ABSTRACT
OBJECTIVE:
Healthcare systems are being challenged to manage increasing numbers of nonhealing wounds. Wound dressings are one of the first lines of defense in wound management, and numerous options exist. The oxidized regenerated cellulose (ORC)/collagen dressing may offer healthcare providers a robust and cost-effective tool for use in a variety of wounds.
DESIGN:
A multidisciplinary panel meeting was convened to discuss the use of ORC/collagen dressings in wound care and provide practice recommendations. A literature search was conducted to provide a brief review of the peer-reviewed studies published between January 2000 and March 2016 to inform the meeting.
SETTING:
A 2-day panel meeting convened in February 2017.
PARTICIPANTS:
Healthcare providers with experience using ORC/collagen dressings. This multidisciplinary panel of 15 experts in wound healing included podiatrists, wound care specialists (doctors, certified wound care nurses, and research scientists), and an orthopedist.
RESULTS:
The literature search identified 58 articles, a majority of which were low levels of evidence (69.3% were level 3 or lower). Panel members identified wound types, such as abrasions, burns, stalled wounds, diabetic foot ulcers, and pressure injuries, where ORC/collagen dressing use could be beneficial. Panel members then provided recommendations and technical pearls for the use of ORC/collagen dressings in practice. Barriers to ORC/collagen dressing use were discussed, and potential resolutions were offered.
CONCLUSIONS:
An ORC/collagen dressing can be a critical tool for clinicians to help manage a variety of wounds. Clinical and economic studies comparing standard-of-care dressings and plain collagen dressings to ORC/collagen dressings are needed.
KEYWORDS: collagen, complex wounds, dressings, oxidized regenerated cellulose, wound care, wound healing
INTRODUCTION
Healthcare systems are being challenged to manage increasing numbers of nonhealing wounds. Chronic wounds affect more than 6.5 million people in the United States.1 This trend has also been observed in other developed countries, such as Denmark (affecting an estimated 1% of the population),2 Sweden (prevalence: 2.4 per 1000 people),3 and the United Kingdom (prevalence: 3.55–3.7 per 1000 people).3 As populations increase and age, the incidence of chronic wounds is also forecasted to increase,4,5 further stressing healthcare systems and providers.
Wound healing and tissue regeneration are a complicated series of biochemical processes that create an orderly healing cascade with 4 key phases: hemostasis, inflammation, proliferation, and remodeling.6 If this process becomes unbalanced, healing stalls and results in chronic, nonhealing wounds. Acute wounds progress through healing in a predictable time frame, culminating in an epithelialized wound.7–9 Chronic wounds start out as acute wounds and, unless their chronic causes are removed, either fail to progress through the wound healing process and (most often) stall in the inflammatory phase, or proceed through the repair process without establishing a sustained anatomic and functional result.7–9
Chronic wounds are often influenced by a number of patient and/or wound environment factors. Patient factors such as age, comorbid conditions (diabetes, obesity, tobacco use, nutrition, etc), and medication use can contribute to the development of nonhealing wounds.1,10–12 However, patient assessment and active management of patient comorbidities can help mitigate some of the risk factors that impair wound healing.13 Wound factors including excess bioburden and biofilms, impaired perfusion, persistent inflammation, repeated trauma, elevated levels of reactive oxygen species and/or proteases, presence of edema and/or lymphedema, and moisture level of the wound environment also contribute to stalled wound healing.14–16 Wound bed preparation can reduce barriers to healing through the removal of devitalized tissue and edema fluid, management of infection/inflammation, rebalance of wound bed moisture, and promotion of re-epithelialization.17–19 Wound evaluation prior to and during treatment is critical in the management of wound healing. By accurately assessing the presence and degree of severity of wound healing barriers, an individualized treatment plan can optimize the potential for wound healing.20
The overall goal for wound care is the management of patients in the most cost-effective and clinically efficient manner while improving the patient’s quality of life. Numerous wound care products are commercially available to healthcare providers to achieve this goal; wound dressings are one of the first-line treatments in wound management. Collagen dressings are 1 tool for clinicians to help manage a variety of wounds. A multidisciplinary panel meeting was convened to discuss the use of oxidized regenerated cellulose (ORC)/collagen dressings in wound care and provide practice recommendations to guide care.
Definitions
Collagen is the major component of soft tissue and contains 3 proteins wrapped around each other in a triple-helix structure.21 Collagen makes up a large portion of the extracellular matrix, acts as a structural scaffold in tissues, and affects cellular functions such as differentiation, migration, and synthesis of proteins.21
While 4 types of collagen exist naturally, the types most often used in collagen dressings are type 1 or a combination of type 1 and denatured collagen.21 While there are a number of collagen-containing dressings available, most collagen dressings today contain bovine, ovine, or porcine collagen that has been treated to make it nonantigenic.
An ORC/collagen dressing (PROMOGRAN Matrix; Systagenix, an ACELITY Company, Gargrave, United Kingdom) is 45% ORC and 55% bovine collagen in a sterile, freeze-dried composite.
Oxidized regenerated cellulose/collagen/silver-ORC dressings (PROMOGRAN PRISMA Matrix; Systagenix, an ACELITY Company) are composed of 44% ORC, 55% bovine collagen, and 1% silver-ORC in a sterile, freeze-dried composite. An important difference between ORC/collagen/silver-ORC and other silver-containing dressings is the low concentration of ionic silver (1%) in combination with ORC within the dressing. Because these dressings contain ionic silver, the use of sterile saline to premoisten the dressing prior to use does not alter the properties of the dressing by precipitating out silver chloride as seen in other silver dressings.
Matrix metalloproteinases and reactive oxygen species in the wound environment
In order for normal wound healing to progress, inflammation is required. This stage is carefully balanced to remove devitalized tissue and damaged portions of the extracellular matrix, allowing for repair. However, when the matrix metalloproteinases (MMPs), reactive oxygen species, and protease inhibitors in inflammation become unbalanced, healing is delayed.
METHODS
Literature search
A literature search for peer-reviewed articles published between January 2000 and March 2016 was conducted. Keywords included ORC/collagen dressings and ORC/collagen/silver-ORC dressing. PubMed, Science Direct, EMBASE, Cochrane Reviews, and other sources (such as QUOSA) were utilized. Abstracts, posters, and off-topic publications were excluded from the results. The level of evidence for each published article included was determined using the Sullivan et al22 categorization.
Panel meeting
A multidisciplinary panel of 15 experts convened February 3 to 4, 2017, in Dallas, Texas, to discuss and provide recommendations for the use of ORC/collagen dressings in wound care. Panel members included podiatrists, wound care specialists (such as doctors, certified wound care nurses, and research scientists), and an orthopedist. Panel members received a booklet of peer-reviewed studies selected by the meeting sponsor (ACELITY, San Antonio, Texas) from the literature search for review prior to the meeting. The booklet included recently published literature on ORC/collagen dressings.
The meeting was moderated by one of the panel members and recorded. Panel members presented case studies that highlighted their individual clinical experience and provided suggestions for ORC/collagen dressing use in practice. Following the presentations, the panelists discussed ORC/collagen dressing use. Follow-up communications were conducted via e-mail and continued throughout the development of this article. All panel members approved the final recommendations.
