2.5 ng ml−1), as well as in type 2 diabetes patients compared with type 1 diabetes patients (P<0.0001). GSH was also decreased in both type 1 diabetes (1.61±0.06 μmol) and type 2 diabetes (1.63±0.05 μmol) patients compared with controls (2.62±0.19 μmol). In contrast, TBARS concentration was higher in type 1 (2.85±0.15 μmol) and type 2 diabetes (2.98±0.10 μmol) patients compared with controls (0.88±0.05 μmol) (P<0.0001 for type 1 and type 2 diabetes vs controls for both markers).
Figure 1.
Serum concentrations of SOD3, GSH and TBARS in recently diagnosed diabetes patients and individuals with normal glucose tolerance (controls). Each spot represents one individual. The horizontal line represents the mean, while the whiskers show the SD. P values adjusted for age, sex, BMI and HbA1c are given. (T1D, type 1 diabetes; T2D, type 2 diabetes).
The entire study population showed a positive correlation of SOD3 with peroneal, median and ulnar MNCV, sural, median and ulnar SNCV, median and ulnar SNAP, and an inverse correlation of SOD3 with NDS and DSPN (all P<0.05), which remained significant after adjustment for age, sex, BMI and HbA1c for all parameters, except for median MNCV and SNAP (Table 2). In the type 1 diabetes group, SOD3 concentrations showed positive correlations with peroneal MNCV, sural, median and ulnar SNCV, and median SNAP and an inverse association with DSPN (all P<0.05), which remained significant after adjustment for all parameters, except for median SNCV. In the type 2 diabetes group, only a positive correlation of SOD3 concentrations with ulnar MNCV and an inverse correlation with DSPN were observed (both P<0.05), which remained significant after adjustment. No associations of GSH and TBARS with neurophysiological parameters were found. SOD3 concentrations were markedly higher in patients without DSPN compared to those with DSPN.
Table 2. Correlations of serum SOD3 concentration with neurophysiological and clinical measures and diabetic sensorimotor polyneuropathy (DSPN).
|
All participants |
Type 1 diabetes |
Type 2 diabetes |
||||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| Peroneal MNCV | 0.153 | 0.006a | 0.213 | 0.028a | 0.073 | 0.287 |
| Median MNCV | 0.188 | 0.001 | 0.162 | 0.096 | 0.103 | 0.133 |
| Ulnar MNCV | 0.176 | 0.002a | 0.091 | 0.351 | 0.171 | 0.012a |
| Sural SNCV | 0.114 | 0.040a | 0.204 | 0.035a | 0.040 | 0.561 |
| Sural SNAP | 0.048 | 0.388 | 0.159 | 0.101 | −0.104 | 0.130 |
| Median SNCV | 0.174 | 0.002a | 0.219 | 0.024 | 0.071 | 0.304 |
| Median SNAP | 0.161 | 0.004 | 0.295 | 0.002a | 0.001 | 0.993 |
| Ulnar SNCV | 0.162 | 0.004a | 0.231 | 0.018a | 0.124 | 0.071 |
| Ulnar SNAP | 0.162 | 0.004a | 0.142 | 0.148 | 0.050 | 0.467 |
| NSS* | −0.081 | 0.146 | −0.116 | 0.234 | −0.015 | 0.830 |
| NDS* | −0.109 | 0.05 | −0.021 | 0.827 | −0.022 | 0.744 |
| DSPN* | −0.216 | 0.0001a | −0.296 | 0.002a | −0.143 | 0.036a |
Abbreviations: MNCV, motor nerve conduction velocity; NDS, Neuropathy Disability Score; NSS, Neuropathy Symptom Score; SNAP, sensory nerve action potential; SNCV, sensory nerve conduction velocity; TDT, thermal detection threshold; VPT, vibration perception threshold.
Pearson correlation coefficients were used to determine correlations between two variables (*Spearman correlation coefficients were applied). P<0.05 (bold) was considered significant.
Association remained significant after multiple linear regression analyses with adjustments for age, sex, BMI, smoking, known diabetes duration and HbA1c.
