Abstract
Background
To investigate associations of kidney cancer mortality with modifiable risk factors of obesity, physical activity, and smoking.
Methods
We evaluate baseline data from US National Health Information Survey from 1998 through 2004 linked to mortality data reporting deaths through 2006. The primary outcome variable was kidney cancer-specific mortality and primary exposure variables were self-reported physical activity and body mass index (BMI). We utilized multivariable adjusted Cox proportional hazards regression models, with delayed entry to account for age at survey interview.
Results
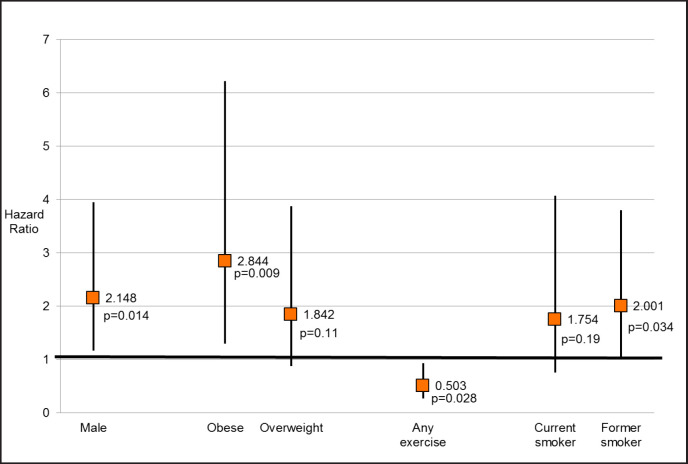
Among 222,163 individuals with complete follow-up data we identified 71 kidney cancer-specific deaths. In multivariate analyses, individuals who reported “any physical activity” were 50% less likely [adjusted hazard ratio (adjusted HR) 0.50, 95% CI 0.27–0.93, p = 0.028] to die of kidney cancer than non-exercisers, while obese individuals (BMI ≥ 30 kg/m2) were nearly 3 times more likely (adjusted HR 2.84, 95% CI 1.30–6.23, p = 0.009) compared to those of normal weight (BMI < 25 kg/m2). Compared to never smokers, former smokers were twice as likely to die of kidney cancer (adjusted HR 2.00, 95% CI 1.05–3.80, p = 0.034).
Conclusion
Physical activity decreases and obesity increases the risk of kidney cancer mortality.
Key Words: Renal cancer, Mortality, Modifable risk, Exercise, Physical activity, Obesity, Smoking
Introduction
Although kidney cancer is an important public health problem, it remains an understudied target for cancer prevention and control. In the USA, it is the fifth most common cancer in men and the seventh most common cancer in women, with an estimated 63,990 new cases diagnosed annually [1]. Kidney cancer is estimated to have a worldwide incidence of 270,000 cases yearly and nearly 116,000 deaths [2]. Despite improvements in detection and treatment, renal cell carcinoma (RCC) remains a potentially lethal cancer, with a 5-year disease-specific mortality of 26% [3]. According to the American Cancer Society, the kidney cancer survival ranges from 81 (Stage I) to 8% (Stage IV), whereas the majority of kidney cancer diagnosed is Stage I [4].
Yet translational clinical trials of kidney cancer prevention have as yet not been undertaken. A potential means of decreasing incidence, morbidity and mortality of kidney cancer is through modulation of modifiable lifestyle factors of disease, notably adiposity, physical activity, and tobacco use [5]. Obesity is a prominent risk factor for incident and prevalent RCC. Lowrance et al. [6] found that for every 1 kg/m2 increase in body mass index (BMI) there was a 4% increase in the odds of having conventional clear-cell RCC histology. However, one manuscript investigating American Association of Retired Persons (AARP) Diet and Health Study cohort noted that increasing exercise was associated with increased risk of kidney cancer-specific mortality [7]. While there are ample observational data describing associations of these modifiable factors with incident and prevalent disease, there remains a paucity of information on the effects of these factors on kidney cancer survival. Herein we investigated associations of kidney cancer mortality with adiposity, exercise, and smoking in a large cohort of USA adults utilizing the National Health Information Survey (NHIS) survey data.
