Abstract
Chromatin assembly factor 1 subunit A (CHAF1A) is the largest subunit of the chromatin assembly factor 1 (CAF-1) complex that is implicated in the assembly of nucleosomes on newly synthesized DNA. The aim of the present study was to determine its expression and biological function in non-small cell lung cancer (NSCLC). The current study examined the levels of CHAF1A expression in 22 samples of NSCLC and corresponding normal lung tissues. Subsequently, endogenous CHAF1A expression in H1299 NSCLC cells was knocked down via lentiviral delivery of CHAF1A-targeting short hairpin RNA (shRNA), and cell proliferation, colony formation and cell cycle distribution were measured. The results demonstrated that levels of CHAF1A mRNA level were ~3-fold greater in NSCLC samples compared with adjacent normal tissues (P<0.05). shRNA-mediated silencing of CHAF1A significantly inhibited the proliferation and colony formation of H1299 cells, compared wirh the delivery of control shRNA (P<0.05). Furthermore, CHAF1A shRNA-transduced cells exhibited a significant increase in the percentage of S-phase cells and a significant decrease in the percentage of cells at the G0/G1 and G2/M phases, compared with control cells (P<0.05). Additionally, CHAF1A knockdown significantly decreased the expression of cyclin D1, cyclin-dependent kinase 2 and S-phase kinase-associated protein 2, and increased the expression of p21 and p27. This indicates that CHAF1A is upregulated in NSCLC and that its silencing suppresses the proliferation and colony formation of NSCLC cells, potentially by inducing G0/G1 cell cycle arrest. CHAF1A may therefore represent a potential therapeutic target to treat NSCLC.
Keywords: cell cycle arrest, chromatin assembly factor 1 subunit A, growth, lung cancer
Introduction
Lung cancer is the leading cause of cancer-associated mortality globally (1). Non-small cell lung cancer (NSCLC), accounts for ~85% of all types of lung cancer and patient prognosis is poor (2). Despite marked improvements in cancer detection and treatment, the 5-year overall survival rate for NSCLC is only ~16% (3). It is thus important to understand the molecular mechanisms involved in the growth of NSCLC to facilitate the development of novel anticancer agents to improve patient outcomes.
The chromatin assembly factor 1 (CAF-1) complex, composed of chromatin assembly factor 1 subunit A (CHAF1A; also known as p150), p60 and p48 subunits, has been implicated in the assembly of nucleosomes on newly replicated DNA (4). CAF-1-mediated chromatin assembly serves a pivotal role in DNA repair, protecting DNA from excessive degradation (5). The interaction between CAF-1 and proliferating cell nuclear antigen aids in recruiting and stabilizing CAF-1 at the site of DNA replication (6). It has been reported that expression of a dominant-negative mutant of CHAF1A induces S-phase cell cycle arrest, which is accompanied by DNA damage (7). A loss-of-function mutation in CAF-1/p150 impairs the organization of heterochromatin in pluripotent embryonic cells (8). CAF-1 is an important regulator of somatic cell identity and its absence leads to a more accessible chromatin structure, increasing the expression of pluripotency-specific genes (9). Several previous studies have suggested that there is an association between CAF-1 overexpression and aggressive parameters of human cancer (10–12). For instance, CAF-1/p60 overexpression is an independent poor prognostic indicator of laryngeal carcinoma (10). In high-grade gliomas, CAF-1/p60 overexpression contributes to tumor aggressiveness and shorter overall survival (11). Barbieri et al (13) indicated that CHAF1A overexpression predicts poor prognosis and stimulates the development of neuroblastoma. Another study demonstrated that upregulation of CHAF1A promotes the proliferation of colon cancer cells (14). In glioblastoma (15) and ovarian cancer (16) cells, CHAF1A also offers a growth advantage. However, the expression and biological significance of CHAF1A in NSCLC remains unclear. Therefore, the present study examined the expression of CHAF1A in NSCLC and adjacent normal tissues and investigated its function in the growth of NSCLC cells.
Materials and methods
Tissue samples
Tumor and corresponding normal tissue specimens were collected from 22 patients with NSCLC who underwent surgical resection between October 2014 and January 2015 at the Department of Thoracic Surgery of the Second Affiliated Hospital of Shanxi Medical University (Taiyuan, China). There were 20 males and 2 females, with a median age of 67 years (range, 47–78 years). Patients who received radiotherapy or chemotherapy prior to surgery were excluded. Written informed consent was obtained from each patient and the study was approved by the local Institutional Review Board of the Second Affiliated Hospital of Shanxi Medical University (Taiyuan, China).