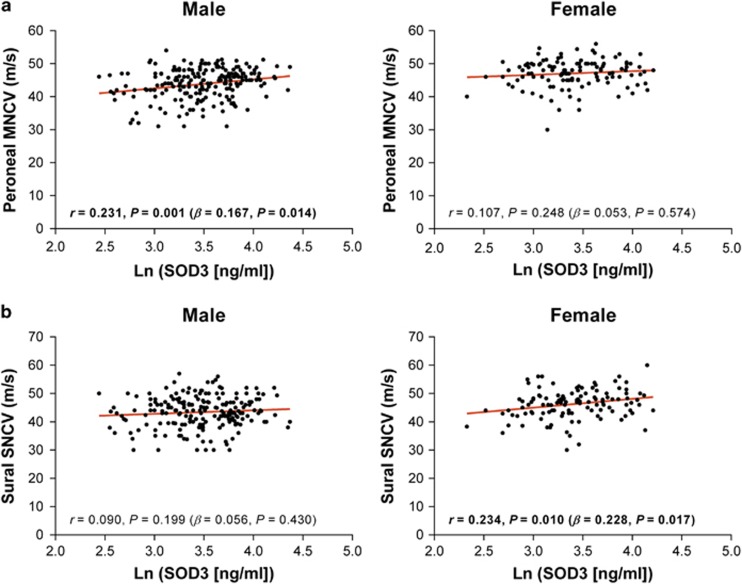
Gender-specific correlations of serum SOD3 concentrations with neurophysiological and clinical parameters and DSPN were also observed (Table 3 and Figure 2). In men, SOD3 correlated positively with peroneal, median, and ulnar MNCV, median SNCV, median SNAP and ulnar SNAP and inversely with NDS and DSPN (all P<0.05). In contrast, women showed only positive correlations of SOD3 with sural and ulnar SNCV and an inverse correlation with DSPN (all P<0.05). For all three parameters, the associations observed in women remained significant after adjustment, while in men, the associations remained significant for peroneal, median, and ulnar MNCV and DSPN. There was no difference between men and women in mean SOD3 concentration (34.6±0.9 vs 32.6±1.2 ng ml−1).
Table 3. Gender-specific correlations of serum SOD3 concentrations with neurophysiological and clinical parameters and DSPN.
|
Male (n=203) |
Female (n=119) |
|||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Peroneal MNCV | 0.231 | 0.001a | 0.107 | 0.248 |
| Median MNCV | 0.269 | 0.0001a | 0.074 | 0.422 |
| Ulnar MNCV | 0.245 | 0.0004a | 0.112 | 0.224 |
| Sural SNCV | 0.090 | 0.199 | 0.234 | 0.010a |
| Sural SNAP | −0.009 | 0.895 | −0.171 | 0.063 |
| Median SNCV | 0.197 | 0.005 | 0.151 | 0.105 |
| Median SNAP | 0.170 | 0.016 | 0.171 | 0.066 |
| Ulnar SNCV | 0.130 | 0.065 | 0.246 | 0.008a |
| Ulnar SNAP | 0.212 | 0.003 | 0.148 | 0.113 |
| NSS* | −0.122 | 0.083 | −0.015 | 0.869 |
| NDS* | −0.143 | 0.041 | −0.071 | 0.443 |
| DSPN* | −0.225 | 0.001a | −0.199 | 0.030a |
Abbreviations: MNCV, motor nerve conduction velocity; NDS, Neuropathy Disability Score; NSS, Neuropathy Symptom Score; SNAP, sensory nerve action potential; SNCV, sensory nerve conduction velocity; TDT, thermal detection threshold; VPT, vibration perception threshold.
Pearson correlation coefficients were used to determine correlations between two variables (*Spearman correlation coefficients were applied). P<0.05 (bold) was considered significant.
Association remained significant after multiple linear regression analyses with adjustments for age, sex, BMI, smoking, known diabetes duration and HbA1c.
Figure 2.
Examples of gender-specific correlations of SOD3 with electrophysiological parameters in recently diagnosed diabetes patients. (a) Correlation of SOD3 concentrations with peroneal motor nerve conduction velocity (MNCV) in male, but not female, recently diagnosed diabetes patients. (b) Correlation of SOD3 concentrations with sural sensory nerve conduction velocity (SNCV) in female, but not male, patients. Each spot represents one individual. Analyses were adjusted for age, sex, BMI, smoking and HbA1c.
