Abstract
Objectives
Epstein-Barr virus (EBV) is considered an important environmental factor in SLE aetiology, but the relationship between SLE and EBV in the Filipino population is unknown. We tested associations between SLE, lupus-associated autoantibodies and seropositivity for EBV and other herpes viruses in the Filipino population.
Methods
Sera from Filipino patients with SLE (n=233), unaffected first-degree relatives (FDRs, n=543) and unrelated controls (n=221) were tested for antibodies against EBV, cytomegalovirus (CMV) and herpes simplex viruses (HSV-1 and HSV-2) by standardised ELISAs. Humoral specificity against EBV nuclear antigen (EBNA)-1 was compared by solid-phase epitope mapping. Autoantibodies were detected by a bead-based multiplex assay. Results were analysed by Fisher's exact test, Student's t-test, χ2 test and one-way analysis of variance, as appropriate for the question.
Results
Filipino patients with SLE had increased seroprevalence and elevated antibody concentrations against EBV viral capsid antigen (EBV-VCA), CMV, HSV-1 and HSV-2 compared with unrelated controls (p<0.05). Seropositivity for anti-EBV early antigen (EA), a marker of EBV reactivation, was dramatically increased in patients with SLE compared with unrelated controls (92.3% vs 40.4%; OR 17.15(95% CI 10.10, 30.66), p<0.0001) or unaffected FDRs (49.4%; OR 12.04(7.42, 20.74), p<0.0001), despite similar seroprevalence of EBV-VCA in patients and FDRs. In patients with SLE, EBV-EA seropositivity correlated with lupus-associated autoantibodies (p<0.001), most notably with autoantibodies against dsDNA, chromatin, Sm, SmRNP and RNP A (p<0.01). Patient and unrelated control sera reacted to the highly repetitive glycine and alanine domain of EBNA-1. An epitope spanning EBNA-1410-420 was identified in sera of patients with SLE and showed limited binding by FDR and control sera.
Conclusions
Filipino patients with SLE have elevated prevalence and concentrations of antibodies against EBV, CMV, HSV-1 and HSV-2 antigens, along with altered anti-EBNA-1 specificities. EBV reactivation is more common among Filipino patients with SLE compared with healthy Filipinos and may contribute to SLE pathogenesis in this population.
Keywords: systemic lupus erythematosus, infections, autoimmunity
Introduction
Although SLE, a prototypical multiorgan autoimmune disease, has been studied for more than a century, its complex aetiology is incompletely understood. Genetic risk1 is strongly associated with SLE and likely interacts with environmental influences, such as infectious agents, to initiate overt clinical disease.2 Consistent with the role of genetic factors in SLE, disease severity varies between racial groups, with African-American, Hispanic and Asian patients disproportionately affected by major organ involvement.3 4 However, these populations are oftentimes under-represented in SLE research. In particular, little is known about the aetiology and pathogenesis of SLE among Filipinos, a racially diverse and heterogeneous population with Malay, Chinese, Indian and Spanish ancestries.
In the 1970s, a multiethnic survey study estimated that the prevalence of SLE among Filipino individuals in Hawaii, USA, was 19.9 per 100 000 individuals, compared with 5.8 per 100 000 in white individuals.5 The increased disease burden in Filipinos may be partially due to genetic differences, since SLE risk alleles and haplotypes in the major histocompatibility complex (MHC) region are more common and have a greater effect size in the Filipino population.6 In addition, anti-dsDNA, which is associated with renal disease, is more common among Filipino patients with SLE and their first-degree relatives (FDRs) compared with their counterparts in African-American cohorts.7 As in other populations, antinuclear antibodies, anti-dsDNA, anti-Ro/SSA and antichromatin are more common in Filipino patients with SLE compared with FDRs and healthy controls,7 but other lupus-associated autoantibodies have not been studied in the Filipino population. Endemic infections in the Philippines contribute to increased morbidity and early mortality among Filipino patients with SLE,8–10 and infections may also influence SLE pathogenesis.
