Abstract
Brain microvascular endothelial cells (BMECs) are the primary component of the blood-brain barrier (BBB). Tight junction (TJ) proteins, including claudin, occludin and zonula occludens (ZO)-1, ZO-2 and ZO-3, maintain the structural integrity of BMECs. Ethanol activates the assembly and disassembly of TJs, which is a process that is regulated by protein kinase C (PKC). In addition, ethanol treatment leads to the loss of structural integrity, which damages the permeability of the BBB and subsequently affects central nervous system homeostasis, thus allowing additional substances to enter the brain. However, the mechanisms underlying ethanol-induced loss of BBB structure remain unknown. It has been hypothesized that long-term exposure to ethanol reduces the expression of claudin-5, occludin and ZO-1 via the PKC signaling pathway, thereby affecting BBB structural integrity. In the current study, the human cerebral microvascular endothelial cell line, HCMEC/D3, was treated with 50, 100, 200 and 400 mM ethanol for 24, 48 and 72 h. Cell viability was determined using an MTS assay. The expression of claudin-5, occludin and ZO-1 protein and mRNA was measured using western blot analysis and reverse transcription-quantitative polymerase chain reaction, respectively. Following the pretreatment of HCMEC/D3 cells with the PKCα-specific inhibitor, safingol (10 µmol/l), the expression of claudin-5, occludin, ZO-1 and phosphorylated (p)-PKCα was measured using western blot analysis, and PKCα localization was determined by immunofluorescence. With increasing concentrations of ethanol, the expression of claudin-5, occludin and ZO-1 protein decreased, while the expression of claudin-5, occludin and ZO-1 mRNA increased. Exposure to ethanol significantly increased the expression of p-PKCα, whereas no significant effect on the expression of PKCα was observed. Following 48 h treatment with 200 mM ethanol, the expression of claudin-5, occludin and ZO-1 protein was significantly decreased when compared with the control. By contrast, the expression of p-PKCα was increased, and increased translocation of PKCα from the cytoplasm to the nuclear membrane and nucleus was observed. In addition, the results demonstrated that safingol significantly reversed these effects of ethanol. In conclusion, long-term exposure to ethanol downregulates the expression of claudin-5, occludin and ZO-1 protein in HCMEC/D3 s, and this effect may be mediated via activation of PKCα.
Keywords: ethanol, blood-brain barrier, human cerebral microvascular endothelial cells, tight junction, protein kinase Cα
Introduction
The blood-brain barrier (BBB) is formed by brain microvascular endothelial cells (BMECs) in conjunction with pericytes and astrocytes (1). The BBB strictly regulates the passage of ions, molecules, leukocytes and nutrients in and out of the brain, and serves an important role in maintaining homeostasis of the central nervous system (2,3). BMECs are the primary component of the BBB. Tight junctions (TJs) are composed of transmembrane proteins, including claudins, occludin and intracellular proteins such as zonula occludens (ZO)-1, ZO-2 and ZO-3, which maintain the structural integrity of BMECs (4,5). Claudin-5 is expressed abundantly in the cerebral microvascular endothelial system and is essential for the assembly of TJs (6,7). Occludin maintains the tightness of TJs, and ZO proteins link claudins, occludin and adhesion molecules to the actin cytoskeleton in order to facilitate signaling between the intracellular actin cytoskeleton and extracellular binding proteins, thereby adjusting the TJ barrier function (8–10).
Ethanol alters endothelial cell function through a number of diverse mechanisms. The exposure of human dermal MECs to ethanol inhibits tumor necrosis factor-induced nuclear translocation of nuclear factor-κB, thereby suppressing endothelial cells activation (11). Ethanol activates Ca2+-activated K+ channels to improve endothelial cell function (12). Low levels of ethanol induce and sustain increased surface-localized endothelial cell fibrinolysis, thereby conferring cardioprotection by reducing the risk of thrombosis, myocardial infarction, cardiovascular mortality and morbidity (13). Chronic exposure to ethanol stimulates pulmonary artery endothelial cell nitric oxide production through phosphatidylinositol 3-kinase- and heat shock protein 90-dependent mechanisms, which contributes to ethanol-mediated susceptibility to lung injury (14). Ethanol activates BMEC myosin light chain kinase (MLCK), leading to the phosphorylation of MLC, occludin and claudin-5, and the subsequent impairment of the BBB (15). In addition, it has been demonstrated that oxidative stress stimulates inositol-1,4,5-triphosphate receptor-gated intracellular Ca2+ release, leading to the activation of MLCK and potentially protein tyrosine kinase (PTK); these alterations contribute to the loss of BBB integrity (16). Ethanol metabolites, acetaldehyde and reactive oxygen species lead to BBB injury by inducing alterations in the basement membrane and TJ proteins via the activation of PTK and matrix metalloproteinases (17). Therefore, the ethanol-mediated modulation of endothelial cell signaling pathways is complex and diverse and its mechanism of action remains unclear.
