Abstract
The present study investigated the clinical significance of the novel biomarker neutrophil gelatinase-associated lipocalin (NGAL) for the early diagnosis of acute renal injury (AKI). Thirty-eight critically ill patients with acute renal injury treated at Zhengzhou No. 7 People's Hospital between December 2015 and November 2016 served as the AKI group (observation group). At the same time, 38 critically ill patients without acute renal injury were also selected as the non-AKI group (control group). Serum NGAL and creatinine (SCr) levels were measured by enzyme-linked immunosorbent assay (ELISA) at 2, 8, 12 and 24 h post-operation, whereas particle-enhanced turbidimetric immunoassay (PETIA) was used to quantify the levels of cysteine protease inhibitor cystatin C (CysC) in serum at 2, 8, 12 and 24 h post-operation. The correlations between indicators were also analyzed, with ROC curves used to evaluate the diagnostic values of NGAL, SCr and CysC in AKI. No significant differences in SCr levels were found between the two groups at different time-points after operation (P>0.05), but NGAL and CysC levels in the observation group were significantly higher than in the control group (P<0.05). Pearson correlation coefficient analysis showed NGAL and CysC were positively correlated with Scr levels. For NGAL in early diagnosis, the area under the AKI curve was 0.904, the sensitivity was 90.2% and the specificity was 89.5%; for CysC in early diagnosis, the area under the AKI curve was 0.806, the sensitivity was 79.2% and the specificity was 78.5%; for SCr in early diagnosis, the area under the AKI curve was 0.634, the sensitivity was 64.2% and the specificity was 62.5%. Therefore, NGAL demonstrated a satisfactory early predictive value for AKI and can be used as a biomarker for early AKI diagnosis.
Keywords: acute renal injury, neutrophil gelatinase-associated lipocalin, serum creatinine, cysteine protease inhibitor cystatin C
Introduction
Acute renal injury (AKI), also known as acute renal failure, refers to the clinical syndrome of a sudden decline in renal function during the first 3 months of a disease course (1). AKI can be caused by a variety of factors, with clinical manifestations including fluid and electrolyte balance and acid and alkaline balance disorders as well as azotemia (2). AKI incidence in critically ill patients is high (up to 30–50%), and AKI severity is positively correlated with patient mortality, with AKI is one of the leading causes of death in critically ill patients (3). AKI can increase the burden of disease and the treatment costs of critically ill patients. At present, the pathophysiological mechanism of AKI is not yet fully understood, so improving treatment methods is a challenge. Therefore, AKI has become one of the most difficult medical problems present throughout the world (4,5). SCr is often used as a diagnostic index for AKI, but SCr levels are affected by many factors (blood volume, muscle mass and protein intake), leading to its uncertain association with acute renal cell injury. As such, SCr cannot be used to accurately and timely reflect the occurrence of AKI (6). Early detection and timely treatment is the key for the treatment of AKI, so the identification of novel biomarkers of AKI for early diagnosis is particularly important. As a member of lipocalin superfamily, NGAL was considered to be one of the ideal and important biomarkers in the diagnosis of AKI (7). CysC is a member of the cysteine protease inhibitor superfamily and is frequently used as a marker for the diagnosis of AKI. In this study, we examined and analyzed NGAL levels in critically ill patients after surgery. The present study provided a theoretical basis for the early diagnosis of AKI.
Materials and methods
General information
Thirty-eight critically ill patients with acute renal injury treated at Zhengzhou No. 7 People's Hospital between December 2015 and November 2016 were selected to serve as AKI group (observation group). At the same time, 38 critically ill patients without acute renal injury were selected as the non-AKI group (control group). Inclusion criteria were as follows: i) critically ill patients with surgical treatment; ii) ICU stay ≥24 h; iii) patients provided informed consent. Exclusion criteria were as follows: i) patients had received nephrotoxic drugs within the week prior to selection; ii) patients had received conventional dialysis before admission; iii) patients with heart failure and malignant tumors. There were no significant differences in general information parameters between the two groups (P>0.05; Table I). The study was approved by the Ethics Committee of Zhengzhou No. 7 People's Hospital.
Table I.
