Abstract
Objective
A large goiter may cause compression of the trachea. The aim of this study was to investigate the impact of thyroidectomy on tracheal anatomy and airflow and to correlate this with changes in health-related quality of life (HRQoL) in patients with benign nodular goiter.
Methods
Magnetic resonance images of the neck and respiratory flow-volume curves, including both inspiration and expiration, were performed prior to and 6 months following surgery. HRQoL was measured by selected scales from the thyroid-specific patient-reported outcome (ThyPRO). Cohen's effect size (ES) was calculated as mean change divided by standard deviation at baseline. ES of 0.2–0.5 were defined as small, 0.5–0.8 as moderate, and values >0.8 as large.
Results
Sixty-five patients completed all examinations. Median goiter volume was 58 mL (range, 14–642 mL) before surgery with surgical removal of a median of 43 g (range, 8–607 g). Six months after surgery, tracheal narrowing and deviation were diminished by a median of 26% (ES = 0.67, p < 0.001) and 33% (ES = 0.61, p < 0.001), respectively. Correspondingly, each 10% decrease in goiter volume resulted in 1.0% less tracheal narrowing (p < 0.001). Concomitantly, a small improvement was seen in forced inspiratory flow at 50% of forced vital capacity (ES = 0.32, p < 0.001). A reduction in tracheal narrowing was associated with improvements in the Impaired Daily Life scale (0.33 points per 1% decrease in tracheal narrowing, p = 0.03) of the ThyPRO questionnaire.
Conclusions
In patients with symptomatic benign nodular goiter, thyroidectomy resulted in substantial improvements in tracheal anatomy and improvements in inspiratory flow, which were followed by gains in HRQoL. This information is pertinent when counseling patients before choice of treatment.
Keywords: Goiter, Thyroidectomy, Tracheal anatomy, Airflow, Upper airway obstruction, Respiratory function
Introduction
Benign nodular goiter is common in the adult population [1]. While iodization programs and alterations in smoking behavior have reduced the impact of the major environmental triggers, awareness of therapeutic modalities remains important [5], especially in genetically susceptible individuals [2, 3, 4]. The treatment strategy depends on a number of factors, including risk or fear of malignancy, as well as patient discomfort and preference, with the overall goal of improving health-related quality of life (HRQoL). Current treatment options comprise surgery [6], shown to substantially improve HRQoL [7], radioiodine [8, 9], ethanol sclerotherapy [10, 11], and ultrasound-guided interventional ablation, with laser or radiofrequency as the most used modalities [5, 11, 12].
A goiter may cause compression of the trachea and the surrounding structures, and can occasionally lead to acute respiratory insufficiency [13, 14, 15, 16]. Nevertheless, due to its slow development and patient adaptation, tracheal compression often remains unrecognized [13, 17, 18]. Several studies have shown that thyroidectomy improves tracheal airflow [19, 20]. However, with the exception of case studies reporting the risk of tracheomalacia and collapsed airways following thyroidectomy [20, 21, 22], no data are available on the change in tracheal anatomy after surgery [6] or on how this affects HRQoL.
As for tracheal parameters, the therapy options for goiter reduction can be expected to differ in efficacy, due to differences in, for example, magnitude of goiter reduction, relief of tracheal compression, and amount of posttreatment fibrosis. Therefore, the choice of the optimal treatment for the individual patient is not straightforward. The aim of this study was to investigate, longitudinally, the effect of thyroidectomy on tracheal anatomy and tracheal airflow in patients with benign nodular goiter, and to correlate these changes with HRQoL.
Materials and Methods
Participants
In our tertiary referral center, consecutive benign nodular goiter patients planned for thyroid lobectomy or total thyroidectomy were asked to participate in the study. Nodular goiter was defined as the presence, at ultrasound investigation, of an enlarged thyroid gland (thyroid volume >18 mL in women and >25 mL in men [23]) with 1 or more nodules. Serum levels of total thyroxine (T4) and total triiodothyronine (T3) had to be within the normal range (67–134 nmol/L and 1.35–2.33 nmol/L, respectively), but both subclinically hypo- and hyperthyroid patients were eligible. Patients were referred from a geographically well-defined catchment area of 1.2 million inhabitants and were included from November 1, 2014 to April 30, 2016. They were followed up for 6 months with the last visit on March 2, 2017. Exclusion criteria were (1) previous surgery to the neck; (2) suspicion of thyroid cancer, as these patients follow a fast track program [24]; (3) age below 20 years, as the trachea still develops in young men until this age [25], or age above 80 years; and (4) neuromuscular diseases including diabetes mellitus.
Assessment of Thyroid and Tracheal Anatomy
We performed magnetic resonance imaging (MRI) of the neck (Achieva D-stream; Phillips, Eindhoven, The Netherlands) in both sagittal and horizontal planes, from the epiglottis to the base of the carina. This was carried out a median of 13 days (range, 1–90) before and a median of 180 days (range, 148–210) after surgery (equaling 6 months). T1- and T2-weighted images were constructed at a slide thickness of 7 mm while the scanner operated at 1.5 T. Repetition time was 680 ms, echo time 12 ms, and completion time 12–15 min. Patients were instructed to avoid swallowing during image construction.
