Abstract
The present study aimed to explore the underlying developmental mechanism of mothers against decapentaplegic homolog (Smad) signaling in the development of mandibular condylar cartilage. To achieve this, the expression levels of Smad2, 3, 4 and 7, and phosphorylated Smad2/3 were investigated at different time points in developing mandibular condylar cartilage. Mandibular condyles from C57BL/6J mice were dissected at the prenatal and postnatal stages. Serial sections were made and the distributions of Smad proteins were examined using immunohistochemical techniques intermittently between day 14.5 of gestation and postnatal day 7. All Smad proteins examined in the present study were expressed in the condylar blastema and during early chondrogenesis. At the postnatal stage, Smad2 and 4 were localized in proliferative and mineralized hypertrophic chondrocytes. Smad3 and 7 were expressed in proliferative and hypertrophic chondrocytes, including pre-hypertrophic and mineralized hypertrophic chondrocytes. Later, positive immunoreactivity of Smad3 reduced at postnatal day 7. A similar expression pattern to Smad3 was observed for p-Smad2/3, but p-Smad2/3 was located in the nuclei of proliferative chondrocytes. These results suggest that Smad signaling members are involved in the development of mandibular condylar cartilage. In addition, the spatial and temporal expression of these Smads indicate that Smad signaling is involved in regulating the differentiation of chondrocytes and endochondral ossification, in order to maintain normal chondrogenesis and morphogenesis of mandibular condylar cartilage.
Keywords: mothers against decapentaplegic homolog signaling pathway, transforming growth factor β signaling, temporomandibular joint, mandibular condylar cartilage, development
Introduction
As a vital and regional growth site, mandibular condylar cartilage is important to the function of the temporomandibular joints and the morphogenesis of maxillofacial areas (1). Therefore, an abundance of studies have focused on the biological and pathological mechanisms of mandibular condylar cartilage formation. Unlike other articular cartilage within limb joints, mandibular condylar cartilage is considered a secondary cartilage and its growth has been categorized into two phases, maturation and mineralization (2). Due to the cellular and phenotypic responses of chondrocytes, in histology mandibular condylar cartilage is consecutively categorized from the outside in; the layers are as follows: Surface articular, resting chondrocyte, proliferative chondrocyte, (non-mineralized) hypertrophic chondrocyte and mineralized hypertrophic chondrocyte (3). The morphogenesis of the mandibular condyle is determined by chondrogenesis within each of the layers.
Various growth factors and signaling pathways are involved in the formation of mandibular condylar cartilage, including Notch signaling members, insulin-like growth factors and transforming growth factor β (TGF-β) superfamily members (4–6). Among these factors and members, the TGF-β/bone morphogenic protein (BMP) signaling pathway is noteworthy. Data from previous studies has revealed that the TGF-β/BMP signaling pathway, which is specifically transmitted through mothers against decapentaplegic homolog (Smad) proteins, is involved in the proliferation and differentiation of chondroblasts, and the ossification of the extracellular matrix, in order to regulate the chondrogenesis of mandibular condylar cartilage (7–9). Smads are intracellular proteins that transduce the extracellular signals from TGF-β ligands to the nucleus, where they activate downstream gene transcription (1–4). Previous studies have demonstrated that signal transduction via the Smad signaling pathway is crucial in regulating TGF-β-mediated gene transcription and exerts versatile regulatory functions on chondrogenesis (9,10). TGF-β family members bind to their corresponding receptors and trigger receptor phosphorylation, activating specific receptor-regulated Smad (R-Smad) proteins to initiate intracellular signaling. R-Smads combine with the common mediator Smad4 to form a complex; following phosphorylation, the complex translocates into nuclei and interacts with transcription factors to trigger the expression of specific chondroblast genes (11). Smad2 and 3 are inhibitory mediators of TGF-β signaling during the maturation of chondrocytes (12). Additionally, Smad3 inhibits the terminal differentiation of chondrocytes, which is essential for maintaining the morphology of the formed articular cartilage (13). In Smad7 conditional knockout models, chondrocyte differentiation was inhibited and malformed cartilage was observed as Smad7 disrupted the formation of Smad2/3 and 4 complexes (14).