LITERATURE SEARCH RESULTS
Publications and levels of evidence
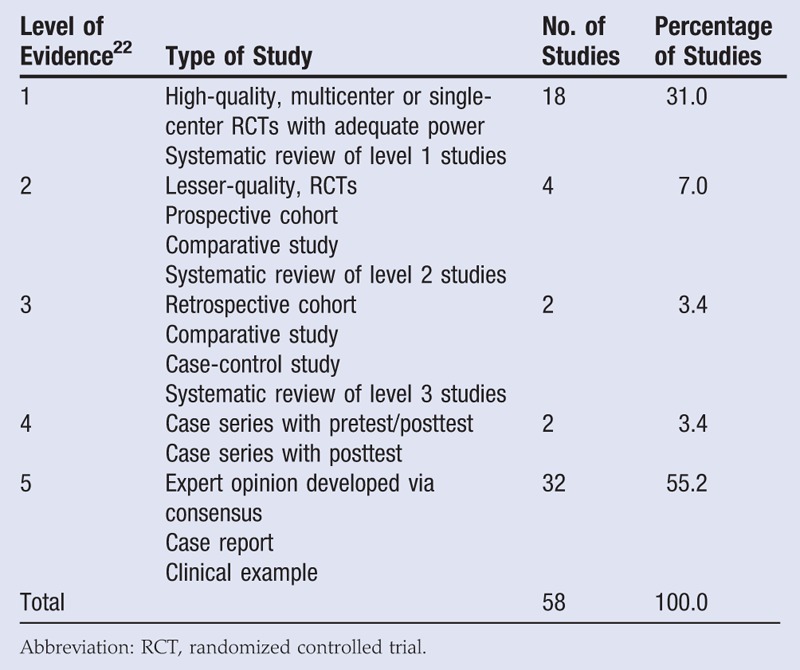
The literature search for peer-reviewed publications on ORC/collagen dressings initially yielded 333 publications. After abstracts, posters, and off-topic publications were removed, 58 articles remained. Using the Sullivan et al22 level of evidence categorization, 31.0% (18 articles) were level 1 (randomized controlled trials [RCTs] or systematic reviews), 7.0% (4 articles) were level 2 (RCTs, prospective cohort study, comparative study, or systematic reviews), 3.4% (2 articles) were level 3 (retrospective cohort, comparative study, or case-control study), 3.4% (2 articles) were level 4 (case series), and a majority (55.2%, 32 articles) were level 5 (expert opinion, case report, or clinical example) (Table 1).
Table 1.
LITERATURE SEARCH RESULTS
The literature search identified 5 systematic reviews. However, these reviews did not focus solely on ORC/collagen dressings (which are unique collagen dressings because of the addition of ORC), but rather on collagen dressings as a whole. All 5 reviews reported that because of insufficient evidence, it was unclear whether collagen-based dressings provide any clinical or cost benefit in the treatment of venous leg ulcers (VLUs) or diabetic foot ulcers (DFUs).23–27
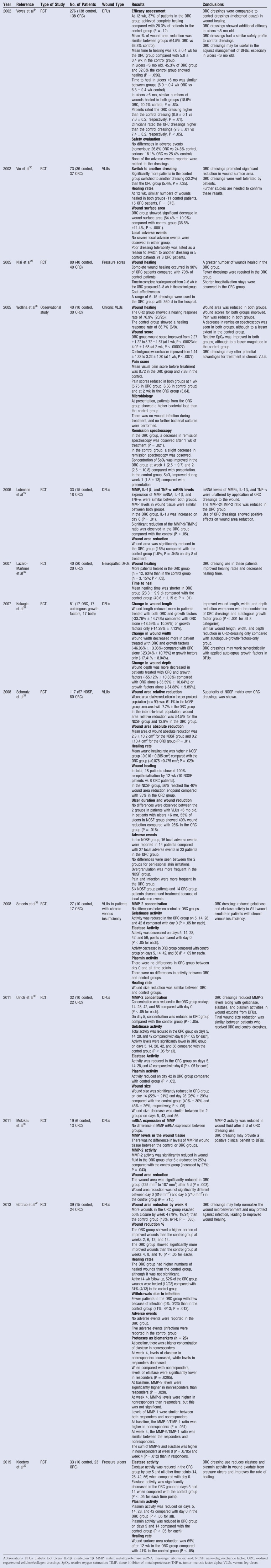
Twelve RCTs and 1 observational study discussing ORC/collagen dressings have been published since 2002 (Table 2). These studies examined the use of ORC/collagen dressings to treat pressure ulcers,28,29 VLUs,30–33 and DFUs.34–40 These trials have reported reduced elastase, MMPs, and reactive oxygen species in the wound29,32,38,39; improved wound area reduction29,30,35,40; increased number of healed wounds28,36; and shortened time to heal36 in patients treated with ORC/collagen dressings compared with controls (standard of care). One study noted that compared with control dressings, fewer ORC/collagen dressings were needed during the course of treatment and that patients in the intervention group had a shortened hospital length of stay.28 Several studies indicated comparable improvements in wound healing30,33,34,40 and wound area reductions32–34,37,38 between ORC/collagen dressings compared with either controls,32–34,38,40 autologous growth factors,37 or nonadherent dressing.30 One trial compared ORC/collagen dressings to a nano-oligosaccharide factor (lipidocolloid dressing impregnated with nano-oligosaccharide factor) in VLUs.31 In this study, wound area reduction and healing rates were significantly improved in the patients who received the intervention.31
Table 2.
OVERVIEW OF LEVEL 1 EVIDENCE
Several prospective studies were identified by the literature search describing the use of ORC/collagen dressings in a variety of wound types.41-44 The Alfieri et al41 study examined the use of ORC/collagen dressings versus iodine-soaked gauze (control) in 98 patients with a stoma at a surgical site. Surgical site infections were not seen in either group; however, there was reduced or no bacterial contamination in the second and third bacterial culture swabs in 60% (n = 33) of the ORC/collagen dressing group compared with 25% (n = 12) of the control group.41 A 52-patient study reported a positive clinical response to treatment consisting of antimicrobial and foam dressings followed 2 to 3 weeks later by ORC/collagen and foam dressings. Multiple different wound types, such as surgical, mixed ulcer, pressure ulcer, DFU, VLU, and mixed arterial/venous ulcer, were included in the study. A trend toward rapid healing and wound reduction after treatment initiation was observed along with improved patient satisfaction as treatment progressed toward wound healing.42 The use of ORC/collagen dressings has also been examined in 25 skin graft donor sites in patients with multiple comorbidities and chronic leg ulcers.43 Complete re-epithelialization was observed between 10 and 34 days postoperatively in all wounds. Two patients developed signs of infection after the first dressing change; however, antibiotics were not needed.43 An article by Mees et al44 describes a treatment pathway in 62 patients with postoperative infected abdominal wounds. These wounds were first treated antiseptically and then with antimicrobial advanced wound dressings until the wound improved to stage 1, followed by ORC/collagen/silver-ORC dressings in combination with alginate dressings. Hydropolymer foam dressings were used as a secondary dressing for all dressings utilized. In the event that high levels of exudate were observed, treatment was switched from dressings to negative-pressure wound therapy (NPWT) until levels of exudate were reduced. At the 3-year follow-up, recurrent incisional hernia was reported in 18.4% of the patients who received only dressings compared with 27.3% in patients treated with dressings followed by NPWT. This difference in recurrent incision hernias was due to the increased complexity of the wounds in the NPWT group.44 These results allowed for the establishment of a treatment pathway at the authors’ institution that included the use of advanced wound dressings early to help manage infection and to protect the wounds from reinfection followed by NPWT use.44
One small case study and 3 retrospective studies were identified in the literature search. The small case study (n = 4) reported the use of ORC/collagen dressings in patients with rheumatoid arthritis with leg ulcers and pyoderma gangrenosum with inflammatory features.45 Despite all patients failing previous treatments, the application of ORC/collagen dressings led to complete wound healing in 2 patients, wound improvement in the remaining 2 patients, and reduction of patient-reported pain. A large retrospective study (N = 974) from Snyder et al20 compared healing rates and estimated costs between ORC/collagen dressings and saline-soaked gauze dressings (control) in a variety of wound types (DFUs, surgical wounds, VLUs, pressure ulcers, and traumatic wounds). In the ORC/collagen dressing group (n = 873), 95% of the wounds completely healed within 38.6 days. Of the control group (n = 101), only 7.2% of the wounds were healed by 38.6 days, and 43% were healed after 6 months of treatment. Study authors estimated that after 2 months of treatment the cost of using ORC/collagen dressings was $2145, compared with $7350 in the control group.20 A retrospective study from Lazaro-Martinez et al46 examined data from an RCT with 40 neuropathic DFU patients. Here, 12 of 20 wounds treated with ORC/collagen dressings healed compared with 3 of 20 with standard-of-care dressings. The authors estimated an average cost of $561.48 for ORC/collagen dressings compared with $2577.65 for standard-of-care dressings over a 6-week period, saving $2280.13 per patient.46 The last retrospective study examined data from an RCT of 276 DFU patients that monitored wound healing over 12 weeks of treatment with either ORC/collagen dressing (n = 138) or moistened gauze (control, n = 138).47 In 58% of patients, wounds that had reduced by more than 53% in size by week 4 were more likely to be fully healed by the 12-week follow-up. Ultimately, this response was not affected by differences in treatment.47
Twelve case reports were identified and described the use of ORC/collagen dressings in below-the-knee amputation,48 lower-extremity wounds,49,50 hydroxyurea-induced leg ulcers,51 leg ulcers,52,53 pressure ulcers,54 surgical wounds,55–58 and DFUs.59 In these reports, ORC/collagen dressings were used either alone or in combination with other advanced wound therapies after wounds failed standard-of-care treatment. In a majority of these case reports, wounds improved or fully healed between 6 weeks and 8.5 months of ORC/collagen dressing use.48,50–52,54–59 One patient with a lower-extremity wound did not show any wound improvement after 2 months of using ORC/collagen dressings.49 This patient was later diagnosed with a pyogenic granuloma (a benign vascular skin growth that can develop after minor injuries or burns), and treatment was changed to local wound excision followed by split-thickness skin grafting.49 One case report discussed the presence of allergic contact dermatitis after application of ORC/collagen dressings in a patient with recurrent leg ulcers.53 This was the only article that described allergic contact dermatitis following the use of ORC/collagen dressings, indicating that this reaction was specific to the patient and not indicative of the population as a whole.