Cell culture
The human lung cancer cell lines A549, H1299, H1688 and H1975, human embryonic kidney 293T cells and normal human lung epithelial BEAS-2B cells were purchased from the American Type Culture Collection (Manassas, VA, USA). All cells were routinely maintained in RPMI 1640 medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 mg/ml streptomycin at 37°C in an atmosphere of humidified air with 5% CO2. Upon reaching 90% confluence, cells were subcultured every three days.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues or cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reversely transcribed to first-strand cDNA using the Reverse Transcription system (catalog no. A3500; Promega Corporation, Madison, WI, USA). DNase (Tiangen Biotech Co., Ltd., Beijing, China) was employed to eliminate DNA prior to reverse transcription. PCR amplifications were performed using the SYBR Green Real-Time PCR Master mix (catalog no. 4309155; Invitrogen; Thermo Fisher Scientific, Inc.). The cycling conditions consisted of an initial denaturation at 95°C for 5 min and 40 cycles of denaturation at 95°C for 20 sec, annealing at 59°C for 20 sec and elongation at 72°C for 40 sec. The PCR primers were as follows: Human CHAF1A forward, 5′-AGGGAAGGTGCCTATGGTG-3′ and reverse, 5′-CAGGGACGAATGGCTGAGTA-3′; human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-CAGGGACGAATGGCTGAGTA-3′ and reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′. Relative CHAF1A mRNA expression was determined according to the 2−ΔΔCq method following normalization to GAPDH (17). Each assay was repeated three times.
Construction of recombinant lentivirus encoding CHAF1A small hairpin (sh)RNA
Knockdown of CHAF1A expression in H1299 cells was performed using lentivirus-mediated RNA interfering technology, as previously described (18). In brief, CHAF1A-targeting and negative control shRNAs were synthesized by GeneChem Co., Ltd (Shanghai, China). The sequence of CHAF1A shRNA was as follows: 5′-CCGGCCGACTCAATTCCTGTGTAAATTCAAGAGATTTACACAGGAATTGAGTCGGTTTTTG-3′. Annealed DNA oligonucleotides were inserted into the GV115 lentivirus vector (GeneChem Co., Ltd.) and the construct was verified by DNA sequencing, performed by Sangon Biotech Co., Ltd. (Shanghai, China). For production of recombinant lentivirus, the GV115-CHAF1A shRNA-expressing plasmid and lentivirus packaging vectors were co-transfected into 293T cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h post-transfection, lentivirus-containing supernatant was collected, purified and titered as previously described (18). A non-specific shRNA-expressing lentivirus was also prepared and used as a negative control.
Infection of cells with CHAF1A-expressing lentivirus
H1299 cells were seeded at 4×105 cells/well onto 6-well plates and incubated in RPMI 1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C overnight prior to transduction. Cells were infected with lentiviral particles expressing control or CHAF1A shRNA at a multiplicity of infection of 5. As the recombinant lentivirus is able to co-express intended shRNAs and green fluorescent protein (GFP), transduction efficiency was monitored by measuring GFP-positive cells under a fluorescence microscope.
Measurement of cell proliferation using the Cellomics method
H1299 cells were seeded onto 96-well plates at a density of 2×103 cells/well and cultured for 5 days at 37°C. Plates were scanned and analyzed daily using the Cellomics ArrayScan VTI (Thermo Fisher Scientific Inc.) (19). The system is an automated fluorescence-imaging microscope that analyzes the intensity and distribution of fluorescence in each individual cell. Cells with green fluorescence were counted at five random fields at ×20 magnification.
Colony formation assay
H1299 cells were seeded onto 6-well plates (400 cells/well) and cultured for 14 days. The wells were stained with Giemsa and the number of colonies containing >50 cells were counted. The assay was repeated three times in triplicate.
Cell cycle analysis by flow cytometry
H1299 cells (8×104 in RPMI 1640 medium) were fixed with ice-cold 70% ethanol and suspended in staining solution containing 0.5 mg/ml RNase, 0.05% Triton X-100 and 10 µg/ml propidium iodide (PI; Sigma Aldrich; Merck KGaA, Darmstadt, Germany). Following 1 h incubation at 37°C in the dark, cells were immediately analyzed using a flow cytometer with CellQuest v3.3 software (BD Biosciences, San Jose, CA, USA).