General patient information.
| Items | Observation group (n=38) | Control group (n=38) | t/χ2 | P-value |
|---|---|---|---|---|
| Sex (male/female) | 21/17 | 20/18 | 0.046 | 0.829 |
| Age range (years) | 40–75 | 35–75 | ||
| Average age (years) | 48.76±7.48 | 49.07±7.86 | 0.176 | 0.861 |
| MAP (mmHg) | 96.87±12.37 | 97.35±11.58 | 0.175 | 0.862 |
| WBC (109/l) | 16.06±5.93 | 15.84±5.74 | 0.164 | 0.869 |
| Serum BUN (mmol/n) | 29.03±5.83 | 28.14±5.35 | 0.693 | 0.490 |
| Type of damage (n, %) | ||||
| Acute tubular necrosis | 15 (39.47) | 16 (42.11) | 0.047 | 0.827 |
| Acute glomerular injury | 14 (36.84) | 15 (39.47) | 0.053 | 0.818 |
| Acute interstitial nephritis | 9 (23.68) | 7 (18.42) | 0.076 | 0.781 |
Experimental methods
Experimental equipment and reagents
Main equipment consisted of a microplate reader (Jiangsu Potebio Biotechnology Co., Ltd., Jiangsu, China), automatic biochemical instrument (Precise, Beijing, China), centrifuge (Beijing Guangan Medical Equipment Factory), pipettes (Dragon Laboratory Instruments Ltd., Beijing, China), EP tubes and centrifuge tubes (Haimen Innovative Experimental Equipment Factory, Jiangsu, China). Experimental reagents consisted of NGAL ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA), CysC kits (Beijing Strong Biotechnologies, Inc., Beijing, China) and SCr kits (BioSino, Beijing, China).
Specimen collection
Blood samples were extracted from the radial artery of all patients at 2, 8, 12 and 24 h post-operation. The blood samples were centrifuged for 5 min, and the supernatant was transferred into EP tubes and kept at −80°C for storage.
Indicator detection
NGAL and Scr levels were detected using ELISA according to the manufacturers instructions. In brief: i) blood stored at −80°C was thawed at room temperature (20°C); ii) samples and standards were diluted (dilution ratio 1:5) and transferred to the plate; iii) samples were incubated in the plate for 30 min at 37°C and then washed with phosphate-buffered saline (PBS) 5 times for 15 sec for each time; iv) enzyme-conjugated reagent (50 µl) was added and incubated at 37°C for 30 min, followed by four 15-sec washes; v) color developer A and B were added and the samples were incubated at room temperature in the dark for 15 min. The termination solution was then added, and the OD value at 450 nm was measured using a microplate reader within 15 min. These readings were used to calculate NGAL and Scr levels.
CysC levels were detected using PETIA, under the principle that serum CysC can bind to latex particles coated with goat anti-human CysC polyclonal antibodies (dilution 1:500; cat. no. PD-RM-0006-M0001; Biomart, Wuhan, China), resulting in increased turbidity at 600 nm proportional to CysC levels. The main parameters of the assay were as follows: main wavelength, 600 nm; reaction methods, point end assay; reaction direction, positive; temperature, 37°C. The difference in OD value of the calibration solution (OD value at 5 min later - OD value at 1 min later) was calculated, and an absorbance-concentration curve for the calibration solution was established. The difference in sample OD value was then calculated and CysC level was calculated according to the absorbance-concentration curve.
Evaluation criteria
Serum NGAL and Scr levels were measured by ELISA at 2, 8, 12 and 24 h post-operation and serum CysC levels were measured by PETIA at the same time-points.
Statistical analysis
Data were processed using the SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) software. Measurement data were expressed as mean ± standard deviation (false) and comparisons between groups were performed using the t-test. Measurement data were expressed by ratio and comparison between the groups were performed using χ2 test. Pearson correlation coefficient analysis was carried out for correlation analysis. Diagnostic value was analyzed by ROC curve. P<0.05 was considered to be statistically significant.
Results
Serum NGAL levels
The serum NGAL level in the observation group was significantly higher than in the control group at 2, 8, 12 and 24 h after operation (P<0.05; Table II).
Table II.