Twenty-one review articles were identified by the literature search; a majority suggested that while ORC/collagen dressings may be used in the care of chronic wounds, more high-level evidence is needed.60–75 Two reviews concluded that ORC/collagen dressings alone or in combination with standard wound care treatment may be beneficial in DFUs.76,77 Two reviews focused on the mechanisms of action for collagen-based wound dressings. These articles indicated that collagen served as a substrate for elevated MMP levels.21,78 Potential cost-effectiveness of ORC/collagen dressings in the treatment of DFUs was the focus of 2 articles.79,80 Both concluded that ORC/collagen dressings may be cost-effective because of improved healing rates; however, more studies are needed.
Impact of ORC/collagen and ORC/collagen/silver-ORC on wound healing
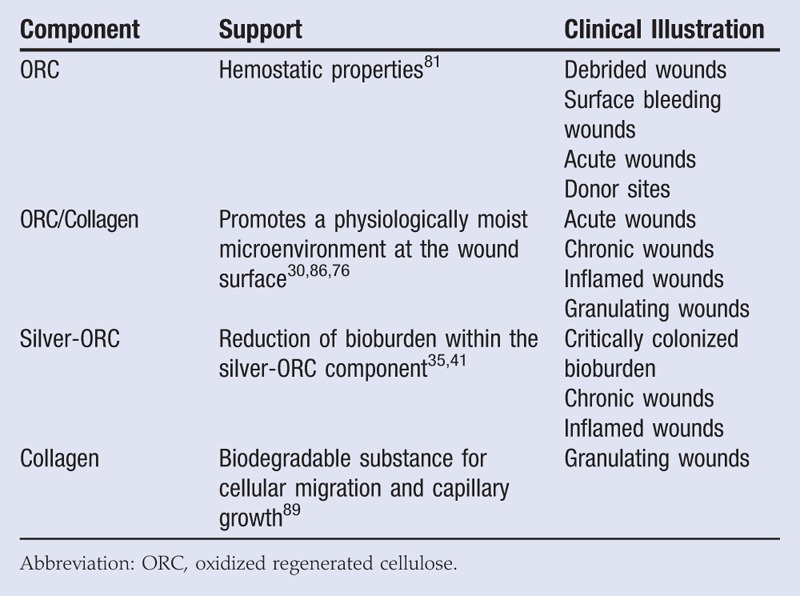
Studies have reported the effects of ORC/collagen and ORC/collagen/silver-ORC in 3 of the 4 wound healing phases (hemostasis, inflammation, and repair). This is summarized in Table 3.
Table 3.
ORC/COLLAGEN AND ORC/COLLAGEN/SILVER-ORC COMPONENTS
Immediately following injury, the body works to attain hemostasis and avoid exsanguination.6 Recently, Cheng et al81 reported that ORC has hemostatic properties, which may be useful for wounds that are bleeding.
During the inflammatory phase, the body works to create a barrier against potential microorganisms and remove foreign particles and damaged tissue through the actions of MMPs, reactive oxygen species, and proteases, such as elastase.6,82 Unfortunately, under certain conditions, these products of healing continue to remain elevated and do not revert to normal levels, stalling wound healing. Published literature has found elevated levels of MMPs and reactive oxygen species in chronic wounds.83–85
In laboratory testing, ORC/collagen absorbs wound exudate from DFUs,86 which helps promote a moist microenvironment at the wound surface, and is conducive to granulation tissue formation, re-epithelialization, and optimal wound healing. A study from Hart et al87 investigated the effects of ORC/collagen on fibroblast migration and proliferation and on accelerated wound repair in a diabetic mouse model. In this study, ORC/collagen resulted in measurable improvements in the histologic appearance of wound tissues.87 A single-blind RCT examined wound size reduction and biochemistry of wound fluid in DFU patients receiving ORC/collagen or standard wound care.35 After 8 days, wound size reduction was greater (P = .045), and wound fluid biochemistry indicated a more favorable moist wound environment in wounds that received ORC/collagen.
The presence of bioburden can create a continuous inflammatory state in the wound, altering the ability of a wound to progress through the inflammation stage of wound healing. In the presence of common wound pathogens and human dermal fibroblasts in vitro, ORC/collagen/silver-ORC was shown to reduce bacterial bioburden/growth and help support a moist wound environment conducive to healing.88 Reducing the bacterial bioburden within the ORC/collagen/ORC-silver dressings may result in a reduced risk of infection. Gottrup et al40 compared ORC/collagen/silver-ORC with standard of care for DFUs and monitored the number of wounds withdrawn from the study because of infection. That number was greater in the standard-of-care group (P = .012).40
During the repair phase of wound healing, fibroblast migration, collagen synthesis, angiogenesis, and granulation tissue formation occur.6 The collagen component serves as a biodegradable substance for cellular migration and capillary growth necessary for the repair process.89 The effect of ORC/collagen on dermal and epidermal healing and growth factor concentration in acute wounds was examined in an in vivo rat model. Full-thickness excision wounds that received ORC/collagen and a hydrocolloid dressing displayed improved re-epithelialization (P < .05) compared with control wounds (hydrocolloid dressing only) and displayed higher levels of growth factor concentrations.90
PANEL MEETING RESULTS
Representative case studies
During the meeting, panel members presented case studies that were representative of their use of ORC/collagen dressings in their practices. Two of these are described below.
Case 1. A 94-year-old woman presented to the emergency room with a lower-extremity traumatic wound resulting from a fall 2 weeks earlier. Medical history included atrial fibrillation, cholecystectomy, hysterectomy, and appendectomy. In the emergency room, the patient was placed on antibiotics and referred to wound care. Her ankle-brachial pressure index was in reference range. The wound was infected and in need of debridement and antimicrobial dressings, with the goal to promote granulation tissue. The ORC/collagen/silver-ORC dressings offered the antimicrobial and granulation tissue promotion properties needed to address the wound requirements.