Western blot analysis
Protein samples were prepared in ice-cold radioimmunoprecipitation assay buffer supplemented with protease/phosphatase inhibitors (Sigma Aldrich; Merck KGaA) for 30 min. Lysates were cleared by centrifugation at 10,000 × g for 10 min at 4°C. Protein samples (50 µg in 10 µl loading buffer per lane) were resolved by 12% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% fat-free milk at 37°C for 1 h and incubated with primary antibodies at 4°C overnight. The primary antibodies used are summarized as follows: Against cyclin D1 (sc-70899; 1:500 dilution), cyclin-dependent kinase 2 (CDK2) (sc-6248; 1:500 dilution), S-phase kinase-associated protein (SKP)2 (sc-74477; 1:800 dilution), p21 (sc-6246; 1:1,000 dilution), p27 (sc-53906; 1:1,000 dilution), and β-actin (sc-47778; 1:2,000 dilution) (all Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The membranes were then incubated with horseradish peroxidase-conjugated anti-mouse IgG (1:5,000 dilution; A9044; Sigma-Aldrich; Merck KGaA) at room temperature for 1 h. Immunoreactive signals were visualized by chemifluorescence using ECL Plus Western Blotting Detection reagents (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Protein signals were quantified using Quantity One software (version 4.6.2; Bio-Rad Laboratories, Hercules, CA, USA). Each assay was repeated three times.
Statistical analysis
Data are presented as mean ± standard deviation. All statistical calculations were performed using SPSS 11.7 software (SPSS, Chicago, IL, USA). Differences between the means were analyzed using Student's t-test or one-way analysis of variance followed by Tukey's multiple comparison test. P<0.05 was considered to indicate a statistically significant difference.
Results
CHAF1A is upregulated in NSCLC tissues relative to adjacent normal tissues
The expression of CHAF1A was examined in 22 samples of NSCLC and corresponding normal lung tissue. RT-qPCR demonstrated that CHAF1A mRNA levels were 2.9-fold greater in NSCLC samples compared with adjacent normal tissues (P<0.05; Fig. 1A). Significantly increased expression of endogenous CHAF1A was further confirmed in all 4 of the NSCLC cell lines by RT-qPCR (P<0.05; Fig. 1B).
Figure 1.
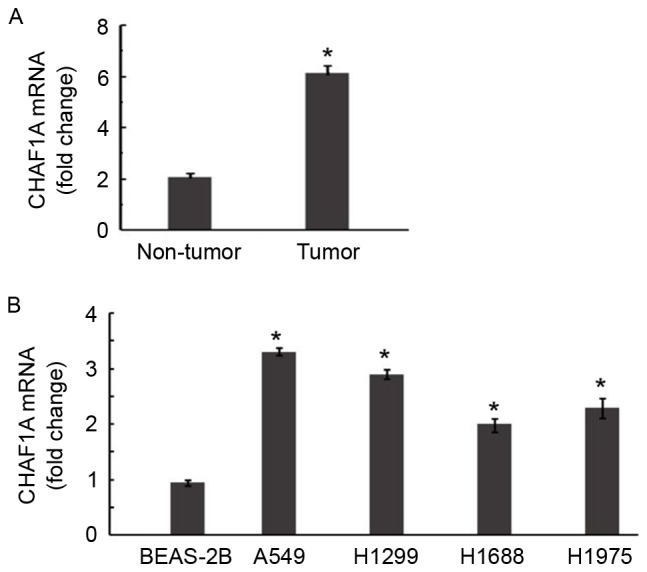
CHAF1A is upregulated in NSCLC. (A) RT-qPCR of the level of CHAF1A mRNA in 22 pairs of NSCLC and adjacent nontumorous lung tissues. *P<0.05 vs. non-tumor lung tissues. (B) RT-qPCR of the level of CHAF1A mRNA in the human lung cancer cell lines A549, H1299, H1688 and H1975, and the normal human lung epithelial cell line BEAS-2B. *P<0.05 vs. BEAS-2B cells. Data are representative of three experiments performed in triplicates (n=9). Data are presented as mean ± standard deviation. NSCLC, non-small cell lung carcinoma; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; CHAF1A, chromatin assembly factor 1 subunit A.