Discussion
This study demonstrates that patients with well-controlled, recent-onset type 1 or type 2 diabetes exhibit markedly decreased serum SOD3 and GSH concentrations and increased TBARS levels, but only SOD3 levels are associated with early changes in motor and sensory nerve conduction attributes and the presence of DSPN defined by electrophysiological criteria. This novel finding is in line with the hypothesis suggesting that a single hyperglycemia-induced process of overproduction of superoxide by the mitochondrial electron transport chain plays a pivotal role in the pathogenesis of experimental diabetic neuropathy.6 Since these associations were found despite favorable glycemic control, with mean HbA1c levels of 6.8 and 6.5% in type 1 and type 2 diabetes, respectively, it is conceivable that only mild degrees of hyperglycemia are sufficient to trigger the neuropathic process.
SOD3 is a secretory extracellular isoform and the major SOD located in the vascular extracellular space, where it plays an important role in maintaining endothelial function.16 In vitro, SOD3 secreted in response to inflammatory cytokines by human mesenchymal stem cells promotes neuronal survival.17 Therefore, decreased SOD3 concentration could lead to endothelial dysfunction and neurotoxicity mediated by inflammatory cytokines18 and be, at least partially, responsible for the development of early DSPN in recent-onset type 1 and type 2 diabetes patients.
Interestingly, SOD3 concentrations were more strongly associated with electrophysiological parameters in men than in women. Moreover, after adjustment for potential confounders, lower SOD3 levels were associated with lower MNCV in men and lower SNCV in women. These findings are in line with other studies demonstrating gender differences in the risk factors associated with diabetes complications including DSPN.19, 20 The reasons for the differences between the genders are still unclear. Although HDL cholesterol levels were considerably higher in women than men (1.51±0.40 vs 1.23±0.35 mmol l−1; P<0.0001), this finding cannot explain the difference, since higher HDL cholesterol was not associated with higher NCV. Future studies are needed to determine the mechanisms (for example, hormone composition or metabolic status) responsible for these discrepancies.
Insufficient cellular antioxidative defense mechanisms could be involved in processes of neuronal damage. Nerve damage and neuronal apoptosis are associated with reduced GSH concentrations and elevated oxidized lipoprotein concentrations.7 There are no published studies assessing the association between oxidative stress and DSPN in recent-onset diabetes. Previous studies showed that among patients with long-term type 2 diabetes, those with DSPN had lower cellular GSH and SOD levels compared with those without DSPN,21, 22 whereas other studies showed higher erythrocyte SOD levels in individuals with type 1 and type 2 diabetes compared with control subjects.23, 24 Thus, our study including recently diagnosed diabetes patients does not confirm these reports. Reasons for these discrepancies could be the different study populations and samples analyzed. While these studies used circulating mononuclear cells to measure the levels of oxidative stress markers, we used serum samples.
The strengths of the present study are the large study sample size and the use of electrophysiological criteria to confirm the presence of DSPN used in addition to scores for clinical symptoms and signs. However, due to the cross-sectional nature of the present analysis, it was not possible to assess the predictive value of reduced antioxidative defense for incident DSPN.
In conclusion, patients with recently diagnosed type 1 and type 2 diabetes show evidence of systemic oxidative stress despite good glycemic control. Reduced SOD3 concentration was associated with diminished nerve conduction attributes and DSPN, pointing to a role of impaired extracellular antioxidative defense of superoxide in the evolution of early DSPN. Prospective studies should determine the long-term predictive value of SOD3 serum levels in the development and progression of DSPN.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We wish to thank the staff of the GDS, especially M Behler, B Ringel and M Schroers-Teuber, for their excellent technical support. The German Diabetes Study (GDS) was initiated and financed by the German Diabetes Center, which is funded by the German Federal Ministry of Health (Berlin, Germany), the Ministry of Innovation, Science, Research and Technology of the state North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD eV).
Footnotes
The authors declare no conflict of interest.