To estimate the volumes of the trachea (from the epiglottis to the carina) and the thyroid gland, the cross-sectional areas of the interior trachea and the thyroid, respectively, were manually traced at each horizontal slide and multiplied with the slide thickness. From these images, the smallest cross-sectional area of the trachea (SCAT) was identified. Next, this area was compared to the cross-sectional area of the trachea 2 cm above the carina (CCAT), as this tracheal parameter is considered constant [25]. Tracheal narrowing was assessed as the (CCAT-SCAT)/CCAT ratio, reflecting possible upper airway obstruction. The maximum tracheal encirclement by the thyroid in degrees, maximum tracheal deviation from the midline, and maximum horizontal diameter of the largest thyroid nodule were assessed. Finally, the cervical or substernal goiter position was assessed according to the clinical definition [26], requiring a portion of the goiter to be permanently retrosternal in a nonhyperextended neck.
Respiratory Function
Flow-volume loops were recorded before and 6 months after surgery, using a precision pneumotachograph (MasterScreen PFT; Jaeger/CareFusion, Hoechberg, Germany). The participants were positioned in a chair with lumbar support and asked not to flex or extend the neck. Each patient performed a forced maximal expiration, immediately followed by a forced maximal inspiration. The procedure was repeated at least 3 times. Medication for pulmonary disease (used by 4 patients) was allowed up to 5 h prior to the examination. From the flow-volume curves the forced vital capacity (FVC), forced expiratory volume in the first second of forceful exhalation (FEV1), peak flow (PEF), forced expiratory flow at 50% of FVC (FEF50%), forced inspiratory flow at 50% of FVC (FIF50%), FEV1/PEF, and FEF50%/FIF50% parameters were obtained and evaluated. All data were entered twice manually and compared for incongruence using REDCap® based at Vanderbilt University Medical Center [27].
Health-Related Quality of Life
Changes in HRQoL were measured by the thyroid-related patient-reported outcome (ThyPRO) instrument, recently recommended as the only validated tool for evaluating HRQoL in patients with benign thyroid diseases [28]. The questionnaire has been extensively validated for use in multiple languages, including Danish [29, 30, 31]. Individual scales of the questionnaire can be selected for measuring specific areas of interest [32]. We therefore selected the Goiter Symptom, Tiredness, Anxiety, and Impaired Daily Life scales as relevant in this setting. The reduced version of the questionnaire thus consisted of 31 items, and the answer to each item was rated on a 5-point Likert scale from 0 (not at all) to 4 (very much), referring to the last 4 weeks. Each scale was scored as a summary score (with reversal of positively formulated items) and linearly transformed to a range of 0–100 points, with higher scores indicating poorer health status. The questionnaire was administered to the patients at the initial consultation and at the day of follow-up, without supervision from health care personnel.
Some of the presented HRQoL data have previously been included in our recent study, where HRQoL after thyroidectomy was the focus [7].
Surgical Procedure
A consultant in head and neck surgery performed a total extracapsular thyroidectomy or a lobectomy, applying the same standardized technique in all patients. The procedure was performed under general anesthesia using the NIM EMG tube (Medtronic, USA) for monitoring of the recurrent laryngeal nerve during surgery. In the absence of complications, patients were discharged on the first postoperative day after lobectomy. On the first day after total thyroidectomy, 100 µg of levothyroxine (LT4) was administered, and calcium blood levels were monitored daily. These patients were discharged when ionized calcium levels approached the normal range, usually within 48 h after surgery. The initial postsurgical follow-up was 2–4 weeks after surgery. The second follow-up, including determination of the thyroid function, was 6–8 weeks after surgery. If necessary, the LT4 dose was adjusted, aiming at euthyroidism (i.e., serum TSH within the reference range).
The study was approved by The Regional Scientific Ethics Committee for Southern Denmark (S-20130096) and registered at the Danish Data Protection Agency and at www.clinicaltrial.gov (NCT03072654).
Statistics
A paired t test was used to compare tracheal, respiratory, and ThyPRO parameters before and after surgery. Some variables (i.e., thyroid volume and FEF50%/FIF50%) were positively skewed and therefore logarithmically transformed to adjust for nonnormality. Effect size (ES) was estimated for the 1-group sample using baseline standard deviation (SD). It was calculated as mean change/SDBaseline. In accordance with Cohen [33], ES of 0.2–0.5 were defined as small, 0.5–0.8 as moderate, and values >0.8 as large.
Multiple linear regression analyses were used for evaluating the effect of age, sex, and thyroid volume on the preoperative tracheal parameters. Goiter location (cervical/substernal) was included in the initial model, but it had no independent effect and was therefore excluded in the final model. Multiple regression analyses were also used to assess the relation between the baseline parameters and the changes in the tracheal dimensions and respiratory function after surgery. In addition, changes in ThyPRO scales were compared with changes in parameters of tracheal anatomy and airflow. Changes in serum TSH and the need for LT4 substitution after surgery showed no impact on the respiratory function, and were therefore omitted in the final model.
The sample size was calculated to 58 patients based on paired samples, with changes in SCAT of 0.15 ± 0.4 cm2 [34, 35, 36]. To compensate for a possible loss to follow-up, we included 75 participants.