Based on the aforementioned evidence, the functions of Smad proteins are key to understanding the effects of the TGF-β/Smad signaling pathway on the development of articular cartilage. However, to the best of our knowledge, there have been few studies on the mechanism of Smad signaling in the development of mandibular condylar cartilage. The present study investigated the spatial and temporal protein expression of Smad2, 3, 4 and 7, and phosphorylated (p-)Smad2/3, during the development of mandibular condylar cartilage. The results of the present study may provide novel insights into the development of mandibular condylar cartilage and form a basis for further studies.
Materials and methods
Animals and tissues preparation
A total of 5 male and 20 female C57BL/6J mice (5 weeks old; weight, 25–30 g) were obtained from the Experimental Animal Laboratory of Sichuan University (Chengdu, China) and provided with access to food and water ad libitum under climate-controlled conditions (25°C; 55% humidity; 12 h light/dark cycle). At night, 3 females and 1 male were transferred into a cage and left to mate. At 7 am the next day, the vaginal plug was examined to confirm gestation, which was designated as embryonic day (E) 0.5. Pregnant mice were anaesthetized with ether and sacrificed by cervical dislocation at three selected time points (E14.5, E15.5 and E16.5). Postnatal newborn mice were sacrificed in the same way at postnatal day (P) 0 (following birth), 4 and 7. The mandibular condyles of the fetuses and newborn mice were dissected. Following fixation in 4% paraformaldehyde at room temperature for 24 h, the specimens were embedded in paraffin. Subsequently, 5-µm-thick serial sections were cut and mounted on poly-L-lysine-coated glass slides. All procedures performed on the animals were approved by the Ethics Committee of West China College of Stomatology, Sichuan University (Chengdu, China).
Immunohistochemistry (IHC)
Primary antibodies directed against Smad2, 3, 4 and 7, and p-Smad2/3, were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The tissue sections were heated overnight at 60°C, deparaffinized in xylene, rehydrated in ethanol, and incubated for 20 min in 3% hydrogen peroxide (H2O2) in methanol at room temperature to block endogenous peroxidase activity. Sections were incubated in the dark for 30 min at 37°C with 3% H2O2 in trypsin-PBS (0.1 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and subsequently incubated overnight at 4°C with the following primary antibodies: Mouse anti-Smad2 (cat. no. sc-101153), 3 (cat. no. sc-101154), 4 (cat. no. sc-7966) and 7 (cat. no. sc-365846), and goat anti-p-Smad2/3 (cat. no. sc-11769) at a dilution of 1:300. Negative control sections were incubated with PBS instead of primary antibodies. Subsequently, the sections were incubated with the appropriate secondary antibodies at 37°C for 30 min, as follows: Biotin-conjugated secondary rabbit anti-goat antibodies (cat. no. sc-2774; Santa Cruz Biotechnology, Inc.) or goat anti-mouse antibodies (Biotin-Streptavidin HRP Detection system; cat. no. sp-9002; OriGene Technologies, Inc., Beijing, China). Secondary antibodies were used at a dilution of 1:50. The peroxidase activity was visualized by immersing the sections in 3,3′-diaminobenzidine at room temperature for 3–10 min. Lastly, images were captured using an optical microscope (magnification, ×100, 160 or 400).
Results
Histological observation
At E14.5, mesenchymal cells began to condense at the distal upper portion of developing mandible (Fig. 1A). At E15.5, the condensation of the mesenchymal cells was more recognizable but without defined cartilaginous layers of condylar architectures (Fig. 1B). In the central region of the developing condylar cartilage, differentiated chondrocytes were first observed. At E16.5, the condylar cartilage was evident and the cartilaginous cells exhibited layer-separated growth, including undifferentiated outer layer and differentiated inner layer chondrocytes (Fig. 1C).
