Abstract
Objectives:
We aimed to determine whether average and trimester-specific exposures to ambient measures of nitrogen dioxide (NO2) and particular matter (PM2.5) were associated with elevated cord blood concentrations of immunoglobulin E (IgE) and two epithelial cell produced cytokines: interleukin-33 (IL-33) and thymic stromal lymphopoietin (TSLP).
Methods:
This study utilized data and biospecimens from the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. There were 2001 pregnant women recruited between 2008 and 2011 from 10 Canadian cities. Maternal exposure to NO2 and PM2.5 was estimated using land use regression and satellite-derived models.
Results:
We observed statistically significant associations between maternal NO2 exposure and elevated cord blood concentrations of both IL-33 and TSLP among girls but not boys.
Conclusions:
Maternal NO2 exposure may impact the development of the newborn immune system as measured by cord blood concentrations of two cytokines.
Learning Objectives
Summarize previous findings on the association between air pollution and childhood allergic disease, including the role of in utero versus childhood exposure.
Discuss the new findings on maternal pollutant exposures associated with specific types of immune system biomarkers in umbilical cord blood.
Identify other characteristics affecting the reported associations between pollution exposure and cord blood cytokine levels.
The role of maternal exposure to environmental contaminants on the developing fetal immune system is not clear. It has been suggested that fetal exposure to some environmental contaminants can promote life-long changes to the developing immune system that would have an effect on immune system responses resulting in an increased risk of an allergic phenotype in childhood and beyond.1–3 Results from research related to the health effects of childhood exposures tend to be more conclusive than studies evaluating effects of fetal exposures. Authors of a comprehensive review concluded that “evidence is sufficient to support a causal association between traffic-related air pollution exposure and exacerbation of childhood asthma.”4 These authors also concluded that the evidence was suggestive but not sufficient to support a causal association between traffic exposure and incident asthma.4 Authors of cohort studies in France,5,6 Netherlands,7,8 Sweden,9 Germany,10 China,11 and North America,12–15 have reported that childhood air pollution exposure is significantly associated with exacerbation or development of childhood asthma or allergic disease. Systematic reviews have reported that traffic-related air pollution is associated with severity of childhood asthma symptoms16 and increased risk of incident asthma, allergies, and sensitization.17
The body of literature regarding air pollution exposure and childhood asthma and allergic disease has limited ability to disentangle the role of in utero versus childhood exposure in allergic disease etiology.13,14 This distinction has relevance for identifying critical windows of exposure and effective prevention strategies. Recent studies have reported that NO2 and PM2.5 may affect placental function18 and gene expression,19 a process that may influence fetal development. Authors of birth cohort studies have reported associations between prenatal air pollution exposure and cord blood levels of immunoglobulin E (IgE),20 interleukin-10 (IL-10),21 and certain lymphocytes.22,23 However, to date, no studies have examined the association between in utero air pollution exposure and epithelial cell derived cytokines. It has been observed that epithelial cells have important roles in the regulation of both innate and adaptive immunity.24,25 As epithelial cells line the respiratory tract and skin, these cells may represent the point of first contact for many environmental contaminants.26 Unlike lymphopoietic cell derived cytokines, epithelial cell derived cytokines function in the absence of a mature immune system.27 In addition, common air pollutants such as NO2 have been shown to impact epithelial cell function.28,29
IL-33 and thymic stromal lymphopoietin (TSLP) are two epithelial cell derived cytokines that participate in allergic disease and type 2 inflammation.30 These mediators are known to have a critical role in the etiology of atopic dermatitis that is often the first manifestation of allergic disease in childhood.31 These two cytokines may have different mechanisms, as TSLP is thought to have a role in childhood allergy and asthma,32 whereas IL-33 has been linked to mechanisms underlying food allergy.33,34 We have previously demonstrated that these biomarkers are detectable at birth and associated with self-reported traffic exposure.35 Previous studies have examined the association between in utero environmental contaminant exposure and cord blood IgE concentrations as a biomarker for infant allergy20,36,37 and this may also serve as an indicator of fetal susceptibility to air pollution.20
The aim of the present study was to investigate the association between maternal exposure to ambient measures of PM2.5 and NO2 and cord blood concentrations of IgE, TSLP, and IL-33. The secondary objective is to determine the nature of these associations by trimester of exposure.
METHODS
Study Population and Data Sources
Details on the study design as well as the subpopulation for the current analysis have been published elsewhere.35,38 As described elsewhere, data and biospecimens were obtained from the Maternal-Infant Research on Environmental Chemicals (MIREC) Study, a trans-Canada prospective cohort study of pregnant women recruited in the first trimester from 10 Canadian cities between 2008 and 2011.38 As previously described, cord blood samples that were determined to be contaminated with maternal blood based on an elevated immunoglobulin A (IgA) concentration (≥10 μg/mL) were excluded from the analysis.39 We excluded preterm infants and multiple births, as these infants have lower levels of the immune system biomarkers of interest in this study and children born preterm have been shown to have an increased risk of asthma in childhood.40,41 All participants gave informed consent upon recruitment and research ethics board approval was obtained from Health Canada, St. Justine's Hospital (Montreal, QC), and the Izaak Walton Killam (IWK) Health Centre (Halifax, NS).
Air Pollution Exposure
Details regarding development of the air pollution models and methodology for determining prenatal exposure histories have been described previously.42 Briefly, information on participant's residential location [based on the forward sortation area (FSA) (ie, the first three letters of the postal code)] was collected from questionnaires administered in the 1st and 3rd trimesters. Air pollution concentrations were based on the population-weighted geographical coordinates of the FSA in which the mothers lived during pregnancy. If a subject moved during pregnancy, pollution estimates were reassigned according to their new FSA.
Estimates of prenatal exposure to PM2.5 were derived from monthly surfaces of a land use regression (LUR) model that was developed using satellite-based PM2.5 estimates as well PM2.5 measurements from fixed-site monitoring stations.43,44 Our estimates of NO2 were derived from a national LUR model developed using satellite NO2 estimates and geographic predictors43 scaled temporally with ground monitors from the National Air Pollution Surveillance (NAPS) network.45 This scaling was done by first calculating a location-specific scaling factor at each fixed monitoring location for each month of the study period. A spatially resolved scaling surface was then created for each month within the study period through spatial interpolating of these location-specific monthly scaling factors using an inverse distance weighting (IDW) interpolation. This approach was applied to create monthly NO2 estimates for FSA locations within 25 km of a NAPS station that measured NO2 during the study period. Monthly scaling surfaces were then combined with annual LUR estimates to create monthly NO246 and to estimate mean pregnancy and trimester-specific NO2 exposure. We used a 25 km buffer for temporal adjustment with ground-based monitoring stations based on prior literature.46 We conducted a sensitivity analysis by using the NO2 estimates from a national NO2 LUR model43,44 (without the inclusion of the temporally scaled NAPS data). The national model had an R2 of 0.73 and a root mean square error of 2.9 parts per billion (ppb). Pregnancy and trimester-level exposure estimates were calculated from monthly estimates for each calendar year on the basis of the proportion of pregnancy or trimester in that year. We used the temporally scaled exposure assessment model as our primary means of exposure assessment, as it facilitated trimester-specific model estimates.