NGAL level comparison (ng/ml).
| Group | Cases | 2 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|
| Observation | 38 | 81.78±3.48 | 83.93±3.23 | 85.29±3.48 | 89.29±3.48 |
| Control | 38 | 23.04±3.98 | 21.05±3.32 | 18.06±3.59 | 17.64±3.59 |
| t-value | 68.941 | 83.683 | 82.889 | 88.339 | |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Serum SCr levels
No significant difference in SCr level was found between observation and control groups at 2, 8, 12 and 24 h after operation (P<0.05; Table III).
Table III.
SCr level comparison (µmol/l).
| Group | Cases | 2 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|
| Observation | 38 | 72.18±3.54 | 70.84±3.28 | 73.87±3.25 | 78.78±3.74 |
| Control | 38 | 72.05±3.72 | 71.03±3.62 | 74.25±3.17 | 79.04±3.69 |
| t-value | 0.156 | 0.240 | 0.516 | 0.305 | |
| P-value | 0.876 | 0.811 | 0.607 | 0.761 |
Serum CysC levels
The serum CysC level in the observation group was significantly higher than in the control group at 2, 8, 12 and 24 h after operation (P<0.05; Table IV).
Table IV.
CysC level comparison (mg/l).
| Group | Cases | 2 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|
| Observation | 38 | 4.08±0.43 | 4.23±0.28 | 4.39±0.38 | 4.59±0.47 |
| Control | 38 | 2.83±0.78 | 2.57±0.37 | 1.73±0.49 | 1.03±0.52 |
| t-value | 8.651 | 22.054 | 26.444 | 31.309 | |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
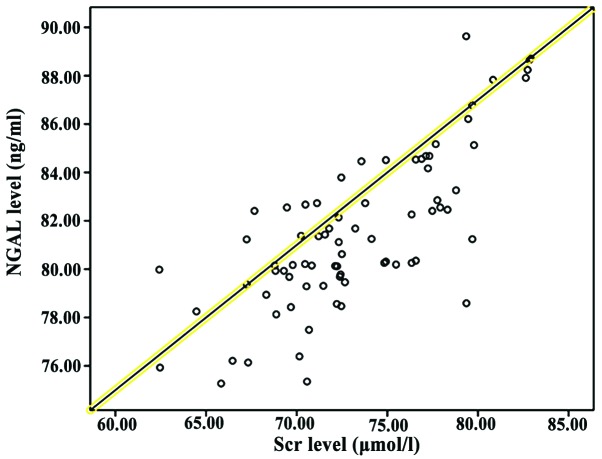
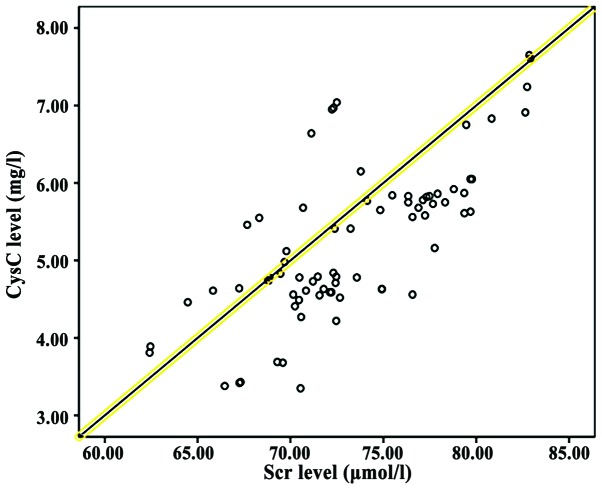
Correlation analysis between NGAL, CysC and Scr
Pearson correlation coefficient analysis showed that NGAL and CysC levels were positively correlated with SCr levels (P<0.05; Table V and Figs. 1 and 2).
Table V.
Correlation between NGAL and CysC levels and SCr level.
| Indication | r | P-value |
|---|---|---|
| NGAL | 0.518 | 0.013 |
| CysC | 0.501 | 0.027 |
Figure 1.
Correlation between NGAL and SCr levels. Pearson correlation coefficient analysis showed that NGAL levels were positively correlated with SCr levels (P<0.05).
Figure 2.