Materials and Methods
Study Population
The NHIS is an annual representative cross-sectional household interview survey performed in the USA. The surveys are collected through personal household interviews conveyed by the USA Census Bureau. The NHIS is a survey of the civilian non-institutionalized population in all 50 states and the District of Columbia. Surveys are administered throughout the year and compiled into annual data sets. This paper uses data from the adult (18+ years of age) population. Response rates for the adult survey in this time period averaged 73.8% (range 69.2–80.4%). Data are collected in an in-person interview. Data for all family members are collected for the Family Core questionnaire; data for children and family members not present for the interview are collected from a knowledgeable adult family member. One adult is randomly selected for the Sample Adult survey; all data are from self-report. Data from the Sample Adult surveys were used for this study. On average 42,000 households including 100,000 people per year. We used baseline data from 1998 through 2004 linked to mortality data reporting deaths through 2006. The data was collected through stratified sampling schemes that oversampled for ethnic subgroups.
Exposure and Outcome Variables
The primary outcome variable was kidney cancer-specific mortality. Mortality was assessed using data from the NHIS linked mortality public-use files. Kidney cancer deaths were grouped as “Malignant neoplasms of kidney and renal pelvis (ICD-10 categories C64-C65)”
The primary exposure variables were self-reported physical activity (“no exercise” versus “light, moderate or vigorous exercise in ≥ 10 minute-bouts”), obesity as measured by BMI in kg/ m2, and smoking (never, former, and current). During the survey respondents were asked: “How often do you do vigorous activities for at least 10 minutes that cause heavy sweating or large increases in breathing or heart rate?”; response categories included “never”, “unable to do this activity”, or a specification of the number of times within a reported time unit (days, weeks, months or years). Those who performed such activities were then asked “How long do you do these vigorous activities each time?” and could specify a continuous number in minutes or hours. Respondents were then asked “How often do you do light or moderate activities for at least 10 minutes that cause only light sweating or a slight to moderate increase in breathing or heart rate?” with the same response categories as for vigorous exercise and the same follow-up question on length of each session. Responses were converted to average minutes of vigorous or moderate exercise/week and respondents were scored on whether they exercised at all, or met recommended standards. Therefore, we investigated physical activity in context of the Physical Activity Guidelines for Americans developed in 2008 as an objective for Healthy People 2020, which is a USA Department of Health and Human Services program to support prevention of disease and a healthier nation. The physical activity requirements consist of 150 min/week of moderate-intensity aerobic, 75 min/week of vigorous-intensity aerobic or an equivalent combination of the 2 goals.
Statistical Analysis
We utilized multivariable adjusted Cox proportional hazards regression models, with delayed entry to account for age at survey interview. Cox models were adjusted for race/ethnicity (non-Hispanic white versus all others), sex, BMI [overweight or obese versus normal weight; underweight (BMI < 18.5 kg/m2) individuals were removed from the analysis], current or ever use of cigarettes, and diagnosis of kidney cancer or any other cancer at time of survey. Thus, our models present the effect of exercise on kidney cancer mortality adjusting for previously identified risk factors of obesity (BMI) and smoking. Epidemiological data from the prostate, lung, colorectal, and ovarian cancer screening trial suggest that age, male gender, obesity, smoking, and hypertension were main risk factors for kidney cancer [5]. This formed the bases of our analysis in order to focus on modifiable risk factors. Analyses were adjusted for the complex NHIS multistage sampling methodology using survey weights.
Results
Among 222,163 individuals with complete follow-up data, of whom 96,715 (48%) were men and 146,014 (73%) non-Hispanic whites, we identified 71 kidney cancer-specific deaths. The mean age is 45.1 years (95% CI = 44.9–45.3) with a mean BMI of 26.7 kg/m2 (95% CI = 26.7–26.8). Those who died from kidney cancer were more likely to be men (p = 0.01); ethnicity was not associated with kidney cancer death (p = 0.86). There were no associations of ethnicity or gender with kidney cancer mortality (table 1). Table 2 describes the amount of exercise and reported via survey and the number subjects who died from kidney cancer. Individuals who reported “any physical activity” were 53% less likely to die of kidney cancer than non-exercisers in multivariable analysis (fig. 1). Compared to those of normal weight (BMI < 25 kg/m2), obese individuals (BMI ≥ 30 kg/m2) were nearly 3 times more likely to die of kidney cancer. Overweight individuals were nearly twice as likely to die of kidney cancer, but this association did not attain significance (p = 0.11). Compared to never smokers, former smokers were twice as likely to die of kidney cancer. There were no significant associations of current smoking with kidney cancer death (fig. 1).