Results
Patient Characteristics
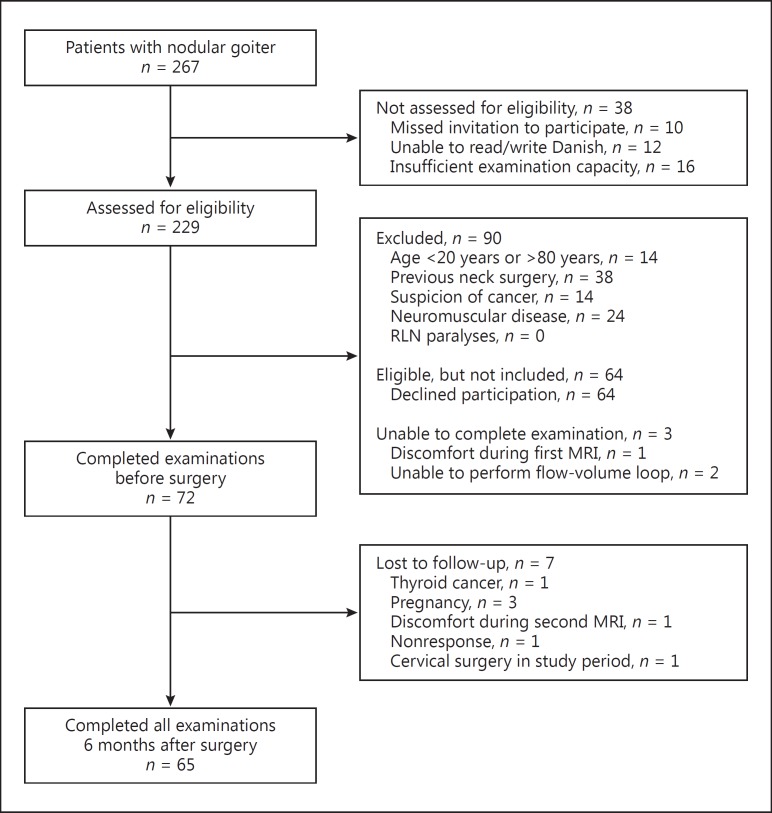
Out of 267 consecutive nodular goiter patients, 229 were assessed for eligibility. A detailed account of the flow leading to inclusion is provided in Figure 1. Sixty-five of 72 (90%) included patients completed the study. The mean age of included patients was 55 ± 13 years versus 55 ± 16 years in the nonincluded patients (n = 195, p = 0.64) (Table 1). Seventy-four percent of the included patients were females compared to 87% of the nonincluded patients (p = 0.049).
Fig. 1.
Flow diagram of patient inclusion and follow-up. RLN, recurrent laryngeal nerve.
Table 1.
Sociodemographic, clinical, and surgical characteristics for patients with benign nodular goiter (n = 65)
| Age, years | 55±13 |
| Sex | |
| Female | 48 (74) |
| Male | 17 (26) |
| Goiter location | |
| Cervical | 51 (78) |
| Intrathoracic | 14 (22) |
| Largest nodule, mm | 33 (9–101) |
| Pre-existing pulmonary disease | |
| Yes | 4 (6) |
| No | 61 (94) |
| Previous radioiodine | |
| Yes | 6 (9) |
| No | 59 (91) |
| Surgery | |
| Total thyroidectomy | 20 (31) |
| Thyroid lobectomy | 45 (69) |
| Mass of removed goiter, g | 43 (8–607) |
| Thyrotropin | |
| Before surgery, mU/L | 0.98 (0.02–3.90) |
| After surgery, mU/L | 2.35 (0.22–10.60) |
| Postoperative LT4 | |
| Total thyroidectomy | 20 (100) |
| Thyroid lobectomy | 7 (16) |
| RLN paralyses | |
| 6 months after surgery | 0 (0) |
| Permanent hypocalcemia | |
| 6 months after surgery | 1 (2) |
Values are n (%), mean ± SD, or median (range), as appropriate. RLN, recurrent laryngeal; LT4, levothyroxine.
Before surgery, the median goiter volume, as assessed by MRI, was 58 mL (range, 14–642 mL) (Table 2), and a median of 43 g (range, 8–607 g) was surgically removed. Men had larger goiters compared to women (mean difference, 135 mL, p < 0.001), but there was no gender difference in the distribution of surgical procedures.
Table 2.