Protein kinase C (PKC) is a serine/threonine kinase present in the tissues and cells of humans and animals. The PKC family consists of several isoforms, which are classified into the following three major subfamilies: Classic (α, β and γ), atypical (δ, ε, η and θ) and novel (ξ and λ). Safingol is an optical isomer (an L-threo enantiomer) of dihydrosphingosine, which is a specific inhibitor of PKC (18). PKC serves a role in increasing and decreasing TJ permeability. PKCα, PKCβ and PKCξ isoforms are primarily expressed in vascular endothelial cells and the central nervous system, and affect the opening of TJs (19–21). Treatment with the PKC agonist, phorbol 12-myristate 13-acetate, may lead to the disintegration of TJs (22). Thrombin regulates the permeability of pulmonary MECs via the PKC signaling pathway (23). Ethanol increases the permeability of airway epithelial TJs in BEAS-2B and normal human bronchial epithelial cells by activating PKCα (24). Persistent activation of PKCα leads to loss of barrier function in the TJ complex (25). During ischemia and hypoxia reperfusion, activation of the PKC signaling molecule increases BBB permeability and decreases the expression of TJ proteins (26,27). This suggests that PKC may serve a role in the ethanol-induced alterations in TJs and thus cell permeability.
It has been hypothesized that ethanol impairs BBB structure by influencing the expression of TJ proteins via the PKCα signaling pathway in BMECs. In the current study, the human cerebral microvascular endothelial cell line, HCMEC/D3, was treated with ethanol, and the expression of claudin-5, occludin and ZO-1 mRNA and protein was detected. Following pretreatment of HCMEC/D3s with the PKCα-specific inhibitor safingol, alterations in the expression of claudin-5, occludin and ZO-1 protein were determined, and the PKCα phosphorylation status and its localization were detected.
Materials and methods
Chemicals and reagents
Dimethyl sulfoxide (cat no. D4540) and safingol (cat no. D4681) were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). CellTiter 96® AQueous One Solution reagent was purchased from Promega Corporation (Madison, WI, USA). Normal donkey serum (cat no. 017-000-121) was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Rabbit anti-PKCα (cat no. sc-208) and mouse anti-β-actin (cat no. sc-47778) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit anti-occludin (cat no. ab31721) was purchased from Abcam (Cambridge, UK). Polyvinylidene difluoride (PVDF) membranes, rabbit anti-claudin-5 (cat no. ABT45), rabbit anti-ZO-1 (cat no. AB2272) and rabbit anti-phosphorylated (p)-PKCα (cat no. 07-790) were purchased from EMD Millipore (Billerica, MA, USA). Alexa Fluor® 488-conjugated donkey anti-rabbit IgG (cat no. A-21206) was purchased from Invitrogen; Thermo Fisher Scientific, Inc. RNAiso Plus (cat no. D9108), PrimeScript™ RT reagent kit (cat no. DRR037) and SYBR® Premix Ex Taq™ II (Tli RNaseH Plus; cat no. RR820A) were purchased from Takara Bio, Inc. (Otsu, Japan). Peroxidase-conjugated anti-mouse (cat no. ZB-2305) and anti-rabbit (cat no. ZB-2301) secondary antibodies were purchased from ZSGB-Bio (Beijing, China). All additional chemicals and supplies were purchased from Beyotime Institute of Biotechnology (Haimen, China).
Cell model and experimental grouping
HCMEC/D3s were purchased from BeNa Culture Collection (Beijing, China). HCMEC/D3s were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. Cultures were incubated at 37°C in a humidified 5% CO2 atmosphere.
The experimental groups were as follows: i) HCMEC/D3 cells were treated with 50, 100, 200 or 400 mM ethanol for 24, 48 and 72 h, and fresh ethanol-containing medium was replaced every 24 h to maintain a constant ethanol concentration; ii) when HCMEC/D3 cells had reached 80–90% confluence in normal medium, the cells were cultured in serum-free RPMI-1640 medium for 24 h prior to pretreatment with 10 µmol/l PKCα-specific inhibitor safingol for 1 h, followed by treatment with 200 mM ethanol for 48 h. Cells in the 200 mM ethanol-treated group were treated with ethanol alone, and cells in the control group were cultured in RPMI-1640 medium only.
Cell viability assay
The viability of HCMEC/D3 cells treated with or without ethanol was determined using an MTS assay. Cells were seeded in 96-well plates at a density of 4,000 cells/well in triplicate. Following 24 h culture, the medium was refreshed with RPMI-1640 and 50, 100, 200 or 400 mM ethanol was added. Culturing was then continued for 24, 48 and 72 h. CellTiter 96® AQueous One Solution reagent was added to each well and cells were incubated at 37°C for 2 h in the dark. The absorbance was read at a wavelength of 490 nm to determine the reduction of MTS by viable cells.