Correlation between NGAL and CysC levels. Pearson correlation coefficient analysis showed that NGAL levels were positively correlated with CysC levels (P<0.05).
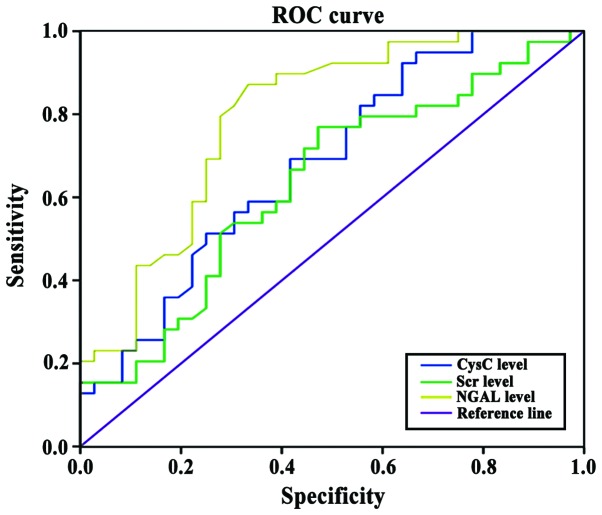
Comparison of NGAL, CysC and SCr for the early diagnosis of AKI
The potential of NGAL, CysC and SCr for use as an early diagnostic marker of AKI was evaluated. For NGAL, the area under the AKI curve was 0.904, the sensitivity was 90.2% and the specificity was 89.5%; for CysC, the area under the AKI curve was 0.806, the sensitivity was 79.2% and the specificity was 78.5%; for Scr, the area under the AKI curve was 0.634, the sensitivity was 64.2% and the specificity was 62.5% (Fig. 3).
Figure 3.
Comparison of NGAL, CysC and Scr for early diagnosis of AKI. The area below the AKI curve of NGAL for early diagnosis of AKI was larger than that of CysC, and the area below the AKI curve of CysC for early diagnosis of AKI was larger than that of SCr.
Discussion
AKI is a new concept proposed by the International Renal Disease and Emergency Medicine community to replace the term ‘acute renal failure’. Infectious diseases are the main causes of AKI, and other causes may include cardiogenic shock, hypovolemia, septic shock and major surgery. AKI is common for critically ill patients in the ICU. The mortality rate of AKI is high and AKI is also an independent risk factor for death in critically ill patients (8). The renal function of AKI patients can be significantly reduced within a short time, and the resultant inability to excrete metabolites reduces the stability of the internal environment. The diagnostic criteria used to identify AKI are: sudden changes in renal structure or function within 48 h identified by imaging, or hematuria during routine examination found within 3 months, with an increase in SCr content greater than 26.4 µmol/l or 50% of the base value; urine output below 0.5 ml/(kg·h) for more than 6 h, but not including patients with dehydration and obstructive nephropathy (9). Compared with the term ‘acute renal failure’, the term AKI covers a wider range, including renal damage from slight changes in renal function to final renal function loss. AKI can accurately reflect the developmental nature of nephropathy (10). Although the incidence of AKI is high, the awareness rate is generally low. Early symptoms of AKI are not typical, and this, coupled with the fact that diagnoses are affected by a variety of factors, results in low diagnostic sensitivity. This in turn delays diagnosis and increases mortality in critically ill patients (11). Most AKI cases are reversible, and early diagnosis and treatment can prevent the development of renal failure. Therefore, diagnosis and treatment are keys in reducing the mortality rate in critically ill patients with AKI (12).
Urine output and changes in SCr levels are usually used to reflect changes in glomerular filtration rate and renal function decline in order to provide a basis for the diagnosis of AKI. Renal tubular damage can destroy the renal hypertonic environment and reduce the ability to concentrate urine, resulting in non-oliguric nephropathy (13). In addition, urine output, which is affected by diuretics and bladder capacity, cannot reflect renal function in real-time. Therefore, for the diagnosis of AKI, urine output and SCr levels cannot properly reflect renal injury. This misses the optimal time-point for treatment and increases patient pain and systemic economic burden (14). The results of this study showed that there was no significant difference in serum SCr level between the two groups at 2, 8, 12 and 24 h after operation (P>0.05). This was because serum SCr level is affected by nutritional status, muscle metabolism, and preoperative medication. Renal compensatory and reserve capacity is strong under normal conditions. SCr levels can only be increased slightly when the glomerular filtration rate is reduced by 50% or more. In addition, SCr accumulation takes time, and SCr levels cannot reach a stable state within a short period (6). Therefore, SCr cannot be used as a marker to accurately reflect short-term changes in renal function.