Immune System Biomarkers
As previously described, immune system biomarkers were measured in the plasma of umbilical cord blood samples (details in).35 TSLP concentrations were measured using a commercial antibody kit (Biolegend, San Diego, CA). IL-33 concentrations were analyzed using antibodies from an R & D systems duoset (Minneapolis, MN). Total IgE and total IgA concentrations were analyzed using ELISA kits (EBioscience, San Diego, CA).
Statistical Analysis
On the basis of our previous work, the percentage of detectable samples was 42%, 52%, and 18% for TSLP, IL-33, and IgE, respectively. As a result, each immune system biomarker was categorized as binary variables. A composite variable was developed to identify samples with elevated concentrations of both TSLP and IL-33 (IL-33/TSLP), as these cytokines are highly correlated (Spearman correlation coefficient = 0.8). TSLP and IL-33 were categorized as elevated at the 80th percentile (TSLP = 554 pg/mL; IL-33 = 879 pg/mL) because there are no pre-existing thresholds. Elevated concentrations of the composite IL-33/TSLP variable were defined as those samples that had elevated concentrations (≥80%ile) of both TSLP and IL-33. In recognition of the arbitrary nature of the 80th percentile threshold, we also conducted analyses between average pollutant exposure and IL-33 and TSLP dichotomized at the level of detection (LOD). On the basis of established cut-off values for IgE where odds of childhood allergic disease are increased, values at least 1.2 ng/mL (0.5 kU/L) were considered elevated.47,48
Mixed effect logistic regression models were used to determine associations between average prenatal exposure to ambient air pollutants and binary measures of immune system biomarkers. This model accounted for potential correlations and other characteristics that distinguish subjects within the same recruitment site and FSAs. FSA and study recruitment site were included in the model as nested random effects. Ambient concentrations of PM2.5 and NO2 were evaluated as continuous variables (1 unit increases) and as quartiles in logistic regression analysis. Confounders were a priori identified as maternal age and income on the basis of our previous analysis of predictors of the immune system biomarkers.35 Due to the strong association between maternal allergy and the immune system biomarkers, and to avoid overadjustment, we did not adjust for this variable. In addition, though cigarette smoking is a risk factor for allergic disease, it was not associated with the immune system biomarkers, possibly due to the low prevalence of smoking in the MIREC population, and, therefore, not included in the multivariate models. Therefore, we conducted sensitivity analyses excluding mothers with a history of maternal allergy and excluding current smokers. We tested for effect modification by sex by evaluating the P value of the sex–exposure interaction term and stratifying results by sex.49 We also examined the influence of including both pollutants in a model on effect estimates. Results are presented for average exposure throughout pregnancy as well as by trimester. In the trimester-specific analysis, only the joint IL-33/TSLP results are shown, as the individual IL-33 and TSLP results were similar.
Restricted cubic spline analyses were performed on all associations with significant results in analyses by both average pregnancy and trimester-specific exposure. This component of the analysis facilitated examination of dose–response relationships using continuous measures of exposure. Knots were specified at the 25th, 50th, and 75th percentiles; Akaike information criterion values were comparable to default knot choices and offered the advantage of comparison with the quartile results. Analyses were performed using SAS v. 9.3 (SAS Institute Inc., Cary, NC) and R v. 3.2.2 (R Foundation, Vienna, Austria).
RESULTS
The number of cord blood samples that met the inclusion criteria (term, singleton birth) was 1253. We excluded 171 samples from the NO2 analysis due to residence beyond 25 km of a NAPs station. Baseline maternal and infant characteristics are summarized in Table 1. Only a small number of participants used allergy medications (4.3%) or smoked during pregnancy (4.9%). Most participants were 30 years of age or over and had a household income of greater than $50,000 (Table 1). Median PM2.5 levels for the duration of pregnancy were 8.34 μg/m3 with an interquartile range (IQR) of 3.22 μg/m3. Median NO2 levels for the duration of pregnancy were 14.15 ppb (IQR 12.99). Average NO2 and PM2.5 were moderately correlated (Spearman correlation coefficient r = 0.40). Correlations among the trimester-specific measures of each pollutant were moderate (PM2.5 ranged from 0.59 to 0.62; NO2 ranged from 0.79 to 0.83). Trimester-specific and average measures were highly correlated for each pollutant (0.82 to 0.86 for PM2.5 and 0.88 to 0.95 for NO2). Effect modification by infant sex was observed in the NO2 and IL-33/TSLP model (product term P = 0.01) and the PM2.5 and IgE model (product term P = 0.05). Although no effect modification by sex was observed between NO2 and IgE or PM2.5 and IL-33/TSLP (both P > 0.10), we present all results stratified by infant sex.
TABLE 1.
Study Participant Characteristics, MIREC Study, Canada, 2008–2011 (n = 1253)∗
| Characteristic | n (%) |
| Maternal age, yrs | |
| ≤29 | 328 (26.2) |
| 30–34 | 451 (36.0) |
| ≥35 | 474 (37.8) |
| Household income ($CAD) | |
| ≤30,000 | 90 (7.5) |
| 30,001–50,000 | 117 (9.7) |
| 50,001–100,000 | 513 (42.6) |
| >100,000 | 485 (40.3) |
| Smoking | |
| Never or quit before pregnancy | 1102 (88.0) |
| Quit when pregnancy confirmed | 90 (7.2) |
| Current | 61 (4.9) |
| Pre-pregnancy BMI† | |
| Underweight (<18.5) | 26 (2.2) |
| Normal (18.5–24.9) | 708 (60.5) |
| Overweight (25–29.9) | 268 (22.9) |
| Obese (≥30) | 168 (14.4) |
| Maternal allergy‡ | |
| No | 1199 (95.7) |
| Yes | 54 (4.3) |
| Parity | |
| Nulliparous | 524 (41.9) |
| Primiparous | 511 (40.9) |
| Multiparous | 216 (17.3) |
| Infant sex | |
| Male | 671 (62.2) |
| Female | 582 (46.5) |
| Birth weight, g | |
| ≤2500 | 11 (0.9) |
| 2501–4000 | 1050 (83.8) |
| >4000 | 192 (15.3) |
*Subgroup total may not equal 1253 due to missing data.