The wound was debrided with a curette (Figure 1A), and ORC/collagen/silver-ORC dressing (PROMOGRAN PRISMA Matrix) was placed, followed by the application of an antimicrobial dressing (WIC Silver Cavity Wound Filler; PolyMem, Fort Worth, Texas) and a 4-layer compression wrap. Dressings were changed once a week according to physician instructions. Here, the ORC/collagen/silver-ORC dressing provided a way to achieve hemostasis following the debridement. The wound showed improvement and reduced cellulitis after 7 days of treatment (Figure 1B). A second curette debridement was performed. After 14 days of treatment, healthy granulation tissue was observed in the wound (Figure 1C). The wound was fully healed after 21 days of treatment (Figure 1D). The ORC/collagen/silver-ORC dressing treatment was discontinued, and the patient was discharged from care with compression stockings.
Figure 1.
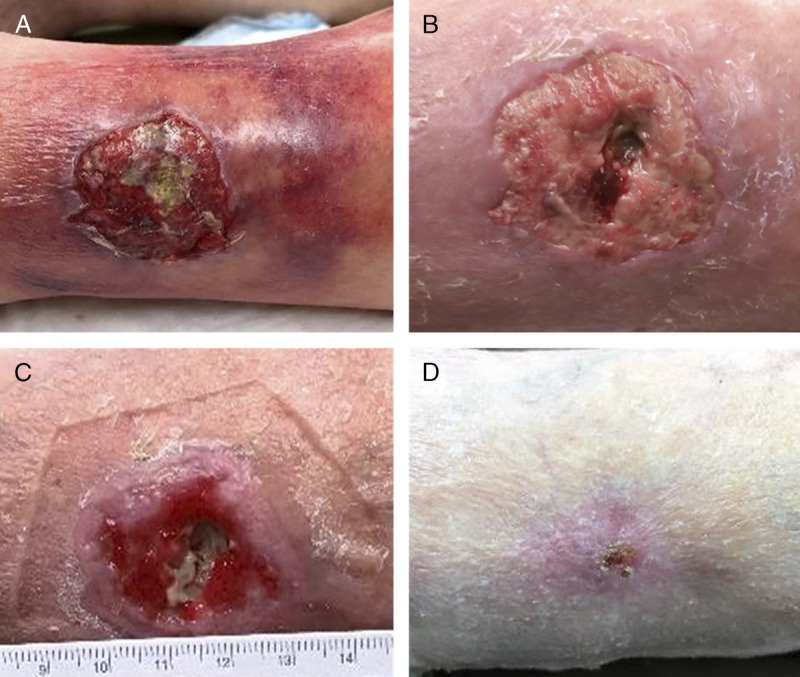
USE OF ORC/COLLAGEN/SILVER-ORC DRESSINGS IN A LOWER-EXTREMITY TRAUMATIC WOUND
Lower-extremity traumatic wound after debridement (A), after 7 days of treatment with ORC/collagen/silver-ORC dressings (B), after 14 days of treatment with ORC/collagen/silver-ORC dressings (C), and fully healed after 21 days of treatment with ORC/collagen/silver-ORC dressing (D). Photos courtesy of Janis Harrison.
Case 2. A 60-year-old man, with a history of diabetes mellitus, peripheral artery disease, and hypertension, presented with a left foot DFU and underlying osteomyelitis involving the second and third toes. The patient underwent surgical debridement with wedge resection of digits 2 and 3 (Figure 2A). Hyperbaric oxygen therapy (HBOT) and NPWT (V.A.C. Therapy; KCI, an ACELITY Company, San Antonio, Texas) were initiated. After 2 weeks of HBOT and NPWT, the wound showed signs of granulation tissue development (Figure 2B). Both HBOT and NPWT were discontinued after 5 weeks (Figure 2C), and treatment with ORC/collagen dressings (PROMOGRAN Matrix Wound Dressing) was initiated.
Figure 2.
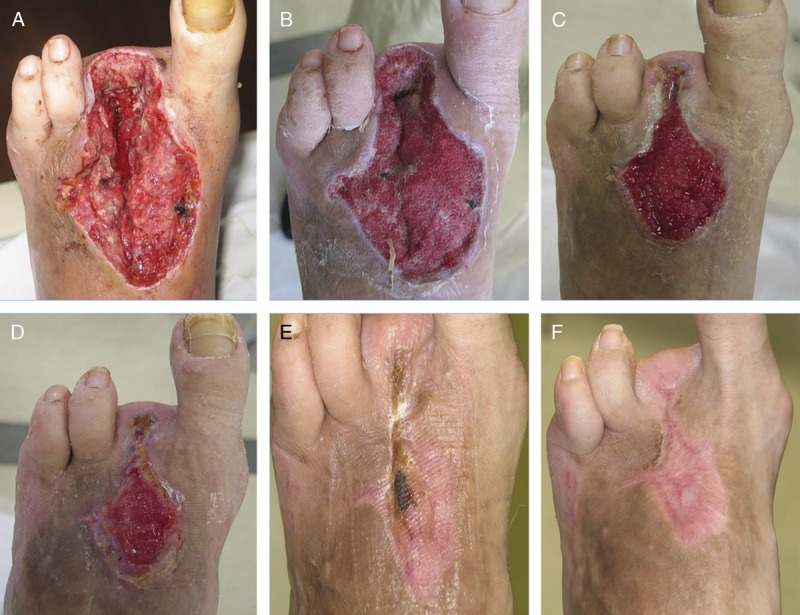
HYPERBARIC OXYGEN THERAPY, NEGATIVE-PRESSURE WOUND THERAPY, AND ORC/COLLAGEN DRESSING USE IN A DIABETIC FOOT ULCER
Wound after surgical amputation and debridement (A), after 2 weeks of hyperbaric oxygen therapy (HBOT) and negative-pressure wound therapy (NPWT) (B), after 5 weeks of therapy with HBOT and NPWT (C), after 3 weeks of treatment with ORC/collagen dressings (D), wound fully healed after 7 weeks of treatment with ORC/collagen dressing (E), and wound fully healed at 3-month follow-up visit (F). Photos courtesy of Jeffery Niezgoda.
The ORC/collagen dressings were used upon discontinuation of NPWT to help manage and prevent exacerbation of significant tissue inflammation and foster healthy granulation tissue formation. After 3 weeks of treatment with ORC/collagen dressings, wound re-epithelialization began to occur (Figure 2D). After 7 weeks of treatment, the wound was fully healed (Figure 2E) and remained healed at the 3-month follow-up visit (Figure 2F).
Panel member recommendations
The following recommendations are based on the practical experience of the panel members in their own day-to-day patient care. As such, wound care providers should remain up to date on the latest ORC/collagen and ORC/collagen/silver-ORC dressing research.
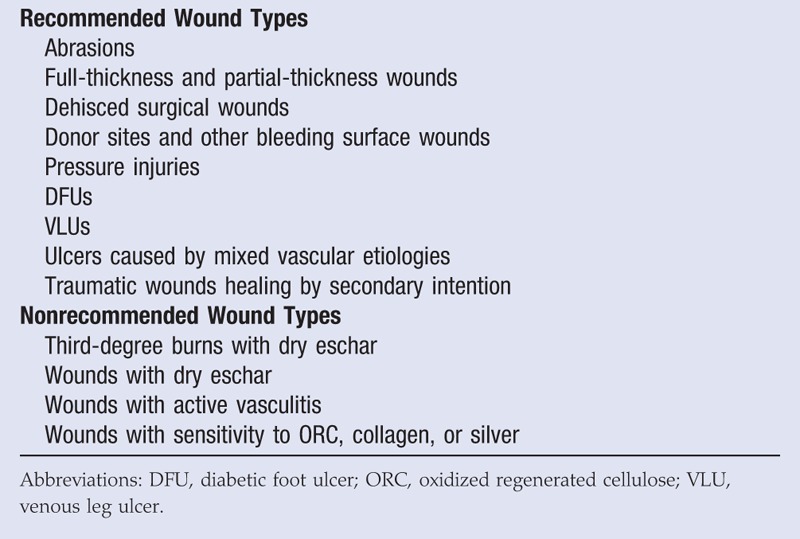
Oxidized regenerated cellulose/collagen dressings are versatile and do not require wounds to be of a certain duration prior to application. As such, the panel members provided a list of wound types where they would and would not typically use ORC/collagen dressings (Table 4). The panel members agreed that the presence of comorbidities and risk factors for poor wound healing, such as diabetes, might warrant the use of ORC/collagen dressings.