Silencing of endogenous CHAF1A in NSCLC cells via infection with CHAF1A shRNA-expressing lentivirus
To determine the biological roles of CHAF1A in NSCLC growth, its expression was knocked down in NSCLC cells using lentivirus-mediated shRNA technology. Microscopic examination of GFP expression following lentivirus infection revealed a similar transduction efficiency of ~80% for control and CHAF1A shRNA (Fig. 2A). However, compared with the delivery of control shRNA, CHAF1A shRNA transduction significantly decreased levels of CHAF1A mRNA in H1299 cells by 84.7±1.2% (P<0.05; Fig. 2B).
Figure 2.
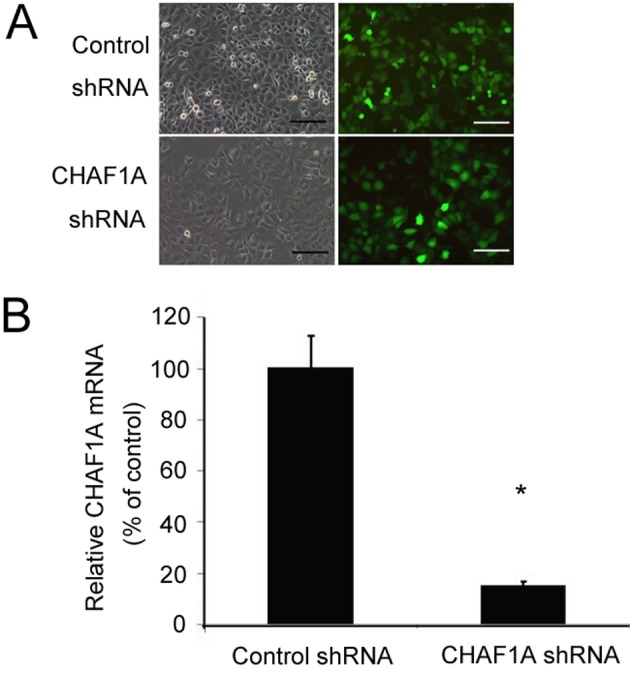
Silencing of CHAF1A in H1299 cells via lentiviral delivery of CHAF1A-targeting shRNA. (A) Light (left) and fluorescence (right) view of H1299 cells transduced with control shRNA- or CHAF1A shRNA-expressing lentivirus. Scale bar=60 µm. (B) Reverse transcription-quantitative polymerase chain reaction analysis of the level of CHAF1A mRNA in H1299 cells transduced with control shRNA- or CHAF1A shRNA-expressing lentivirus. *P<0.05 vs. control shRNA. Data are representative of three experiments performed in triplicate (n=9). Data are presented as mean ± standard deviation. CHAF1A, chromatin assembly factor 1 subunit A; shRNA, short hairpin RNA.
Knockdown of CHAF1A impairs the growth and colony formation of NSCLC cells
The effect of CHAF1A downregulation on the growth of NSCLC cells was subsequently assessed. During a 5-day culture period, H1299 cells transduced with CHAF1A-shRNA lentivirus and control lentivirus were counted daily using the Cellomics method. The number of cells was significantly lower in the CHAF1A shRNA group compared with the control group on day 5 (162±10 vs. 523±8 cells/field; P<0.05; Fig. 3). The colony formation assay further demonstrated that the delivery of CHAF1A shRNA significantly reduced the number of colonies formed by H1299 cells following 14-day incubation (21±9 vs. 84±12 colonies/dish; P<0.05; Fig. 4). These results indicate that the suppression of NSCLC cell growth is mediated by CHAF1A silencing.
Figure 3.