Figure 1.
Histological observation of developing mandibular condylar cartilage between E14.5 and E16.5. (A) At E14.5 mesenchymal cells were present in the developing mandible. Magnification, ×100. (B) At E15.5 chondrocytes were present in the developing mandible. Magnification, ×160. (C) At E16.5 the condylar cartilage was first clearly observed and the cartilaginous cells presented as two layers as follows: Differentiated inner chondrocytes and undifferentiated outer chondrocytes. Magnification, ×160. The dotted line marks the edge of the developing mandibular ramus and condyle. Mc, mandibular condyle (condylar primordium); M, mandible; TPe, temporalis muscle; Md, mastoid process; Dc, differentiated chondrocytes; E, embryonic day.
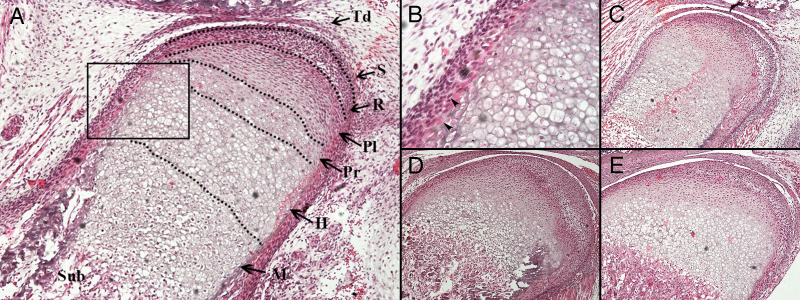
At E17.5, a sequential arrangement of chondroblast layers was observed (Fig. 2A and B). Osteoblasts were identified at the periphery of the hypertrophic chondrocytes. After E17.5, endochondral ossification progressed and the length of the mandibular condyle increased (Fig. 2C and D). The primary structure of mature condylar cartilage had taken shape by P7 (Fig. 2E).
Figure 2.
Histological observation of the developing mandibular condylar cartilage between E17.5 and P7. (A) At E17.5, four layers of chondrocytes were arranged regularly and possessed good continuity. The dotted line marks the edge of the developing mandibular ramus and condyle. The boxed part is Fig. 2B. Magnification, ×100. (B) A magnification of a section from Fig. 2A. The arrows indicate perichondral osteoblasts at the perichondrium/periosteum of the developing condyle. Magnification, ×400. (C) At P0 each layer of the mandibular condylar cartilage was observed. At (D) P4 and (E) P7, the length of the hypertrophic chondrocyte layers in the vertical axis decreased, while the subchondral bone increased in volume. Magnification, ×100. Td, temporomandibular joint disk; S, surface articular layer; R, resting chondrocyte layer; Pl, proliferative chondrocyte layer; Pr, pre-hypertrophic chondrocyte layer; H, hypertrophic chondrocyte layer (non-mineralized hypertrophic chondrocyte zone); M, mineralized hypertrophic chondrocyte layer; Sub, subchondral bone; E, embryonic day; P, postnatal day.
IHC results
The IHC results (Figs. 3 and 4) demonstrated that positive products of Smad2, 3, 4 and 7 were observed in the cytoplasm of coagulating mesenchymal cells at the initial stage of condylar development at E14.5 and E15.5. Notably, the expression patterns of these products were not exactly accordant since the expression of Smad4 was localized to the central section of the mesenchymal condensation. At E16.5, the mesenchymal cells located in the center of the developing condylar cartilage differentiated into chondrocytes and the preliminary morphology of the mandibular condylar cartilage was formed. During these processes, Smad2, 3, 4 and 7 were identified in differentiating chondrocytes. However, Smad3 was mainly located in the differentiated chondrocytes, while Smad4 was identified in the undifferentiated chondrocytes.
Figure 3.