Table 4.
PANEL RECOMMENDATIONS FOR ORC/COLLAGEN DRESSING USE
The use of ORC/collagen dressings was recommended throughout the clinical treatment pathway for wound care. Recommendations included the application of ORC/collagen dressings following sharp debridement, as part of wound bed preparation, prior to application of advanced modalities, and as an adjunct to advanced wound healing modalities.37,42,43,58,81 Panel members also suggested using ORC/collagen/silver-ORC dressings in wounds with signs of infection,40,41 as long as the infection is managed with appropriate debridement and antibiotic treatment.
All panel members agreed that the frequency of application depends on the amount of wound exudate present. For low-exudating to nonexudating wounds, ORC/collagen and ORC/collagen/silver-ORC dressings should be reapplied every 72 hours or as necessary. However, for moderately exudating wounds, the dressings may need to be reapplied more frequently.
Prior to initiating wound treatment, patients must be assessed for underlying factors that may be contributing to impaired wound healing (Table 5). Barriers to wound healing in the wound environment must also be addressed.17–19 In order to ensure that wound healing is progressing, continuous monitoring of the wound is necessary. If the wound has not responded or stops responding to treatment, the wound care plan should be reevaluated.20 For example, in DFUs, a 50% wound area reduction after 4 weeks of treatment is a predictor of wound healing.47,91,92 If after 4 weeks the wound area has not reduced by 50%, the patient and wound should be reassessed, and new treatment initiated.
Table 5.
PANEL-RECOMMENDED WOUND MANAGEMENT PARAMETERS

These dressings also can be used adjunctively with other advanced wound products.37 Panel members have applied alginate, foam, or nonadherent dressings as secondary coverings over the ORC/collagen dressings.42 Some wounds may require skin grafts for closure. In these patients, panel members utilize ORC/collagen/silver-ORC dressings during the wound bed preparation stages prior to epidermal or split-thickness skin grafting.58 The ORC/collagen dressings can also be used over donor site wounds.43 Patients with medium-depth donor site wounds can show complete re-epithelialization of the donor site wound between 10 and 34 days.43
For a variety of complex wound types and complex patients, ORC/collagen dressings have shown positive clinical results,28,30,34,36,40 making them an ideal choice for inclusion in the available wound dressing armamentarium in patient care locations and facilities. The panel members recommend that facilities stock multiple products in the medical station system to enable quick and easy access to wound care products. Panel member experience has shown that when ORC/collagen dressings are stocked as items on the nursing units, as well as in surgery or intensive care units, the staff nurse has the product readily at his/her disposition.
In long-term-care or skilled nursing facilities, wound care products that are stored are usually basic, first-choice products that address antimicrobial activity and/or moisture balance. Cost is an enormous factor in such care settings, and dressings perceived to be more economical are usually chosen. However, smaller up-front costs do not necessarily translate to less expensive patient care if the wound does not heal. The most expensive wound dressing is one that does not promote wound healing. Durable medical equipment suppliers can be used to obtain dressings for specific patients if their medical insurance covers these supplies.
Technical pearls
The following technical pearls are based on the practical experience of the panel members in their own day-to-day patient care. As such, wound care providers should remain up to date on the latest ORC/collagen and ORC/collagen/silver-ORC dressing research.
Oxidized regenerated cellulose/collagen dressings can be used for either infected or inflamed wounds. This is important because in chronic, nonhealing wounds it can be difficult to distinguish between an infected and an inflamed wound. Clinical signs of infected wounds include fever, purulent drainage, presence of necrotic tissue, pain, and an elevated white blood cell count. Infected wounds may display erythema greater than 0.5 cm around the ulcer, as well as pain, cellulitis, edema, local warmth, and induration, and granulation tissue is usually spongy or friable (Figure 3). Patients with infected wounds can benefit from the initiation of appropriate infection management algorithms, including the use of antibiotics and antimicrobial dressings. Immunocompromised patients may not mount a sufficient physiologic response to infection; as such, secondary signs such as pain in an otherwise painless foot and wound deterioration should be considered as signs of infection. Panel members recommend the use of ORC/collagen/silver-ORC dressings as part of the treatment regimen in mildly infected or critically colonized wounds.40,41
Figure 3.
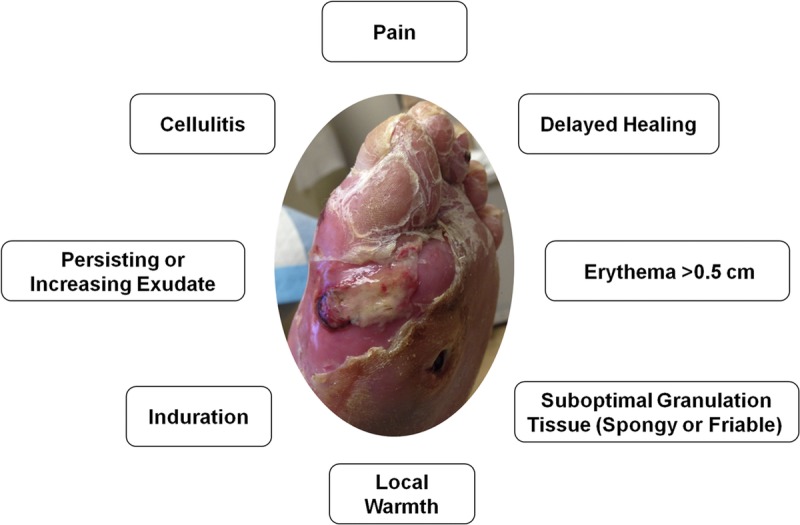
CLINICAL SIGNS OF WOUND INFECTION
Photo courtesy of Stephanie Wu.
In contrast, signs of an inflamed wound include pain, localized erythema less than 0.5 cm around the ulcer, induration, and edema (Figure 4). Granulation tissue is also either absent, spongy, or friable. Patients with inflamed wounds typically do not respond or see only marginal benefit from infection management protocols. The use of ORC/collagen dressings is also recommended for inflamed wounds.
Figure 4.
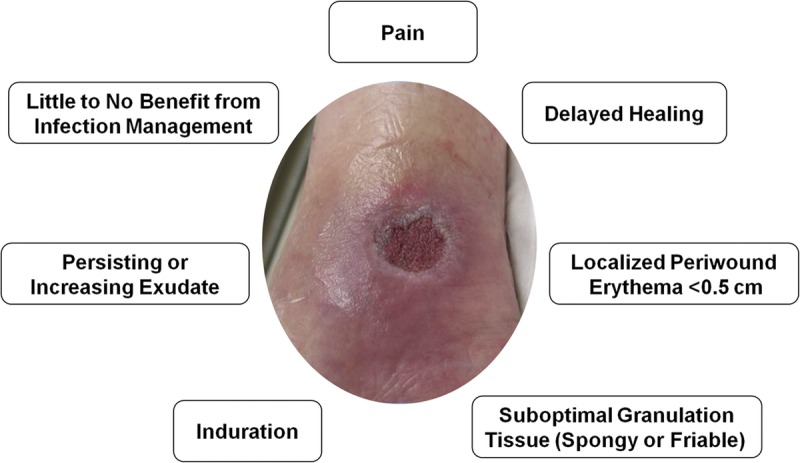
CLINICAL SIGNS OF WOUND INFLAMMATION
Photo courtesy of Jeffery Niezgoda.