Table 1.
Demographics
| Covariates | Total |
Kidney cancer death |
|||
|---|---|---|---|---|---|
| No. | % | CI | No. | % | |
| Total | 22,2163 | – | – | 71 | 0.031 |
| Age at survey, years | |||||
| < 60 | 167,262 | 75.2 | 0.4 | 20 | 0.0119 |
| 60–74 | 34,514 | 15.5 | 0.3 | 24 | 0.0695 |
| 75+ | 20,387 | 9.1 | 0.2 | 27 | 0.1177 |
| Race/ethnicity | |||||
| Non-Hispanic White | 146,014 | 65.7 | 0.6 | 54 | 0.0369 |
| Hispanic | 37,439 | 16.8 | 0.4 | 9 | 0.0240 |
| African American | 30,197 | 13.5 | 0.5 | 7 | 0.0231 |
| Asian/Pacific Islander | 5,492 | 2.8 | 0.2 | 1 | 0.0182 |
| Other | 3,021 | 1.4 | 0.1 | 0 | 0.0000 |
| Sex | |||||
| Male | 96,715 | 43.5 | 0.3 | 48 | 0.0496 |
| Female | 125,448 | 56.5 | 0.3 | 23 | 0.0183 |
| BMI, kg/m2 | |||||
| < 20 (Underweight) | 4,229 | 1.9 | 0.1 | 1 | 0.0236 |
| 20–25 (Normal) | 85,023 | 38.3 | 0.3 | 20 | 0.0235 |
| 25.1–30 (Overweight) | 74,752 | 33.6 | 0.3 | 27 | 0.0361 |
| > 30 (Obese) | 48,502 | 21.8 | 0.3 | 21 | 0.0432 |
| Get recommended exercise | 101,657 | 45.8 | 0.5 | 12 | 0.0118 |
| Current cigarette smoker | 50,062 | 22.5 | 0.3 | 14 | 0.0279 |
| Former cigarette smoker | 48,361 | 21.8 | 0.3 | 34 | 0.0703 |
| Diagnosed with other cancer | 15,415 | 6.9 | 0.1 | 23 | 0.1492 |
Table 2.
Amount of exercise compared to kidney cancer deaths.
| Exercise level | Frequency | Percent of sample (%) | Kidney cancer deaths |
|---|---|---|---|
| Unable | 4,116 | 1.6 | 6 |
| No exercise | 116,390 | 50 | 53 |
| Exercise, below standards | 21,530 | 10.5 | 4 |
| Exercise, meet standards | 30,935 | 14.5 | 5 |
| Exercise, exceed standards by 2× | 49,192 | 23.3 | 3 |
| Total | 222,163 | 100 | 71 |
Fig. 1.
HR for kidney cancer mortality. Box and whisker plot representing the HR and confidence intervals for mortality and the corresponding risk factors.
Discussion
We observed that obesity increases and physical activity decreases the risk of death from kidney cancer. These statements; however, are in direct conflict with recent reports stating the opposite. One highly popularized article noted a protective effect of obesity in kidney cancer [8]. The data were collected from 1,975 patients from 19 centers in the International Metastatic Renal Cell Carcinoma Database Consortium and validated in a separate cohort of over 4,000 patients enrolling in metastatic kidney cancer clinical trials. The authors concluded that the “obesity paradox” lead to significant improvement in survival and attributed the benefit to the reduction in fatty acid synthase gene expression in obese patients. Another theory is that the inverse relationship between obesity and mortality is due to a less aggressive disease subtype [9]. Additionally, obesity in metastatic kidney cancer patients may provide an advantage if weight loss is a factor for morality and was not examined in this study.