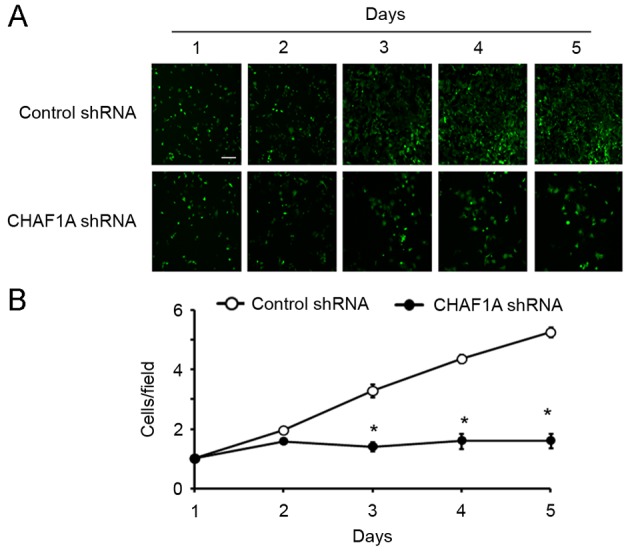
Silencing of CHAF1A inhibits the proliferation of H1299 cells. Cells transduced with control shRNA- or CHAF1A shRNA-expressing lentivirus were seeded onto 96-well plates (2×103 cells/well) and analyzed daily using the Cellomics ArrayScan for 5 days. (A) Panels indicate representative images of cells with green fluorescence. Scale bar= 60 µm. (B) Proliferation curves of the two groups. *P<0.05 vs. Control shRNA. Data are representative of three experiments performed in triplicate (n=9). Data are presented as mean ± standard deviation. CHAF1A, chromatin assembly factor 1 subunit A; shRNA, short hairpin RNA.
Figure 4.
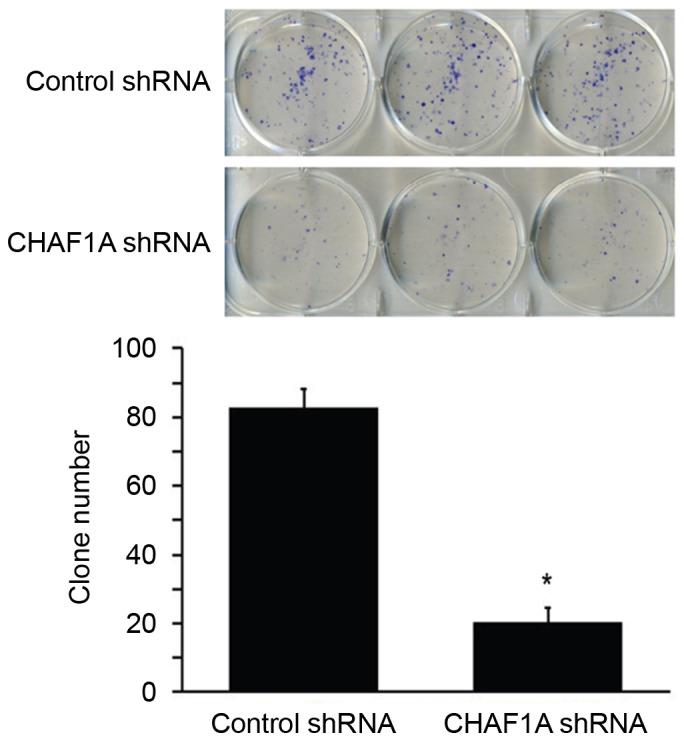
Colony formation of H1299 cells transduced with control shRNA- or CHAF1A shRNA-expressing lentivirus following 14 day culture. Following staining with Giemsa, the number of colonies consisting of >50 cells was determined. *P<0.05 vs. control shRNA. Data are representative of three experiments performed in triplicate (n=9). Data are presented as mean ± standard deviation. CHAF1A, chromatin assembly factor 1 subunit A; shRNA, short hairpin RNA.
CHAF1A silencing induces cell cycle arrest at the S-phase
Finally, it was assessed whether the anti-growth effect of CHAF1A downregulation is associated with the induction of cell cycle arrest. Cell cycle progression was analyzed by flow cytometry following PI staining. As presented in Fig. 5A and B, the percentage of cells entering the S-phase was significantly decreased in CHAF1A shRNA-transduced cells relative to control cells (28±0.9 vs. 43±1.2%; P<0.05). By contrast, the percentage of cells at the G0/G1 phase was significantly increased in CHAF1A shRNA-transduced cells compared with the control (57±0.8 vs. 40±0.9%; P<0.05). Western blot analysis revealed that CHAF1A silencing significantly downregulated the expression of cyclin D1, CDK2, and SKP2 (P<0.05, but significantly increased the expression of p21 and p27 (P<0.05; Fig. 5C). This indicates that knockdown of CHAF1A leads to cell cycle arrest during S-phase.
Figure 5.
