Abstract
The gene encoding the high glycine/tyrosine keratin-associated protein 20-2 (KAP20-2) gene has been described in humans, but has not been identified in any livestock species. A search for similar sequences in the caprine genome using the human KAP20-2 gene (KRTAP20-2) revealed a homologous sequence on chromosome 1. Three different banding patterns representing distinct sequences (A–C) in Longdong cashmere goats were identified using polymerase chain reaction-single stranded conformational polymorphism (PCR-SSCP) analysis. These sequences shared high sequence similarity with the human and mouse KRTAP20-2 sequences, suggesting that A–C are caprine variants of the human and mouse genes. Four single nucleotide polymorphisms (SNPs) were identified, and three of them were non-synonymous. KRTAP20-2 was found to be expressed in secondary hair follicles, but not in heart, liver, lung, kidney, spleen, or longissimus dorsi muscle. The presence of A was associated with increased cashmere fibre weight, while the presence of B was associated with a decrease in cashmere fibre weight and curly fibre length. Goats with genotype AA had a higher cashmere fibre weight and a higher curly fibre length than those with genotypes AB or BB. These results indicate that caprine KRTAP20-2 variation may have value as a genetic marker for improving cashmere fibre weight.
Keywords: cashmere fibre weight, keratin-associated protein 20-2 (KAP20-2) gene, length of the curly fibre, Longdong cashmere goat, tissue expression, variation
1. Introduction
Cashmere goat fibre is a heterogeneous fleece comprised of wool and cashmere fibres, which are produced by the primary and secondary hair follicles, respectively. Due to its characteristics of being finer, lighter, softer, stronger, and having better elasticity and insulating properties, cashmere prices are typically more stable and higher than wool and mohair prices, and it is therefore considered a luxury product. Of all the cashmere traits of value, weight and mean fibre diameter are the most important traits economically, and they underpin most of the commercial return to cashmere producers [1]. It is well known that variation in cashmere traits is controlled by both genetic and environmental factors, so the identification of genes that regulate cashmere quantity and quality offers an opportunity to improve cashmere production.
Morphologically, cashmere fibre is composed of an external cuticular sheath and an inner cortex. Keratins and keratin-associated proteins (KAPs) are believed to play an important role in defining the physico-mechanical properties of the fibre, and fibre growth involves the expression of both the keratin and KAP genes [2].
In sheep, the KAPs can be classified into three broad groups according to their amino acid composition: the high sulphur (HS; ≤30 mol% cysteine), the ultra-high sulphur (UHS; >30 mol% cysteine) and the high glycine/tyrosine (HGT; 35–60 mol% glycine and tyrosine) KAPs [3]. To date, over 100 KAPs have been identified across species, and they have been categorized into 27 families [4].
Up to now, research into the KAP genes (KRTAPs) has been primarily undertaken in humans and sheep. For example, at least 80 KRTAPs have been categorised into 25 families in humans [5,6]. In sheep, 29 KRTAPs from 13 families have been identified [3,7,8]. Only 10 KAP genes have been described in goats, despite there being 17 caprine KRTAP sequences in the NCBI GenBank database. The published caprine KRTAP sequences include KRTAP1-1 [9], KRTAP1-4 [10], KRTAP6-2 [11], KRTAP7-1 [12], KRTAP8-1 [13], KRTAP8-2 [12], KRTAP9-2 [14], KRTAP11-1 [15], KRTAP13-1 [16], and KRTAP13-3 [9].
Although only a few KRTAPs have been identified in goats, two KRTAPs (KRTAP13-1 and KRTAP8-2) have been reported to be associated with cashmere quality and quantity traits, including fibre diameter, weight, and the length of the curly fibre [16,17]. In the closely related species Ovis aries (sheep), more studies of KRTAP variation have been undertaken [3,18], and its association with wool traits has been described, including associations between variation in KRTAP1-2 [19], KRTAP6-1 [20,21], KRTAP6-3 [22], KRTAP8-2 [23], and KRTAP22-1 [7], and variation in commercially important wool traits. This suggests that the identification of new caprine KRTAPs, variation in these genes, and any effect of observed variation on cashmere traits are worthy of investigation.
The KAP20 family has been described in humans [24]. It consists of two members, KAP20-1 and KAP20-2, and the genes producing these KAPs are expressed in the matrix and pre-cortex of developing hair fibres [25]. In this study, we describe the identification of a caprine KAP20 gene, and reveal variation in that gene using polymerase chain reaction-single stranded conformational polymorphism (PCR-SSCP). We also confirm the expression of this gene using reverse transcription-PCR (RT-PCR), and investigate associations between variation in the gene and variation in cashmere traits in Longdong cashmere goats.
2. Materials and Methods
2.1. Animals, Animal Tissues and Data Collected
All animal work was conducted according to the guidelines for the care and use of experimental animals established by the Ministry of Science and Technology of the People’s Republic of China (Approval number 2006–398), and was approved by the Animal Care Committee of Gansu Agricultural University.
A total of 373 Longdong cashmere goats from the progeny of 11 unrelated sires were investigated. The goats were reared at the Yusheng Cashmere Goat Breeding Company in Huan County, Gansu Province. At 12 months of age (first combing), the combed cashmere weight and the length of the curly cashmere fibre from the mid-side region were measured. Samples were also collected from the mid-side region to enable measurement of the mean fibre diameter of the fibre at the Inner Mongolia Agricultural University, Inner Mongolia, China. Blood samples from these goats were collected onto Munktell TFN paper (Munktell Filter AB, Falun, Sweden).
Three separate three-year-old Longdong cashmere goats in the catagen phase of fibre growth were slaughtered, and tissue samples were collected from each, including samples of skin, heart, liver, lung, kidney, spleen, and longissimus dorsi muscle. The tissue samples were immediately frozen in liquid nitrogen and stored at −80 °C. Secondary hair follicles were separated from the skin tissue using the method described by Jin et al. [12].