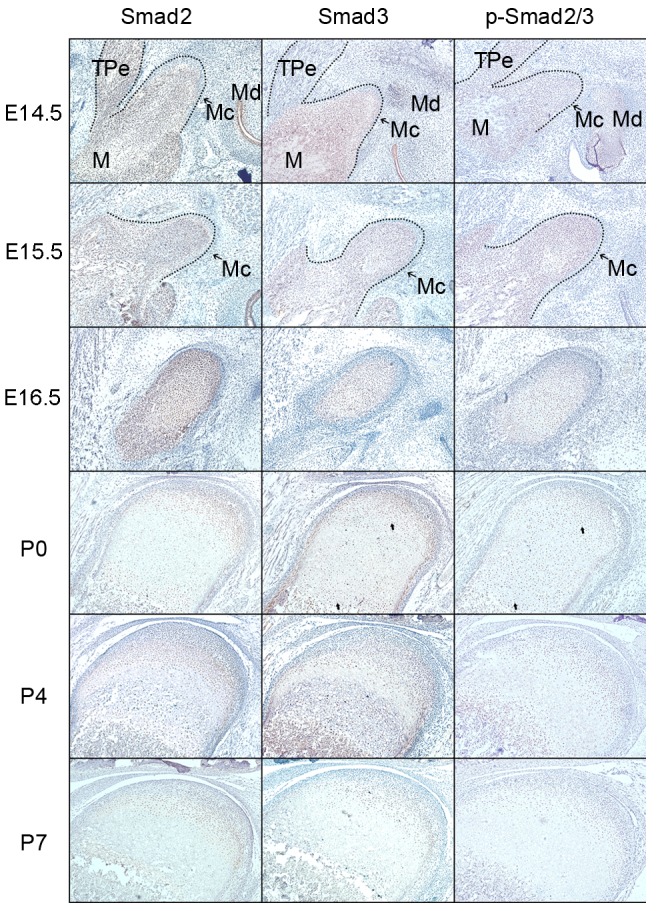
Protein expression of Smad2, Smad3 and p-Smad2/3 in the developing condylar cartilage between E14.5 and P7. The arrows demonstrate the distinct expression of Smad3 and p-Smad2/3 in the cytoplasm or nuclei of proliferative and hypertrophic chondrocytes. The dotted line marks the edge of the developing mandibular ramus and condyle. E14-E16: Magnification, ×160. P0-P7: Magnification, ×100. E, embryonic day; P, postnatal day.
Figure 4.
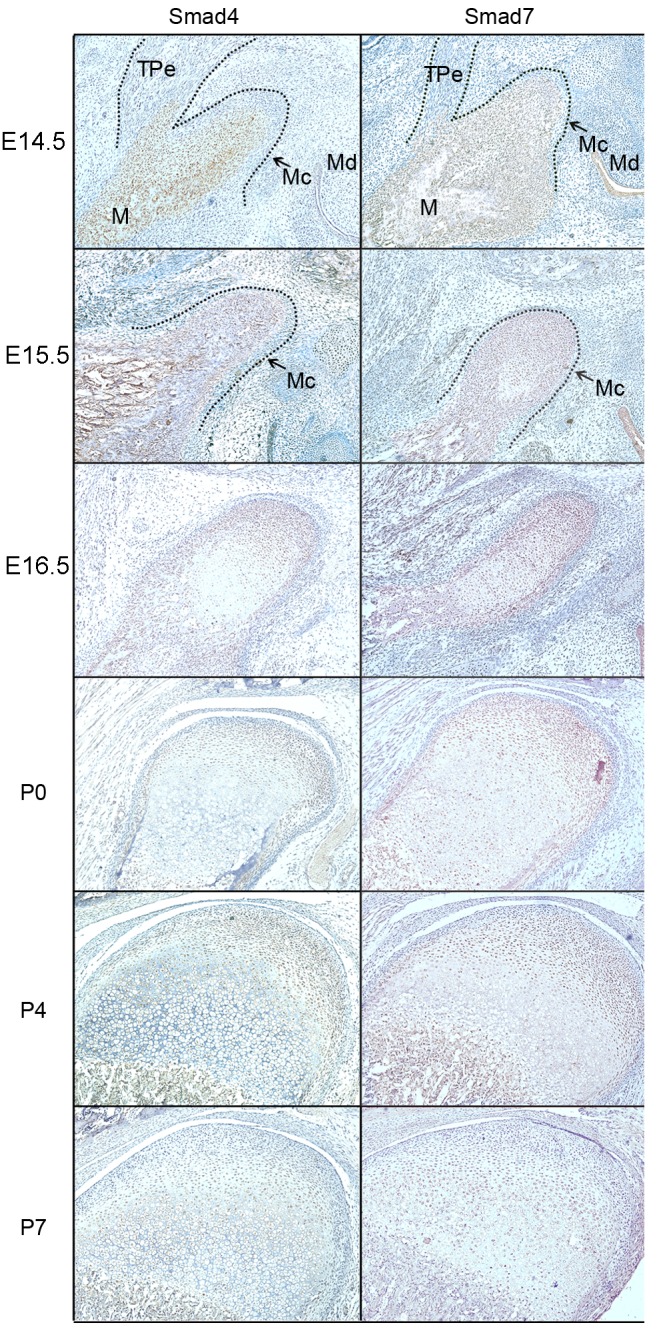
Smad4 and Smad7 protein expression in the developing condylar cartilage between E14.5 and P7. The dotted line marks the edge of the developing mandibular ramus and condyle. E14-E16: Magnification, ×160. P0-P7: Magnification, ×100. E, embryonic day; P, postnatal day.
From P0, the chondrocytes underwent proliferation and differentiation to elongate the condylar cartilage, which corresponded with chondrogenesis in the mandibular condyles. Smad2 and 4 expression was associated with condylar cartilage growth; their expression was identified in proliferative and mineralized hypertrophic chondrocytes at the postnatal stage, but was reduced in the hypertrophic zone. Smad3 and 7 were expressed in all proliferative and hypertrophic chondrocytes of the developing condylar cartilage, including pre-hypertrophic and mineralized hypertrophic chondrocytes. Although Smad3 and 7 were expressed in the cytoplasm of proliferative chondrocytes, Smad3 was observed in the nuclei while Smad7 was observed in the cytoplasm of mineralized hypertrophic chondrocytes. At P7, the expression of Smad3 in hypertrophic and mineralized hypertrophic chondrocytes was reduced.
As a direct downstream activator of the TGF-β/Smad signaling pathway, p-Smad2/3 was expressed in different regions of the developing condylar cartilage, which was similar to the observed expression pattern of Smad3. During the chondrogenesis stage of condylar cartilage development (prior to E16.5), p-Smad2/3 was primarily expressed in the cytoplasm