Protein extraction and western blot analysis
Total cellular protein was extracted using radioimmunoprecipitation assay buffer (cat no. P0013B; Beyotime Institute of Biotechnology) supplemented with 10 mg/ml phenylmethanesulfonyl fluoride and phosphatase inhibitors, and samples were centrifuged at 11,430 × g for 15 min at 4°C. The protein concentration of the supernatant was determined using a BCA protein assay kit. Protein samples (50 µg) was subjected to 10% SDS-PAGE and transferred to PVDF membranes. Following blocking with 8% non-fat milk in Tris-buffered saline-0.1% Tween-20 (TBST) at room temperature for 2 h, membranes were incubated at 4°C overnight with rabbit anti-claudin-5 polyclonal antibody (dilution, 1:5,000), rabbit anti-occludin polyclonal antibody (dilution, 1:2,000), rabbit anti-ZO-1 polyclonal antibody (dilution, 1:5,000), rabbit anti-PKCα polyclonal antibody (dilution, 1:800) or rabbit anti-p-PKCα polyclonal antibody (dilution, 1:500). Mouse anti-β-actin monoclonal antibody (dilution, 1:5,000) was used for relative protein quantification. Membranes were then washed three times with TBST and incubated with the corresponding peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (dilution, 1:5,000) at room temperature for 2 h. Chemiluminescence was visualized with luminol reagent, and images were captured and analyzed using an electrophoresis gel imaging analysis system (Tanon 5500; Tanon Science and Technology Co., Ltd., Shanghai, China). Band densities were analyzed semi-quantitatively using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Extraction of total RNA and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using RNAiso Plus according to the manufacturer's protocol. The PrimeScript™ RT reagent kit was subsequently used to synthesize cDNA in a total reaction volume of 10 µl, consisting of 2 µl PrimerScript buffer (5X), 0.5 µl PrimeScript RT Enzyme Mix I, 0.5 µl Oligo dT Primer, 0.5 µl Random hexamers, 4.5 µl RNase-free dH2O and 2 µl total RNA (0.4 µg). The cDNA was amplified by qPCR with sequence-specific primer pairs (Table I). qPCR was performed using SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) and an Applied Biosystems 7500 Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). Samples were analyzed in triplicate. The reaction mixture (20 µl) consisted of 10 µl SYBR Premix ExTaq, 0.8 µl forward primer, 0.8 µl reverse primer, 0.4 µl Rox reference dye, 6 µl RNase-free dH2O and 2 µl cDNA. The thermal cycling parameters were as follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec, followed by 95°C for 15 sec, 60°C for 30 sec and 95°C for 15 sec. Relative quantification of claudin-5, occludin, ZO-1 and the β-actin endogenous reference gene was performed using the 2−∆∆Cq comparative threshold cycle method (28). The primers for claudin-5, occludin, ZO-1 and β-actin were designed using web-based Integrated DNA Technologies SciTools Real-Time qPCR software (Oligo Analyser version 3.1; idtdna.com/Scitools/Applications/RealTimePCR; Integrated DNA Technologies, Inc., Coralville, IA, USA).
Table I.
Primer sequences used for reverse transcription-quantitative polymerase chain reaction.
| Genes | Forward (5′-3′) | Reverse (5′-3′) | GenBank ID |
|---|---|---|---|
| Claudin-5 | TTTCCCTAACTTCAGCTGCC | CCCTCTTTGAAGGTTCGGG | NM_001130861 |
| Occludin | GCAAAGTGAATGACAAGCGG | CACAGGCGAAGTTAATGGAAG | NM_002538 |
| ZO-1 | TGCTGAGTCCTTTGGTGATG | AATTTGGATCTCCGGGAAGAC | NM_003257 |
| β-actin | CTAACTTGCGCAGAAAACAAGAT | TTCCTGTAACAACGCATCTCATA | NM_001101 |
ZO-1, zonula occludens-1.
Immunofluorescence staining
Cells were cultured on glass cover slips in 24-well plates and incubated with RPMI-1640 medium containing 200 mM ethanol or 200 mM ethanol plus safingol for 48 h. Cells were then washed with PBS and fixed in 4% (volume/volume) paraformaldehyde for 20 min at room temperature. Following washing, cells were permeabilized in 0.5% Triton X-100, washed again and incubated with 2% normal donkey serum in PBS for 2 h at room temperature. The cells were subsequently immunostained with antibodies against PKCα (dilution, 1:100) at 4°C overnight. Following washing, the primary antibody was detected with the Alexa Fluor® 488-conjugated donkey anti-rabbit IgG (dilution, 1:200) for 2 h at room temperature under light protection. The immunofluorescence images were viewed under a fluorescence microscope (Leica DM4000 B; Leica Microsystems GmbH, Wetzlar, Germany). Cells were incubated with rabbit non-immune IgG or PBS instead of the primary antibody for the negative control and no positive signal was detected.
Statistical analysis
All data are presented as the mean ± standard deviation, and were analyzed using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons among different groups were evaluated using a one-way analysis of variance with a Bonferroni post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of long-term ethanol exposure on cell viability
An MTS assay was used to assess the effects of long-term ethanol exposure on cell viability. Compared with the respective controls, cell viability was slightly increased following 24, 48 and 72 h treatment with 50 mM ethanol and slightly decreased following 24 and 48 h treatment with 100 mM ethanol and following 24 h treatment with 200 mM ethanol; however, these differences were not statistically significant (Fig. 1). Cell viability was significantly decreased following 72 h of treatment with 100 mM ethanol (83.6±4.5%), following 48 and 72 h of treatment with 200 mM ethanol (75.8±3.4 and 70.4±5.3%, respectively) and following 24, 48 and 72 h of treatment with 400 mM ethanol (72.1±3.9, 55.1±4.1 and 40.6±3.9%, respectively; all P<0.05), when compared with their respective untreated controls (Fig. 1).
Figure 1.