Wound debridement is an essential component of wound healing. Occasionally, minor bleeding may occur after debridement. The ORC/collagen dressing should then be applied, followed by a secondary dressing. Oxidized regenerated cellulose has hemostatic properties81 that may be beneficial for bleeding wounds. At subsequent dressing changes, ORC/collagen dressings are reapplied. It is not necessary to remove any residual ORC/collagen dressing from the wound during dressing changes, because the dressing transforms into a biodegradable gel upon contact with fluid/exudate. It is important during dressing reapplication not to vigorously cleanse or disturb the wound bed, as this can disrupt wound healing and developing granulation tissue. Lightly pouring sterile saline over the wound can gently cleanse the wound bed.93
Panel member experience supports use of the ORC/collagen dressings early in wound care treatment for the purposes of wound bed preparation.58 If the wound care plan involves grafting, the panel members recommend using ORC/collagen dressings prior to and after the first wound dressing change. The use of ORC/collagen dressings prior to grafting serves to promote the formation of granulation tissue, optimizing the wound bed for graft take. In addition, panel members have used ORC/collagen dressings once NPWT was discontinued, until the wound was healed or ready for epidermal grafting. After the first dressing change, panel members apply ORC/collagen dressings to maintain a moist wound environment and promote wound re-epithelialization.
Perceived barriers and resolutions
Several barriers to the use of ORC/collagen dressings exist (Table 6). These barriers include a lack of knowledge and difficulty in home health or other healthcare facilities stocking ORC/collagen dressings. While facilities that utilize a central supply delivery system try to be effective, the system can fail for many reasons, including staffing issues, prioritization issues, or a lack of training.
Table 6.
PERCEIVED BARRIERS TO ORC/COLLAGEN DRESSING USE
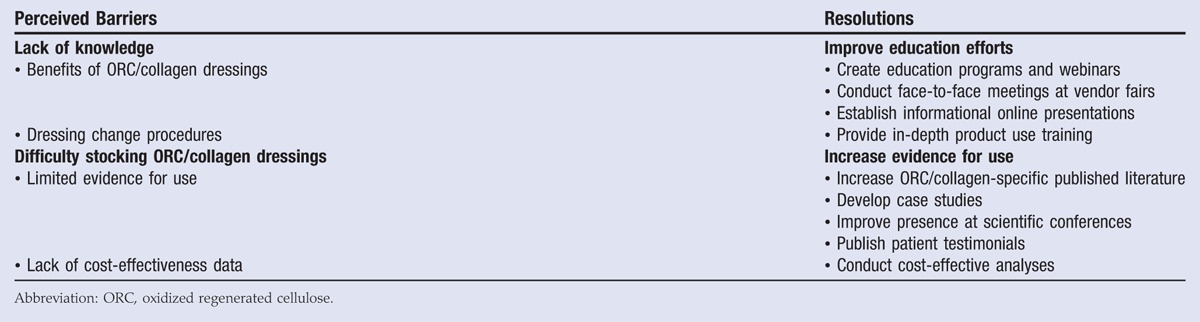
In order to overcome barriers to ORC/collagen dressing use, improved education efforts are recommended, especially those that feature why, when, and how to use the ORC/collagen dressings. Similarly, an increase in published manuscripts, case studies, poster presentations at scientific conferences, and patient testimonials can add to the current knowledge base and provide evidence for use of ORC/collagen dressings.
Value analysis
Products for wound care, such as collagen, have specific Healthcare Common Procedure Coding System codes. However, if a company has secured a code for the product, it does not guarantee appropriate coverage or payment. For example, collagen dressings coded A6021, A6023, and A6024 must be predominantly collagen. Any collagen dressing using these codes but with other components in greater amounts than collagen will not be reimbursed for use.
Per the Centers for Medicare & Medicaid Services, use of more than 1 type of wound filler or more than 1 type of wound cover in a single wound is rarely medically necessary, and if used, the reason must be well documented. This policy affects the outpatient clinical setting more than inpatient acute care and post–acute care settings. For example, in an outpatient clinical setting, use of collagen on an ulcer for promotion of granulation tissue and epithelialization may not be reimbursed if other dressings were also used to manage heavy exudate or to reduce the frequency of dressing changes. The same treatment combination in an inpatient setting with the goal of accelerating healing and reducing complications and length of stay would be reimbursable. In addition, while outpatient facilities can order dressings directly from durable medical equipment companies to be delivered to the patient at his/her home/residence, ORC/collagen dressings may not be covered. As such, it is often difficult to convince outpatient facilities that stocking ORC/collagen dressings is beneficial.
Ultimately, the value of the product or modality should be measured in outcome per cost and not cost in isolation of the overall benefit to the patient. Patient-centered care must consider the effectiveness and efficiency of a product. Studies have shown increased rates of healing with ORC/collagen dressings in chronic wounds such as pressure ulcers, DFUs, and VLUs.30,34,36 A large retrospective study estimated that after 2 months of therapy, the cost of using ORC/collagen dressings was US $2145 compared with US $7350 in patients who were treated with saline gauze dressings.20 Value analysis should be periodically re-examined to ensure that wound care products are still cost-efficient and clinically effective.
Limitations and future research recommendations
The paucity of published evidence for the use of ORC/collagen and ORC/collagen/silver-ORC dressings is a limitation. As such, the recommendations for use of ORC/collagen or ORC/collagen/silver-ORC dressings were based on panel member experience but supported by literature when possible. Further, these recommendations were derived during an open consensus that could be biased by the social interactions of the participants. Future studies comparing standard-of-care dressings and plain collagen dressings to ORC/collagen dressings should be conducted. In addition, large economic studies comparing the cost of care between standard-of-care dressings, plain collagen dressings, and ORC/collagen dressings should be performed.
CONCLUSIONS
A literature search and panel meeting were conducted to review the use of ORC/collagen dressings in wound care and provide practice recommendations. The literature search indicated that while positive clinical outcomes have been observed, limited evidence exists and future research is necessary to validate potential clinical and economic benefits from ORC/collagen and ORC/collagen silver-ORC dressings. Panel members made recommendations on wound types that may benefit from ORC/collagen or ORC/collagen/silver-ORC use. These included abrasions, full- and partial-thickness wounds, donor sites, ulcers, and traumatic wounds healing by secondary intention. Panel members also recommended optimizing both the patient and the wound prior to treatment to help reduce barriers to wound healing. Technical pearls were discussed, including the differences between an inflamed and infected wound, how often to apply ORC/collagen dressings, and preferences for use during the wound care clinical treatment pathway. An ORC/collagen dressing can be a critical tool for clinicians to help manage a variety of wounds. However, more clinical and economic studies comparing standard-of-care dressings and plain collagen dressings with ORC/collagen dressings are needed.
Footnotes
The authors have disclosed that they have no other financial relationships related to this article.