Thyroid, tracheal, and airflow parameters for patients with benign nodular goiter before and after surgery (n = 65)
| Parameters | Cervical goiter (n = 51) |
Substernal goiter (n = 14) |
Overall (n = 65) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before surgery | after surgery | p value | effect size | before surgery | after surgery | p value | effect size | be fore surgery | after surgery | % change | p value | effect size | |
| Thyroid volume, mL | 52 | 12 | <0.001 | 1.22 | 242 | 0 | <0.001 | 1.70 | 58 | 10 | 77 | <0.001 | 0.76 |
| (14–232) | (0–49) | (99–642) | (0–70) | (14–642) | (0–70) | ||||||||
| Thyroid tracheal | 300 | 138 | <0.001 | 7.96 | 305 | 0 | <0.001 | 6.90 | 300 | 133 | 59 | <0.001 | 7.22 |
| encirclement, ° | (253–353) | (0–282) | (226–360) | (0–169) | (226–360) | (0–282) | |||||||
| Tracheal narrowing, % | 35 | 29 | 0.001 | 0.49 | 62 | 30 | <0.001 | 1.69 | 39 | 29 | 26 | <0.001 | 0.67 |
| (0–59) | (0–61) | (40–90) | (7–54) | (0–90) | (0–54) | ||||||||
| Tracheal deviation, mm | 10±5 | 7±4 | <0.001 | 0.54 | 20±10 | 9±5 | <0.001 | 1.15 | 12±7 | 8±4 | 33 | <0.001 | 0.61 |
| Tracheal volume, cm3 | 17.7±5.3 | 19.0±5.5 <0.001 | 0.23 | 19.9±5.4 | 25.1±7.0 | <0.001 | 0.96 | 18.2±5 | 20.3±6 | 10 | <0.001 | 0.39 | |
| SCAT, cm2 | 1.6±0.5 | 1.8±0.5 | <0.001 | 0.48 | 1.1±0.6 | 2.0±0.6 | <0.001 | 1.74 | 1.5±0.5 | 1.8±0.5 | 17 | <0.001 | 0.73 |
| CCAT, cm2 | 2.4±0.6 | 2.5±0.7 | 0.06 | 0.15 | 3.0±0.8 | 3.0±0.7 | 0.49 | 0.08 | 2.5±0.7 | 2.6±0.7 | 3 | 0.17 | 0.09 |
| FVC, L | 4.1±0.9 | 4.0±0.9 | 0.001 | 0.10 | 3.7±0.9 | 3.7±0.8 | 0.74 | 0.02 | 4.0±0.9 | 3.9±0.9 | 2 | 0.004 | 0.08 |
| FEVb L | 3.2±0.7 | 3.1±0.7 | 0.05 | 0.08 | 2.8±0.7 | 2.8±0.7 | 0.39 | 0.05 | 3.1±0.7 | 3.0±0.7 | 0 | 0.14 | 0.06 |
| PEF, L/min | 7.9±2.1 | 8.0±1.8 | 0.60 | 0.05 | 5.9±1.9 | 7.6±2.0 | 0.004 | 0.92 | 7.5±2.2 | 8.0±1.9 | 2 | 0.03 | 0.19 |
| FEF50%, L/min | 3.7±1.5 | 3.5±1.3 | 0.14 | 0.09 | 3.0±1.2 | 2.7±1.1 | 0.01 | 0.22 | 3.5±1.4 | 3.4±1.3 | 7 | 0.03 | 0.11 |
| FIF50%, L/min | 5.1±1.3 | 5.5±1.5 | 0.02 | 0.31 | 3.6±1.1 | 4.6±1.3 | 0.02 | 0.98 | 4.8±1.4 | 5.3±1.5 | 7 | <0.001 | 0.32 |
| FIF50%/FIF50% | 0.7±0.3 | 0.7±0.3 | 0.01 | 0.21 | 0.9±0.4 | 0.6±0.3 | 0.006 | 0.72 | 0.8±0.3 | 0.7±0.3 | 13 | 0.001 | 0.29 |
| FEV1/PEF | 6.8±1.5 | 6.5±1.0 | 0.07 | 0.21 | 8.1±1.8 | 6.3±1.5 | 0.007 | 1.03 | 7.1±1.6 | 6.5±1.2 | 2 | 0.002 | 0.39 |
Values are median (range) or mean ± SD, unless otherwise specified. Comparison of values before and after surgery, using a paired t test. Median percent change before and 6 months after surgery. Effect sizes 0.2–0.5 were defined as small, 0.5–0.8 as moderate, and >0.8 as large. Tracheal narrowing (possible upper airway obstruction) is defined as (CCAT-SCAT)/CCAT, where SCAT is the smallest cross-sectional area of the trachea, and CCAT is the cross-sectional area 2 cm above the carina. FVC, forced vital capacity; FEV1, forced expiratory volume in the first second of forceful exhalation; PEF, peak flow; FEF50%, forced expiratory flow at 50% of FVC; FIF50%, forced inspiratory flow at 50% of FVC.
Fourteen of 65 (22%) patients had a substernal goiter, and these differed from patients with cervical goiters by having significantly larger goiter volumes (median, 242 mL [99–642 mL] vs. 52 mL [14–232 mL], p < 0.001), being older (median, 66 years [46–77 years] vs. 52 years [20–77 years], p = 0.003), and more often being male (50 vs. 20%, p = 0.02).