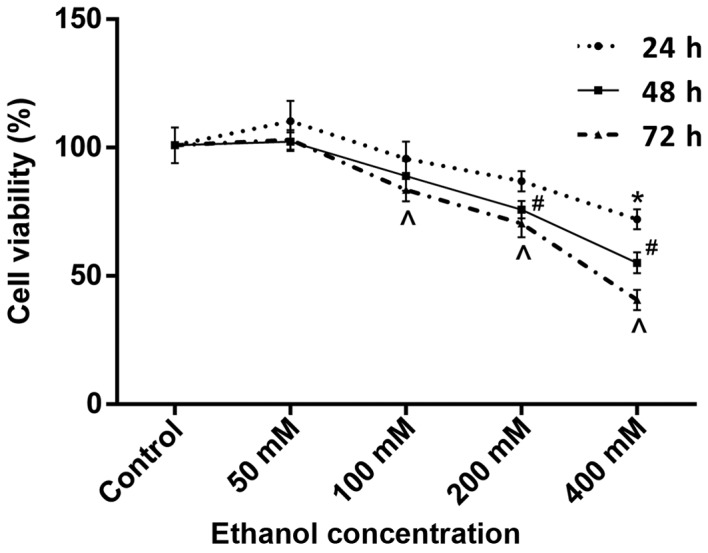
Cell viability following treatment with different doses of ethanol. Cell viability was determined using an MTS assay. Data are presented as the mean ± standard deviation and experiments were performed in triplicate. *P<0.05 vs. control at 24 h; #P<0.05 vs. control at 48 h; ^P<0.05 vs. control at 72 h.
Effect of long-term ethanol exposure on the expression of claudin-5, occludin and ZO-1
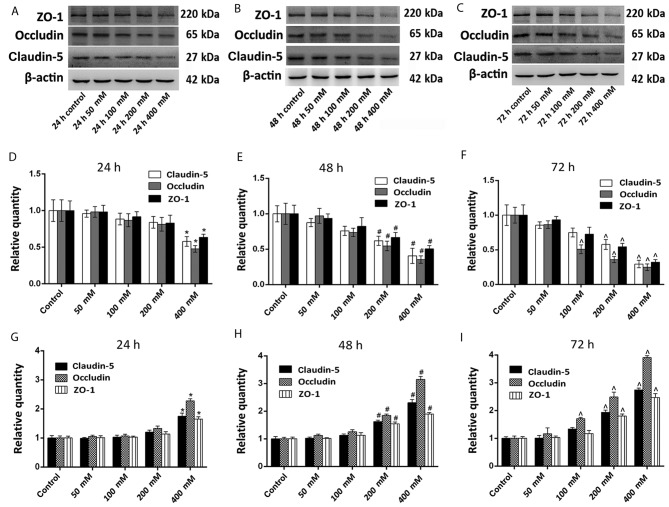
Western blotting was used to evaluate the effects of long-term ethanol exposure on the expression of claudin-5, occludin and ZO-1. Overall, the protein expression levels of claudin-5, occludin and ZO-1 were decreased following treatment with all concentrations of ethanol for 24, 48 or 72 h (Fig. 2A-C).
Figure 2.
Effect of ethanol treatment on the expression of claudin-5, occludin and ZO-1. Protein expression levels were measured using western blotting, and mRNA expression levels were determined using reverse transcription-quantitative polymerase chain reaction. (A) Western blotting analysis of claudin-5, occludin, ZO-1 and β-actin expression following ethanol treatment at (A) 24, (B) 48 and (C) 72 h. Quantitative analysis of western blotting results, which were normalized to β-actin expression at (D) 24, (E) 48 and (F) 72 h. Relative expression of claudin-5, occludin and ZO-1 mRNA at (G) 24, (H) 48 and (I) 72 h. The results (n=5) are presented as the mean ± standard deviation. *P<0.05 vs. control at 24 h; #P<0.05 vs. control at 48 h; ^P<0.05 vs. control at 72 h. ZO-1, zonula occludens-1.
Compared with their respective controls, the protein expression levels of claudin-5 and ZO-1 were significantly decreased following 48 and 72 h of treatment with 200 mM ethanol, and following 24, 48 and 72 h of treatment with 400 mM ethanol (all P<0.05; Fig. 2D-F). The protein expression of occludin was significantly decreased following 72 h treatment with 100 mM ethanol, following 48 and 72 h of treatment with 200 mM ethanol, and at 24, 48 and 72 h following treatment with 400 mM ethanol when compared with the untreated controls (all P<0.05; Fig. 2D-F). The protein expression levels of claudin-5 after 24, 48 and 72 h of treatment with 50 and 100 mM ethanol, and 24 h of 200 mM ethanol treatment were markedly decreased compared with their respective controls. The protein expression levels of occludin after 24, 48 and 72 h of treatment with 50 mM ethanol, 24 and 48 h of 100 mM ethanol treatment, and 24 h of 200 mM ethanol treatment were markedly decreased compared with their respective controls. The protein expression levels of ZO-1 after 24, 48 and 72 h of treatment with 50 and 100 mM ethanol, and 24 h of 200 mM ethanol treatment were markedly decreased compared with their respective controls. Although these protein levels decreased, the differences were not significant.