of coagulating mesenchymal cells in the condylar blastema. Notably, with the differentiation and maturation of chondrocytes, the expression of p-Smad2/3 relocated from the cytoplasm to the nucleus of proliferative chondrocytes and mineralized hypertrophic chondrocytes at P0 and P4, and reduced in hypertrophic and mineralized hypertrophic chondrocytes at P7. Notably, p-Smad2/3 was expressed in the cytoplasm of proliferative chondrocytes at P7.
Discussion
Previous studies have reported that the TGF-β signaling pathway serves an important role in cartilaginous remodeling, osteoarthritis, osteochondromas and hyperplasia (15–17). The expression of Smads has been identified to be associated with the function of TGF-β signaling (18,19). Typically, Smad2, 3 and 4 are considered to be the intracellular mediator Smads that transduce TGF-β signaling to the nuclei. During the differentiation of chondrocytes, Smad2 and 3 form a complex with Smad4, which enters the nuclei and modulates gene expression (5). As inhibitors of chondrocyte differentiation, Smad2 and 3 are activated by TGF-β in chondrocytes, and are involved in the reduction of chondrocyte terminal differentiation, which mediates the suppressive effect of TGF-β on the maturation of chondrocytes (15). Previous studies have reported that Smad2 and 3 are co-expressed during the development of different organs. During the development of Meckel's cartilage, Smad2 and 3 were identified to be expressed in differentiated chondrocytes (18). The present study demonstrated that the expression of Smad3 markedly overlapped with Smad2 expression during chondrogenesis and the maturation of proliferative chondrocytes. Similarly, in the epiphyseal growth plate of limb joints, Smad2 and 3 are markedly expressed in proliferating chondrocytes and maturing chondrocytes (12). Such coexpression implies a functional synergy of Smad2 and 3. When p-Smad2/3 signaling is absent, chondrocytes exit their quiescent state and undergo anomalous terminal differentiation (18).