2.2. Search for Caprine Sequences Homologous to the Human KAP20-2 Gene
The coding region of a human KRTAP20-2 sequence (GenBank accession no. NM_181616) was used to BLAST search the caprine genome assembly GCF_001704415.1 [26]. The sequence that shared the greatest similarity with the human KRTAP20-2 sequence was presumed to be caprine KRTAP20-2.
2.3. PCR-SSCP Analysis of Caprine KRTAP20-2
The goat genome sequence was used to design PCR primers (Table 1). The entire coding region of the putative caprine KRTAP20-2 was amplified. These primers were synthesized by Takara Biotechnology Company Limited (Dalian, China). Goat genomic DNA for PCR amplification was purified from 1.2 mm punches of dried blood spots collected on TFN cards using a two-step washing procedure [27]. Amplifications were performed in a 20-μL reaction consisting of the genomic DNA purified from one 1.2-mm punch of dried blood, 2.0 μL of 10 × PCR buffer (Supplied with the DNA polymerase enzyme), 0.25 μM of each primer, 150 μM of each deoxynucleotides (dNTPs) (Takara), 2.5 mM Mg2+, 0.5 U of Taq DNA polymerase (Takara), and double-distilled water (ddH2O) to make up the volume. The thermal profile consisted of 2 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 59 °C, and 30 s at 72 °C, with a final extension of 5 min at 72 °C. Amplification was carried out in Bio-Rad S1000 thermal cyclers (Bio-Rad, Hercules, CA, USA).
Table 1.
PCR primers used in this study.
| Gene | Sequence (5′-3′) | Amplicon Size (bp) | Purpose of Primers |
|---|---|---|---|
| KRTAP20-2 | CAGACTATAGAGACAGATTCC CCAATTAGTTGAGTTTCTCTG |
273 | Gene identification and SSCP analysis |
| KRTAP20-2 | TGGAAACTACTATGGCGGCC TATCTTCTGCAACAGGATGG |
156 | Reverse transcription (RT)-PCR |
| β-actin | AGCCTTCCTTCCTGGGCATGGA GGACAGCACCGTGTTGGCGTAGA |
113 | RT-PCR |
bp: base pairs; SSCP: single stranded conformational polymorphism.
A 0.7-μL aliquot of each amplicon was mixed with 7 μL of loading dye (98% formamide, 10 mM ethylenediaminetetraacetic acid (EDTA), 0.025% bromophenol blue, 0.025% xylene-cyanol), and after denaturation at 95 °C for 5 min, samples were rapidly cooled on wet ice, and then loaded on 16 cm × 18 cm, 12% acrylamide:bisacrylamide (37.5:1) (Bio-Rad) gels. Electrophoresis was performed using Protean II xi cells (Bio-Rad) for 17 h in 0.5 × TBE at 210 V and 16.5 °C. The gels were silver-stained according to the method of Byun et al. [28].
2.4. Sequencing of Alleles and Sequence Analyses
Amplicons that were identified as homozygous by SSCP were directly sequenced in both directions at the Beijing Genomics Institute, Beijing, China. Alleles that were only in a heterozygous form were sequenced using an approach described by Gong et al. [29]. Briefly, a band corresponding to the allele was excised as a gel slice from the polyacrylamide gel, macerated, and then used as a template for re-amplification with the original primers. This second amplicon was then sequenced directly.
Sequence alignments, translations, and comparisons were carried out using DNAMAN version 5.2.10 (Lynnon BioSoft, Vaudreuil, QC, Canada). The BLAST algorithm was used to search the NCBI GenBank (http://www.ncbi.nlm.nih.gov/) databases. Potential phosphorylation sites were predicted using the NetPhos 3.1 Server [30], and a phylogenetic tree was constructed using the predicted amino acid sequences of the three new goat KRTAP20-2 sequences and other HGT–KAPs, including: NM_001193399 (sheep KAP6-1), KT725832 (sheep KAP6-2), NM_181604 (human KAP6-2), KT725837 (sheep KAP6-3), KT725840 (sheep KAP6-4), KT725845 (sheep KAP6-5), AY510121 (goat KAP7-1), X05638 (sheep KAP7-1), NM_181606 (human KAP7-1), AY510122 (goat KAP8-1), X05639 (sheep KAP8-1), NM_175857 (human KAP8-1), AY510123 (goat KAP8-2), KF220646 (sheep KAP8-2), NM_181607 (human KAP19-1), NM_181609 (human KAP19-3), NM_181610 (human KAP19-4), NM_181611 (human KAP19-5), NM_00130312 (human KAP19-6), NM_181614 (human KAP19-7), AB096964 (human KAP19-8), NM_181615 (human KAP20-1), NM_181616 (human KAP20-2), NM_001163615 (mouse KAP20-2), NM_181619 (human KAP21-1), NM_181617 (human KAP21-2), KX377616 (sheep KAP22-1), and NM_181620 (human KAP22-1), using MEGA version 7.0.
2.5. Expression of Caprine KRTAP20-2 in Selected Tissues
Total RNA from the seven tissue samples collected was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and the quality and concentration of RNA extracted were checked using 2% agarose gels electrophoresis and UV spectrophotometry. Reverse transcription was performed to produce cDNA using the PrimeScript™ RT Reagent Kit with gDNA Eraser (Perfect Real Time) (Takara), and following the manufacturer’s instructions. The amplification of the cDNA was carried out using another set of PCR primers located within the KRTAP20-2 coding region (TGGAAACTACTATGGCGGCC and TATCTTCTGCAACAGGATGG; Table 1). This enabled amplification of a shorter fragment. This amplification used the same conditions and thermal profile described above for the genomic amplification, but the genomic DNA was replaced by 0.8 μL of the cDNA. The goat β-actin gene was chosen as an internal reference standard, with the PCR primers for the amplification of this sequence described in Table 1. PCR products were examined by electrophoresis in 1.0% agarose gels.