Tracheal Anatomy
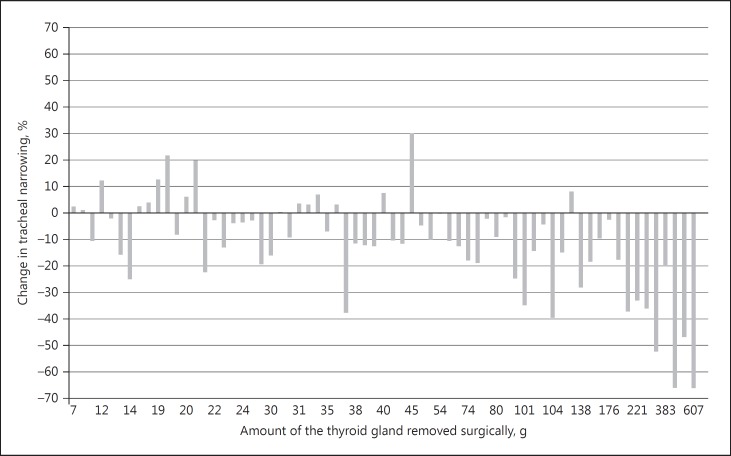
Prior to surgery, the median tracheal narrowing was 39% (0–90%) compared to 29% (0–54%) after surgery, reflecting a postsurgical increase in SCAT (Table 2). The individual changes are shown in Figure 2. In a multiple regression analysis, tracheal narrowing at baseline correlated strongly with initial goiter volume (1.5% increase in tracheal narrowing per 10% increase in goiter volume, p < 0.001). The SCAT and tracheal deviation also correlated with the goiter volume, with a 3.5-mm2 decrease in SCAT (p < 0.001), and a 0.4-mm increase in tracheal deviation (p < 0.001) per 10% increase in goiter volume. Tracheal deviation also increased with age (0.1 mm/year, p = 0.046). The tracheal volume did not significantly correlate with presurgical goiter volume. A substernal location correlated strongly with both the largest thyroid nodule diameter and the goiter volume. The median tracheal encirclement by the thyroid was 300° (range, 226–360°) before surgery, and was most pronounced among patients with the largest goiters (0.8°/mL, p = 0.01).
Fig. 2.
The distribution of the amount of the thyroid gland removed surgically and the postsurgical change in tracheal narrowing (n = 65). Tracheal narrowing (degree of upper airway obstruction) is defined as the (CCAT-SCAT)/CCAT ratio, where SCAT is the smallest cross-sectional area of the trachea, and CCAT is the cross-sectional area 2 cm above the carina.
After surgery, tracheal narrowing (ES = 0.67, p < 0.001), SCAT (ES = 0.73, p < 0.001), and tracheal deviation (ES = 0.61, p < 0.001) improved moderately, whereas tracheal volume only underwent a small increase (ES = 0.39, p < 0.001). Patients with a substernal goiter experienced the largest improvements in tracheal parameters (Table 2).
Using multiple regression analyses, the improvements in tracheal parameters all correlated strongly with the goiter shrinkage. Thus, each 10% decrease in goiter volume resulted in an increase of 1.9% in SCAT (p < 0.001), a decrease of 1.0% in tracheal narrowing (p < 0.001), a 0.3-mm decrease in tracheal deviation (p < 0.001), and an increase of 0.13 mL in tracheal volume (p < 0.001). The volume of the remaining thyroid tissue after hemithyroidectomy had no influence on the results.
Tracheal Airflow
Tracheal narrowing correlated significantly with the initial respiratory parameters, as a 1% increase in tracheal narrowing led to a decrease of 0.03 L/min in PEF (p = 0.03) and a decrease of 0.02 L/min in FIF50% (p = 0.01). The pronounced improvements in tracheal anatomy following surgery were reflected in tracheal airflow changes (Table 2). A small, but highly significant, improvement was found in FIF50% (ES = 0.32), whereas FVC (ES = 0.08) was almost unaltered. Small declines in the FEF50%/FIF50% (ES = 0.29) and FEV1/PEF (ES = 0.39) ratios were seen. Improvements in tracheal narrowing correlated with improvements in PEF, since a 1% reduction in tracheal narrowing was followed by an increase of 0.03 L/min in PEF (p = 0.003), and a 0.03 decrease in the FEV1/PEF ratio (p = 0.01). Nineteen of 65 patients had an FEV1/PEF ratio >8.0, indicating the presence of upper airway obstruction [30]. This number was significantly reduced to 7 patients following surgery (p = 0.009).
Health-Related Quality of Life
The Goiter Symptom score showed a large improvement from 40 ± 21 to 10 ± 11 points (ES = 1.37, p < 0.001) 6 months after surgery. The Tiredness scale and the Anxiety scale changed moderately after surgery from 48 ± 25 to 35 ± 24 points (ES = 0.52, p < 0.001) and from 20 ± 21 to 7 ± 12 points (ES = 0.59, p < 0.001), respectively. The Impaired Daily Life scale improvement from 13 ± 19 to 7 ± 16 points was insignificant (ES = 0.29, p = 0.48). However, using multiple regression analyses, the relief in tracheal narrowing was positively correlated with improvement in the Impaired Daily Life scale, corresponding to an additional decrease of 0.33 points in the Impaired Daily Life scale per 1% less tracheal narrowing (p = 0.03) after surgery. This reflects that patients with the largest compression also experience the most pronounced improvements in Impaired Daily Life. Tracheal and airflow baseline parameters did not correlate with HRQoL, nor did airflow changes following surgery.
Discussion
This is the first study demonstrating considerable postsurgical tracheal decompression and improvement of the tracheal anatomy, as assessed by MRI. These improvements correlated strongly with the initial goiter volume and with the volume of the removed benign thyroid tissue, as patients with the largest goiters experienced the most pronounced improvements in both tracheal anatomy and airflow after thyroidectomy.
In parallel with the decompression of the trachea following surgery, the tracheal airflow also improved, especially in patients with large (substernal) goiters. According to Poisseulle's law, air flow is four-fold proportional to the tracheal radius. Any expansion of the tracheal lumen will thus lead to a considerable improvement in air flow rate. However, a negative transmural pressure gradient across the tracheal wall may lead to a partial collapse of the tracheal rings encircling a stenosis, which is a risk mainly during inspiration [6]. Our results are fully in accordance with this physical principle, since the tracheal decompression obtained by surgery resulted in an improvement, especially in the inspiratory airflow, while the expiratory parameter FEF50% in fact decreased minutely.