Three of 4 previous prospective investigations regarding the incidence of renal cancer and obesity noted a healthy weight to have a protective effect [10,11,12,13]. The 1 null study was from George et al. [10] utilizing the National Institutes of Health-AARP Diet and Health Study noting that “sitting time” was not associated with an increase in kidney cancer. However, there is a possibility that exercise and accompanying weight loss may potentially prevent kidney cancer death.
We noted that exercise individuals who reported “any physical activity” were 53% less likely to die of kidney cancer than non-exercisers in the multi-variable analysis. The rising number of RCC cases is believed to be a result of improved imaging techniques and increased incidental tumor discovery [14]. Moreover, RCC can also be attributed to the increased prevalence of associated risk factors such as obesity and hypertension [5]. Therefore, lifestyle interventions can be an inexpensive, preventative and adjunctive program tailored to the individual patient and may affect outcomes. The majority of studies investigating physical activity and renal cancer outcomes noted a benefit to exercise in either incidence or mortality of renal cancer. We identified a total of 10 articles relevant to physical activity and the effect on RCC. Four studies were longitudinal studies investigating the association of physical activity and incident kidney cancer [7,10,11,12,13,15]. Overall, there is not an obvious trend with exercise and indecent renal cancer. Only 2 studies concluded a protective effect, one of which only showed the protective effect in women [12,13]. Three studies reported a relative risk ranging from 0.66 to 0.87 comparing the highest level of exercise to low, leisure, or regular daily activity [11,12]. Any activity level seemed to have a small protective effect in all studies. Only 1 study obtained a hazard ratio (HR) describing a sitting time more than 9 hours compared to less than 3 hours was associated with an increase in incident renal cancer (OR 1.11) [10]. Washio et al. [15] examined the occupations of 46 kidney cancer deaths noting reduced HR when their work was associated with more standing and moving in a Japan Collaborative Cohort Study for Evaluation of Cancer Risk. However, Arem et al. [7] noted that increasing exercise was associated with increased risk of kidney cancer-specific mortality in the AARP Diet and Health Study cohort. Our findings align with JACC study rather than the AARP.
Case control studies examining the association of kidney cancer and physical activity utilized various descriptions of physical activity, such as a level of activity (low, medium, high), activities per week, or quartiles [16,17,18,19,20]. Most studies show a protective effect of exercise reducing kidney cancer prevalence in general. One study noted a protective effect only in men, likely due to the higher incidence in this population [20]. Only 1 study investigated renal cancer-specific mortality noting exercise correlating with a reduced mortality rate [15]. This study was a small study carried out in Japan. Our manuscript will be the largest study to date investigating physical activity and RCC-specific mortality.
The World Cancer Research Fund estimates that one-quarter to one-third of new cancer cases in the US in 2015 will be related to increased adiposity or obesity, physical inactivity, and poor nutrition [21]. Preventive behavioral interventions, including improved diet and increased activity, may attenuate social and economic burden of some cancers [22]. Addressing weight management in RCC survivors is important given the potential link between increased adiposity and recurrence [23].
Additionally, since most of these patients undergo surgical removal of all or part of the affected kidney, the presence of weight-related comorbidities such as obesity, diabetes, and hypertension can accelerate the process of renal decline and renal failure. Obesity is a risk factor for chronic kidney disease (CKD), progression of CKD, and end-stage renal disease [24]. Controlling for other factors, obese patients tend to have higher risk of CKD even after partial nephrectomy [25]. A meta-analysis of the benefits of intentional weight loss in patients with CKD and glomerular hyperfiltration showed that nonsurgical weight loss reduced proteinuria and blood pressure [26]. Exercise also benefits those who are already on the spectrum of CKD [27].
For the majority of Americans who do not smoke, the most important behaviors to reduce cancer risk are to maintain a healthy weight, be physically active on a regular basis, and eat a mostly plant-based diet, consisting of a variety of vegetables and fruit, whole grains, and limited amounts of red and processed meat [28]. While epidemiologic studies strongly support the protective association of these lifestyle factors on cancer prevention, studies specific to RCC are less common. If successful, we may identify a potential means of decreasing the morbidity and mortality of RCC through accomplishing behavior change aimed at modifiable risk factors of disease. If successful, the strategy outlined in this proposal represents a relatively low-cost intervention that could beneficially impact adherence to lifestyle goals and potentially reduce morbidity and mortality from RCC.