2.6. Statistical Analyses
All analyses were performed using IBM SPSS Statistics version 24.0 (IBM, New York, NY, USA). General linear mixed-effects models (GLMMs) were used to assess whether the presence or absence (coded as 1 or 0 respectively) of KRTAP20-2 alleles was associated with various cashmere traits in the 373 Longdong cashmere goats studied. For genotypes with a frequency >5% (thus providing adequate sample size), a second set of GLMMs were used to ascertain the effect of genotype on various cashmere traits. To reduce the probability of false positive results during the multiple comparisons in these models, a Bonferroni correction was applied. Sire and gender were found to affect (p < 0.05) of all the fibre traits, so they were included in the models as a random and fixed factor, respectively. Birth rank was not found to affect cashmere fibre traits, and was not included in the models. Only the main effects were tested.
3. Results
3.1. Identification of Caprine KRTAP20-2
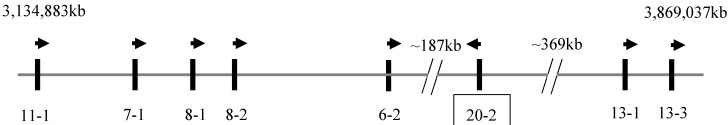
A BLAST search of the caprine genome assembly GCF_001704415.1 using the human KRTAP20-2 coding sequence (NM_181616) revealed a region on goat chromosome 1 (nt 3486283_3486471) that contained a 189-bp open reading frame, and that had 75% nucleotide identity with the human KRTAP20-2 sequence. Seven previously described caprine KAP genes were also identified near this region; these were KRTAP11-1, KRTAP7-1, KRTAP8-1, KRTAP8-2, KRTAP6-2, KRTAP20-2, KRTAP13-1, and KRTAP13-3, in order from the centromere to the telomere (Figure 1).
Figure 1.
Location of the putative KRTAP20-2 (boxed), together with seven other KRTAPs on goat chromosome 1. The vertical bars represent the keratin-associated protein (KAP) genes, and the arrows indicate the direction of transcription. The numbers below these bars are the KAP gene names (e.g., 11-1 represents KRTAP11-1). The spacing of the genes is only approximate, and is based on the caprine genome assembly, as are the nucleotide coordinates [26].
3.2. Detection of Allelic Variation in Caprine KRTAP20-2
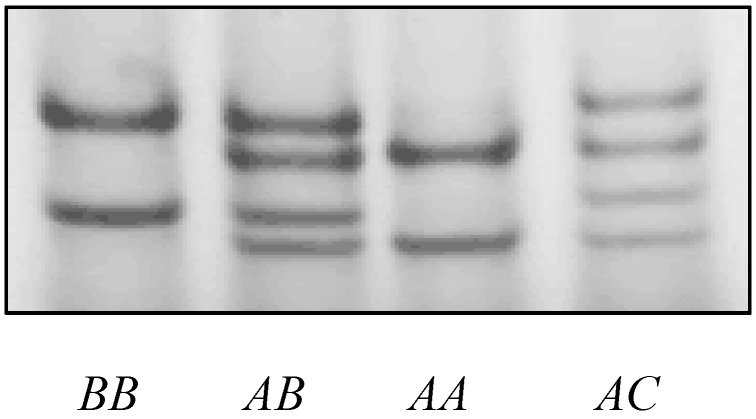
Amplicons of the predicted size (273 bp) were obtained using the SSCP analysis of DNA in the goat blood samples. Three different PCR-SSCP patterns were detected (Figure 2). Either one pattern, or a combination of two patterns, was observed for each goat. Sequencing of amplicons representing the three unique SSCP patterns, revealed three different alleles (named A to C), which differed at the nucleotide level within the 189-bp coding sequence. Allele B was identical to the caprine genome assembly, while the A and C alleles differed from the deposited genome sequence.
Figure 2.
Representative PCR-SSCP patterns for the caprine KAP20-2 gene. Four genotypes are shown, and there are three unique banding patterns, which correspond to three alleles A–C.
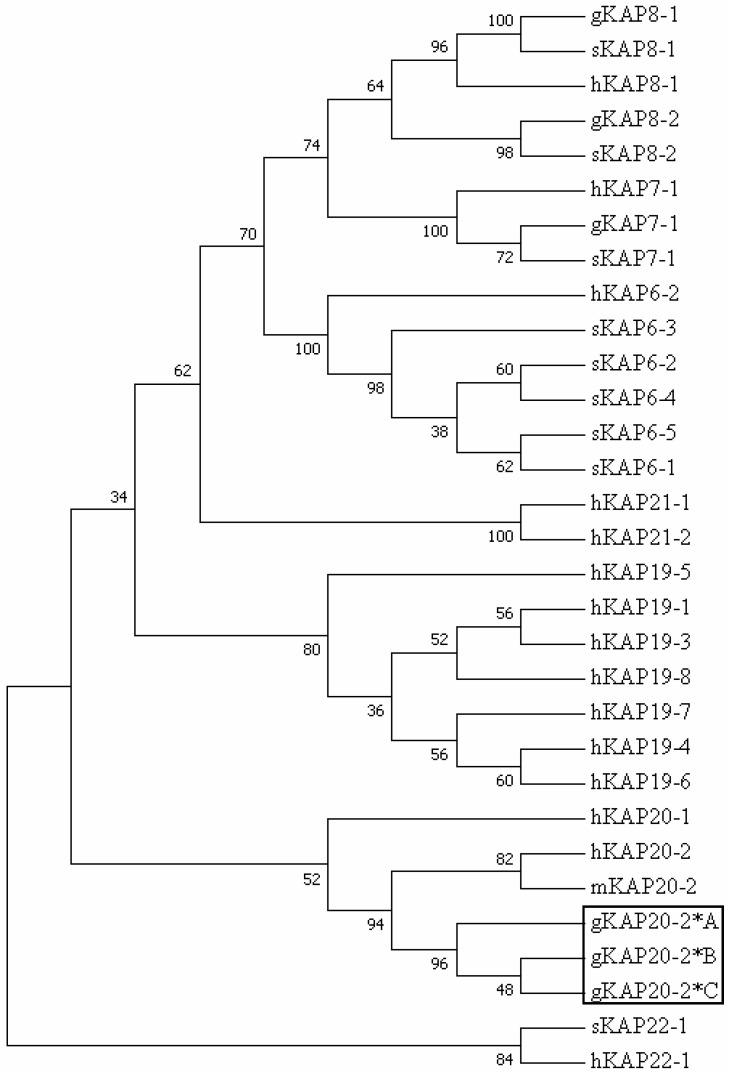
Phylogenetic analysis revealed that the predicted amino acid sequences of the DNA sequences identified were more closely related to KAP20-2 sequences from human and mouse than other HGT-KAP sequences that have been identified in goats, sheep, and humans (Figure 3). This suggests that these goat sequences represent alleles of caprine KRTAP20-2. The alleles were named CAPHI-KRTAP20-2*A to CAPHI-KRTAP20-2*C according to the nomenclature proposed by Gong et al. [31], and the sequences were deposited in GenBank with accession numbers MF973462–MF973464, respectively.