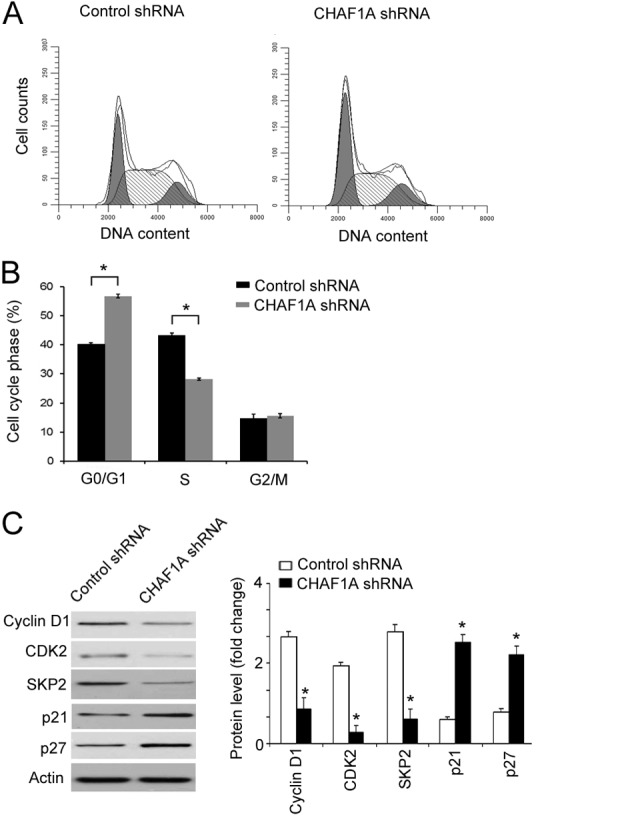
Effect of CHAF1A silencing on cell cycle progression in H1299 cells. (A and B) Cells transduced with control shRNA- or CHAF1A shRNA-expressing lentivirus were stained with PI and assessed by flow cytometry. (A) Representative histograms of cell cycle distribution. (B) Quantification of the percentage of cells at each cell cycle phase. (C) Western blot analysis of indicated proteins. Bar graphs indicate densitometric analysis of protein signals from three independent experiments. *P<0.05 vs. Control shRNA. Data are representative of three experiments performed in triplicates (n=9). Data are presented as mean ± standard deviation. PI, propidium iodide; CHAF1A, chromatin assembly factor 1 subunit A; shRNA, short hairpin RNA; CDK, cyclin dependent kinase; SKP, S-phase kinase associated protein.
Discussion
The CAF-1 complex serves a critical role in DNA replication and repair and its deregulation contributes to the pathogenesis of cancer (5,8,9). The CAF-1 subunit p60 is frequently upregulated in human cancer and contributes to aggressive phenotypes (10,11). Mascolo et al (20) reported that overexpression of CAF-1/p60 is significantly associated with node and/or distant metastases in cutaneous melanoma. The current study examined the expression of CHAF1A, the largest subunit of CAF-1, in NSCLC. Data from the present study indicated that CHAF1A expression was significantly elevated in NSCLC tissues compared with adjacent normal lung tissues. To the best of our knowledge, this is the first report confirming the overexpression of CHAF1A in NSCLC, although upregulation of CHAF1A has been identified in aggressive neuroblastoma (13) and colon cancer (14), suggesting its involvement in the pathogenesis of cancer.
Having identified that CHAF1A is upregulated in NSCLC, the current study sought to determine its biological roles in this disease. Lentivirus-mediated delivery of CHAF1A shRNA resulted in efficient knockdown of endogenous CHAF1A expression in H1299 cells. Notably, it was determined in the current study using the Cellomics method that CHAF1A silencing significantly suppressed the proliferation of H1299 cells. Furthermore, CHAF1A downregulation impaired the colony formation capacity of H1299 cells, resulting in the formation of fewer colonies following 14 day culture. In accordance with the results of the present study, it has been determined that CHAF1A silencing inhibits the tumorigenicity and growth of colon cancer cells (14). The results of the present study indicate that CHAF1A is required for the growth of NSCLC cells. This provides a rationale for targeting CHAF1A as a potential therapeutic strategy for NSCLC.