Patients experienced significant improvements in HRQoL after surgery. However, the improvement in the Impaired Daily Life scale was associated only with the decompression of the trachea, and much less with the airflow improvement. This divergent finding is puzzling, considering the fact that the luminal area of the trachea is a surrogate parameter in this context, whereas a compromised airflow more directly gives rise to symptoms, and potentially affects the HRQoL. One explanation may be that the ES related to the changes in the airflow parameters were of much smaller magnitudes than those found for the tracheal dimensions. In addition, many other factors - beyond the physical dimensions of the thyroid gland and the related neck structures - are involved in the increase in HRQoL experienced by patients undergoing thyroid surgery, as we have recently shown [7].
Many of our patients demonstrated tracheal compression on MRI, and the improvements in tracheal narrowing correlated well with a reduced FEV1/PEF ratio used as an indirect measure of upper airway obstruction. However, these results were not consistent with a fixed criterion for upper airway obstruction (FEV1/PEF >8 [37]), as 7 patients still met this criterion after surgery, in contrast to 19 patients before surgery. This ratio is thought to be an important indicator of upper airway obstruction in patients with goiter [17, 38]. However, the criterion is based on nonphysiological data using mouth pieces with very small orifices equaling a SCAT of 0.5 cm2 [39] - only found in our patients with goiters above 300 mL. Therefore, the formal criterion for upper airway obstruction, as previously defined, might not be applicable to the majority of patients with symptomatic nodular goiter.
Changes in tracheal parameters and respiratory function have also been investigated using other methods for goiter shrinkage. Radioiodine treatment, with or without recombinant human TSH (rhTSH) prestimulation, showed improvements in SCAT of 4–33% at 6–12 months following therapy [8, 34, 35, 36]. These figures are comparable to those obtained in the present surgical series, demonstrating a median improvement in SCAT of 17% in the entire cohort. On the other hand, our cohort consists of patients with much smaller goiters than those included in the radioiodine studies [34, 35, 36]. In view of the 23–40% goiter shrinkage following radioiodine [8, 34, 35, 40], which could be further increased to 53% with rhTSH prestimulation [33], this difference in efficacy is well explained [33, 34, 40].
While the strengths of our study include a reasonable population size (based on power calculations), consecutive patients, and well-validated techniques for the evaluation of the end points, some limitations also need to be addressed. First, our study was conducted in a tertiary referral center. Although our center receives patients with symptomatic goiter from a large region in an unselected manner, some selection bias is inevitable, as also evidenced by the inclusion of just 72 of the initial 267 patients with symptomatic nodular goiter. A comparison of removed thyroid volumes between included and nonincluded patients would have been optimal. However, we had no access to such data from the majority of nonincluded patients. Second, this being an observational study, the lack of a control group hinders an evaluation of the degree to which the pertinent variables were normalized by surgery. However, although we do not know the natural history, had these patients not been operated on, previous studies suggest annual goiter growth rates of up to 20% [5]. Patients with diabetes were excluded as patients also participated in other substudies of esophageal motility requiring normal nerve function [41]. This might have introduced bias and a reduction in external validity. However, our results are applicable to the majority of patients with nodular goiter. Finally, the manual tracing on the MRI images were not performed blindly as the study protocol requires continuously comparing images before and after surgery to assess the area of interest. The degree to which this biases our volume estimates remains unknown.
We conclude that thyroidectomy, in patients with symptomatic benign nodular goiter, yields substantial improvements in tracheal anatomy, and that this correlates with an increase in HRQoL. Improvement, although to a lesser degree, was also seen in the respiratory function, and mainly in the inspiratory parameters. Our findings are highly relevant for the dialogue with patients about the pros and cons of the available methods for goiter shrinkage.
Disclosure Statement
The study was economically supported by The Region of Southern Denmark, The Department of Clinical Research, University of Southern Denmark, and The Fund for Advancements of Medical Sciences. There are no conflicts of interest to report. L.H. was supported by an unrestricted grant from the Novo Nordisk Foundation, and S.B. was supported by grants from Odense University Hospital's Research Council.