Figure 3.
Maximum parsimony tree of the high glycine/tyrosine keratin-associated proteins (HGT-KAPs) identified in goats, sheep and humans, together with the mouse KAP20-2 sequence. The tree was constructed using the predicted amino acid sequences. The numbers at the forks indicate the bootstrap confidence values. The caprine KAPs are indicated with a prefix “g”, while the sequences of sheep, human, and mouse are indicated with “s”, “h” and “m”, respectively. The three newly identified goat KAP20-2 sequences are shown in a box, and the GenBank accession numbers for the other HGT-KAPs are: NM_001193399 (sKAP6-1), KT725832 (sKAP6-2), NM_181604 (hKAP6-2), KT725837 (sKAP6-3), KT725840 (sKAP6-4), KT725845 (sKAP6-5), AY510121 (gKAP7-1), X05638 (sKAP7-1), NM_181606 (hKAP7-1), AY510122 (gKAP8-1), X05639 (sKAP8-1), NM_175857 (hKAP8-1), AY510123 (gKAP8-2), KF220646 (sKAP8-2), NM_181607 (hKAP19-1), NM_181609 (hKAP19-3), NM_181610 (hKAP19-4), NM_181611 (hKAP19-5), NM_00130312 (hKAP19-6), NM_181614 (hKAP19-7), AB096964 (hKAP19-8), NM_181615 (hKAP20-1), NM_181616 (hKAP20-2), NM_001163615 (mKAP20-2), NM_181619 (hKAP21-1), NM_181617 (hKAP21-2), KX377616 (sKAP22-1), and NM_181620 (hKAP22-1).
Four single nucleotide polymorphisms (SNPs) (c.27C>T, c.37C>T, c.125T>C, and c.126G>A) were identified among the three sequences. These SNPs were all located in the coding sequence, and three of them were non-synonymous. SNP c.37C>T would result in a putative amino acid change of p.His13Tyr, whereas the other two non-synonymous SNPs (c.125T>C and c.126G>A) were located within the same codon, and would result in an amino acid change of p.Met42Thr (Table 2).
Table 2.
Nucleotide substitution and alleles of the caprine KAP20-2 gene.
| Substitution 1 | Allele | Amino Acid Change 1 | ||
|---|---|---|---|---|
| A | B | C | ||
| c.27C>T | C | T | C | Silent |
| c.37C>T | C | T | T | p.His13Tyr |
| c.125T>C | T | C | C | p.Met42Thr |
| c.126G>A | G | A | A | p.Met42Thr |
1 Numbering of nucleotide and amino acid positions follows the guidelines of the Human Genome Variation Society (HGVS) nomenclature (http://varnomen.hgvs.org/).
3.3. Amino Acid Sequence Analyses
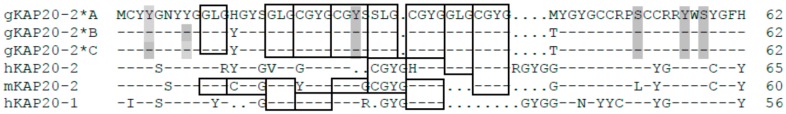
The three caprine KRTAP20-2 sequences would all encode polypeptides of 62 amino acid residues. These polypeptides contained a high content of glycine (32.26%), and moderate levels of tyrosine (20.97–22.58%) and cysteine (14.52%). The theoretical isoelectric points (pI) of the three putative polypeptides were all 7.26 and for these notional caprine KAP20-2 polypeptides, between five and six residues were predicted to be potentially phosphorylated (Figure 4).
Figure 4.
Alignment of KAP20-n sequences from goat, human, and mouse. The amino acid sequences are predicted from the nucleotide sequences, and are shown in one-letter code. Dashes represent amino acids identical to the top sequence, and dots have been introduced to improve the alignment. The length of each sequence is shown on the right. Residues that may be phosphorylated in the caprine KAP20-2 sequences are shaded. Boxes indicate the repeats of (G/S)LG and CGY(G/S). The goat sequences are indicated with a prefix “g”, while the sequences of human and mouse are indicated with “h” and “m”, respectively.
3.4. Expression of KRTAP20-2 in Different Tissues
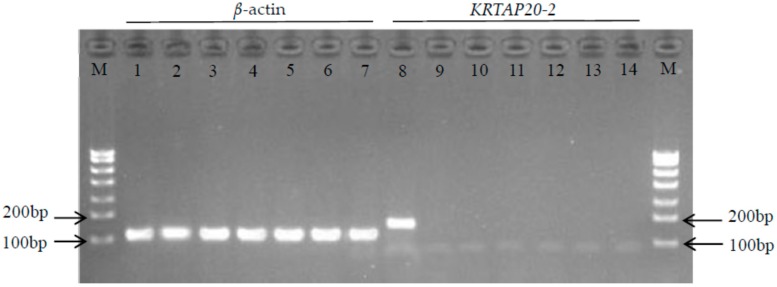
The RT-PCR analysis of KRTAP20-2 expression in the seven different tissues retrieved from the Longdong cashmere goats revealed that the gene was expressed at high levels in secondary hair follicles, but expression was not detected in the other six tissues (heart, liver, lung, kidney, spleen, and longissimus dorsi muscle) (Figure 5).