†World Health Organization Classification (WHO, 2006).
‡Defined as use of maternal allergy medication.
Among boys, no statistically significant associations were observed between NO2 or PM2.5 and any of the immune system biomarkers; odds ratios (ORs) were close to the null value and not suggestive of an inverse or positive relation (Table 2). Among girls, statistically significant associations were observed between the top two quartiles of NO2 (>14.16 ppb) and elevated IL-33, TSLP, and TSLP/IL-33 (Table 3). Compared with women with NO2 exposure levels in the lowest quartile, those in the highest quartile (>20.76 ppb) had an elevated odds of high cord blood IL-33/TSLP [OR = 3.42, 95% confidence interval (95% CI): 1.61 to 7.27], TSLP (OR = 3.00; 95% CI: 1.55 to 5.82), and IL-33 concentrations (OR = 3.20; 95% CI: 1.60 to 6.40). One unit (ppb) increases in NO2 were also statistically significantly associated with IL-33, TSLP, and IL-33/TSLP (OR of elevated IL-33/TSLP per 1 ppb increase in NO2 = 1.05, 95% CI: 1.02 to 1.08). When IL-33 and TSLP were dichotomized at the LOD, the associations with mean NO2 were attenuated [fourth quartile NO2: IL-33 OR = 1.25 (95% CI: 0.76 to 2.05); TSLP OR = 1.38 (95% CI: 0.84 to 2.29)]. No statistically significant associations were observed between PM2.5 and IL-33 or TSLP whether measured independently or jointly. Elevated (fourth quartile) PM2.5 exposure (>9.23 μg/m3) was associated with significantly increased odds of IgE concentrations among girls (OR = 2.57, 95% CI: 1.24 to 5.32). Maternal PM2.5 exposure, measured as a continuous variable, was positively associated with elevated IgE (OR per 1 μg/m3 PM2.5 = 1.14, 95% CI: 1.00 to 1.31). Inclusion of both pollutants in one model did not produce any notable changes in results.
TABLE 2.
Mixed-Level Logistic Regression Analysis of Average Ambient Air Pollutants and Cord Blood Immune System Biomarkers Among Boys, Odds Ratios, and 95% Confidence Intervals (n = 574)∗
| Pollutant† | IgE ≥0.5 ku/L | IL-33 ≥80%ile | TSLP ≥80%ile | IL-33/TSLP ≥80%ile |
| NO2, ppb | ||||
| ≤7.77 | 1.0 | 1.0 | 1.0 | 1.0 |
| 7.78 to ≤14.15 | 1.26 (0.61–2.62) | 0.80 (0.43–1.49) | 1.00 (0.54–1.84) | 0.91 (0.46–1.80) |
| 14.16 to ≤20.76 | 0.90 (0.41–1.99) | 0.80 (0.44–1.45) | 0.93 (0.51–1.69) | 0.86 (0.44–1.67) |
| >20.76 | 1.10 (0.50–2.43) | 0.90 (0.50–1.64) | 0.71 (0.38–1.34) | 0.95 (0.49–1.86) |
| 1 | 1.00 (0.96–1.04) | 1.01 (0.98–1.04) | 0.99 (0.96–1.02) | 1.01 (0.98–1.04) |
| PM2.5, μg/m3 | ||||
| ≤6.01 | 1.0 | 1.0 | 1.0 | 1.0 |
| 6.02 to ≤8.34 | 1.44 (0.74–2.79) | 0.86 (0.48–1.55) | 0.91 (0.51–1.63) | 0.90 (0.48–1.69) |
| 8.44 to ≤9.23 | 0.81 (0.36–1.80) | 1.58 (0.90–2.77) | 1.41 (0.80–2.48) | 1.53 (0.83–2.81) |
| >9.23 | 0.72 (0.33–1.60) | 1.32 (0.75–2.32) | 0.99 (0.55–1.76) | 0.94 (0.50–1.78) |
| 1 | 0.95 (0.81–1.10) | 1.09 (0.98–1.21) | 1.03 (0.92–1.14) | 1.02 (0.91–1.15) |
IgE, immunoglobulin E; IL, interleukin; TSLP, thymic stromal lymphopoietin.
*Adjusted for maternal age, household income (center and FSA are random effects).
†Pollutant concentrations are average exposure levels throughout pregnancy.
TABLE 3.
Mixed-Level Logistic Regression Analysis of Average Ambient Air Pollutants and Cord Blood Immune System Biomarkers Among Girls, Odds Ratios, and 95% Confidence Intervals (n = 507)∗
| Pollutant† | IgE ≥0.5 ku/L | IL-33 ≥80%ile | TSLP ≥80%ile | IL-33/TSLP ≥80%ile |
| NO2, ppb | ||||
| ≤7.77 | 1.0 | 1.0 | 1.0 | 1.0 |
| 7.78 to ≤14.15 | 1.28 (0.63–2.61) | 2.31 (1.14–4.65) | 2.25 (1.16–4.38) | 1.91 (0.86–4.21) |
| 14.16 to ≤20.76 | 1.93 (0.93–3.99) | 2.51 (1.19–5.28) | 2.87 (1.43–5.76) | 2.77 (1.24–6.20) |
| >20.76 | 1.02 (0.47–2.17) | 3.20 (1.60–6.40) | 3.00 (1.55–5.82) | 3.42 (1.61–7.27) |
| 1 | 1.01 (0.98–1.04) | 1.04 (1.04–1.08) | 1.04 (1.01–1.07) | 1.05 (1.02–1.08) |
| PM2.5, μg/m3 | ||||
| ≤6.01 | 1.0 | 1.0 | 1.0 | 1.0 |
| 6.02 to ≤8.43 | 1.97 (0.93–4.20) | 1.26 (0.61–2.60) | 1.52 (0.78–2.97) | 1.35 (0.64–2.82) |
| 8.44 to ≤9.23 | 1.37 (0.64–2.92) | 1.53 (0.79–2.98) | 1.44 (0.75–2.78) | 1.44 (0.70–2.95) |
| >9.23 | 2.57 (1.24–5.32) | 1.25 (0.57–2.70) | 1.14 (0.55–2.37) | 1.04 (0.47–2.31) |
| 1 | 1.14 (1.00–1.31) | 1.07 (0.93–1.23) | 1.03 (0.89–1.18) | 1.02 (0.88–1.18) |
IgE, immunoglobulin E; IL, interleukin; TSLP, thymic stromal lymphopoietin.
*Adjusted for maternal age, household income (center and FSA are random effects).
†Pollutant concentrations are average exposure levels throughout pregnancy.