Limitations of the study largely stem from bias of survey data. Surveys only provide data for that time point and there can be variation in answers depending on question format, which did have different versions over the years. Personal in-home surveyors conducted the survey at the time of the US Census, which provides complete data and accurate collection and limits selection bias. Our study also lacks the specific grade and stage of kidney cancer. We are unable to investigate specific comorbidities that may have a confounding effect on obesity and exercise. Only cause of death data was available for linkage, which is subject to documentation error or multiple causes. The cause of death specifically from kidney cancer may have less contributing comorbidities and likely strengthens the associations we identified. Moreover, due to small numbers there may be interactions between smoking, obesity, and exercise that we are unable to identify or analyze separately.
In conclusion, despite limitations of survey data we present the largest study to date investigating physical activity and RCC-specific mortality. We show there may be benefits to exercise program in preventing kidney cancer mortality. Further studies will need to provide the more specific recommendations regarding the type of exercise and length of time performed. We also do not address physical activity for the specific purpose of maintaining a healthy weight, which may be important. We propose utilizing exercise programs not only to prevent cancer, but may be proposed as an adjunctive therapy for patients on active surveillance for their small renal mass. Further study is warranted on this important public health topic.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society Cancer Facts & Figures 2016. Atlanta, Ga, 2016.
- 5.Lotan Y, Karam JA, Shariat SF, Gupta A, Roupret M, Bensalah K, Margulis V. Renal cell carcinoma risk estimates based on participants in the prostate, lung, colorectal, and ovarian cancer screening trial and national lung screening trial. Urol Oncol. 2016;34:167. doi: 10.1016/j.urolonc.2015.10.011. e9–16. [DOI] [PubMed] [Google Scholar]
- 6.Lowrance WT, Thompson RH, Yee DS, Kaag M, Donat SM, Russo P. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. BJU Int. 2010;105:16–20. doi: 10.1111/j.1464-410X.2009.08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arem H, Moore SC, Park Y, Ballard-Barbash R, Hollenbeck A, Leitzmann M, Matthews CE. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. Int J Cancer. 2014;135:423–431. doi: 10.1002/ijc.28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X, Lee JL, Rini BI, Srinivas S, Bjarnason GA, Ernst S, Wood LA, Vaishamayan UN, Rha SY, Agarwal N, Yuasa T, Pal SK, Bamias A, Zabor EC, Skanderup AJ, Furberg H, Fay AP, de Velasco G, Preston MA, Wilson KM, Cho E, McDermott DF, Signoretti S, Heng DY, Choueiri TK. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol. 2016;34:3655–3663. doi: 10.1200/JCO.2016.66.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishihara R, VanderWeele TJ, Shibuya K, Mittleman MA, Wang M, Field AE, Giovannucci E, Lochhead P, Ogino S. Molecular pathological epidemiology gives clues to paradoxical findings. Eur J Epidemiol. 2015;30:1129–1135. doi: 10.1007/s10654-015-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George SM, Moore SC, Chow WH, Schatzkin A, Hollenbeck AR, Matthews CE. A prospective analysis of prolonged sitting time and risk of renal cell carcinoma among 300,000 older adults. Ann Epidemiol. 2011;21:787–790. doi: 10.1016/j.annepidem.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahabir S, Leitzmann MF, Pietinen P, Albanes D, Virtamo J, Taylor PR. Physical activity and renal cell cancer risk in a cohort of male smokers. Int J Cancer. 2004;108:600–605. doi: 10.1002/ijc.11580. [DOI] [PubMed] [Google Scholar]
