Abstract
IgA-dominant infection-related glomerulonephritis (IRGN) is a distinct morphologic variant of IRGN, characterized by dominant or codominant glomerular deposits of IgA, mostly in elderly and patients with diabetes. More cases are being reported in recent times due to increased awareness of the disease entity and increased rate of Staphylococcus infection. It usually presents as rapidly progressive renal failure with proteinuria, and treatment guidelines for this disease entity are not well defined. We report here 12 cases of IgA-dominant IRGN seen over a period of 5 years from a single center. Clinical features, biopsy findings, treatment, and outcomes were analyzed. Out of 12 patients, eight were males. The mean age of presentation was 52.4 ± 21 years. Skin was the most common site of infection seen in six patients. Gross hematuria was seen in 4 patients and 11 had nephrotic proteinuria. Eleven had low serum C3. Only two patients had diabetes. Methicillin-resistant Staphylococcus aureus (MRSA) was the most common organism isolated in six patients. Most common histopathology was crescentic glomerulonephritis seen in seven patients, followed by endocapillary proliferation in three and diffuse proliferative glomerulonephritis in two. Hemodialysis was done in eight patients and six patients received steroid therapy. End-stage renal disease developed in three patients, chronic kidney disease in three, and three patients died due to sepsis. Various infections including MRSA and Escherichia coli were associated with IgA-dominant IRGN both in patients with diabetes and nondiabetics. Suspicion and recognition of the disease is important as it has therapeutic and prognostic implications.
Keywords: Diabetic, elderly, IgA dominant, infection-related glomerulonephritis, methicillin-resistant Staphylococcus aureus
Introduction
IgA-dominant infection-related glomerulonephritis (IRGN) is caused not only by Staphylococcus but also by other organisms.[1] It is characterized by proliferative glomerulonephritis by light microscopy (LM) with dominant or codominant deposits of IgA in immunofluorescence (IF) along with hump-like subepithelial deposits in electron microscopy (EM). The treatment is controversial. Antibiotics form the mainstay of therapy with surgical drainage if required. Here, we report 12 cases of IgA-dominant IRGN seen over a period of 5 years.
Materials and Methods
We conducted a prospective observational study from January 2011 to December 2015 at our institution. We included patients with a diagnosis of IgA-dominant IRGN for analysis. Detailed history, clinical examination, laboratory investigations, renal biopsy, treatment, and outcome were documented. Laboratory investigations included urine analysis, urine protein creatinine ratio (uPCR), complete hemogram, renal function tests, serum electrolytes, liver function tests, serum antinuclear antibodies, serum complements (C3, C4 levels), antinuclear cytoplasmic antibodies, urine and blood culture tests, antistreptolysin titers, viral markers (HIV, hepatitis B surface antigen, and anti-hepatitis C virus), and other case-based relevant tests. Percutaneous renal biopsy was done and specimens sent for LM and IF. Our criteria for IgA-dominant glomerulonephritis was the presence of dominant or codominant deposits of IgA in IF and diffuse proliferative glomerulonephritis (DPGN) with or without crescents. Steroid was given in selected patients after controlling infection. Intravenous methylprednisolone of 250 mg/day for 3 days, 1 mg/kg/day of oral prednisolone for 4 weeks, and rapidly tapered over 2 months.
Results
A total of 12 out 230 IRGN patients had IgA-dominant IRGN with the incidence of 5% out of IRGN. Eight were males with male:female ratio of 2:1. Clinical features including age, sex, biopsy features, treatment given, and outcomes were analyzed [Table 1].
Table 1.