When stratified by trimester of exposure, results were similar for boys with no significant associations and effect estimates tending to be close to the null value (Table 4). Among girls, the magnitude of the association between NO2 and IL-33/TSLP was strongest when exposure was measured during the third trimester (third trimester NO2 >20.76 ppb; OR IL-33/TSLP = 4.39, 95% CI: 2.00 to 9.64). First trimester PM2.5 concentrations in the fourth quartile (>9.23 μg/m3) were significantly associated with elevated levels of IgE (OR = 2.37, 95% CI: 1.23 to 4.58) among girls. Second trimester third quartile PM2.5 exposure was associated with statistically significantly increased odds of high IL-33/TSLP (OR = 2.16, 95% CI: 1.03 to 4.53) among girls (Table 5).
TABLE 4.
Mixed-Level Logistic Regression Analysis of Quartiles of Ambient Air Pollutants by Trimester of Exposure and Cord Blood Immune System Biomarkers Among Boys, Odds Ratio, and 95% Confidence Intervals∗
| Pollutant | N (%) | IgE ≥0.5 ku/L | IL-33/TSLP ≥80%ile |
| NO2, ppb | |||
| Trimester 1 | |||
| ≤7.77 | 81 (15.5) | 1.0 | 1.0 |
| 7.78 to ≤14.15 | 142 (27.2) | 0.95 (0.42–2.20) | 1.08 (0.52–2.26) |
| 14.16 to ≤20.76 | 133 (25.4) | 1.13 (0.47–2.69) | 0.82 (0.38–1.78) |
| >20.76 | 167 (31.9) | 1.31 (0.57–3.03) | 0.91 (0.43–1.91) |
| Trimester 2 | |||
| ≤7.77 | 90 (16.6) | 1.0 | 1.0 |
| 7.78 to ≤14.15 | 138 (25.5) | 1.12 (0.53–2.36) | 1.17 (0.56–2.45) |
| 14.16 to ≤20.76 | 183 (33.8) | 0.67 (0.30–1.47) | 0.70 (0.33–1.49) |
| >20.76 | 131 (24.2) | 0.54 (0.22–1.30) | 1.18 (0.56–2.52) |
| Trimester 3 | |||
| ≤7.77 | 125 (22.2) | 1.0 | 1.0 |
| 7.78 to ≤14.15 | 135 (24.0) | 1.30 (0.64–2.64) | 0.63 (0.30–1.31) |
| 14.16 to ≤20.76 | 174 (31.0) | 0.59 (0.26–1.35) | 1.05 (0.55–2.00) |
| >20.76 | 128 (22.8) | 0.79 (0.33–1.89) | 0.99 (0.50–1.97) |
| PM2.5, μg/m3 | |||
| Trimester 1 | |||
| ≤6.01 | 178 (26.5) | 1.0 | 1.0 |
| 6.02 to ≤8.43 | 203 (30.3) | 1.09 (0.59–2.02) | 0.79 (0.44–1.41) |
| 8.44 to ≤9.23 | 115 (17.1) | 1.50 (0.72–3.13) | 0.93 (0.48–1.79) |
| >9.23 | 175 (26.1) | 0.93 (0.46–1.89) | 0.98 (0.55–1.75) |
| Trimester 2 | |||
| ≤6.01 | 193 (28.8) | 1.0 | 1.0 |
| 6.02 to ≤8.43 | 167 (24.9) | 1.21 (0.64–2.28) | 0.79 (0.43–1.47) |
| 8.44 to ≤9.23 | 124 (18.5) | 0.97 (0.46–2.06) | 1.08 (0.58–2.03) |
| >9.23 | 187 (27.9) | 0.60 (0.29–1.27) | 1.19 (0.67–2.09) |
| Trimester 3 | |||
| ≤6.01 | 194 (28.9) | 1.0 | 1.0 |
| 6.02 to ≤8.43 | 198 (29.5) | 1.31 (0.72–2.40) | 1.75 (0.99–3.09) |
| 8.44 to ≤9.23 | 123 (18.3) | 0.81 (0.38–1.73) | 1.00 (0.50–2.02) |
| >9.23 | 156 (23.2) | 1.05 (0.52–2.09) | 1.34 (0.72–2.48) |
IgE, immunoglobulin E; IL, interleukin; TSLP, thymic stromal lymphopoietin.
*Adjusted for maternal age and income (center and FSA are random effects).
TABLE 5.
Mixed-Level Logistic Regression Analysis of Air Pollutants by Trimester of Exposure and Cord Blood Immune System Biomarkers Among Girls, Odds Ratios, and 95% Confidence Intervals∗
| Pollutant | N (%) | IgE ≥0.5 ku/L | IL-33/TSLP ≥80%ile |
| NO2, ppb | |||
| Trimester 1 | |||
| ≤7.77 | 118 (24.9) | 1.0 | 1.0 |
| 0.78 to ≤14.15 | 133 (28.1) | 1.81 (0.80–4.07) | 0.82 (0.35–1.95) |
| 14.16 to ≤20.76 | 102 (21.6) | 2.39 (1.03–5.55) | 1.36 (0.56–3.29) |
| >20.76 | 120 (25.4) | 1.67 (0.72–3.86) | 1.89 (0.84–4.27) |
| Trimester 2 | |||
| ≤7.77 | 118 (24.3) | 1.0 | 1.0 |
| 7.78 to ≤14.15 | 145 (29.9) | 1.56 (0.69–3.48) | 1.27 (0.58–2.76) |
| 14.16 to ≤20.76 | 105 (21.6) | 3.37 (1.49–7.62) | 2.45 (1.13–5.32) |
| >20.76 | 117 (24.1) | 1.13 (0.47–2.74) | 1.90 (0.88–4.08) |
| Trimester 3 | |||
| ≤7.77 | 146 (29.4) | 1.0 | 1.0 |
| 7.78 to ≤14.15 | 136 (27.4) | 1.45 (0.71–2.97) | 2.41 (1.08–5.37) |
| 14.16 to ≤20.76 | 113 (22.8) | 1.43 (0.67–3.05) | 2.71 (1.19–6.16) |
| >20.76 | 131 (20.4) | 1.63 (0.77–3.47) | 4.39 (2.00–9.64) |
| PM2.5, μg/m3 | |||
| Trimester 1 | |||
| ≤6.01 | 169 (29.1) | 1.0 | 1.0 |
| 6.02 to ≤8.43 | 158 (27.2) | 1.47 (0.72–2.96) | 0.93 (0.45–1.90) |
| 8.44 to ≤9.23 | 98 (16.9) | 1.26 (0.55–2.86) | 0.81 (0.35–1.85) |
| >9.23 | 155 (26.7) | 2.37 (1.23–4.58) | 1.34 (0.66–2.70) |
| Trimester 2 | |||
| ≤6.01 | 148 (25.5) | 1.0 | 1.0 |
| 6.02 to ≤8.43 | 166 (28.6) | 1.84 (0.89–3.78) | 1.59 (0.79–3.19) |
| 8.44 to ≤9.23 | 113 (19.5) | 1.75 (0.80–3.85) | 2.16 (1.03–4.53) |
| >9.23 | 153 (26.4) | 1.92 (0.92–4.00) | 1.10 (0.51–2.36) |
| Trimester 3 | |||
| ≤6.01 | 170 (29.3) | 1.0 | 1.0 |
| 6.02 to ≤8.43 | 166 (28.6) | 1.05 (0.53–2.08) | 1.04 (0.52–2.09) |
| 8.44 to ≤9.23 | 123 (21.2) | 1.52 (0.76–3.03) | 1.72 (0.85–3.48) |
| >9.23 | 121 (20.9) | 1.60 (0.80–3.18) | 1.27 (0.62–2.61) |
IgE, immunoglobulin E; IL, interleukin; TSLP, thymic stromal lymphopoietin.