Several viral pathogens have been associated with SLE in non-Filipino populations.11 To date, the strongest of these associations is with Epstein-Barr virus (EBV),12 13 a member of the herpes virus family that infects B cells and is linked to SLE through molecular mimicry,14 15 bystander activation16 and epitope spreading.17 18 The initial active EBV infection is followed by a lifelong latent infection with potential for reactivation, thus providing opportunities for both acute and long-lasting effects on the immune system.19 Latent EBV infection requires EBV nuclear antigen (EBNA)-1.20 21 Several EBNA-1 epitopes, such as PPPGRRP and (GR)x, exhibit cross-reactivity with early autoantigens in SLE, such as Sm B′ (PPPGMRPP) and Sm D1 (GR)x, respectively.22–24 Furthermore, several studies have shown an increased prevalence of antibodies against EBV early antigen (EBV-EA), a marker of EBV reactivation,25–27 as well as increased EBV viral loads28 in patients with SLE, suggesting that EBV reactivation may present a chronic immune stimulus that affects the disease course in SLE. A limited number of studies have implicated another herpes virus, cytomegalovirus (CMV), in SLE,29 30 although the effects of CMV on SLE development and autoantibody production remain unclear.31
Increased rates of EBV and CMV seropositivity have been described in patients with SLE compared with healthy controls,12 32–34 but this has not previously been examined in the Filipino population. To better understand the role of herpes virus infections in SLE among the Filipino population, we compared the seroprevalence of EBV, CMV and herpes simplex viruses (HSV-1 and HSV-2) in Filipino patients with SLE, their unaffected FDRs and unrelated healthy Filipino controls. We also evaluated the effects of herpes virus seropositivity on American College of Rheumatology (ACR) classification criteria and autoantibody positivity in Filipino patients with SLE.
Methods
Participants
Experiments were performed in accordance with the Declaration of Helsinki and were approved by the Institutional Review Boards of the Oklahoma Medical Research Foundation, University of Santo Tomas Hospital and Cedars-Sinai Medical Center. Each study participant provided written informed consent.
Demographic information on all study participants and clinical characteristics of patients with SLE included in this study has previously been published.7 Briefly, this study included a total of 233 Filipino patients with SLE who met at least 4 of the 11 ACR SLE classification criteria,35 36 543 of these patients’ unaffected FDRs (parents and siblings), and 221 unaffected, unrelated controls, matched by sex and age ±5 years. A thorough clinical interview and physical examination were performed for each of the enrolled subjects. FDRs with three ACR SLE classification criteria were excluded from the study. Control subjects with evidence of recent infection or with the presence of personal or a family history of systemic autoimmune rheumatic disease were also excluded from the study. All control subjects completed the Connective Tissue Disease Screening Questionnaire37 at the time of enrolment. Healthy control individuals were recruited from Filipino medical and nursing students, medical staff and allied health personnel at the University of Santo Tomas Hospital. Demographic information, standardised clinical information and serum samples were collected at the time of enrolment. Sera were stored at −20°C until time of use.
Viral serology testing
Patients with SLE, unaffected FDRs and matched controls were tested for evidence of previous infection with EBV, CMV, HSV-1 and HSV-2 by standardised ELISAs for IgG responses to EBV viral capsid antigen (VCA), EBV-EA, CMV immediate early antigen, HSV-1 (strain F) and HSV-2 (strain G), following the manufacturer's instructions (Wampole, Cranbury, NJ). Results and analyses are presented as units of the international standardised ratio, a semiquantitative measure designed to detect seroconversion.38
Standard solid-phase ELISAs were used to measure IgG responses against EBNA-1 in all subjects. Polystyrene microtitre 96-well plates were coated with 2 µg of antigen EBNA-1 (EBNA-1 mosaic; BiosPacific, Emeryville, CA), following our reported protocol.23 Briefly, serum samples (at 1:100 and 1:1000 dilutions) were incubated at room temperature for 2 hours followed by incubation with an antihuman IgG secondary antibody conjugated to alkaline phosphatase (Jackson Immunoresearch Laboratories, West Grove, PA). All wells received 1 µg of 4-nitrophenyl phosphate disodium salt hexahydrate (Sigma, St. Louis, MO), and optical density (OD)39 was measured at 410 nm. Plates were standardised across assays by normalising a known positive control to an OD of 1.0 at an absorbance of 410 nm.
Autoantibody detection
Serum samples were screened for autoantibody specificities using the BioPlex 2200 multiplex system (Bio-Rad Technologies, Hercules, CA). The BioPlex 2200 ANA kit uses fluorescently labelled magnetic beads for the simultaneous, semiquantitative detection of autoantibodies recognising dsDNA, chromatin, ribosomal P, 52 kD Ro/SSA, 60 kD Ro/SSA, La/SSB, Sm, the Sm/RNP complex, RNP A, RNP 68, centromere B, Scl-70 and Jo-1.40 Centromere B, Scl-70 and Jo-1, which are not specifically associated with SLE, were excluded from these analyses. Anti-dsDNA (IU/mL) has a previously determined positive cut-off of 10 IU/mL; an Antibody Index (AI) value (range 0–8) is reported by the manufacturer to reflect the fluorescence intensity of each of the other autoantibody specificities with a positive cut-off at AI=1.0. The AI scale is standardised relative to calibrators and control samples provided by the manufacturer.