Compelling evidence indicates that CAF-1 activity is required for efficient replication of euchromatic DNA (21). Loss of CAF-1 results in cell cycle arrest at the S-phase (21,22). Takami et al (23) reported that the depletion of each subunit of the CAF-1 complex delays S-phase progression, followed by accumulation in late S/G2 phase and aberrant mitosis. Quivy et al (24) demonstrated that the interaction between CHAF1A and heterochromatin protein 1 is indispensable for pericentric heterochromatin replication and S-phase progression in mouse cells. In agreement with these studies, the current study revealed that a knockdown of CHAF1A significantly increased the percentage of S-phase cells and decreased the percentages of cells at the G0/G1 and G2/M phases, indicating that it induced cell cycle arrest at the S-phase. At the molecular level, both p21 and p27 are well-defined CDK inhibitors that serve an important role in controlling cell cycle progression (25). SKP2 functions as a positive regulator of cell cycle progression by promoting p27 degradation (26). These results provide an explanation for the reduced proliferation observed in CHAF1A-depleted H1299 cells.
Certain limitations of the present study should be noted. Firstly, no information is available concerning the association of CHAF1A expression with the clinicopathological parameters and prognosis of NSCLC. Secondly, validation of the in vivo effect of CHAF1A knockdown on the growth of NSCLC in animal models is required. Finally, the exact mechanism for the regulation of cell cycle arrest by CHAF1A in NSCLC cells remains unclear and should be investigated.
In conclusion, the results of the current study demonstrate that CHAF1A is upregulated in NSCLC and that its depletion hinders the growth of NSCLC cells. This potentially occurs through the induction of cell cycle arrest at the S-phase. The results of the current study warrants further investigations into the effect of depletion of CHAF1A on the tumorigenicity and growth of NSCLC cells in animal models.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.NSCLC Meta-analysis Collaborative Group, corp-author. Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–1571. doi: 10.1016/S0140-6736(13)62159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng YW, Li RM, Zhang XW, Ren XB. Current adoptive immunotherapy in non-small cell lung cancer and potential influence of therapy outcome. Cancer Invest. 2013;31:197–205. doi: 10.3109/07357907.2013.775294. [DOI] [PubMed] [Google Scholar]
- 4.Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Mjelle R, Hegre SA, Aas PA, Slupphaug G, Drabløs F, Saetrom P, Krokan HE. Cell cycle regulation of human DNA repair and chromatin remodeling genes. DNA Repair (Amst) 2015;30:53–67. doi: 10.1016/j.dnarep.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Zhang K, Gao Y, Li J, Burgess R, Han J, Liang H, Zhang Z, Liu Y. A DNA binding winged helix domain in CAF-1 functions with PCNA to stabilize CAF-1 at replication forks. Nucleic Acids Res. 2016;44:5083–5094. doi: 10.1093/nar/gkw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye X, Franco AA, Santos H, Nelson DM, Kaufman PD, Adams PD. Defective S phase chromatin assembly causes DNA damage, activation of the S phase checkpoint, and S phase arrest. Mol Cell. 2003;11:341–351. doi: 10.1016/S1097-2765(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 8.Houlard M, Berlivet S, Probst AV, Quivy JP, Héry P, Almouzni G, Gérard M. CAF-1 is essential for heterochromatin organization in pluripotent embryonic cells. PLoS Genet. 2006;2:e181. doi: 10.1371/journal.pgen.0020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheloufi S, Elling U, Hopfgartner B, Jung YL, Murn J, Ninova M, Hubmann M, Badeaux AI, Ang C Euong, Tenen D, et al. The histone chaperone CAF-1 safeguards somatic cell identity. Nature. 