Figure 5.
RT-PCR results of β-actin and KRTAP20-2 in seven tissues of Longdong cashmere goats. M: marker. Lanes 1–7 and 8–14 are expression results of β-actin and KRTAP20-2 in seven tissues, respectively. Numbers below lanes are tissues studied: 1 and 8: secondary hair follicle; 2 and 9: heart; 3 and 10: liver; 4 and 11: lung; 5 and 12: kidney; 6 and 13: spleen; 7 and 14: longissimus dorsi muscle.
3.5. Phenotypic Correlations between the Various Cashmere Traits
Cashmere weight had a moderately high positive correlation (0.3 < |r| ≤ 0.7) with the length of the curly fibre (r = 0.490), while mean fibre diameter had a weak positive correlation (|r| ≤ 0.3) with cashmere weight (r = 0.280) and the length of the curly fibre (r = 0.216) (Table 3).
Table 3.
Pearson correlation coefficients, r, were calculated to test the strength of the associations between the various traits.
| Trait | Cashmere fibre weight | Mean fibre diameter |
| Mean fibre diameter | 0.280, p < 0.001 | |
| Curly fibre length | 0.490, p < 0.001 | 0.216, p < 0.001 |
3.6. Allele and Genotype Frequencies of KRTAP20-2 in the Longdong Cashmere Goats
The frequencies of the three KRTAP20-2 alleles in the 373 Longdong cashmere goats were: A: 68.37%, B: 28.95%, and C: 2.68%. Five genotypes (AA, AB, BB, AC, and BC) were detected. Of these, AA, AB, and BB were the most common, with a combined frequency of 94%. The remaining two genotypes (AC and BC) occurred at a frequency less than 5%, and genotype CC was not observed.
3.7. Associations between KRTAP20-2 Variation and Cashmere Traits
Of the three alleles detected in the Longdong cashmere goats, allele C was present at a frequency of less than 5%; given this low frequency and potential for bias, its association with cashmere traits was not investigated. In the presences/absence models, the presence of A was associated with increased cashmere fibre weight (present: 416 ± 2.8 g; absent: 378 ± 5.4 g; p < 0.001), while the presence of B was found to be associated with a decrease in cashmere fibre weight (present: 392 ± 3.8 g; absent: 422 ± 3.1 g; p < 0.001) and the length of the curly fibre (present: 4.1 ± 0.04 cm; absent: 4.2 ± 0.04 cm; p = 0.021). No association with fibre diameter was detected for either A or B (Table 4).
Table 4.
Association of KRTAP20-2 alleles with various cashmere traits (Mean ± SE) 1 in Longdong cashmere goats.
| Cashmere Trait (Unit) | Allele | Absent | Present | p Value 1 | ||
|---|---|---|---|---|---|---|
| Mean ± SE | n | Mean ± SE | n | |||
| Cashmere fibre weight (g) | A | 378 ± 5.4 | 64 | 416 ± 2.8 | 309 | <0.001 |
| B | 422 ± 3.1 | 218 | 392 ± 3.8 | 155 | <0.001 | |
| Mean fibre diameter (μm) | A | 13.6 ± 0.06 | 64 | 13.6 ± 0.03 | 309 | 0.581 |
| B | 13.6 ± 0.03 | 218 | 13.6 ± 0.04 | 155 | 0.748 | |
| Curly fibre length (cm) | A | 4.1 ± 0.06 | 64 | 4.2 ± 0.03 | 309 | 0.328 |
| B | 4.2 ± 0.04 | 218 | 4.1 ± 0.04 | 155 | 0.021 |
1 Estimated marginal means and standard errors (SE) of those means derived from general linear mixed-effects models that included “gender” as a fixed factor, and “sire” as a random factor. p < 0.05 are in bold.
For AA, AB, and BB goats, genotype was found to have an effect on some cashmere traits. Goats with genotype AA had a higher cashmere fibre weight (p < 0.001), and the length of the curly fibre (p = 0.032). No associations were found between the KRTAP20-2 genotype and mean fibre diameter (Table 5).
Table 5.
The effect of the KRTAP20-2 genotype on various cashmere traits (Mean ± SE) 1 in Longdong cashmere goats.
| Cashmere Trait (Unit) | Mean ± SE | p Value | ||
|---|---|---|---|---|
| AA (n = 201) | AB (n = 91) | BB (n = 61) | ||
| Cashmere fibre weight (g) | 422 ± 3.2 a | 402 ± 4.6 b | 375 ± 5.5 c | <0.001 |
| Mean fibre diameter (μm) | 13.6 ± 0.03 | 13.6 ± 0.05 | 13.6 ± 0.06 | 0.793 |
| Curly fibre length (cm) | 4.2 ± 0.04 a | 4.1 ± 0.05 b | 4.1 ± 0.06 b | 0.032 |
1 Estimated marginal means and SE of those means derived from general linear mixed-effects models that included “gender” as a fixed factor and “sire” as a random factor. A Bonferroni correction was applied to correct for multiple comparisons. Means within rows that do not share a superscript letter (a, b or c) are significantly (p < 0.05) different and bolded.
4. Discussion
This study reports the identification of a new caprine KAP gene encoding a HGT-KAP protein, and the association between variation in the sequence of that gene, and some cashmere fibre traits. The putative caprine KRTAP20-2 was located at a previously unannotated position of chromosome 1, and it shared the highest homology with the KRTAP20-2 sequences from human and mouse. Based on this, it was concluded that it represents the caprine KRTAP20-2 sequences. The identification of KRTAP20-2 brings the total number of caprine KAP genes described in the published literature from 10 to 11. A number of ovine KRTAPs have been identified using a similar investigative approach, including KRTAP8-2 [32], KRTAP15-1 [8], KRTAP22-1 [7], and KRTAP24-1 [33], and this suggests that the approach described in the study is robust as regards identifying KRTAPs in goats as well as sheep.