RT-qPCR analysis was employed to verify the effect of long-term ethanol exposure on TJ proteins by measuring the expression of claudin-5, occludin and ZO-1 mRNA. As demonstrated in Fig. 2G-I, the expression of claudin-5 and ZO-1 mRNA was significantly increased following 48 and 72 h of treatment with 200 mM ethanol, and following 24, 48 and 72 h of treatment with 400 mM ethanol compared with the untreated controls (all P<0.05). The expression of occludin mRNA was significantly increased following 72 h of treatment with 100 mM ethanol, following 48 and 72 h of treatment with 200 mM ethanol and following 24, 48 and 72 h of treatment with 400 mM ethanol when compared with untreated controls (all P<0.05; Fig. 2G-I). The expression of claudin-5 mRNA after 24, 48 and 72 h of treatment with 50 and 100 mM ethanol, and 24 h of 200 mM ethanol treatment was markedly decreased compared with their respective controls. The expression of occludin mRNA after 24, 48 and 72 h of treatment with 50 mM ethanol, 24 and 48 h of 100 mM ethanol treatment, and 24 h of 200 mM ethanol treatment was markedly decreased compared with their respective controls. The expression of ZO-1 mRNA at 24, 48 and 72 h of treatment with 50 and 100 mM ethanol, and 24 h of 200 mM ethanol treatment was markedly decreased compared with their respective controls. Although these protein levels decreased, the differences were not significant.
Effect of the ethanol-induced increase in p-PKCα expression on claudin-5, occludin and ZO-1 expression
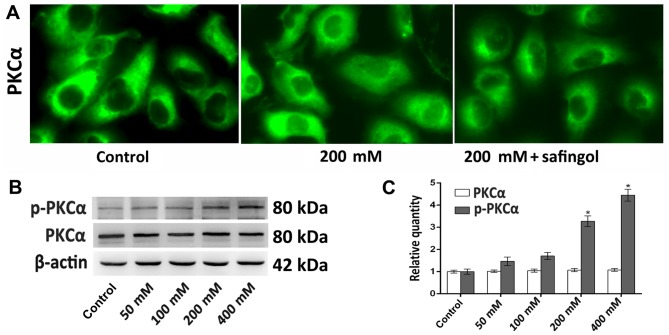
Immunofluorescence and western blotting analyses were employed to determine ethanol-induced PKCα activation by measuring PKCα and/or p-PKCα expression. Typically, PKCα is diffusely distributed throughout the cytoplasm and is localized in the nucleus in small amounts (29). Following ethanol treatment, immunofluorescence staining demonstrated that PKCα translocated from the cytoplasm to the perinuclear region and the nucleus, while safingol reversed this (Fig. 3A). The western blotting results indicated that PKCα expression in cells treated with all concentrations of ethanol was not significantly different following 48 h, where as p-PKCα expression was significantly increased in the 200 and 400 mM ethanol treatment groups compared with the control groups (both P<0.05; Fig. 3B and C).
Figure 3.
Alterations in the expression of PKCα following ethanol treatment. PKCα expression was detected by immunofluorescence staining and western blotting analyses. (A) Representative immunofluorescence staining images of PKCα following 48 h treatment with 200 mM ethanol or 200 mM ethanol plus safingol (magnification, ×400). (B) Western blotting of PKCα and p-PKCα expression following treatment with different concentrations of ethanol for 48 h. (C) Quantitative analysis of PKCα and p-PKCα protein expression. The results (n=5) are presented as the mean ± standard deviation. *P<0.05 vs. control. p-PKCα, phosphorylated-protein kinase Cα.
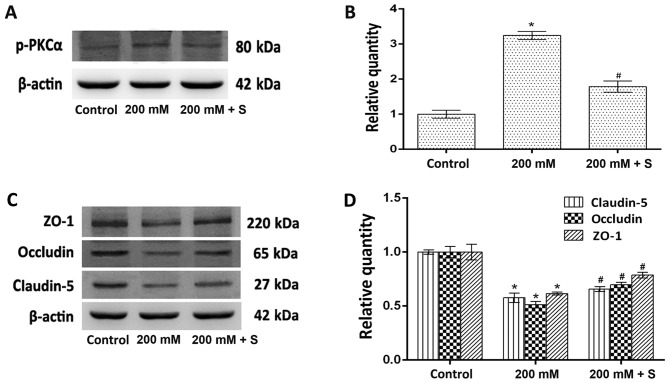
The role of PKCα in the expression of TJ proteins was determined by measuring the protein expression levels of claudin-5, occludin, ZO-1 and p-PKCα following treatment with ethanol and safingol by western blotting. The expression of p-PKCα in cells treated with 200 mM ethanol was significantly increased when compared with untreated controls (P<0.05; Fig. 4A and B). By contrast, the protein expression levels of claudin-5, occludin and ZO-1 were significantly decreased when compared with controls (all P<0.05; Fig. 4C and D). In the ethanol plus safingol treatment group, the expression of p-PKCα was significantly decreased (P<0.05; Fig. 4A and B), and the expression of claudin-5, occludin and ZO-1 was significantly increased when compared with the ethanol-only treated group (all P<0.05; Fig. 4C and D).
Figure 4.
Effect of the ethanol-induced increase in p-PKCα expression on claudin-5, occludin and ZO-1 expression. (A) Western blotting analysis of p-PKCα and β-actin protein expression. (B) Quantitative analysis of p-PKCα protein expression relative to β-actin expression. (C) Western blotting analysis of claudin-5, occludin and ZO-1 protein expression. (D) Quantitative analysis of claudin-5, occludin and ZO-1 protein expression relative to β-actin expression. The results (n=5) are presented as the mean ± standard deviation. *P<0.05 vs. control; #P<0.05 vs. 200 mM ethanol. p-PKCα, phosphorylated-protein kinase Cα; ZO-1, zonula occludens-1; S, safingol.