Clinical and laboratory data, treatment, and outcomes of patients
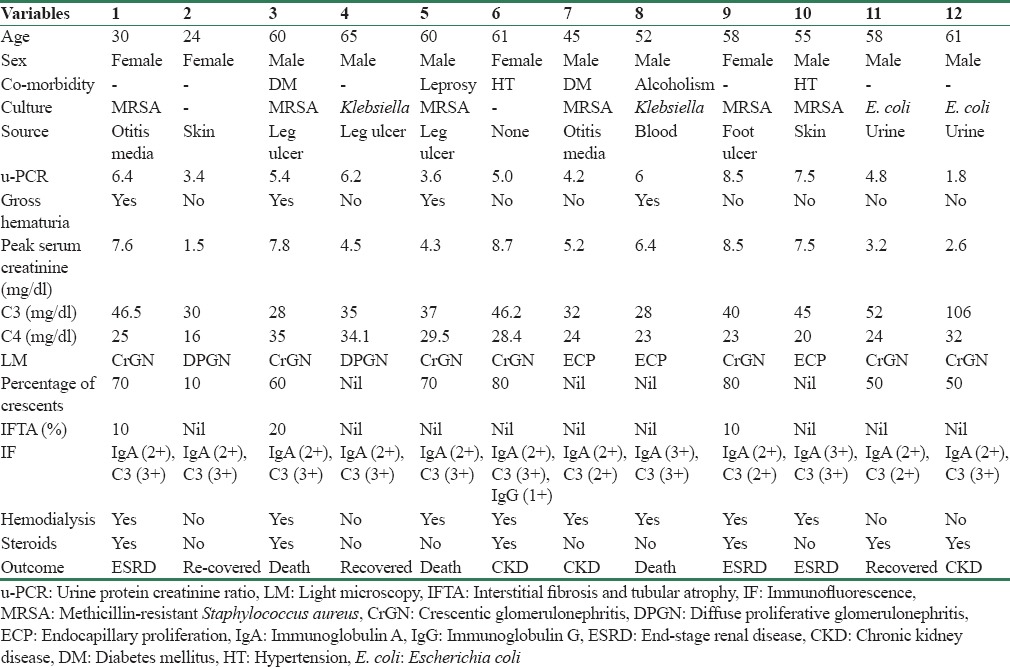
The mean age of presentation was 52.4 ± 21 years. The most common site of infection was skin (50%). Rapidly progressive glomerulonephritis was the typical presentation (92%) in all our patients except one. Gross hematuria was seen in 4 (33%) and 11 (92%) had nephrotic range proteinuria with mean uPCR of 5.23. The median time lag between the infection and onset of clinical manifestation was 6.5 days. The mean serum creatinine at presentation was 2.26 mg/dl and mean peak serum creatinine was 5.65 mg/dl. All but one (92%) had low C3 levels. Serum C4 was normal in all patients. Two patients had diabetes (17%), two had hypertension (17%), one had Hansen's disease (8%), one had alcoholism (8%), and remaining 6 (50%) had no associated comorbidities. Methicillin-resistant Staphylococcus aureus (MRSA) was the most common organism (50%) implicated, followed by Escherichia coli (17%) and Klebsiella (17%). No organism was isolated in two patients.
In LM, crescentic glomerulonephritis (CrGN) was seen in seven patients (58%), endocapillary proliferative glomerulonephritis (ECP) in three (25%), and DPGN in two (17%) patients. IF showed dominant or codominant deposits of IgA and C3 in all patients. IgG was present in only one patient. All biopsies were negative for kappa and lambda. EM done only in four selected patients (patients - 2, 3, 6, and 12) due to logistic reasons, showed electron dense deposits in subepithelial and hinge region consistent with IRGN. Eight patients required hemodialysis. We used steroids alone in four patients and steroid along with cyclophosphamide in two patients. All patients who had documented infection received antibiotics according to culture and sensitivity. Three patients died due to sepsis, three progressed to end-stage renal disease (ESRD), and three became chronic kidney disease (CKD) patients. Three recovered completely at a mean period of 32 days, had normal C3 at 3 months, but all of them had persistent subnephrotic proteinuria and hypertension at follow-up.
Discussion
The epidemiology of IRGN is undergoing striking changes, not only in developed countries but also in developing countries like India. Spector et al.[2] in 1980 reported three patients with mesangial proliferative and DPGN with IF showing IgA dominant in one. This was followed by Koyama et al. who reported ten cases of glomerulonephritis following MRSA infections.[3] Patients typically present with severe renal failure, proteinuria, and hematuria.