*Adjusted for maternal age and income (center and FSA are random effects).
In the cubic spline analysis, the overall association between the NO2 and high IL33/TSLP among girls was significant (P = 0.004). The P value testing the null hypothesis of linearity was 0.09. The spline curve plateaus at approximately the 75%ile (20.76 ppb), though CIs are wide and crossing the null value at this concentration (Fig. 1). The spline curve of the relation between PM2.5 and IgE among girls did not have a significant overall association (P overall association = 0.15) and did not significantly deviate from linearity (P = 0.77) (Fig. 2).
FIGURE 1.
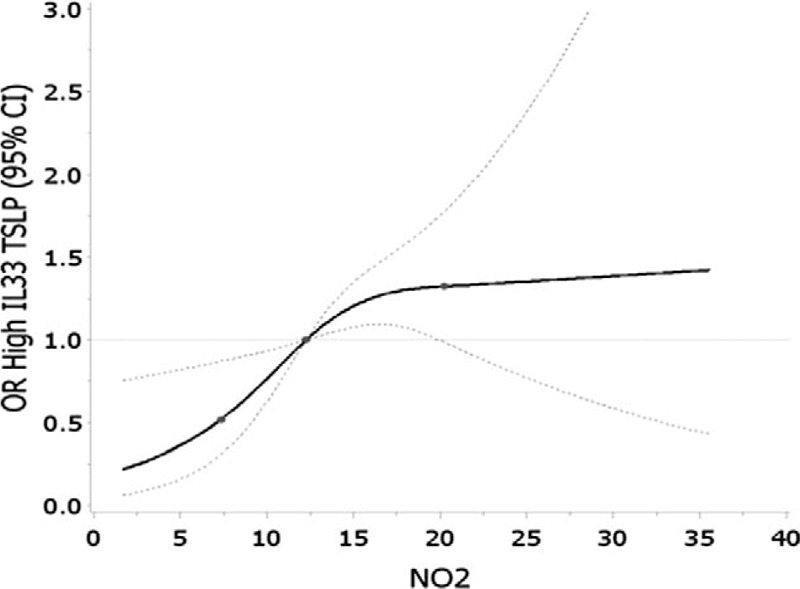
Restricted cubic spline analysis of average NO2 (ppb) and elevated IL-33 and TSLP among girls (≥80th%ile). Adjusted for maternal age and income. Knots set at 25th, 50th, and 75th percentiles. Error bars indicate 95% confidence intervals.
FIGURE 2.
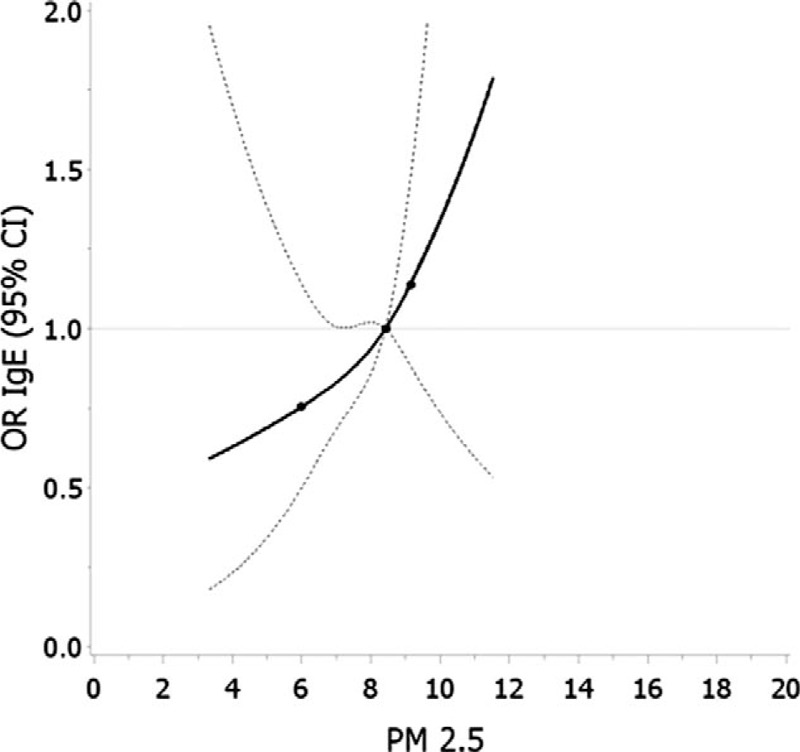
Restricted cubic spline analysis of average PM2.5 (μg/m3) and elevated IgE (≥0.5 ku/L) among girls. Adjusted for maternal age and income. Knots set at 25th, 50th, and 75th percentiles. Error bars indicate 95% confidence intervals.
In the sensitivity analysis, excluding women with a history of maternal allergy or current smokers had no impact on the results (data not shown in tables). In addition, using the LUR model of NO2 exposure assessment did not materially change the ORs from the mean pregnancy exposure models. Trimester-specific values estimated from the LUR model were highly correlated. As a result, trimester-specific ORs were similar to each other (data not shown).