Discussion
The increased permeability of the BBB as a result of BBB dysfunction is well studied in individuals with alcoholism (30) and animal models (31). A previous study treated BMECs with ethanol at various time points and demonstrated a progressive decline in transendothelial electrical resistance and an increase in monocyte migration across the BBB (15). Although the mechanisms of ethanol-induced BBB leakage are not fully understood, ethanol treatment is associated with a decrease in the expression of total occludin and claudin-5 without alterations in ZO-1 content in primary bovine BMECs (15). The results of the current study are consistent with these observations, as a reduction in occludin and claudin-5 expression in HCMEC/D3s was observed. However, although the expression of ZO-1 was not affected in this previous study, a decrease in the expression of ZO-1 was observed in HCMEC/D3 s treated with ethanol in the current study. This is likely due to differences between ethanol administration methods, including exposure time and concentration, as well as the cell type and species under investigation.
TJ proteins, claudin-5, occludin and ZO-1 are important for the formation and maintenance of a functional BBB (4,5). Tissues or cells that lose the ability to regulate the functions of TJs become more susceptible to various injuries, including inflammation and viral and bacterial infections (32,33).
The aim of the present study was to investigate the effect of ethanol on TJ proteins in HCMEC/D3s, and determine the primary signaling pathways involved. It was demonstrated that the ethanol-induced decrease in the protein expression levels of claudin-5, occludin and ZO-1 was concentration-dependent, and treatment with the PKCα-specific inhibitor, safingol, reversed these effects. These results indicate that ethanol may mediate its effects via a PKCα-dependent mechanism. The regulation of TJ proteins is not fully understood; therefore what is know about the regulation of TJs is just speculation. Due to the ability of ethanol to activate PKCα by Rho guanosine triphosphatases or filamentous actin (34,35), it has been hypothesized that PKCα is the major factor by which ethanol mediates its effects. A previous study implicated PKCα as a major component involved in increased TJ permeability (36). The observed reversal of the effects of ethanol treatment on occludin, claudin-5 and ZO-1 expression by safingol treatment in current study supports this hypothesis.
The effect of ethanol on HCMEC/D3 viability was determined using an MTS assay and the expression of claudin-5, occludin and ZO-1 was measured using western blotting and RT-qPCR analyses. The MTS assay revealed that ethanol exposure reduced HCMEC/D3 viability in a concentration- and time-dependent manner. The viability of cells in the low-dose ethanol group (50 mM) was increased when compared with the control, which may be associated with self-protection via enhanced antioxidant capacity (37,38). The aforementioned studies demonstrated that low concentrations of ethanol may reduce asymmetric dimethylarginine and increase dimethylarginine dimethylaminohydrolase activity, which lead to increased cell viability. Western blotting analysis demonstrated a significant reduction in the expression of claudin-5, occludin and ZO-1 protein in the cells 24 h after treatment with 400 mM ethanol, and 48 and 72 h after treatment with 200 and 400 mM ethanol; the protein level of occludin also significantly decreased after 72 h of treatment with 100 mM ethanol. This ethanol-induced impairment of TJ protein expression was reversed in cells pretreated with safingol. By contrast, the expression of claudin-5, occludin and ZO-1 mRNA in the cells was increased after 24 h of treatment with 400 mM ethanol, and 48 and 72 h of treatment with 200 and 400 mM ethanol; the expression of occludin mRNA was also significantly increased after 72 h of treatment with 100 mM ethanol. The decreased level of protein may induce a negative feedback loop, thus leading to increased mRNA levels or the binding of the specific protein to the relevant gene mRNA, inhibiting its translation. This may stabilize the mRNA so that the protein content is reduced, while the levels of mRNA are increased. RNA-binding proteins (RBPs) influence TJ mRNA stability and translation. CUG-binding protein 1 (CUGBP1) and T-cell intracellular antigen-related protein (TIAR) are negative regulators of RBPs. The co-localization of CUGBP1 and tagged occludin mRNA in processing bodies represses occludin translation and stabilizes the expression of its mRNA (39,40). The mRNA encoding the membrane-associated TJ protein, ZO-1, has an affinity for TIAR, which represses ZO-1 translation and stabilizes its mRNA expression (39). However, the specific mechanism of action is unclear and further studies are required. Claudin-5, occludin and ZO-1 proteins are important for TJ formation, therefore the observed decrease in the expression of TJ proteins may be associated with ethanol-induced BBB leakage.
PKCα is a typical PKC isoform primarily expressed in vascular endothelial cells and the central nervous system (41). PKCα increases and decreases TJ permeability (21). It is highly unlikely that increased PKC activity is solely due to increased protein expression (21), and additional intracellular mechanisms may be responsible for altering PKC activity. For example, activated PKC translocates from the cytoplasm to the perinuclear region and nucleus (42,43). In addition, PKC is itself phosphorylated, which indicates its activation (44). The translocation and/or phosphorylation of various PKC isoforms are important for changes in their respective activities. In the current study, immunofluorescence staining results demonstrated that PKCα translocated from the cytoplasm to the perinuclear region and nucleus in cells exposed to ethanol. In addition, western blotting analysis indicated that PKCα expression was not significantly altered and p-PKCα expression was significantly increased by ethanol treatment. These results suggest that ethanol induced an increase in PKCα activity by increasing its conversion from an inactive to an active form, rather than by increasing its expression. The PKCα inhibitor experiments indicated that PKCα was the most likely mechanism by which ethanol induces TJ impairment. Increased PKCα activity and altered claudin-5, occludin and ZO-1 protein levels were observed in ethanol-treated cells. These ethanol-induced alterations were all reversed by pretreating HCMEC/D3 s with the PKCα-specific inhibitor, safingol. The increased activity of PKCα and the associated decrease in the expression of TJ proteins, as well as the reversal of ethanol-induced alterations by PKCα inhibition, further the understanding of the regulation of ethanol-induced TJ impairment.