Nasr et al.[4] first described five cases of acute postinfectious glomerulonephritis (APIGN) in 2003 with diffuse ECP glomerulonephritis, intense deposits of IgA as dominant or codominant immunoglobulin, and mesangial and subepithelial deposits on EM and were termed IgA-dominant APIGN. Predisposing conditions for IgA-dominant IRGN include old age, diabetes, S. aureus infections, malignancy, intravenous drug abuse, alcoholism, HIV infection, and atopic dermatitis. Earlier the term, staphylococcal-associated glomerulonephritis was used interchangeably for IgA-dominant postinfectious glomerulonephritis. However, recently, it was found that organisms other than MRSA also can cause similar pathology. The infection may not be clinically obvious, and often the diagnosis may be overlooked.[5]
The consensus for diagnosis of IRGN requires three out of the following five criteria: (1) clinical or laboratory evidence of infection preceding or at the onset of glomerulonephritis; (2) depressed serum complement; (3) ECP and exudative glomerulonephritis; (4) C3-dominant or codominant glomerular IF staining; and (5) hump-shaped subepithelial deposits shown on EM.[6] We followed the same five criteria for diagnosis of IgA-dominant IRGN except the fourth criteria where IgA-dominant and codominant IF is needed for diagnosis.
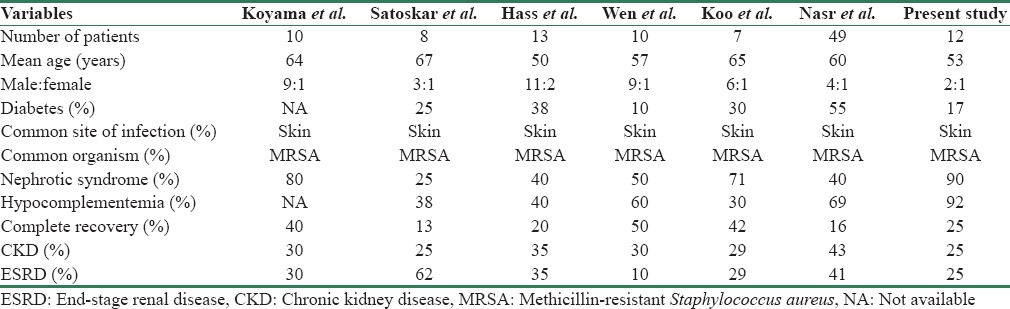
Table 2 shows various studies published in IgA-dominant IRGN.[3,6,7,8,9,10] Previous reports suggest that IgA-dominant glomerulonephritis is common in elderly diabetic individuals and male predominance.[11] In our series, all but two patients were nondiabetics. Recently, a pooled analysis of 78 cases was published.[12] Skin infections and visceral abscesses were frequently encountered source of infection, and Staphylococcus was the most common pathogen. After treatment with antibiotics/supportive treatment, renal function improved in 54.5%, 11.7% had persistent renal dysfunction, 19.5% developed ESRD, and 14.3% died.
Table 2.
Comparison of published series of immunoglobulin A-dominant infection-related glomerulonephritis
The pathogenesis of IgA-dominant glomerulonephritis is poorly understood. Theories including superantigens by Koyama et al. where superantigens directly stimulate antibody production without Major histocompatibility antigen (MHC) restriction were proposed.[3] Coagulase-negative Staphylococcus which does not produce enterotoxin suggests involved antigens other than superantigens.[1] Abnormal immune response, increased serum IgA in response to subclinical infections, and advanced end glycation products have been proposed as the underlying mechanisms responsible for increased incidence in patients with diabetes.[13,14] The pathogenesis of the IgA predominant deposition is not well understoo and is likely to involve specific host–pathogen interactions. Increased serum IgA levels in some reported cases suggest activation of selective IgA immune responses. Superantigens bypass normal antigen presentation by binding directly to the major histocompatibility class II molecules on antigen-presenting cells and to the variable portion of the beta chain of T-cells. This leads to massive T-cell activation and production of high amounts of pro-inflammatory cytokines including those with class-switching functions.
In one study, diffuse ECP glomerulonephritis was found in all cases, and three patients also had CrGN. EM revealed large subepithelial hump-shaped deposits in all cases.[9] In our series, CrGN (58%) was the most common histopathology. Four patients did not have any crescents, and none of the patients had any chronic changes in the form of interstitial fibrosis and tubular atrophy.