2015;528:218–224. doi: 10.1038/nature15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesolella M, Iorio B, Landi M, Cimmino M, Ilardi G, Iengo M, Mascolo M. Overexpression of chromatin assembly factor-1/p60 predicts biological behaviour of laryngeal carcinomas. Acta Otorhinolaryngol Ital. 2017;37:17–24. doi: 10.14639/0392-100X-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Tayrac M, Saikali S, Aubry M, Bellaud P, Boniface R, Quillien V, Mosser J. Prognostic significance of EDN/RB, HJURP, p60/CAF-1 and PDLI4, four new markers in high-grade gliomas. PLoS One. 2013;8:e73332. doi: 10.1371/journal.pone.0073332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polo SE, Theocharis SE, Grandin L, Gambotti L, Antoni G, Savignoni A, Asselain B, Patsouris E, Almouzni G. Clinical significance and prognostic value of chromatin assembly factor-1 overexpression in human solid tumours. Histopathology. 2010;57:716–724. doi: 10.1111/j.1365-2559.2010.03681.x. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri E, De Preter K, Capasso M, Chen Z, Hsu DM, Tonini GP, Lefever S, Hicks J, Versteeg R, Pession A, et al. Histone chaperone CHAF1A inhibits differentiation and promotes aggressive neuroblastoma. Cancer Res. 2014;74:765–774. doi: 10.1158/0008-5472.CAN-13-1315. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Cui F, Yu F, Peng X, Jiang T, Chen D, Lu S, Tang H, Peng Z. Up-regulation of CHAF1A, a poor prognostic factor, facilitates cell proliferation of colon cancer. Biochem Biophys Res Commun. 2014;449:208–215. doi: 10.1016/j.bbrc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Peng H, Du B, Jiang H, Gao J. Over-expression of CHAF1A promotes cell proliferation and apoptosis resistance in glioblastoma cells via AKT/FOXO3a/Bim pathway. Biochem Biophys Res Commun. 2016;469:1111–1116. doi: 10.1016/j.bbrc.2015.12.111. [DOI] [PubMed] [Google Scholar]
- 16.Xia D, Yang X, Liu W, Shen F, Pan J, Lin Y, Du N, Sun Y, Xi X. Over-expression of CHAF1A in Epithelial Ovarian Cancer can promote cell proliferation and inhibit cell apoptosis. Biochem Biophys Res Commun. 2017;486:191–197. doi: 10.1016/j.bbrc.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Liu WB, Jia WD, Xu GL, Ma JL, Ren Y, Chen H, Sun SN, Huang M, Li JS. Embryonic morphogen nodal is associated with progression and poor prognosis of hepatocellular carcinoma. PLoS One. 2014;9:e85840. doi: 10.1371/journal.pone.0085840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchser WJ, Laskow TC, Pavlik PJ, Lin HM, Lotze MT. Cell-mediated autophagy promotes cancer cell survival. Cancer Res. 2012;72:2970–2979. doi: 10.1158/0008-5472.CAN-11-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascolo M, Vecchione ML, Ilardi G, Scalvenzi M, Molea G, Di Benedetto M, Nugnes L, Siano M, De Rosa G, Staibano S. Overexpression of Chromatin Assembly Factor-1/p60 helps to predict the prognosis of melanoma patients. BMC Cancer. 2010;10:63. doi: 10.1186/1471-2407-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klapholz B, Dietrich BH, Schaffner C, Hérédia F, Quivy JP, Almouzni G, Dostatni N. CAF-1 is required for efficient replication of euchromatic DNA in Drosophila larval endocycling cells. Chromosoma. 2009;118:235–248. doi: 10.1007/s00412-008-0192-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Tan JL, Ingouff M, Sundaresan V, Berger F. Chromatin assembly factor 1 regulates the cell cycle but not cell fate during male gametogenesis in Arabidopsis thaliana. Development. 2008;135:65–73. doi: 10.1242/dev.010108. [DOI] [PubMed] [Google Scholar]
- 23.Takami Y, Ono T, Fukagawa T, Shibahara K, Nakayama T. Essential role of chromatin assembly factor-1-mediated rapid nucleosome assembly for DNA replication and cell division in vertebrate cells. Mol Biol Cell. 2007;18:129–141. doi: 10.1091/mbc.E06-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quivy JP, Gérard A, Cook AJ, Roche D, Almouzni G. The HP1-p150/CAF-1 interaction is required for pericentric heterochromatin replication and S-phase progression in mouse cells. Nat Struct Mol Biol. 2008;15:972–979. doi: 10.1038/nsmb.1470. [DOI] [PubMed] [Google Scholar]
- 25.Fillies T, Woltering M, Brandt B, Van Diest JP, Werkmeister R, Joos U, Buerger H. Cell cycle regulating proteins p21 and p27 in prognosis of oral squamous cell carcinomas. Oncol Rep. 2007;17:355–359. [PubMed] [Google Scholar]
- 26.Shin JS, Hong SW, Lee SL, Kim TH, Park IC, An SK, Lee WK, Lim JS, Kim KI, Yang Y, et al. Serum starvation induces G1 arrest through suppression of Skp2-CDK2 and CDK4 in SK-OV-3 cells. Int J Oncol. 2008;32:435–439. [PubMed] [Google Scholar]