- 12.Moore SC, Chow WH, Schatzkin A, Adams KF, Park Y, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Physical activity during adulthood and adolescence in relation to renal cell cancer. Am J Epidemiol. 2008;168:149–157. doi: 10.1093/aje/kwn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007;166:932–940. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 14.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 15.Washio M, Mori M, Khan M, Sakauchi F, Watanabe Y, Ozasa K, Hayashi K, Miki T, Nakao M, Mikami K, Ito Y, Kubo T, Wakai K, Tamakoshi A. Diabetes mellitus and kidney cancer risk: the results of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study) Int J Urol. 2007;14:393–397. doi: 10.1111/j.1442-2042.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 16.Shu X, Lin J, Wood CG, Tannir NM, Wu X. Energy balance, polymorphisms in the mTOR pathway, and renal cell carcinoma risk. J Natl Cancer Inst. 2013;105:424–432. doi: 10.1093/jnci/djt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavani A, Zucchetto A, Dal Maso L, Montella M, Ramazzotti V, Talamini R, Franceschi S, La Vecchia C. Lifetime physical activity and the risk of renal cell cancer. Int J Cancer. 2007;120:1977–1980. doi: 10.1002/ijc.22438. [DOI] [PubMed] [Google Scholar]
- 18.Pan SY, DesMeules M, Morrison H, Wen SW. Obesity, high energy intake, lack of physical activity, and the risk of kidney cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2453–2460. doi: 10.1158/1055-9965.EPI-06-0616. [DOI] [PubMed] [Google Scholar]
- 19.Chiu BC, Gapstur SM, Chow WH, Kirby KA, Lynch CF, Cantor KP. Body mass index, physical activity, and risk of renal cell carcinoma. Int J Obes (Lond) 2006;30:940–947. doi: 10.1038/sj.ijo.0803231. [DOI] [PubMed] [Google Scholar]
- 20.Menezes RJ, Tomlinson G, Kreiger N. Physical activity and risk of renal cell carcinoma. Int J Cancer. 2003;107:642–646. doi: 10.1002/ijc.11427. [DOI] [PubMed] [Google Scholar]
- 21.World Cancer Research Fund International Diet, Nutrition, Physical Activity and Kidney Cancer. 2015 [Google Scholar]
- 22.Ekelund U, Ward HA, Norat T, Luan J, May AM, Weiderpass E, Sharp SJ, Overvad K, Ostergaard JN, Tjonneland A, Johnsen NF, Mesrine S, Fournier A, Fagherazzi G, Trichopoulou A, Lagiou P, Trichopoulos D, Li K, Kaaks R, Ferrari P, Licaj I, Jenab M, Bergmann M, Boeing H, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Peeters PH, Monnikhof E, Bueno-de-Mesquita HB, Quiros JR, Agudo A, Sanchez MJ, Huerta JM, Ardanaz E, Arriola L, Hedblad B, Wirfalt E, Sund M, Johansson M, Key TJ, Travis RC, Khaw KT, Brage S, Wareham NJ, Riboli E. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC) Am J Clin Nutr. 2015;101:613–621. doi: 10.3945/ajcn.114.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park YH, Lee JK, Kim KM, Kook HR, Lee H, Kim KB, Lee S, Byun SS, Lee SE. Visceral obesity in predicting oncologic outcomes of localized renal cell carcinoma. J Urol. 2014;192:1043–1049. doi: 10.1016/j.juro.2014.03.107. [DOI] [PubMed] [Google Scholar]
- 24.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 25.Richards KA, Negron E, Cohn JA, Steinberg Z, Eggener SE, Shalhav AL. The impact of body mass index on renal functional outcomes following minimally invasive partial nephrectomy. J Endourol. 2014;28:1338–1344. doi: 10.1089/end.2014.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25:1173–1183. doi: 10.1093/ndt/gfp640. [DOI] [PubMed] [Google Scholar]
- 27.Aucella F, Battaglia Y, Bellizzi V, Bolignano D, Capitanini A, Cupisti A. Physical exercise programs in CKD: lights, shades and perspectives. J Nephrol. 2015;28:143–150. doi: 10.1007/s40620-014-0169-6. [DOI] [PubMed] [Google Scholar]
- 28.Weir HK, Thompson TD, Soman A, Moller B, Leadbetter S, White MC. Meeting the healthy people 2020 objectives to reduce cancer mortality. Prev Chronic Dis. 2015;12:E104. doi: 10.5888/pcd12.140482. [DOI] [PMC free article] [PubMed] [Google Scholar]