IgA nephropathy (IgAN) forms a close differential diagnosis for IgA-dominant IRGN. Serum complements are normal in IgAN whereas hypocomplementemia varies between 35% and 100% in various case reports of IgA-dominant IRGN.[15] Features that favor IgA-dominant IRGN over IgAN include initial presentation in older age or in a diabetic patient, acute renal failure, intercurrent culture-documented staphylococcal infection, hypocomplementemia, diffuse glomerular endocapillary hypercellularity with prominent neutrophil infiltration on LM, stronger IF staining for C3 than IgA, and the presence of subepithelial humps on EM.[16,17] Finally, stronger mesangial staining for lambda light chains than kappa light chains may point to IgAN. Both these entities need to be differentiated as prognosis and treatment outcomes vary.
The profile of IgA deposited in IgA-dominant glomerulonephritis has to be investigated. A case report which investigated IgA profile in a patient with glomerulonephritis associated with Clostridium difficile infection mimicking IgA-dominant glomerulonephritis clarified it as IgAN based on pathogenic mechanism by immune profiling of deposits.[18]
There are no guidelines available for the treatment of IgA-dominant infectious glomerulonephritis, but antibiotic therapy remains mainstay of therapy.[19] The use of immunosuppressant including steroids is controversial as they can aggravate infection.[20] A Recent review by Glassock et al. provides evidence for usage against steroids and other immunosuppressants in staphylococcal glomerulonephritis.[21] We used antibiotics in all patients with documented infection according to culture and sensitivity and steroids in six patients. More randomized controlled trials are needed to define the role of steroids and compare the benefits with the risks associated with steroid therapy. Management of underlying comorbidity, especially diabetes is important as it can exacerbate the underlying infection. The prognosis is poor with <20% of patient's fully recovering renal function, especially in those with underlying comorbidities, crescents in biopsy, and advanced age.
Conclusion
IgA-dominant infection related glomerulonephritis is being increasingly recognized in India owing to increased awareness. Our experience suggests that it occurs often in nondiabetics also. The prognosis is poor as evidenced by increased progression to CKD and mortality. A large study is needed involving pooling of cases from all over the world to better understand the pathogenesis and treatment strategies for this disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Dr. Anila Abraham Kurien, Renopath, Chennai, and Prof. Patrick D. Walker, MD, Director, Nephropath, Arkansas, for their help in evaluation of renal biopsy.
References
- 1.Nasr SH, Radhakrishnan J, D’Agati VD. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013;83:792–803. doi: 10.1038/ki.2012.407. [DOI] [PubMed] [Google Scholar]
- 2.Spector DA, Millan J, Zauber N, Burton J. Glomerulonephritis and Staphylococcal aureus infections. Clin Nephrol. 1980;14:256–61. [PubMed] [Google Scholar]
- 3.Koyama A, Kobayashi M, Yamaguchi N, Yamagata K, Takano K, Nakajima M, et al. Glomerulonephritis associated with MRSA infection: A possible role of bacterial superantigen. Kidney Int. 1995;47:207–16. doi: 10.1038/ki.1995.25. [DOI] [PubMed] [Google Scholar]
- 4.Nasr SH, Markowitz GS, Whelan JD, Albanese JJ, Rosen RM, Fein DA, et al. IgA-dominant acute poststaphylococcal glomerulonephritis complicating diabetic nephropathy. Hum Pathol. 2003;34:1235–41. doi: 10.1016/s0046-8177(03)00424-6. [DOI] [PubMed] [Google Scholar]
- 5.Nasr SH, Share DS, Vargas MT, D’Agati VD, Markowitz GS. Acute poststaphylococcal glomerulonephritis superimposed on diabetic glomerulosclerosis. Kidney Int. 2007;71:1317–21. doi: 10.1038/sj.ki.5002135. [DOI] [PubMed] [Google Scholar]