In the tissues investigated in the study, KRTAP20-2 mRNA was only identified in secondary hair follicles, but not in heart, liver, lung, kidney, spleen, and muscle. This is consistent with findings reported for Liaoning cashmere goats, where KRTAP7-1 and KRTAP8-2 were only expressed in hair follicles and not in heart, liver, spleen, lung, and kidney tissues [12], and the observation of Rogers et al. [34] that a large number of the KAP family members were exclusively expressed in hair follicles. Given that cashmere fibre is produced by secondary hair follicles, it could be inferred that the specific expression of KRTAP20-2 in secondary hair follicles may at least in part be responsible for fibre traits.
Despite the predicted amino acid sequences of caprine KRTAP20-2 having high similarity to KRTAP20-2 sequences from human and mouse, some differences in the sequences exist. Firstly, the human and mouse proteins contain conserved amino-terminal and carboxyl-terminal sequences of M(I/C)YY(R/S)(G/N)YY and RY(W/-)(S/-)(Y/C)GFY) [24], whereas the goat KAP20-2 sequences described here do not precisely contain these conserved sequences. Instead, they have the sequences MCYYGNYY and RYWSYGFH at the amino-terminal and carboxyl-terminal ends, respectively (Figure 4). There is also variability in the number of sequence repeats in the putative amino acid sequences. In the middle region of the human and mouse protein sequence, there is a trimer repeat (G/S)LG and a tetramer repeat CGY(G/S). The goat sequence contains four repeats of (G/S)LG, whereas the human and mouse KAP20-2 sequences have one and two repeats, respectively (Figure 4). The goat sequences also contain four repeats of CGY(G/S), but human KAP20-2 has two repeats, and mouse KAP20-2 has five repeats (Figure 4).
The putative goat KAP20-2 sequences have a lower content of glycine and tyrosine (53.23–54.84 mol%) than human KAP20-2 (61.5 mol%) and mouse KAP20-2 (63.3 mol%). However, the serine content in goat (8.1 mol%) is higher than that in the human (4.6 mol%) and mouse (3.3 mol%) proteins. The cysteine content in goat (14.5 mol%) is also higher than in human (12.3 mol%), but lower than in mouse (16.7 mol%). Finally, human KAP20-2 is the longest polypeptide with 65 amino acids, followed by the goat sequence, with 62 amino acids, and 60 amino acids for the mouse.
It is interesting to note that some of the putative amino acid changes in KAP20-2 would result in a change in the number of potential phosphorylation sites. Although the presence and function of phosphorylation is poorly understood for KAPs, it is known to occur for the keratins, and affects keratin assembly and organization [35]. This in turn influences the resulting fibre structure. If variation in phosphorylation patterns occur, then it is possible that this may influence cashmere traits.
It is noteworthy that four SNPs were detected in the coding sequence of caprine KRTAP20-2, and that most (three out of four) of them were non-synonymous substitutions. When the DNA sequences were compared, it was found that all of the three non-synonymous SNPs (c.37C>T, c.125T>C, and c.126G>A) were in complete linkage, and what is more, two of these SNPs were adjacent and located within the same codon (Table 2). This suggests that these SNPs may have co-evolved, and then been maintained in goats. The co-evolution of SNPs has been observed in the KAP15-1 gene in sheep, and has been suggested to result from gene conversion or non-reciprocal genetic exchange [8]. Further research is needed on the evolution of the KAP genes in ruminants.
The content of HGT-KAPs in fibre vary both between and within species, ranging from more than 30% in echidna quill and 18% in mouse hair to less than 12% in sheep wool. There is less than 1% HGT-KAP in Lincoln sheep wool, and between 4–12% in Merino wool. The wide range in the content of the HGT-KAPs in different type of fibres and breeds of sheep suggests that these proteins may be responsible for some of the variation in fibre properties, which was in part confirmed in this study. Of the three cashmere traits studied, variation in caprine KRTAP20-2 was associated with the combed fibre weight and the length of the curly fibre, but not the mean fibre diameter. The differing effect of the gene on the traits may be a consequence of phenotypic correlations between the traits. These phenotypic correlations are similar to the findings reported by Bishop et al. [36], Zhou et al. [37], and Ma et al. [38], who found moderate correlations between combed cashmere weight and the length of the fibre, mean fibre diameter and combed cashmere weight, and mean fibre diameter and the length of the fibre.
The effect of KRTAP20-2 on cashmere fibre traits is similar to that reported for KRTAP8-2 [17] and KRTAP13-1 [16] in Inner Mongolian cashmere goats and Xinjiang cashmere goats, respectively. Given that these gene are clustered on goat chromosome 1, and that KRTAP20-2 and KRTAP8-2 are HGT-KAP genes, whereas KRTAP13-1 is a HS-KAP gene, the possibility exists that the functional effect detected for these genes may due to tight linkage to other KAP genes. This would require further investigation of other linked KAP genes on the same chromosome.
The effect of the presence of A on cashmere fibre weight in these goats was large, and it suggests that selection for A would be economically valuable. Goats with the genotype AA could notionally increase cashmere fibre weight by 12.5% compared with those with genotype BB (estimated from Table 5). Thus, for a goat with 400 g of combed cashmere fibre weight, the weight improvement might equate to approximately an extra 50 g of cashmere fibre, and without any significant change in fibre diameter. Therefore, KRTAP20-2 may be a useful genetic marker for improved cashmere goat breeding. Further investigation on more goats from different breeds is needed to confirm this finding, as it has been reported that the phenotypic correlations between cashmere fibre traits vary between goats of differing origin [39].
Acknowledgments
This work was financially supported by the Fuxi Young Talents Fund of Gansu Agricultural University (Gaufx-02Y02), University Scientific Research Project of Gansu Province (2017A-029), the Agricultural Science and Technology Innovation Project of Gansu Province (GNCX-2013-35), the Young Graduate Supervisor Fund of Gansu Agricultural University (GAU-QNDS-201601) and the Major Scientific and Technology Project of Qingyang City.