In conclusion, the results of the current study demonstrate that the ethanol-induced decrease in the expression of claudin-5, occludin and ZO-1 proteins and increase in PKCα activity in HCMEC/D3s, are concentration and time-dependent. In addition, pre-treatment of HCMEC/D3s with safingol prior to ethanol exposure reversed these ethanol-induced alterations. The results suggest that ethanol decreases the expression of TJ proteins in HCMEC/D3s via a PKCα-dependent mechanism. Ethanol-induced TJ impairment may lead to the loss of BBB structure and BBB dysfunction, thereby increasing the transport of ions, molecules and leukocytes in and out of the brain.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81172904), the Natural Science Foundation of Liaoning Province (grant no. 201102299) and the Shenyang Scientific and Technological Plan (grant no. F11-264-1-67).
References
- 1.Kamiichi A, Furihata T, Kishida S, Ohta Y, Saito K, Kawamatsu S, Kan C. Establishment of a new conditionally immortalized cell line from human brain microvascular endothelial cells: A promising tool for human blood-brain barrier studies. Brain Res. 2012;1488:113–122. doi: 10.1016/j.brainres.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 3.Haorah J, Schall K, Ramirez SH, Persidsky Y. Activation of protein tyrosine kinases and matrix metalloproteinases causes blood-brain barrier injury: Novel mechanism for neurodegeneration associated with alcohol abuse. Glia. 2008;56:78–88. doi: 10.1002/glia.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citi S, Cordenonsi M. Tight junction proteins. Biochim Biophys Acta. 1998;1448:1–11. doi: 10.1016/S0167-4889(98)00125-6. [DOI] [PubMed] [Google Scholar]
- 5.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/S0166-2236(00)02004-X. [DOI] [PubMed] [Google Scholar]
- 6.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krueger M, Härtig W, Reichenbach A, Bechmann I, Michalski D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One. 2013;8:e56419. doi: 10.1371/journal.pone.0056419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- 10.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: Lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 11.Saeed RW, Varma S, Peng T, Tracey KJ, Sherry B, Metz CN. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. J Immunol. 2004;173:6376–6383. doi: 10.4049/jimmunol.173.10.6376. [DOI] [PubMed] [Google Scholar]
- 12.Kuhlmann CR, Li F, Lüdders DW, Schaefer CA, Most AK, Backenköhler U, Neumann T, Tillmanns H, Waldecker B, Erdogan A, Wiecha J. Dose-dependent activation of ca2+-activated k+ channels by ethanol contributes to improved endothelial cell functions. Alcohol Clin Exp Res. 2004;28:1005–1011. doi: 10.1097/01.ALC.0000130811.92457.0D. [DOI] [PubMed] [Google Scholar]
- 13.Booyse FM, Aikens ML, Grenett HE. Endothelial cell fibrinolysis: Transcriptional regulation of fibrinolytic protein gene expression (t-PA, u-PA, and PAI-1) by low alcohol. Alcohol Clin Exp Res. 1999;23:1119–1124. doi: 10.1111/j.1530-0277.1999.tb04235.x. [DOI] [PubMed] [Google Scholar]
- 14.Polikandriotis JA, Rupnow HL, Hart CM. Chronic ethanol exposure stimulates endothelial cell nitric oxide production through PI-3 kinase- and hsp90-dependent mechanisms. Alcohol Clin Exp Res. 2005;29:1932–1938. doi: 10.1097/01.alc.0000187597.62590.a4. [DOI] [PubMed] [Google Scholar]
- 15.Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 16.Haorah J, Knipe B, Gorantla S, Zheng J, Persidsky Y. Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J Neurochem. 2007;100:324–336. doi: 10.1111/j.1471-4159.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- 17.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GK, Haimovitz-Friedman A, Dhupar SK, Ehleiter D, Maslak P, Lai L, Loganzo F, Jr, Kelsen DP, Fuks Z, Albino AP. Potentiation of apoptosis by treatment with the protein kinase C-specific inhibitor safingol in mitomycin C-treated gastric cancer cells. J Natl Cancer Inst. 1995;87:1394–1399. doi: 10.1093/jnci/87.18.1394. [DOI] [PubMed] [Google Scholar]
- 19.Chen ML, Pothoulakis C, Lamont JT. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to clostridium difficile toxin A. J Biol Chem. 2002;277:4247–4254. doi: 10.1074/jbc.M109254200. [DOI] [PubMed] [Google Scholar]
- 20.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, III, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Citi S, Denisenko N. Phosphorylation of the tight junction protein cingulin and the effects of protein kinase inhibitors and activators in MDCK epithelial cells. J Cell Sci. 1995;108:2917–2926. doi: 10.1242/jcs.108.8.2917. [DOI] [PubMed] [Google Scholar]
- 23.Siflinger Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: A paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L435–L451. doi: 10.1152/ajplung.00106.2002. [DOI] [PubMed] [Google Scholar]