During endochondral ossification, Smad2 and 3 are distinct in their expression patterns. Smad3 has been identified to be predominantly expressed in the perichondrium of developing cartilage undergoing endochondral ossification (20). The present study demonstrated that along with the maturation and mineralization of chondrocytes, Smad3 expression was diminished in hypertrophic and mineralized hypertrophic chondrocytes during the postnatal stages, despite the expression of Smad3 being persistent in the cytoplasm of proliferative chondrocytes. This expression pattern indicates that the decreasing expression of Smad3 was potentially self-regulatory to allow for endochondral ossification during the development of condylar cartilage (21).
Nevertheless, certain studies have revealed that Smad3 is dispensable in the early stages of cartilage formation (13), and that a synergistic and negative regulation exists between Smad2 and 3 signaling in the regulation of chondrocyte differentiation (22). The present study demonstrated that p-Smad2/3 was expressed in the nuclei of postnatal proliferative and mineralized hypertrophic chondrocytes, but not hypertrophic chondrocytes.
Smad proteins continuously move between the cytoplasm and nuclei, thus Smads reach a steady state (23). It is well known that Smad4 combines with Smad2/3 to form a complex, Smad4 is then activated when it undergoes phosphorylation. The present study demonstrated that as a central mediator for the TGF-β/BMP signaling pathway, Smad4, was expressed in proliferative and hypertrophic chondrocytes during the development of condylar cartilage, which was in accordance with the Smad4 expression pattern identified in the epiphyseal growth plate in a previous study (24).
In the present study, Smad7 was markedly expressed in coagulating mesenchymal cells during chondrogenesis, and in proliferative and hypertrophic chondrocytes during maturation of condylar cartilage. As a negative regulator of the TGF-β/Smad signaling pathway, Smad7 controls the function of R-Smads by targeting the TGF-β receptor for degradation (25). A previous study demonstrated that Smad7 antagonized TGF-β signaling in the nucleus by interfering with functional Smad-DNA complex formation, and repressing Smad3/4, Smad2/4 and Smad1/4 complex formation (26). Additionally, an abnormal feedback regulation of Smad7 may cause the pathological growth of chondrocytes (14).
In conclusion, the spatial and temporal expression of Smad2, 3, 4 and 7, and p-Smad2/3, in the development of mandibular condylar cartilage was explored in the present study. The results demonstrated that these proteins were involved in the development of mandibular condylar cartilage. In addition, these Smad proteins were identified to be localized to different regions and thus may exert distinct functions on chondrogenesis and morphogenesis. The results of the present study may stimulate further understanding of the biological function of Smad signaling in the development of mandibular condylar cartilage.