Author Contributions
J.W., H.Z., Y.L. and J.G.H.H. conceived and designed the experiments. J.W., L.C. and Z.H. performed the experiments. J.W., L.C., J.H. and H.Z. analyzed the data; J.H., X.L. and S.L. contributed to the discussion of the results. J.W., H.Z., Y.L. and J.G.H.H. wrote the manuscript. All authors reviewed and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Franck R.R., editor. Silk, Mohair, Cashmere and Other Luxury Fibres. Woodhead Publishing Limited and CRC Press LLC in Association with the Textile Institute; Cambridge, UK: 2001. Appendix 10: Quality assessment of goat hair for textile use; pp. 227–233. [Google Scholar]
- 2.Yu Z., Gordon S.W., Nixon A.J., Bawden C.S., Rogers M.A., Wildermoth J.E., Maqbool N.J., Pearson A.J. Expression patterns of keratin intermediate filament and keratin associated protein genes in wool follicles. Differentiation. 2009;77:307–316. doi: 10.1016/j.diff.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Gong H., Zhou H., Forrest R.H., Li S., Wang J., Dyer J.M., Luo Y., Hickford J.G. Wool keratin-associated protein genes in sheep-a review. Genes. 2016;7:24. doi: 10.3390/genes7060024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan I., Emanuel Maldonado E., Vasconcelos V., O’Brien S.J., Johnson W.E., Antunes A. Mammalian keratin associated proteins (KRTAPs) subgenomes: Disentangling hair diversity and adaptation to terrestrial and aquatic environments. BMC Genom. 2014;15:779. doi: 10.1186/1471-2164-15-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers M.A., Winter H., Langbein L., Wollschläger A., Praetzel-Wunder S., Jave-Suarez L.F., Schweizer J. Characterization of human KAP24.1, a cuticular hair keratin-associated protein with unusual amino-acid composition and repeat structure. J. Investig. Dermatol. 2007;127:1197–1204. doi: 10.1038/sj.jid.5700702. [DOI] [PubMed] [Google Scholar]
- 6.Rogers M.A., Langbein L., Praetzel-Wunder S., Giehl K. Characterization and expression analysis of the hair keratin associated protein KAP26.1. Br. J. Dermatol. 2008;159:725–729. doi: 10.1111/j.1365-2133.2008.08743.x. [DOI] [PubMed] [Google Scholar]
- 7.Li S., Zhou H., Gong H., Zhao F., Wang J., Liu X., Luo Y., Hickford J.G. Identification of the ovine keratin-associated protein 22-1 (KAP22-1) gene and its effect on wool traits. Genes. 2017;8:27. doi: 10.3390/genes8010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Zhou H., Zhu J., Hu J., Liu X., Li S., Luo Y., Hickford J.G. Identification of the ovine keratin-associated protein 15-1 gene (KRTAP15-1) and genetic variation in its coding sequence. Small Rumin. Res. 2017;153:131–136. doi: 10.1016/j.smallrumres.2017.06.007. [DOI] [Google Scholar]
- 9.Andrews M., Visser C., Marle-Köster E.V. Identification of novel variants for KAP1.1, KAP8.1 and KAP13.3 in South African goats. Small Rumin. Res. 2017;149:176–180. doi: 10.1016/j.smallrumres.2017.02.014. [DOI] [Google Scholar]
- 10.Shah R.M., Ganai T.A., Sheikh F.D., Shanaz S., Shabir M., Khan H.M. Characterization and polymorphism of keratin associated protein 1.4 gene in goats. Gene. 2013;518:431–442. doi: 10.1016/j.gene.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M., Wang X., Chen H., Lan X.Y., Guo Y.K., Li J.Y., Wei T.B., Jing Y.J., Liu S.Q., Zhang M.H., et al. The PCR-SSCP and DNA sequencing methods detecting a large deletion mutation at KAP6.2 locus in the cashmere goat. Small Rumin. Res. 2008;75:243–246. doi: 10.1016/j.smallrumres.2007.10.007. [DOI] [Google Scholar]
- 12.Jin M., Wang L., Li S., Xing M.X., Zhang X. Characterization and expression analysis of KAP7.1, KAP8.2 gene in Liaoning new-breeding Cashmere goat hair follicle. Mol. Biol. Rep. 2011;38:3023–3028. doi: 10.1007/s11033-010-9968-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M., Chen H., Wang X., Yu H., Wang M., Wang J., Lan X.Y., Zhang C.F., Zhang L.Z., Guo Y.K., et al. aPCR-SSCP and DNA sequencing detecting two silent SNPs at KAP8.1 gene in the cashmere goat. Mol. Biol. Rep. 2009;36:1387–1391. doi: 10.1007/s11033-008-9325-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Zhao Z.D., Xu H.R., Qu L., Zhao H.B., Li T., Zhang Z.Y. Variation and expression of KAP9.2 gene affecting cashmere trait in goats. Mol. Biol. Rep. 2012;39:10525–10529. doi: 10.1007/s11033-012-1937-9. [DOI] [PubMed] [Google Scholar]
- 15.Jin M., Cao Q., Wang R., Piao J., Zhao F., Piao J. Molecular characterization and expression pattern of novel Keratin-associated protein 11.1 gene in the Liaoning Cashmere goat (Capra hircus) Asian Australas. J. Anim. Sci. 2017;30:328–337. doi: 10.5713/ajas.16.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y., Liu W.J., Zhang F.Q., Shao Y.G., Yu S.G. The polymorphism of a novel mutation of KAP13.1 gene and its associations with cashmere traits on Xinjiang local goat breed in China. Asian. J. Anim. Vet. Adv. 2010;5:34–42. [Google Scholar]