EBNA-1 epitope mapping
All possible unique octapeptides of the EBNA-1 protein overlapping by seven amino acids were synthesised on the ends of polyethylene solid-phase supports in 96-well microtitre plate format using f-moc side-chain protection chemistry (Chiron Mimotopes, Clayton, Victoria, Australia).24 38 Antipeptide assays were conducted utilising a modified ELISA technique which we have previously described in detail.41 42 Briefly, the peptides were blocked in 3% low-fat milk phosphate-buffered saline (PBS). The solid phase peptides were then washed in PBS with 0.05% Tween and incubated with a 1:100 dilution of patient or control sera, washed, and incubated with antihuman IgG conjugated to alkaline phosphatase (Jackson Immunoresearch Laboratories). A final wash was performed, followed by incubation in a p-nitrophenyl phosphate solution to induce a colour change reaction if antibody–peptide interaction was present. The colour change was measured using a micro-ELISA plate reader (DYNEX Technologies, Chantilly, VA) at 410 nm. Positive controls on the same plate were developed and normalised to an OD of 1.0 to standardise results across plates and assays.
The positive cut-off was defined as the mean plus 4 SD of the OD of EBV-VCA-negative, unrelated healthy controls. Moderate binding and strong binding were defined as two and three times the positive cut-off, respectively. An epitope was defined as a series of two or more consecutive peptides with average binding above the positive cut-off in any one group (patients with SLE, FDRs or controls).
Statistical analysis
Frequency and percentage distribution of specific patient demographics and SLE ACR criteria were determined. The per cent of patients, FDRs and controls seropositive for antiviral antibodies or autoantibodies was compared by χ2 test, or by two-sided Fisher's exact test for comparisons in which the assumptions of χ2 test were not met. For comparisons of autoantibody seropositivity, p values were adjusted by the false discovery rate method. ORs with 95% CIs were calculated by the mid-p method. The Mann-Whitney U test (Wilcoxon rank-sum test) was used to determine differences in viral concentration between patients with SLE and control and FDR groups. IQR and median (M) values were reported within each group of patients with SLE, FDRs and controls. ACR criteria and total number of positive autoantibodies were compared by Kruskal-Wallis test. Statistical analyses were performed in Microsoft Excel or in R program.
Results
Demographic and clinical features of the study population
Demographic information and clinical features of the study population have previously been published.7 Briefly, of the 233 Filipino patients with SLE, 94% were female, with a mean age of 29±12 years (range 7–80 years of age). Patients in this study met an average of six ACR classification criteria (range, 4–10). The most common SLE ACR classification criteria observed included: malar rash (81.5%), immunologic criteria (80.7%), photosensitivity (78.4%) and arthritis (73.3%).7 The FDRs of patients with SLE came from 224 families (19 multiplex, 205 simplex) and included 246 parents and 297 siblings. Sixty-two per cent of FDRs were female, with a mean age of 41±17 (range 5–102 years of age).7 Of the 220 unrelated healthy controls, 69% were female, with a mean age of 27±7 years (range 14–69 years of age).
Filipino patients with SLE have elevated seroprevalence of EBV, CMV, HSV-1 and HSV-2 compared with healthy unrelated controls and higher antiviral antibody concentrations compared with FDRs or unrelated controls
To assess rates of previous herpes virus infection in this Filipino cohort, we measured serum antibodies against EBV-VCA as an indicator of EBV infection, as well as antibodies against CMV, HSV-1 and HSV-2 antigens. Compared with FDRs, patients with SLE had similar rates of seropositivity for EBV-VCA, CMV and HSV-1 (p>0.05), while the rate of HSV-2 seropositivity was higher in patients with SLE (OR 2.17 (95% CI 1.52, 3.15), p<0.001) (figure 1). Compared with unrelated controls, patients with SLE had significantly higher seropositivity for anti-CMV (OR 4.76 (95% CI 2.57, 9.46), p<0.001), anti-HSV-1 (OR 7.04 (95% CI 4.45, 11.44), p<0.001) and anti-HSV-2 (OR 7.76 (95% CI 5.11, 11.95), p<0.001), as well as a slight but statistically significant increase in EBV-VCA seropositivity (OR 6.76 (95% CI 1.16, 173.0), p<0.05) (figure 1). These results suggest that Filipino patients with SLE and their FDRs have similar exposure to herpes viruses, and these individuals may have greater exposure to EBV, CMV, HSV-1 and HSV-2 than unrelated, healthy individuals in this Filipino cohort.