DISCUSSION
This study was conducted to determine the association between maternal exposure to ambient air pollutants and umbilical cord blood concentrations of IgE, TSLP, and IL-33. There is a body of literature supporting a causal association between childhood exposure to air pollutants and exacerbation of asthma and literature that is suggestive of an association between air pollution and development of asthma4 as well as recent immunological literature demonstrating the integral role of these immune system biomarkers in the mechanisms underlying childhood allergy.30
We observed that maternal NO2 exposure was associated with significantly increased odds of high cord blood IL-33 and TSLP concentrations among girls. This finding was consistent in analyses of categorical and continuous exposure variables and persisted whether IL-33 and TSLP were analyzed individually or jointly. This association was not, however, observed in analyses wherein TSLP and IL-33 were dichotomized at the LOD. In light of literature suggesting that IL-33 and TSLP are cross-regulated and that IL-33 can induce TSLP production, our finding of an association between NO2 and two cytokines together is not surprising.25 When stratified by trimester of exposure, this association was most pronounced during the third trimester. Given the wide CIs in the NO2 effect estimates as well as the novelty of these findings, replication in further studies is warranted.
As this study is the first of its kind, it was not feasible to conduct direct comparisons with previous epidemiological studies. However, the present findings are consistent with results regarding relations among NO2, epithelial cells, and TSLP. NO2 exposure may induce epithelial cell injury29 and promote release of pro-inflammatory mediators.28 Furthermore, diesel exhaust, which may contain NO2, has been shown to upregulate epithelial cell production of TSLP.50
The present findings are also consistent with literature from animal models reporting that in utero exposure to pollutants, including diesel and particulate matter, promotes susceptibility to asthma and allergic sensitization51–53 and induces the release of pro-inflammatory mediators, such as IL-8, from lung tissue.54 Maternal air pollution exposure may also stimulate placental production of pro-inflammatory cytokines and subsequently influence fetal immune system development.55 We therefore speculate that the observed association between NO2 and IL-33 and TSLP is mediated by air pollution induced inflammatory responses in maternal airway tissue. These maternal responses, which can include release of pro-inflammatory mediators, may promote fetal epithelial cell cytokine production. Given the stronger magnitude of association in the third trimester, it is possible that later gestational NO2 exposure is the critical window for influencing TSLP and IL-33 concentrations. Experimental evidence has suggested that third trimester exposure to the heavy metal lead may be more likely to promote Th2 cytokine response than exposure earlier in pregnancy.56 However, authors of a birth cohort study in the Czech Republic reported that air pollution (PM2.5 and polycyclic aromatic hydrocarbons) exposure during the first trimester was associated with increases in T lymphocytes that may promote autoimmune responses.22 Scientific understanding regarding the critical window of gestational exposure for each pollutant and specific immunological endpoint is limited. Replication of the present findings regarding third trimester NO2 exposure and pro-inflammatory cytokine responses will help address this knowledge gap.
We hypothesize that it is unlikely that the cytokines measured in the present study are produced by maternal epithelial cells. IL-33 is a member of the IL-1 family and previous research has reported that IL-1α and IL-1β do not readily cross the placenta.57,58 TSLP has been shown to be produced by trophoblasts during early gestation.59 However, it is unlikely that maternal TSLP crosses the placenta due to its large molecular weight, which is of comparable size to IL-1, and the absence of known active transport processes.
The observed sex-dependent nature of the association between NO2, IL-33, and TSLP is also consistent with previous research. Studies examining relations between traffic-related pollutants, including NO2, and childhood asthma have shown stronger associations among girls than boys.13,60,61 This potential early life air pollution susceptibility among girls is of particular interest, as boys tend to have a higher prevalence of asthma at younger ages.62 The mechanism underlying these observed sex-dependent differences and susceptibilities at birth warrants further investigation.
Our finding of a stronger, more consistent association with NO2 than PM2.5 may be attributable to the fact that the NO2 exposure estimates are an approximation of traffic-related air pollution exposure, whereas the satellite-derived PM2.5 estimates are reflective of both anthropogenic and biogenic sources of PM2.5. Traffic-related air pollution and correlated exposures,63,64 rather than NO2, therefore may be the underlying causal agent. These correlated exposures include diesel exhaust, polycyclic aromatic hydrocarbons, and certain volatile organic compounds (eg, benzene, toluene, ethylbenzene, and xylene), all of which have been linked to related adverse respiratory and allergic outcomes.65,66 Literature regarding PM2.5 and NO2 exposure has not demonstrated consistent results regarding the relative contributions of these pollutants to childhood allergic disease outcomes. Authors of a prospective birth cohort study in Germany reported that NO2 but not PM2.5 was associated with childhood eczema, whereas PM2.5 but not NO2 was associated with asthmatic bronchitis.10 Authors of another cohort study reported that the association between early life air pollution exposure and certain types of asthma was stronger with NO2 than PM2.5.13
We observed statistically significant associations between PM2.5 exposure, particularly during the first trimester, and IgE among girls. One identified birth cohort study from the Czech Republic reported a statistically significant positive association between PM2.5 exposure during the sixth month of gestation and cord blood IgE; exposure during all other time periods was of a null or inverse relation.20 Herr et al20 examined effect estimates of elevated IgE per 25 μg/m3 increase in PM2.5, which reflects a notably higher ambient concentration than measured in the MIREC cohort (median = 8.34; maximum = 11.57 μg/m3). It is possible that ambient PM2.5 concentrations in the Czech Republic study were sufficiently high to suppress first trimester β-cell production of IgE, whereas the lower concentrations observed in the present study did not result in a similar immunologic response. Replication of the present finding is necessary to determine whether the observed associations between PM2.5 and IgE among girls are a result of chance, confounding, or reflective of a true association.
Strengths of this study included the relatively large sample size in the MIREC study, the inclusion of many potential confounding factors, and the availability of novel immune system biomarkers. The use of spatiotemporal models for NO2 and PM2.5 enabled us to develop average exposure measures over the time span of a pregnancy that captured both detailed spatial and temporal variations in pollutant concentrations.
A primary limitation of our study is the potential exposure misclassification of the air pollutants resulting from the use of three-digit postal codes rather than full residential address. We did not have information on work location, time spent at home, or indoor pollutants. Further, the use of different exposure estimation methods for the two pollutants precluded direct comparison of results for the two pollutants. A second limitation is the lack of clinical outcome data. Although IL-33 and TSLP have been implicated in allergic disease,31 literature on the longitudinal relation from birth to childhood is lacking. Thus, it is not possible to draw definitive conclusions about the risk of developing childhood allergic disease on the basis of these findings. Determining the impacts of the observed results on childhood allergic disease requires further follow-up of the MIREC cohort. Third, considering that high NO2 concentrations are found in regions of high population density, it is possible that the observed results are subject to residual confounding due to co-occurring pollutants or characteristics unique to this subpopulation.