References
- 1.Carlé A, Krejbjerg A, Laurberg P. Epidemiology of nodular goitre. Influence of iodine intake. Best Pract Res Clin Endocrinol Metab. 2014;28:465–479. doi: 10.1016/j.beem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Brix TH, Kyvik KO, Hegedus L. Major role of genes in the etiology of simple goiter in females: a population-based twin study. J Clin Endocr Metab. 1999;84:3071–3075. doi: 10.1210/jcem.84.9.5958. [DOI] [PubMed] [Google Scholar]
- 3.Brix TH, Hansen PS, Kyvik KO, Hegedus L. Cigarette smoking and risk of clinically overt thyroid disease: a population-based twin case-control study. Arch Intern Med. 2000;160:661–666. doi: 10.1001/archinte.160.5.661. [DOI] [PubMed] [Google Scholar]
- 4.Hansen PS, Brix TH, Bennedbaek FN, Bonnema SJ, Kyvik KO, Hegedus L. Genetic and environmental causes of individual differences in thyroid size: a study of healthy Danish twins. J Clin Endocr Metab. 2004;89:2071–2077. doi: 10.1210/jc.2003-031999. [DOI] [PubMed] [Google Scholar]
- 5.Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–132. doi: 10.1210/er.2002-0016. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen JR, Hegedus L, Kruse-Andersen S, Godballe C, Bonnema SJ. The impact of goitre and its treatment on the trachea, airflow, oesophagus and swallowing function. A systematic review. Best Pract Res Clin Endocrinol Metab. 2014;28:481–494. doi: 10.1016/j.beem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen JR, Watt T, Cramon P, Døssing H, Hegedüs L, Bonnema SJ, Godballe C. The quality of life after thyroidectomy in patients with nontoxic nodular goiter - a prospective cohort study. Head Neck Surg. 2017 doi: 10.1002/hed.24886. DOI: 10.1002/hed.24886. [DOI] [PubMed] [Google Scholar]
- 8.Graf H, Fast S, Pacini F, Pinchera A, Leung A, Vaisman M, Reiners C, Wemeau JL, Huysmans D, Harper W, Driedger A, de Souza HN, Castagna MG, Antonangeli L, Braverman L, Corbo R, Duren C, Proust-Lemoine E, Edelbroek MA, Marriott C, Rachinsky I, Grupe P, Watt T, Magner J, Hegedus L. Modified-release recombinant human TSH (MRrhTSH) augments the effect of 131I therapy in benign multinodular goiter: results from a multicenter international, randomized, placebo-controlled study. J Clin Endocr Metab. 2011;96:1368–1376. doi: 10.1210/jc.2010-1193. [DOI] [PubMed] [Google Scholar]
- 9.Bonnema SJ, Hegedus L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;33:920–980. doi: 10.1210/er.2012-1030. [DOI] [PubMed] [Google Scholar]
- 10.Bennedbaek FN, Karstrup S, Hegedus L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid diseases. Eur J Endocrinol. 1997;136:240. doi: 10.1530/eje.0.1360240. [DOI] [PubMed] [Google Scholar]
- 11.Gharib H, Hegedus L, Pacella CM, Baek JH, Papini E. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocr Metab. 2013;98:3949–3957. doi: 10.1210/jc.2013-1806. [DOI] [PubMed] [Google Scholar]
- 12.Papini E, Pacella CM, Hegedus L. Diagnosis of endocrine disease: thyroid ultrasound (US) and US-assisted procedures: from the shadows into an array of applications. Eur J Endocrinol. 2014:170:R133–R146. doi: 10.1530/EJE-13-0917. [DOI] [PubMed] [Google Scholar]
- 13.Menon SK, Jagtap VS, Sarathi V, Lila AR, Bandgar TR, Menon PS, Shah NS. Prevalence of upper airway obstruction in patients with apparently asymptomatic euthyroid multi nodular goitre. Indian J Endocrinol Metab. 2011;15:S127–S131. doi: 10.4103/2230-8210.83351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson M, Davis TM, Bade PG, Castelden WM. Tracheo-oesophageal compression due to rapid thyroid enlargement after radioiodine treatment. Med J Aust. 1995;162:485–486. [PubMed] [Google Scholar]
- 15.Ghai A, Hooda S, Wadherra R, Garg N. Gross tracheal deviation: airway challenges and concerns-two case reports. Acta Anaesthesiol Belg. 2011;62:203–206. [PubMed] [Google Scholar]
- 16.Shaha A, Alfonso A, Jaffe BM. Acute airway distress due to thyroid pathology. Surgery. 1987;102:1068–1074. [PubMed] [Google Scholar]
- 17.Miller MR, Pincock AC, Oates GD, Wilkinson R, Skene-Smith H. Upper airway obstruction due to goitre: detection, prevalence and results of surgical management. Q J Med. 1990;74:177–188. [PubMed] [Google Scholar]
- 18.Torchio R, Gulotta C, Perboni A, Ciacco C, Guglielmo M, Orlandi F, Milic-Emili J. Orthopnea and tidal expiratory flow limitation in patients with euthyroid goiter. Chest. 2003;124:133–140. doi: 10.1378/chest.124.1.133. [DOI] [PubMed] [Google Scholar]
- 19.Thusoo TK, Gupta U, Kochhar K, Hira HS. Upper airway obstruction in patients with goiter studies by flow volume loops and effect of thyroidectomy. World J Surg. 2000;24:1570–1572. doi: 10.1007/s002680010279. [DOI] [PubMed] [Google Scholar]
- 20.Nakadate Y, Fukuda T, Hara H, Tanaka M. Tracheomalacia after reoperation for an adenomatous goiter located in a unique position. J Anesth. 2011;25:745–748. doi: 10.1007/s00540-011-1181-9. [DOI] [PubMed] [Google Scholar]
- 21.Geelhoed GW. Tracheomalacia from compressing goiter: management after thyroidectomy. Surgery. 1988;104:1100–1108. [PubMed] [Google Scholar]