- 24.Simet SM, Wyatt TA, Devasure J, Yanov D, Allen-Gipson D, Sisson JH. Alcohol increases the permeability of airway epithelial tight junctions in beas-2B and NHBE cells. Alcohol Clin Exp Res. 2012;36:432–442. doi: 10.1111/j.1530-0277.2011.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosson D, O'Brien TG, Kampherstein JA, Szallasi Z, Bogi K, Blumberg PM, Mullin JM. Protein kinase C-alpha activity modulates transepithelial permeability and cell junctions in the LLC-PK1 epithelial cell line. J Biol Chem. 1997;272:14950–14953. doi: 10.1074/jbc.272.23.14950. [DOI] [PubMed] [Google Scholar]
- 26.Fleegal MA, Hom S, Borg LK, Davis TP. Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol. 2005;289:H2012–H2019. doi: 10.1152/ajpheart.00495.2005. [DOI] [PubMed] [Google Scholar]
- 27.Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30:1847–1859. doi: 10.1038/jcbfm.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Gao Q, Tan J, Ma P, Ge J, Liu Y, Sun X, Zhou L. PKC alpha affects cell cycle progression and proliferation in human RPE cells through the downregulation of p27kip1. Mol Vis. 2009;15:2683–2695. [PMC free article] [PubMed] [Google Scholar]
- 30.Thomsen H, Kaatsch HJ, Asmus R. Magnetic resonance imaging of the brain during alcohol absorption and elimination-a study of the ‘rising tide phenomenon’. Blutalkohol. 1994;31:178–185. [PubMed] [Google Scholar]
- 31.Phillips SC, Cragg BG. Weakening of the blood-brain barrier by alcohol-related stresses in the rat. J Neurol Sci. 1982;54:271–278. doi: 10.1016/0022-510X(82)90187-3. [DOI] [PubMed] [Google Scholar]
- 32.Wu LL, Chiu HD, Peng WH, Lin BR, Lu KS, Lu YZ, Yu LC. Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta. Crit Care Med. 2011;39:2087–2098. doi: 10.1097/CCM.0b013e31821cb40e. [DOI] [PubMed] [Google Scholar]
- 33.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis; Proc Am Thorac Soc; 2009; pp. 655–659. [DOI] [PubMed] [Google Scholar]
- 34.Slater SJ, Cook AC, Seiz JL, Malinowski SA, Stagliano BA, Stubbs CD. Effects of ethanol on protein kinase C alpha activity induced by association with Rho GTPases. Biochemistry. 2003;42:12105–12114. doi: 10.1021/bi034860e. [DOI] [PubMed] [Google Scholar]
- 35.Slater SJ, Stagliano BA, Seiz JL, Curry JP, Milano SK, Gergich KJ, Stubbs CD. Effects of ethanol on protein kinase C activity induced by filamentous actin. Biochim Biophys Acta. 2001;1544:207–216. doi: 10.1016/S0167-4838(00)00222-3. [DOI] [PubMed] [Google Scholar]
- 36.Clarke H, Ginanni N, Laughlin KV, Smith JB, Pettit GR, Mullin JM. The transient increase of tight junction permeability induced by bryostatin 1 correlates with rapid downregulation of protein kinase C-alpha. Exp Cell Res. 2000;261:239–249. doi: 10.1006/excr.2000.5035. [DOI] [PubMed] [Google Scholar]
- 37.Tan B, Huang H, Wang JJ. Protective effect of low concentration alcohol on human umbilicus vein endothelial cells. Chin J Cardiovasc Review. 2006;5:370–372. [Google Scholar]
- 38.McCarthy ET, Zhou J, Eckert R, Genochio D, Sharma R, Oni O, De A, Srivastava T, Sharma R, Savin VJ, Sharma M. Ethanol at low concentrations protects glomerular podocytes through alcohol dehydrogenase and 20-HETE. Prostaglandins Other Lipid Mediat. 2015;116-117:1–98. doi: 10.1016/j.prostaglandins.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang H, Rao JN, Wang JY. Posttranscriptional regulation of intestinal epithelial tight junction barrier by RNA-binding proteins and microRNAs. Tissue Barriers. 2014;2:e28320. doi: 10.4161/tisb.28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu TX, Rao JN, Zou T, Liu L, Xiao L, Ouyang M, Cao S, Gorospe M, Wang JY. Competitive binding of CUGBP1 and HuR to occludin mRNA controls its translation and modulates epithelial barrier function. Mol Biol Cell. 2013;24:85–99. doi: 10.1091/mbc.E12-07-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chai J, Long B, Liu X, Li Y, Han N, Zhao P, Chen W. Effects of sevoflurane on tight junction protein expression and PKC-α translocation after pulmonary ischemia-reperfusion injury. Exp Mol Med. 2015;47:e167. doi: 10.1038/emm.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polosukhina D, Singaravelu K, Padanilam BJ. Activation of protein kinase C isozymes protects LLCPK1 cells from H2O2 induced necrotic cell death. Am J Nephrol. 2003;23:380–389. doi: 10.1159/000073984. [DOI] [PubMed] [Google Scholar]
- 43.Hu T, Exton JH. A point mutation at phenylalanine 663 abolishes protein kinase C alpha's ability to translocate to the perinuclear region and activate phospholipase D1. Biochem Biophys Res Commun. 2005;333:750–753. doi: 10.1016/j.bbrc.2005.05.184. [DOI] [PubMed] [Google Scholar]
- 44.Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13:1658–1676. [PubMed] [Google Scholar]