- 6.Nasr SH, D’Agati VD. IgA-dominant postinfectious glomerulonephritis: A new twist on an old disease. Nephron Clin Pract. 2011;119:c18–25. doi: 10.1159/000324180. [DOI] [PubMed] [Google Scholar]
- 7.Satoskar AA, Nadasdy G, Plaza JA, Sedmak D, Shidham G, Hebert L, et al. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol. 2006;1:1179–86. doi: 10.2215/CJN.01030306. [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Racusen LC, Bagnasco SM. IgA-dominant postinfectious glomerulonephritis: A report of 13 cases with common ultrastructural features. Hum Pathol. 2008;39:1309–16. doi: 10.1016/j.humpath.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Wen YK, Chen ML. IgA-dominant postinfectious glomerulonephritis: Not peculiar to staphylococcal infection and diabetic patients. Ren Fail. 2011;33:480–5. doi: 10.3109/0886022X.2011.573895. [DOI] [PubMed] [Google Scholar]
- 10.Koo TY, Kim GH, Park MH. Clinicopathologic features of IgA-dominant postinfectious glomerulonephritis. Korean J Pathol. 2012;46:105–14. doi: 10.4132/KoreanJPathol.2012.46.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal V, Kaul A, Ranade RS, Sharma RK. Immunoglobulin A dominant membranoproliferative glomerulonephritis in an elderly man: A case report and review of the literature. Indian J Nephrol. 2015;25:168–70. doi: 10.4103/0971-4065.145425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bu R, Li Q, Duan ZY, Wu J, Chen P, Chen XM, et al. Clinicopathologic features of IgA-dominant infection-associated glomerulonephritis: A pooled analysis of 78 cases. Am J Nephrol. 2015;41:98–106. doi: 10.1159/000377684. [DOI] [PubMed] [Google Scholar]
- 13.Ohmuro H, Tomino Y, Tsushima Y, Shimizu M, Kuramoto T, Koide H. Elevation of serum IgA1 levels in patients with diabetic nephropathy. Nephron. 1993;63:355. doi: 10.1159/000187224. [DOI] [PubMed] [Google Scholar]
- 14.Vázquez-Moreno L, Candia-Plata MC, Robles-Burgueño MR. Hypersialylated macromolecular serum immunoglobulin A1 in type 2 diabetes mellitus. Clin Biochem. 2001;34:35–41. doi: 10.1016/s0009-9120(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 15.Worawichawong S, Girard L, Trpkov K, Gough JC, Gregson DB, Benediktsson H. Immunoglobulin A-dominant postinfectious glomerulonephritis: Frequent occurrence in nondiabetic patients with Staphylococcus aureus infection. Hum Pathol. 2011;42:279–84. doi: 10.1016/j.humpath.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Rajakumar V, Mohamed SA, Kurien AA, Fernando ME. IgA dominant postinfectious glomerulonephritis: Report of two cases. Indian J Nephrol. 2014;24:181–4. doi: 10.4103/0971-4065.132020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph P, Gaut HL. IgA dominant post-infectious glomerulonephritis: Pathology and insights into disease mechanisms. Diagn Histopathol. 2013;19:175–81. [Google Scholar]
- 18.Wallace E, Maillard N, Ueda H, Hall S, Fatima H, Novak J, et al. Immune profile of IgA-dominant diffuse proliferative glomerulonephritis. Clin Kidney J. 2014;7:479–83. doi: 10.1093/ckj/sfu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaba Y, Hiki Y, Aoyama T, Sano T, Matsuo T, Shimizu T, et al. Effective antibiotic treatment of methicillin-resistant Staphylococcus aureus-associated glomerulonephritis. Nephron. 2002;92:297–303. doi: 10.1159/000063309. [DOI] [PubMed] [Google Scholar]
- 20.Eswarappa M, Ravi V, Mysorekar V, Gireesh MS. IgA dominant poststaphylococcal glomerulonephritis: Complete recovery with steroid therapy. Indian J Nephrol. 2014;24:336–7. doi: 10.4103/0971-4065.133051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glassock RJ, Alvarado A, Prosek J, Hebert C, Parikh S, Satoskar A, et al. Staphylococcus-related glomerulonephritis and poststreptococcal glomerulonephritis: Why defining “post” is important in understanding and treating infection-related glomerulonephritis. Am J Kidney Dis. 2015;65:826–32. doi: 10.1053/j.ajkd.2015.01.023. [DOI] [PubMed] [Google Scholar]