REFERENCES
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottrup F. A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am J Surg 2004;187:38S-43S. [DOI] [PubMed] [Google Scholar]
- 3.Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on healthcare providers in Europe. J Wound Care 2009;18:154-61. [DOI] [PubMed] [Google Scholar]
- 4.Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44-5. [PubMed] [Google Scholar]
- 5.Dowsett C, Bielby A, Searle R. Reconciling increasing wound care demands with available resources. J Wound Care 2014;23:552, 554, 556-7, 560-2. [DOI] [PubMed] [Google Scholar]
- 6.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 2009;37:1528-42. [DOI] [PubMed] [Google Scholar]
- 7.Bryant R, Nix D. Acute and Chronic Wounds: Current Management Concepts. 4th ed St Louis, MO: Mosby; 2010. [Google Scholar]
- 8.Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165-70. [DOI] [PubMed] [Google Scholar]
- 9.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy M. Skin and wound care: important considerations in the older adult. Adv Skin Wound Care 2008;21:424-36. [DOI] [PubMed] [Google Scholar]
- 12.Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg 1998;176:39S-47S. [DOI] [PubMed] [Google Scholar]
- 13.Sibbald RG, Woo KY, Ayello E. Wound bed preparation: DIM before DIME. Wound Healing Southern Africa 2008;1:29-34. [Google Scholar]
- 14.Robson MC, Stenberg BD, Heggers JP. Wound healing alterations caused by infection. Clin Plast Surg 1990;17:485-92. [PubMed] [Google Scholar]
- 15.Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997;77:637-50. [DOI] [PubMed] [Google Scholar]
- 16.Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321-5. [DOI] [PubMed] [Google Scholar]
- 17.Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:S1-S28. [DOI] [PubMed] [Google Scholar]
- 18.Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg 2006;117:72S-109S. [DOI] [PubMed] [Google Scholar]
- 19.Sibbald RG, Orsted HL, Coutts PM, Keast DH. Best practice recommendations for preparing the wound bed: update 2006. Adv Skin Wound Care 2007;20:390-405. [DOI] [PubMed] [Google Scholar]
- 20.Snyder RJ, Richter D, Hill ME. A retrospective study of sequential therapy with advanced wound care products versus saline gauze dressings: comparing healing and cost. Ostomy Wound Manage 2010;56:9-15. [Google Scholar]
- 21.Brett D. A review of collagen and collagen-based wound dressings. Wounds 2008;20:347-53. [PubMed] [Google Scholar]
- 22.Sullivan D, Chung KC, Eaves FF, Rohrich RJ. The Level of Evidence Pyramid: indicating levels of evidence in plastic and reconstructive surgery articles. Plast Reconstr Surg 2011;128:311-4. [DOI] [PubMed] [Google Scholar]
- 23.Bouza C, Munoz A, Amate JM. Efficacy of modern dressings in the treatment of leg ulcers: a systematic review. Wound Repair Regen 2005;13:218-29. [DOI] [PubMed] [Google Scholar]
- 24.Greer N, Foman NA, MacDonald R, et al. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med 2013;159:532-42. [DOI] [PubMed] [Google Scholar]
- 25.Nelson EA. Venous leg ulcers. BMJ Clin Evid 2011;2011:1902. [PMC free article] [PubMed] [Google Scholar]
- 26.Westby MJ, Norman G, Dumville JC, Stubbs N, Cullum N. Protease-modulating matrix treatments for healing venous leg ulcers. Cochrane Database Syst Rev 2016;12:CD011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L, Norman G, Dumville JC, O’Meara S, Bell-Syer SE. Dressings for treating foot ulcers in people with diabetes: an overview of systematic reviews. Cochrane Database Syst Rev 2015;CD010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nisi G, Brandi C, Grimaldi L, Calabro M, D’Aniello C. Use of a protease-modulating matrix in the treatment of pressure sores. Chir Ital 2005;57:465-8. [PubMed] [Google Scholar]
- 29.Kloeters O, Unglaub F, de Laat E, van Abeelen M, Ulrich D. Prospective and randomised evaluation of the protease-modulating effect of oxidised regenerated cellulose/collagen matrix treatment in pressure sore ulcers. Int Wound J 2015;13:1231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vin F, Teot L, Meaume S. The healing properties of PROMOGRAN in venous leg ulcers. J Wound Care 2002;11:335-41. [DOI] [PubMed] [Google Scholar]
- 31.Schmutz JL, Meaume S, Fays S, et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. Int Wound J 2008;5:172-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smeets R, Ulrich D, Unglaub F, Woltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J 2008;5:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wollina U, Schmidt WD, Kronert C, Nelskamp C, Scheibe A, Fassler D. Some effects of a topical collagen-based matrix on the microcirculation and wound healing in patients with chronic venous leg ulcers: preliminary observations. Int J Low Extrem Wounds 2005;4:214-24. [DOI] [PubMed] [Google Scholar]
- 34.Veves A, Sheehan P, Pham HT. A randomized, controlled trial of PROMOGRAN (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822-7. [DOI] [PubMed] [Google Scholar]
- 35.Lobmann R, Zemlin C, Motzkau M, Reschke K, Lehnert H. Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing. J Diabetes Complications 2006;20:329-35. [DOI] [PubMed] [Google Scholar]
- 36.Lazaro-Martinez JL, Garcia-Morales E, Beneit-Montesinos JV, Martinez-de-Jesus FR, Aragon-Sanchez FJ. Randomized comparative trial of a collagen/oxidized regenerated cellulose dressing in the treatment of neuropathic diabetic foot ulcers. Cir Esp 2007;82:27-31. [DOI] [PubMed] [Google Scholar]
- 37.Kakagia DD, Kazakos KJ, Xarchas KC, et al. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J Diabetes Complications 2007;21:387-91. [DOI] [PubMed] [Google Scholar]
- 38.Ulrich D, Smeets R, Unglaub F, Woltje M, Pallua N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J Wound Ostomy Continence Nurs 2011;38:522-8. [DOI] [PubMed] [Google Scholar]
- 39.Motzkau M, Tautenhahn J, Lehnert H, Lobmann R. Expression of matrix metalloproteases in the fluid of chronic diabetic foot wounds treated with a protease absorbent dressing. Exp Clin Endocrinol Diabetes 2011;119:286-90. [DOI] [PubMed] [Google Scholar]
- 40.Gottrup F, Cullen BM, Karlsmark T, Bischoff-Mikkelsen M, Nisbet L, Gibson MC. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen 2013;21:216-25. [DOI] [PubMed] [Google Scholar]
- 41.Alfieri S, Di MD, Menghi R, et al. Role of oxidized regenerated cellulose in preventing infections at the surgical site: prospective, randomized study in 98 patients affected by a dirty wound. Minerva Chir 2011;66:55-62. [PubMed] [Google Scholar]
- 42.Braumann C, Guenther N, Menenakos C, et al. Clinical experiences derived from implementation of an easy to use concept for treatment of wound healing by secondary intention and guidance in selection of appropriate dressings. Int Wound J 2011;8:253-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konstantinow A, Fischer TV, Ring J. Effectiveness of collagen/oxidised regenerated cellulose/silver-containing composite wound dressing for the treatment of medium-depth split-thickness skin graft donor site wounds in multi-morbid patients: a prospective, noncomparative, single-centre study [published online ahead of print November 2016]. Int Wound J 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mees J, Mardin WA, Senninger N, Bruewer M, Palmes D, Mees ST. Treatment options for postoperatively infected abdominal wall wounds healing by secondary intention. Langenbecks Arch Surg 2012;397:1359-66. [DOI] [PubMed] [Google Scholar]
- 45.Coelho S, Amarelo M, Ryan S, Reddy M, Sibbald RG. Rheumatoid arthritis-associated inflammatory leg ulcers: a new treatment for recalcitrant wounds. Int Wound J 2004;1:81-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazaro-Martinez JL, Aragon-Sanchez FJ, Garcia-Morales E, Beneit-Montesinos JV, Gonzalez-Jurado M. A retrospective analysis of the cost-effectiveness of a collagen/oxidized regenerated cellulose dressing in the treatment of neuropathic diabetic foot ulcers. Ostomy Wound Manage 2010;56:4-8. [Google Scholar]
- 47.Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003;26:1879-82. [DOI] [PubMed] [Google Scholar]
- 48.Pattison PS, Gordon JK, Mallen JK, Hoerner J. Case report: using dual therapies—negative pressure wound therapy and modified silicone gel liner—to treat a limb postamputation and dehiscence. Wounds 2005;17:233-40. [Google Scholar]