- 22.Gilfillan N, Ball CM, Myles PS, Serpell J, Johnson WR, Paul E. A cohort and database study of airway management in patients undergoing thyroidectomy for retrosternal goitre. Anaesth Intensive Care. 2014;42:700–708. doi: 10.1177/0310057X1404200604. [DOI] [PubMed] [Google Scholar]
- 23.Gutekunst R, Becker W, Hehrmann R, Olbricht T, Pfannenstiel P. Ultrasonic diagnosis of the thyroid gland (in German) Dtsch Med Wochenschr. 1988;113:1109–1112. doi: 10.1055/s-2008-1067777. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen JR, Johansen J, Gano L, Sorensen JA, Larsen SR, Andersen PB, Thomassen A, Godballe C. A “package solution” fast track program can reduce the diagnostic waiting time in head and neck cancer. Eur Arch Otorhinolaryngol. 2014;271:1163–1170. doi: 10.1007/s00405-013-2584-z. [DOI] [PubMed] [Google Scholar]
- 25.Griscom NT. CT measurement of the tracheal lumen in children and adolescents. AJR Am J Roentgenol. 1991;156:371–372. doi: 10.2214/ajr.156.2.1898817. [DOI] [PubMed] [Google Scholar]
- 26.Rios A, Rodriguez JM, Balsalobre MD, Tebar FJ, Parilla P. The value of various definitions of intrathoracic goiter for predicting intra-operative and postoperative complications. Surgery. 2010;147:233. doi: 10.1016/j.surg.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong CK, Lang BH, Lam CL. A systematic review of quality of thyroid-specific health-related quality-of-life instruments recommends ThyPRO for patients with benign thyroid diseases. J Clin Epidemiol. 2016;78:63–72. doi: 10.1016/j.jclinepi.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Watt T, Barbesino G, Bjorner JB, Bonnema SJ, Bukvic B, Drummond R, Groenvold M, Hegedüs L, Kantzer V, Lasch KE, Marcocci C, Mishra A, Netea-Maier R, Ekker M, Paunovic I, Quinn TJ, Rasmussen ÅK, Russell A, Sabaretnam M, Smit J, Törring O, Zivaljevic V, Feldt-Rasmussen U. Cross-cultural validity of the thyroid-specific quality-of-life patient-reported outcome measure, ThyPRO. Qual Life Res. 2015;24:769–780. doi: 10.1007/s11136-014-0798-1. [DOI] [PubMed] [Google Scholar]
- 30.Watt T, Hegedüs L, Groenvold M, Bjorner JB, Rasmussen AK, Bonnema SJ, Feldt-Rasmussen U. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur J Endocrinol. 2010;162:161–167. doi: 10.1530/EJE-09-0521. [DOI] [PubMed] [Google Scholar]
- 31.Watt T, Bjorner JB, Groenvold M, Rasmussen AK, Bonnema SJ, Hegedüs L, Feldt-Rasmussen U. Establishing construct validity for the thyroid-specific patient reported outcome measure (ThyPRO): an initial examination. Qual Life Res. 2009;18:483–496. doi: 10.1007/s11136-009-9460-8. [DOI] [PubMed] [Google Scholar]
- 32.Watt T, Cramon P, Hegedüs L, Bjorner JB, Bonnema SJ, Rasmussen ÅK, Feldt-Rasmussen U, Groenvold M. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J Clin Endocrinol Metab. 2014;99:3708–3717. doi: 10.1210/jc.2014-1322. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. ed 2. Hillsdale: Lawrence Erlbaum; 1988. Statistical Power Analyses for Behavioral Sciences. chapt 2. [Google Scholar]
- 34.Bonnema SJ, Bertelsen H, Mortensen J, Andersen PB, Knudsen DU, Bastholt L, Hegedus L. The feasibility of high dose iodine 131 treatment as an alternative to surgery in patients with a very large goiter: effect on thyroid function and size and pulmonary function. J Clin Endocr Metab. 1999;84:3636–3641. doi: 10.1210/jcem.84.10.6052. [DOI] [PubMed] [Google Scholar]
- 35.Huysmans DA, Hermus AR, Corstens FH, Barentsz JO, Kloppenborg PW. Large, compressive goiters treated with radioiodine. Ann Intern Med. 1994;121:757–762. doi: 10.7326/0003-4819-121-10-199411150-00005. [DOI] [PubMed] [Google Scholar]
- 36.Bonnema SJ, Nielsen VE, Boel-Jorgensen H, Grupe P, Andersen PB, Bastholt L, Hegedus L. Recombinant human thyrotropin-stimulated radioiodine therapy of large nodular goiters facilitates tracheal decompression and improves inspiration. J Clin Endocr Metab. 2008;93:3981–3984. doi: 10.1210/jc.2008-0485. [DOI] [PubMed] [Google Scholar]
- 37.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 38.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 39.Empey D. Patterns of airflow in upper airways obstruction. Thorax. 1972;27:262. doi: 10.1136/thx.27.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnema SJ, Nielsen VE, Boel-Jorgensen H, Grupe P, Andersen PB, Bastholt L, Hegedus L. Improvement of goiter volume reduction after 0.3 mg recombinant human thyrotropin-stimulated radioiodine therapy in patients with a very large goiter: a double-blinded, randomized trial. J Clin Endocrinol Metab. 2007;92:3424–3428. doi: 10.1210/jc.2007-0501. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen JR, Markoew S, Døssing H, Hegedüs L, Bonnema SJ, Godballe C. Changes in swallowing symptoms and esophageal motility after thyroid surgery - a prospective cohort study. World J Surg. 2017 doi: 10.1007/s00268-017-4247-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]