NGAL is a member of lipocalin superfamily with a small molecular weight of only 25 kDa (15). NGAL was first found in human neutrophils. NGAL is very stable, so it can be easily detected in serum. Under normal conditions, low NGAL expression levels can be detected in a variety of tissues (lung, kidney, large intestine and stomach). High expression levels of NGAL can be induced by cell apoptosis after epithelial cell damage (16). The results of this study showed that serum NGAL levels in the observation group were significantly higher than in the control group at 2, 8, 12 and 24 h after operation (P<0.05). A possible mechanism for this phenomenon may be that various factors stimulated renal tubular epithelial cells, leading to increased NGAL expression. NGAL can be absorbed by renal epithelial cells to regulate the expression of apoptosis-related proteins, which in turn promotes cell maturation and induces granulocyte apoptosis (16).
CysC, a member of the cysteine protease inhibitor superfamily, is a type of non-glycosylated alkaline protein secreted by karyocytes. CysC can be synthesized at a very stable rate and released into the circulation. After complete glomerular filtration, CysC will be uptaken and degraded by nearby tubule epithelial cells. Therefore, serum CysC levels can precisely reflect the glomerular filtration rate. As such, CysC can be an ideal marker for the diagnosis of AKI (17–19). Under normal conditions, CysC levels are low. However, CysC levels can significantly increase after renal tubular injury (20). The results of this study showed that the serum CysC levels in the observation group were significantly higher than in the control group at 2, 8, 12,0 and 24 h after operation (P<0.05), indicating that internal environment of the patients was altered after AKI.
Relevant studies have confirmed that increased NGAL and CysC levels appeared 24 to 48 h prior to SCr level increases in the diagnosis of AKI (21). In the present study, we found that for NGAL in early diagnosis, the area under the AKI curve was 0.904, the sensitivity was 90.2% and the specificity was 89.5%; for CysC in early diagnosis, the area under the AKI curve was 0.806, the sensitivity was 79.2% and the specificity was 78.5%; for SCr in early diagnosis, the area under the AKI curve was 0.634, the sensitivity was 64.2% and the specificity was 62.5%. Our findings were consistent with previous studies. In addition, the sensitivity and specificity of NGAL were higher than those of CysC and SCr for the detection of AKI. The results also showed that the levels of NGAL and CysC were positively correlated with SCr levels, indicating that the increased expression of NGAL and CysC were consistent with the upregulation of SCr expression.
In summary, evaluating NGAL levels in critically ill patients may improve the early detection of AKI and facilitate early treatment and improve prognosis.