CONCLUSIONS
We report a positive, statistically significant association between maternal NO2 exposure and elevated (≥80th percentile) cord blood concentrations of the epithelial cell derived cytokines TSLP and IL-33. We also observed a positive association between elevated overall average and first trimester PM2.5 exposure (>9.23 μg/m3) and high IgE among girls (first trimester exposure OR = 2.37, 95% CI: 1.23 to 4.58), although these associations were not statistically significant in spine analysis. These findings suggest that maternal NO2 exposure may impact newborn immune system development as measured by cord blood concentrations of two cytokines. Replication of these observed findings, particularly in a cohort of differing socioeconomic and ethnic background than the MIREC population, will help clarify the public health implications of low-level in utero NO2 exposure. Studies that further elucidate the observed dose–response relationships, the role of timing of exposure, and the physiologic mechanisms will be a valuable contribution to understanding early life influences on immune system function, potential subsequent risk of allergic disease, and differential responses by child sex.
Footnotes
Authors Dodds, Ashley-Martin, Lavigne, Arbuckle, Johnson, Hystad, Crouse, and Marshall have no other relationships/conditions/circumstances that present potential conflict of interest.
The JOEM editorial board and planners have no financial interest related to this research.
This work was funded by a Category A grant (grant #10012) from the Izaak Walton Killam (IWK) Health Centre. The MIREC Study was funded by the Chemicals Management Plan of Health Canada, the Canadian Institutes for Health Research (grant# MOP – 81285), and the Ontario Ministry of the Environment.
The authors report no conflicts of interest.
REFERENCES
- 1.Dietert RR, Lee JE, Hussain I, Piepenbrink M. Developmental immunotoxicology of lead. Toxicol Appl Pharmacol 2004; 198:86–94. [DOI] [PubMed] [Google Scholar]
- 2.Holladay S, Smialowicz R. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect 2000; 108:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luebke RW, Chen DH, Dietert R, Yang Y, King M, Luster MI. The comparative immunotoxicity of five selected compounds following developmental or adult exposure. J Toxicol Environ Health B 2006; 9:1–26. [DOI] [PubMed] [Google Scholar]
- 4.Health Effects Institute. Traffic-Related Air Pollution: a Critical Review of the Literature on Emissions, Exposure, and Health Effects. Final Version of Special Report. No. 17. 2010. Available at: http://pubs.healtheffects.org/getfile.php?u=552 Accessed February 23, 2016. [Google Scholar]
- 5.Annesi-Maesano I, Moreau D, Caillaud D, et al. Residential proximity fine particles related to allergic sensitisation and asthma in primary school children. Respir Med 2007; 101:1721–1729. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Baïz N, Zhang T, Banerjee S, Annesi-Maesano I. Modifiable exposures to air pollutants related to asthma phenotypes in the first year of life in children of the EDEN mother-child cohort study. BMC Public Health 2013; 13:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brauer M, Hoek G, Smit HA, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J 2007; 29:879–888. [DOI] [PubMed] [Google Scholar]
- 8.Gehring U, Wijga AH, Brauer M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med 2010; 181:596–603. [DOI] [PubMed] [Google Scholar]
- 9.Nordling E, Berglind N, Melen E, et al. Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology 2008; 19:401–408. [DOI] [PubMed] [Google Scholar]
- 10.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med 2008; 177:1331–1337. [DOI] [PubMed] [Google Scholar]
- 11.Deng Q, Lu C, Norback D, Bornehag CG, Zhang Y, Lie W, et al. Effects of early life exposure to ambient air pollution and childhood asthma in China. Environ Res 2015; 143:83–92. [DOI] [PubMed] [Google Scholar]
- 12.Brunst KJ, Ryan PH, Brokamp C, Bernstein D, Reponen T, Lockey J, et al. Timing and duration of traffic-related air pollution exposure and the risk for childhood wheeze and asthma. Am J Respir Crit Care Med 2015; 192:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark NA, Demers PA, Karr CJ, Koehorn M, Lencar C, Tamburic L, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect 2010; 118:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel MM, Quinn JW, Jung KH, Hoepner L, Diaz D, Perzanowski M. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ Res 2011; 111:1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankardass K, Jerrett M, Dell SD, Foty R, Stieb D. Spatial analysis of exposure to traffic-related air pollution at birth and childhood atopic asthma in Toronto, Ontario. Health Place 2015; 34:287–295. [DOI] [PubMed] [Google Scholar]
- 16.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect 2010; 118:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 2015; 70:245–256. [DOI] [PubMed] [Google Scholar]
- 18.Van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PY, et al. Air pollution exposure and markers of placental growth and function: the Generation R Study. Environ Health Perspect 2012; 120:1753–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saenen ND, Plusquin M, Bijnens E, Janssen BG, Gyselaers W, Cox B, et al. In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: an ENVIRONAGE Birth Cohort Study. Environ Health Perspect 2015; 123:834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herr CE, Ghosh R, Dostal M, Skokanova V, Ashwood P, Lipsett M, et al. Exposure to air pollution in critical prenatal time windows and IgE levels in newborns. Pediatr Allergy Immunol 2011; 22:75–84. [DOI] [PubMed] [Google Scholar]
- 21.Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Roosli M, et al. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PLoS One 2011; 6:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herr CEW, Dostal M, Ghosh R, Ashwood P, Lipsett M, Pinkerton KE, et al. Air pollution exposure during critical time periods in gestation and alterations in cord blood lymphocyte distribution: a cohort of livebirths. Environ Health 2010; 9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonardi GS, Fletcher T, Armstrong B. Immune biomarkers in relation to exposure to particulate matter: a cross-sectional survey in 17 cities of Central Europe. Inhal Toxicol 2000; 12 suppl 4:1–14. [PubMed] [Google Scholar]
- 24.Proud D, Leigh R. Epithelial cells and airway diseases. Immunol Rev 2011; 242:186–204. [DOI] [PubMed] [Google Scholar]
- 25.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev 2009; 226:172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbas A. Cellular and Molecular Immunology. Philadelphia, PA: Elsevier Saunders; 2015. [Google Scholar]
- 27.Brugman S, Perdijk O, van Neerven RJJ, Savelkoul HFJ. Mucosal immune development in early life: setting the stage. Arch Immunol Ther Exp 2015; 63:251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayyagari VN, Januszkiewicz A, Nath J. Pro-inflammatory responses of human bronchial epithelial cells to acute nitrogen dioxide exposure. Toxicology 2004; 197:149–164. [DOI] [PubMed] [Google Scholar]