References
- Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handbook Clin Neurol 2014; 126: 3–22. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011; 7: 573–583. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 1995; 64: 97–112. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 2002; 33: 337–349. [DOI] [PubMed] [Google Scholar]
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxic 2003; 17: 24–38. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 2004; 25: 612–628. [DOI] [PubMed] [Google Scholar]
- Ziegler D, Sohr CG, Nourooz-Zadeh J. Oxidative stress and antioxidant defense in relation to the severity of diabetic polyneuropathy and cardiovascular autonomic neuropathy. Diabetes Care 2004; 27: 2178–2183. [DOI] [PubMed] [Google Scholar]
- Ziegler D, Buchholz S, Sohr C, Nourooz-Zadeh J, Roden M. Oxidative stress predicts progression of peripheral and cardiac autonomic nerve dysfunction over 6 years in diabetic patients. Acta Diabetol 2015; 52: 65–72. [DOI] [PubMed] [Google Scholar]
- Ziegler D, Strom A, Bruggemann J, Ziegler I, Ringel B, Puttgen S et al. Overexpression of cutaneous mitochondrial superoxide dismutase in recent-onset type 2 diabetes. Diabetologia 2015; 58: 1621–1625. [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Saxena A, Weber KS, Strassburger K, Herder C, Burkart V et al. Cohort profile: the German Diabetes Study (GDS). Cardiovasc Diabetol 2016; 15: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014; 63: 2454–2463. [DOI] [PubMed] [Google Scholar]
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993; 36: 150–154. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Albers JW, Andersen H, Arezzo JC, Biessels GJ, Bril V et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 2011; 27: 620–628. [DOI] [PubMed] [Google Scholar]
- Jelenik T, Séquaris G, Kaul K, Ouwens DM, Phielix E, Kotzka J et al. Tissue-specific differences in the development of insulin resistance in a mouse model for Type 1 diabetes. Diabetes 2014; 63: 3856–3867. [DOI] [PubMed] [Google Scholar]
- Iida S, Chu Y, Francis J, Weiss RM, Gunnett CA, Faraci FM et al. Gene transfer of extracellular superoxide dismutase improves endothelial function in rats with heart failure. Am J Physiol Heart Circ Physiol 2005; 289: H525–H532. [DOI] [PubMed] [Google Scholar]
- Kemp K, Gray E, Mallam E, Scolding N, Wilkins A. Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Rev 2010; 6: 548–559. [DOI] [PubMed] [Google Scholar]
- Schamarek I, Herder C, Nowotny B, Carstensen-Kirberg M, Strassburger K, Nowotny P et al. Adiponectin, markers of subclinical inflammation and nerve conduction in individuals with recently diagnosed type 1 and type 2 diabetes. Eur J Endocrinol 2016; 174: 433–443. [DOI] [PubMed] [Google Scholar]
- Forsberg E, Xu C, Grünler J, Frostegård J, Tekle M, Brismar K et al. Coenzyme Q10 and oxidative stress, the association with peripheral sensory neuropathy and cardiovascular disease in type 2 diabetes mellitus. J Diab Complications 2015; 29: 1152–1158. [DOI] [PubMed] [Google Scholar]
- Schneider MP, Ritt M, Raff U, Ott C, Schmieder RE. Gender is related to alterations of renal endothelial function in type 2 diabetes. Nephrol Dial Transplant 2009; 24: 3354–3359. [DOI] [PubMed] [Google Scholar]
- Kasznicki J, Kosmalski M, Sliwinska A, Mrowicka M, Stanczyk M, Majsterek I et al. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep 2012; 39: 8669–8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MM, Folgado J, Tormo C, Artero A, Ascaso M, Martinez-Hervas S et al. Altered glutathione system is associated with the presence of distal symmetric peripheral polyneuropathy in type 2 diabetic subjects. J Diab Complications 2015; 29: 923–927. [DOI] [PubMed] [Google Scholar]
- Dominguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 1998; 21: 1736–1742. [DOI] [PubMed] [Google Scholar]
- Seghrouchni I, Drai J, Bannier E, Riviere J, Calmard P, Garcia I et al. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. Clin Chim Acta 2002; 321: 89–96. [DOI] [PubMed] [Google Scholar]