- 17.Liu H., Li N., Jia C., Zhu X., Jia Z. Effect of the polymorphisms of keratin associated protein 8.2 gene on fibre traits in Inner Mongolia cashmere goats. Asian Australas. J. Anim. Sci. 2007;20:821–826. doi: 10.5713/ajas.2007.821. [DOI] [Google Scholar]
- 18.Zhou H., Gong H., Wang J., Dyer J.M., Luo Y., Hickford J.G.H. Identification of four new gene members of the KAP6 gene family in sheep. Sci. Rep. 2016;6:24074. doi: 10.1038/srep24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong H., Zhou H., Hodge S., Dyer J.M., Hickford J.G.H. Association of wool traits with variation in the ovine KAP1-2 gene in Merino cross lambs. Small Rumin. Res. 2015;124:24–29. doi: 10.1016/j.smallrumres.2015.01.009. [DOI] [Google Scholar]
- 20.Zhou H., Gong H., Li S., Luo Y., Hickford J.G.H. A 57-bp deletion in the ovine KAP6-1 gene affects wool fibre diameter. J. Anim. Breed. Genet. 2015;132:301–307. doi: 10.1111/jbg.12138. [DOI] [PubMed] [Google Scholar]
- 21.Tao J., Zhou H., Gong H., Yang Z., Ma Q., Cheng L., Ding W., Li Y., Hickford J.G.H. Variation in the KAP6-1 gene in Chinese Tan sheep and its effect on wool traits. Small Rumin. Res. 2017;154:129–132. doi: 10.1016/j.smallrumres.2017.08.001. [DOI] [Google Scholar]
- 22.Li S., Zhou H., Gong H., Zhao F., Wang J., Luo Y., Hickford J.G.H. Variation in the ovine KAP6-3 gene (KRTAP6-3) is associated with variation in mean fibre diameter-associated wool traits. Genes. 2017;8:204. doi: 10.3390/genes8080204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao J., Zhou H., Yang Z., Gong H., Ma Q., Ding W., Li Y., Hickford J.G.H. Variation in the KAP8-2 gene affects crimping and grow rate of wool in Chinese Tan sheep. Small Rumin. Res. 2017;149:77–80. doi: 10.1016/j.smallrumres.2017.01.001. [DOI] [Google Scholar]
- 24.Rogers M.A., Langbein L., Winter H., Ehmann C., Praetzel S., Schweizer J. Characterization of a first domain of human high glycine-tyrosine and high sulfur keratin-associated protein (KAP) genes on chromosome 21q22.1. J. Biol. Chem. 2002;277:48993–49002. doi: 10.1074/jbc.M206422200. [DOI] [PubMed] [Google Scholar]
- 25.Shimomura Y., Ito M. Human hair keratin-associated proteins. J. Investig. Dermatol. Symp. Proc. 2005;10:230–233. doi: 10.1111/j.1087-0024.2005.10112.x. [DOI] [PubMed] [Google Scholar]
- 26.GCF_001704415.1. [(accessed on 26 June 2016)]; Available online: https://www.ncbi.nlm.nih.gov/assembly/GCF_001704415.1.
- 27.Zhou H., Hickford J.G., Fang Q. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006;354:159–161. doi: 10.1016/j.ab.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Byun S.O., Fang Q., Zhou H., Hickford J.G.H. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal. Biochem. 2009;385:174–175. doi: 10.1016/j.ab.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Gong H., Zhou H., Hickford J.G. Diversity of the glycine/tyrosine-rich keratin-associated protein 6 gene (KAP6) family in sheep. Mol. Biol. Rep. 2011;38:31–35. doi: 10.1007/s11033-010-0074-6. [DOI] [PubMed] [Google Scholar]
- 30.Blom N., Gammeltoft S., Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 31.Gong H., Zhou H., McKenzie G.W., Yu Z., Clerens S., Dyer J.M., Plowman J.E., Wright M.W., Arora R., Bawden C.S., et al. An updated nomenclature for keratin-associated proteins (KAPs) Int. J. Biol. Sci. 2012;8:258–264. doi: 10.7150/ijbs.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong H., Zhou H., Dyer J.M., Hickford J.G. The sheep KAP8-2 gene, a new KAP8 family member that is absent in humans. Springerplus. 2014;3:528. doi: 10.1186/2193-1801-3-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou H., Gong H., Yan W., Luo Y., Hickford J.G. Identification and sequence analysis of the keratin-associated protein 24-1 (KAP24-1) gene homologue in sheep. Gene. 2012;511:62–65. doi: 10.1016/j.gene.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 34.Rogers M.A., Langbein L., Praetzel-Wunder S., Winter H., Schweizer J. Human hair keratin-associated proteins (KAPs) Int. Rev. Cytol. 2006;251:209–263. doi: 10.1016/S0074-7696(06)51006-X. [DOI] [PubMed] [Google Scholar]
- 35.Ku N.O., Liao J., Chou C.F., Omary M.B. Implications of intermediate filament protein phosphorylation. Cancer Metastasis. Rev. 1996;15:429–444. doi: 10.1007/BF00054011. [DOI] [PubMed] [Google Scholar]
- 36.Bishop S.C., Russel A.J.F. The inheritance of fibre traits in a crossbred population of Cashmere goats. Anim. Sci. 1996;63:429–436. doi: 10.1017/S1357729800015320. [DOI] [Google Scholar]
- 37.Zhou H.M., Allain D., Li J.Q., Zhang W.G., Yu X.C. Genetic parameters of production traits of Inner Mongolia cashmere goats in China. J. Anim. Breed. Genet. 2002;119:385–390. doi: 10.1046/j.1439-0388.2002.00362.x. [DOI] [Google Scholar]
- 38.Ma N., Li Y., Song Y., Lou Y., Son X. Estimates of genetic parameters of main economic traits in Liaoning cashmere goat. J. Jilin Agric. Univ. 2005;27:446–448. [Google Scholar]
- 39.McGregor B., Butler K. Cashmere production and fleece attributes associated with farm of origin, age and sex of goat in Australia. Anim. Prod. Sci. 2008;48:1090–1098. doi: 10.1071/EA06308. [DOI] [Google Scholar]