- 29.Frampton MW, Boscia J, Roberts NJ, Azadniv M, Torres A, Cox C, et al. Nitrogen dioxide exposure: effects on airway and blood cells. Am J Physiol Lung Cell Mol Physiol 2002; 282:L155–L165. [DOI] [PubMed] [Google Scholar]
- 30.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol 2012; 143:222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Imunol 2011; 2:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauvreau GM, O’Byrne, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 2014; 370:2102–2110. [DOI] [PubMed] [Google Scholar]
- 33.Chu DK, Llop-Guevar A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 2013; 131:187–200. [DOI] [PubMed] [Google Scholar]
- 34.Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, et al. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol 2014; 26:539–549. [DOI] [PubMed] [Google Scholar]
- 35.Ashley-Martin J, Dodds L, Arbuckle TE, Levy AR, Platt RW, Marshall JS. Predictors of interleukin-33, thymic stromal lymphopoietin and immunoglobulin E. Pediatr Allergy Immunol 2015; 26:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grandjean P, Poulsen LK, Heilmann C, Steuerwald U, Weihe P. Allergy and sensitization during childhood associated with prenatal and lactational exposure to marine pollutants. Environ Health Perspect 2010; 118:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okada E, Sasaki S, Sajio Y, Washino N, Miyashita C, Kobayashi S, et al. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res 2012; 112:118–125. [DOI] [PubMed] [Google Scholar]
- 38.Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, et al. Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr Perinatal Epidemiol 2013; 27:415–425. [DOI] [PubMed] [Google Scholar]
- 39.Ownby DR, McCullough J, Johnson CC, Peterson EL. Evaluation of IgA measurements as a method for detecting maternal blood contamination of cord blood samples. Pediatr Allergy Immunol 1996; 7:125–129. [DOI] [PubMed] [Google Scholar]
- 40.Algert CS, Bowen JR, Lain SL, Allen HD, Vivian-Taylor JM, Roberts CL. Pregnancy exposures and risk of childhood asthma admission in a population birth cohort. Pediatr Allergy Immunol 2011; 22:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavigne E, Ashley-Martin J, Dodds L, Arbuckle TE, Hystad P, Johnson M, et al. Air pollution exposure during pregnancy and fetal markers of metabolic function: the MIREC Study. Am J Epidemiol 2016; 183:842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hystad P, Setton E, Cervantes A, Poplawski K, Deschenes S, Brauer M, et al. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect 2011; 119:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hystad P, Villeneuve PJ, Goldberg MS, Crouse DL, Johnson K. Canadian Cancer Registries Epidemiology Research Group. Exposure to traffic-related air pollution and the risk of developing breast cancer among women in eight Canadian provinces: a case–control study. Environ Int 2015; 74:240–248. [DOI] [PubMed] [Google Scholar]
- 45.Johnson M, Macneill M, Grgicak-Mannion A, Nethery E, Xu X, Dales, et al. Development of temporally refined land-use regression models predicting daily household-level air pollution in a panel study of lung function among asthmatic children. J Expo Sci Environ Epidemiol 2013; 231:259–267. [DOI] [PubMed] [Google Scholar]
- 46.Bechle MJ, Millet D, Marshall JD. A national spatiotemporal exposure surface for NO 2: monthly scaling of a satellite-derived land-use regression, 2000–2010. Environ Sci Technol 2015; 49:12297–12305. [DOI] [PubMed] [Google Scholar]
- 47.Pesonen M, Kallio MJT, Siimes MA, Elg P, Bjorksten F, Ranki A, et al. Cord serum immunoglobulin E as a risk factor for allergic symptoms and sensitization in children and young adults. Pediatr Allergy Immunol 2009; 20:12–18. [DOI] [PubMed] [Google Scholar]
- 48.Sadeghnejad A, Karmaus W, Davis S, Kurukulaaratchy RJ, Mathews S, Arshad SH. Raised cord serum immunoglobulin E increases the risk of allergic sensitisation at ages 4 and 10 and asthma at age 10. Thorax 2004; 59:936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kynyk J, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med 2011; 17:6–11. [DOI] [PubMed] [Google Scholar]
- 50.Bleck B, Grunig G, Chiu A, et al. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol 2013; 190:3757–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fedulov AV, Lerne A, Yang Z, et al. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol 2008; 38:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamada K, Suzaki Y, Lerne A, et al. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health A 2007; 70:688–695. [DOI] [PubMed] [Google Scholar]
- 53.Hong X, Liu C, Chen X, et al. Maternal exposure to airborne particulate matter causes postnatal immunological dysfunction in mice offspring. Toxicology 2013; 306:59–67. [DOI] [PubMed] [Google Scholar]
- 54.Sexton KG, Jeffries HE, Kamens RM, et al. Photochemical products in urban mixtures enhance inflammatory responses in lung cells. Inhal Toxicol 2004; 16 suppl 1:107–114. [DOI] [PubMed] [Google Scholar]
- 55.de Melo JO, Soto SF, Katayama IA, Wenceslau CF, Pires AG, Veras MM, et al. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett 2015; 232:475–480. [DOI] [PubMed] [Google Scholar]
- 56.Bunn TL, Parsons PJ, Kao E, Dietert RR. Exposure to lead during critical windows of embryonic development: differential immunotoxic outcome based on stage of exposure and gender. Toxicol Sci 2001; 64:57–66. [DOI] [PubMed] [Google Scholar]
- 57.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol 2005; 106:802–807. [DOI] [PubMed] [Google Scholar]
- 58.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol 204;103:546–550. [DOI] [PubMed] [Google Scholar]
- 59.Guo PF, Du MR, Wu HX, et al. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood 2010; 116:2061–2069. [DOI] [PubMed] [Google Scholar]
- 60.McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect 2006; 114:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Vliet P, Knape M, de Hartog J, Janssen N, Harssema H, Brunekreef B, et al. Motor vehicle exhaust and chronic respiratory symptoms in children living near freeways. Environ Res 1997; 74:122–132. [DOI] [PubMed] [Google Scholar]
- 62.Garner R, Kohen D. Changes in the prevalence of asthma among Canadian children. Health Rep 2008; 19:45–50. [PubMed] [Google Scholar]
- 63.Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MMB, et al. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmos Environ 2008; 34:51–59. [Google Scholar]
- 64.Han X, Naeher LP. A review of traffic-related air pollution exposure assessment studies in the developing world. Environ Int 2006; 32:106–120. [DOI] [PubMed] [Google Scholar]
- 65.Bolden AL, Kwiatkowski CF, Colborn T. New look at BTEX: are ambient levels a problem? Environ Sci Technol 2015; 49:5261–5276. [DOI] [PubMed] [Google Scholar]
- 66.Lemke LD, Lamerato LI, Xu X, et al. Geospatial relationships of air pollution and acute asthma events across the Detroit–Windsor international border: study design and preliminary results. J Expo Sci Environ Epidemiol 2014; 24:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]