References
- 1.Teles F, de Mendonça Uchôa JV, Mendonça Mirelli Barreto D, Costa Falcão Pedrosa A. Acute kidney injury in leptospirosis: The Kidney Disease Improving Global Outcomes (KDIGO) criteria and mortality. Clin Nephrol. 2016;86:303–309. doi: 10.5414/CN108865. [DOI] [PubMed] [Google Scholar]
- 2.Brown JR, Robb JF, Malenka DJ. Abstract 1056: Does ‘safe’ dosing of iodinated contrast prevent contrast-induced acute kidney injury. Cardiology. 2015;131:249. doi: 10.1161/CIRCINTERVENTIONS.109.910638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 4.Wang N, Jiang L, Zhu B, Wen Y, Xi XM. Beijing Acute Kidney Injury Trial (BAKIT) Workgroup: Fluid balance and mortality in critically ill patients with acute kidney injury: A multicenter prospective epidemiological study. Crit Care. 2015;19:371. doi: 10.1186/s13054-015-1085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuartero M, Betbesé A, Sabater J, Ballús J, Ordóñez J. Urinary TIMP2 and IGFBP7 as early biomarkers of acute kidney injury in septic and nonseptic critically ill patients. Crit Care. 2015;19(Suppl 1):191–201. doi: 10.1186/cc14368. [DOI] [Google Scholar]
- 6.Lagos-Arevalo P, Palijan A, Vertullo L, Devarajan P, Bennett MR, Sabbisetti V, Bonventre JV, Ma Q, Gottesman RD, Zappitelli M. Cystatin C in acute kidney injury diagnosis: Early biomarker or alternative to serum creatinine? Pediatr Nephrol. 2015;30:665–676. doi: 10.1007/s00467-014-2987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veighey K, MacAllister R. Clinical applications of remote ischaemic preconditioning in native and transplant acute kidney injury. Pediatr Nephrol. 2015;30:1749–1759. doi: 10.1007/s00467-014-2965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostermann M, Dickie H, Barrett NA. Renal replacement therapy in critically ill patients with acute kidney injury - when to start. Nephrol Dial Transplant. 2012;27:2242–2248. doi: 10.1093/ndt/gfr707. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Zappitelli M, Chawla LS. Novel biomarkers of AKI: The challenges of progress ‘Amid the noise and the haste’. Nephrol Dial Transplant. 2013;28:235–238. doi: 10.1093/ndt/gfs595. [DOI] [PubMed] [Google Scholar]
- 10.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, McGuinness S, Mehrtens J, Myburgh J, Psirides A, et al. SPLIT Investigators; ANZICS CTG: Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: The SPLIT Randomized Clinical Trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 11.Siew ED, Davenport A. The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int. 2015;87:46–61. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karvellas CJ, Durand F, Nadim MK. Acute kidney injury in Cirrhosis. Crit Care Clin. 2015;31:737–750. doi: 10.1016/j.ccc.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Siew ED, Matheny ME. Choice of reference Serum creatinine in defining acute kidney injury. Nephron. 2015;131:107–112. doi: 10.1159/000439144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grynberg K, Polkinghorne KR, Ford S, Stenning F, Lew TE, Barrett JA, Summers SA. Early serum creatinine accurately predicts acute kidney injury post cardiac surgery. BMC Nephrol. 2017;18:93. doi: 10.1186/s12882-017-0504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Geus HR, Betjes MG, Schaick R, Groeneveld JA. Plasma NGAL similarly predicts acute kidney injury in sepsis and nonsepsis. Biomarkers Med. 2013;7:415–421. doi: 10.2217/bmm.13.5. [DOI] [PubMed] [Google Scholar]
- 16.Devarajan P. NGAL for the detection of acute kidney injury in the emergency room. Biomarkers Med. 2014;8:217–219. doi: 10.2217/bmm.13.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yim H, Kym D, Seo DK, Yoon J, Yang HT, Lee J, Cho YS, Hur J, Chun W, Han SW. Serum cystatin C and microalbuminuria in burn patients with acute kidney injury. Eur J Clin Invest. 2015;45:594–600. doi: 10.1111/eci.12452. [DOI] [PubMed] [Google Scholar]
- 18.Bongiovanni C, Magrini L, Salerno G, Gori CS, Cardelli P, Hur M, Buggi M, Di Somma S. Serum cystatin C for the diagnosis of acute kidney injury in patients admitted in the emergency department. Dis Markers. 2015;2015:416059. doi: 10.1155/2015/416059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpon LC, Sugo EK, Carlotti AP. Diagnostic and prognostic value of serum cystatin C in critically ill children with acute kidney injury. Pediatr Crit Care Med. 2015;16:e125–e131. doi: 10.1097/PCC.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 20.Gaygısız Ü, Aydoğdu M, Badoğlu M, Boyacı N, Güllü Z, Gürsel G. Can admission serum cystatin C level be an early marker subclinical acute kidney injury in critical care patients? Scand J Clin Lab Invest. 2016;76:143–150. doi: 10.3109/00365513.2015.1126854. [DOI] [PubMed] [Google Scholar]
- 21.Ghonemy TA, Amro GM. Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery. Saudi J Kidney Dis Transpl. 2014;25:582–588. doi: 10.4103/1319-2442.132194. [DOI] [PubMed] [Google Scholar]