References
- 1.Ranly DM. Craniofacial growth. Dent Clin North Am. 2000;44:457–470. [PubMed] [Google Scholar]
- 2.Inoue H, Nebgen D, Veis A. Changes in phenotypic gene expression in rat mandibular condylar cartilage cells during long-term culture. J Bone Miner Res. 1995;10:1691–1697. doi: 10.1002/jbmr.5650101111. [DOI] [PubMed] [Google Scholar]
- 3.Shen G, Darendeliler MA. The adaptive remodeling of condylar cartilage-a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu T, Nakano K, Tsujigiwa H, Nagatsuka H, Watanabe T, Okafuji N, Kurihara S, Hasegawa H, Nagai N, Kawakami T. Notch signaling in mandibular condylar cartilage development. Eur J Med Res. 2007;12:515–519. [PubMed] [Google Scholar]
- 5.Van der Kraan PM, Davidson EN Blaney, van den Berg WB. A role for age-related changes in TGFbeta signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res Ther. 2010;12:201. doi: 10.1186/ar2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patil AS, Sable RB, Kothari RM. Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J Cell Physiol. 2012;227:1796–1804. doi: 10.1002/jcp.22905. [DOI] [PubMed] [Google Scholar]
- 7.Chai Y, Ito Y, Han J. TGF-beta signaling and its functional significance in regulating the fate of cranial neural crest cells. Crit Rev Oral Biol Med. 2003;14:78–88. doi: 10.1177/154411130301400202. [DOI] [PubMed] [Google Scholar]
- 8.Li TF, O'Keefe RJ, Chen D. TGF-beta signaling in chondrocytes. Front Biosci. 2005;10:681–688. doi: 10.2741/1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–159. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 11.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, Drissi H, O'Keefe RJ. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21:4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwai T, Murai J, Yoshikawa H, Tsumaki N. Smad7 Inhibits chondrocyte differentiation at multiple steps during endochondral bone formation and down-regulates p38 MAPK pathways. J Biol Chem. 2008;283:27154–27164. doi: 10.1074/jbc.M801175200. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O'Keefe RJ. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141:4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 16.Meng Q, Long X, Deng M, Cai H, Li J. The expressions of IGF-1, BMP-2 and TGF-β1 in cartilage of condylar hyperplasia. J Oral Rehabil. 2011;38:34–40. doi: 10.1111/j.1365-2842.2010.02125.x. [DOI] [PubMed] [Google Scholar]
- 17.Cuellar A, Inui A, James MA, Borys D, Reddi AH. Immunohistochemical localization of bone morphogenetic proteins (BMPs) and their receptors in solitary and multiple human osteochondromas. J Histochem Cytochem. 2014;62:488–498. doi: 10.1369/0022155414535781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Bringas P, Jr, Mogharei A, Zhao J, Deng C, Chai Y. Receptor-regulated and inhibitory Smads are critical in regulating transforming growth factor beta-mediated Meckel's cartilage development. Dev Dyn. 2002;224:69–478. doi: 10.1002/dvdy.10088. [DOI] [PubMed] [Google Scholar]
- 19.Flanders KC, Heger CD, Conway C, Tang B, Sato M, Dengler SL, Goldsmith PK, Hewitt SM, Wakefield LM. Brightfield proximity ligation assay reveals both canonical and mixed transforming growth factor-β/bone morphogenetic protein Smad signaling complexes in tissue sections. J Histochem Cytochem. 2014;62:846–863. doi: 10.1369/0022155414550163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdier MP, Seité S, Guntzer K, Pujol JP, Boumédiène K. Immunohistochemical analysis of transforming growth factor beta isoforms and their receptors in human cartilage from normal and osteoarthritic femoral heads. Rheumatol Int. 2005;25:118–124. doi: 10.1007/s00296-003-0409-x. [DOI] [PubMed] [Google Scholar]
- 21.Song B, Estrada KD, Lyons KM. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009;20:379–388. doi: 10.1016/j.cytogfr.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez J, Serra R. Unique and redundant roles of Smad3 in TGF-beta-mediated regulation of long bone development in organ culture. Dev Dyn. 2004;230:685–699. doi: 10.1002/dvdy.20100. [DOI] [PubMed] [Google Scholar]
- 23.Hill CS. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2009;19:36–46. doi: 10.1038/cr.2008.325. [DOI] [PubMed] [Google Scholar]
- 24.Sakou T, Onishi T, Yamamoto T, Nagamine T Sampath, Tk Ten Dijke P. Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Res. 1999;14:1145–1152. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional SmadDNA complex formation. Mol Cell Biol. 2007;27:4488–4499. doi: 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]