- 49.Mason MES. Case study of a patient with a chronic lower extremity wound. Ann Long Term Care 2005;13. [Google Scholar]
- 50.Derbyshire A. The use of combination treatments and dressings for a traumatic wound. Br J Nurs 2004;13:987-93. [DOI] [PubMed] [Google Scholar]
- 51.Romanelli M, Dini V, Romanelli P. Hydroxyurea-induced leg ulcers treated with a protease-modulating matrix. Arch Dermatol 2007;143:1310-3. [DOI] [PubMed] [Google Scholar]
- 52.De Morentin HM, Dodiuk-Gad RP, Brenner S. Klinefelter’s syndrome presenting with leg ulcers. Skinmed 2004;3:274-8. [DOI] [PubMed] [Google Scholar]
- 53.Foti C, Bonamonte D, Conserva A, Angelini G. Allergic contact dermatitis to regenerated oxidized cellulose contained in a matrix employed for wound therapy. Contact Dermatitis 2007;57:47-8. [DOI] [PubMed] [Google Scholar]
- 54.Allam RC. Micronized, particulate dermal matrix to manage a non-healing pressure ulcer with undermined wound edges: a case report. Ostomy Wound Manage 2007;53:78-82. [PubMed] [Google Scholar]
- 55.Guarnera G, Restuccia A. PROMOGRAN and complex surgical lesions: a case report. J Wound Care 2004;13:237-9. [DOI] [PubMed] [Google Scholar]
- 56.Fino P, Fioramonti P, Onesti MG, Passaretti D, Scuderi N. Skin metastasis in patient with hairy cell leukemia: case report and review of literature. In Vivo 2012;26:311-4. [PubMed] [Google Scholar]
- 57.Noble T. Use of a protease-modulating matrix dressing on a non-healing tendo-Achilles surgical wound. J Wound Care 2006;15:368-71. [DOI] [PubMed] [Google Scholar]
- 58.Tausche AK, Sebastian G. Wound conditioning of a deep tissue defect including exposed bone after tumour excision using PROMOGRAN Matrix, a protease-modulating matrix. Int Wound J 2005;2:253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monami M, Mannucci E, Giulio M. Use of an oxidized regenerated cellulose and collagen composite for healing of chronic diabetic foot ulcers: a report of two cases. Diabetes Care 2002;25:1892-3. [DOI] [PubMed] [Google Scholar]
- 60.Papanas N, Eleftheriadou I, Tentolouris N, Maltezos E. Advances in the topical treatment of diabetic foot ulcers. Current Diabetes Reviews 2012;8:209-18. [DOI] [PubMed] [Google Scholar]
- 61.Sweeney IR, Miraftab M, Collyer G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int Wound J 2012;9:601-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khanolkar MP, Bain SC, Stephens JW. The diabetic foot. QJM 2008;101:685-95. [DOI] [PubMed] [Google Scholar]
- 63.Game FL, Jeffcoate WJ. Dressing and diabetic foot ulcers: a current review of the evidence. Plast Reconstr Surg 2016;138:158S-164S. [DOI] [PubMed] [Google Scholar]
- 64.Chaby G, Senet P, Vaneau M, et al. Dressings for acute and chronic wounds: a systematic review. Arch Dermatol 2007;143:1297-304. [DOI] [PubMed] [Google Scholar]
- 65.Woo K, Ayello EA, Sibbald RG. The edge effect: current therapeutic options to advance the wound edge. Adv Skin Wound Care 2007;20:99-117. [DOI] [PubMed] [Google Scholar]
- 66.Hodde JP, Johnson CE. Extracellular matrix as a strategy for treating chronic wounds. Am J Clin Dermatol 2007;8:61-6. [DOI] [PubMed] [Google Scholar]
- 67.Agren MS, Werthen M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int J Lower Extrem Wounds 2007;6:82-97. [DOI] [PubMed] [Google Scholar]
- 68.Barber C, Watt A, Pham C, et al. Influence of bioengineered skin substitutes on diabetic foot ulcer and venous leg ulcer outcomes. J Wound Care 2008;17:517-27. [DOI] [PubMed] [Google Scholar]
- 69.Game FL, Attinger C, Hartemann A, et al. IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2016;32Suppl 1:75-83. [DOI] [PubMed] [Google Scholar]
- 70.Gottrup F, Apelqvist J. Present and new techniques and devices in the treatment of DFU: a critical review of evidence. Diabetes Metab Res Rev 2012;28:64-71. [DOI] [PubMed] [Google Scholar]
- 71.White R, McIntosh C. A review of the literature on topical therapies for diabetic foot ulcers. Part 2: advanced treatments. J Wound Care 2009;18:335-41. [DOI] [PubMed] [Google Scholar]
- 72.Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle) 2014;3:511-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fonder MA, Lazarus GS, Cowan DA, Aronson B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185-206. [DOI] [PubMed] [Google Scholar]
- 74.Gould LJ. Topical collagen-based biomaterials for chronic wounds: rationale and clinical application. Adv Wound Care (New Rochelle). 2016;5:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dumville JC, O’Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;CD009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmes C, Wrobel JS, Maceachern MP, Boles BR. Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: a systematic review. Diabetes Metab Syndr Obes 2013;6:17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tricco AC, Cogo E, Isaranuwatchai W, et al. A systematic review of cost-effectiveness analyses of complex wound interventions reveals optimal treatments for specific wound types. BMC Med 2015;13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ovington LG. Advances in wound dressings. Clin Dermatol 2007;25:33-8. [DOI] [PubMed] [Google Scholar]
- 79.Chow I, Lemos EV, Einarson TR. Management and prevention of diabetic foot ulcers and infections: a health economic review. Pharmacoeconomics 2008;26:1019-35. [DOI] [PubMed] [Google Scholar]
- 80.Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with PROMOGRAN in four European countries. J Wound Care 2002;11:70-4. [DOI] [PubMed] [Google Scholar]
- 81.Cheng W, He J, Wu Y, et al. Preparation and characterization of oxidized regenerated cellulose film for hemostasis and the effect of blood on its surface. Cellulose 2013;20:2547-58. [Google Scholar]
- 82.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514-25. [DOI] [PubMed] [Google Scholar]
- 83.Yager DR, Kulina RA, Gilman LA. Wound fluids: a window into the wound environment? Int J Low Extrem Wounds 2007;6:262-72. [DOI] [PubMed] [Google Scholar]
- 84.Moseley R, Hilton JR, Waddington RJ, Harding KG, Stephens P, Thomas DW. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen 2004;12:419-29. [DOI] [PubMed] [Google Scholar]
- 85.Snyder RJ, Driver V, Fife CE, et al. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Manage 2011;57:36-46. [PubMed] [Google Scholar]
- 86.Cullen B, Watt PW, Lundqvist C, et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol 2002;34:1544-56. [DOI] [PubMed] [Google Scholar]
- 87.Hart J, Silcock D, Gunnigle S, Cullen B, Light ND, Watt PW. The role of oxidised regenerated cellulose/collagen in wound repair: effects in vitro on fibroblast biology and in vivo in a model of compromised healing. Int J Biochem Cell Biol 2002;34:1557-70. [DOI] [PubMed] [Google Scholar]
- 88.Cullen B, Boyle C, Rennison T, Webb Y, Gregory S. ORC/collagen matrix containing silver controls bacterial bioburden while retaining dermal cell viability [abstract]. Presented at the Symposium on Advanced Wound Care; April 30–May 3, 2006, San Antonio, TX. [Google Scholar]
- 89.Duffy GP, McFadden TM, Byrne EM, Gill SL, Farrell E, O’Brien FJ. Towards in vitro vascularisation of collagen-GAG scaffolds. Eur Cell Mater 2011;21:15-30. [DOI] [PubMed] [Google Scholar]
- 90.Jeschke MG, Sandmann G, Schubert T, Klein D. Effect of oxidized regenerated cellulose/collagen matrix on dermal and epidermal healing and growth factors in an acute wound. Wound Repair Regen 2005;13:324-31. [DOI] [PubMed] [Google Scholar]
- 91.Coerper S, Beckert S, Kuper M, Jekov M, Konigsrainer A. Fifty percent area reduction after 4 weeks of treatment is a reliable indicator for healing—analysis of a single-center cohort of 704 diabetic patients. J Diabetes Complications 2009;23:49-53. [DOI] [PubMed] [Google Scholar]
- 92.Snyder RJ, Cardinal M, Dauphinee DM, Stavosky J. A post-hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 2010;56:44-50. [PubMed] [Google Scholar]
- 93.Nicks BA, Ayello EA, Woo K, Nitzki-George D, Sibbald RG. Acute wound management: revisiting the approach to assessment, irrigation, and closure considerations. Int J Emerg Med 2010;3:399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]