Figure 1.
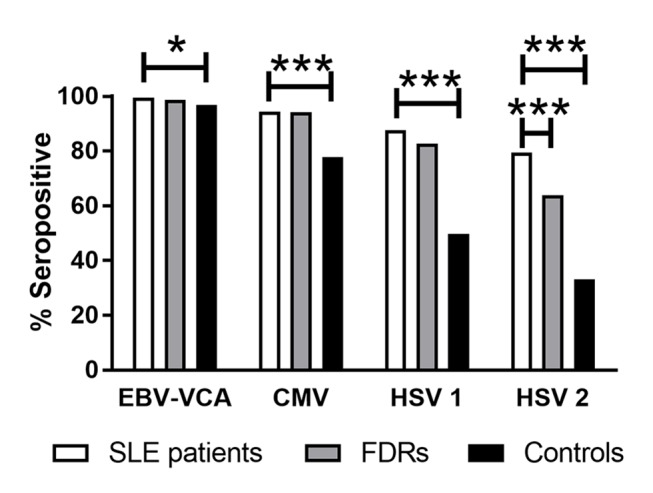
Filipino patients with SLE have an increased seroprevalence of common herpes viruses compared with controls. Bars indicate the percentage of patients with SLE (white), unaffected FDRs (grey) and unrelated healthy controls (black) seropositive for EBV-VCA, CMV immediate early antigen, HSV-1 (strain F) and HSV-2 (strain G). *p<0.05; ***p<0.001. CMV, cytomegalovirus; EBV-VCA, EBV viral capsid antigen; FDR, first-degree relative; HSV, herpes simplex virus.
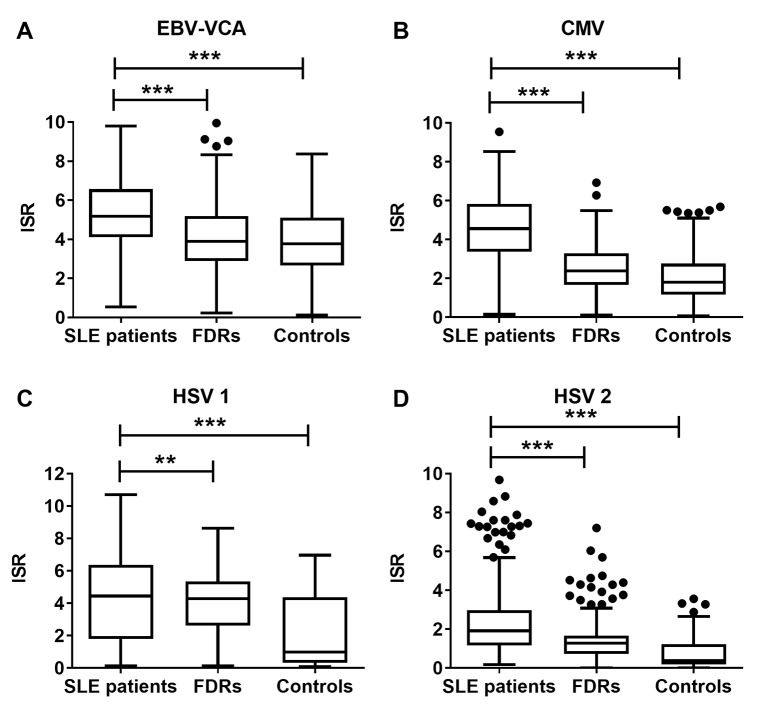
Next, we assessed the relative concentrations of antibodies against EBV-VCA, CMV, HSV-1 and HSV-2 among all individuals who were seropositive against these viruses. Patients with SLE had significantly higher concentrations of each antiviral antibody than unrelated controls or FDRs (figure 2), suggesting that the patients with SLE had an enhanced humoral response towards these viruses.
Figure 2.
Filipino patients with SLE carry greater antiviral antibody concentrations than unaffected relatives or unrelated healthy controls. Antibody concentrations against (A) EBV-VCA, (B) CMV, (C) HSV-1 and (D) HSV-2 were measured as ISR values in patients with SLE, FDRs and unrelated healthy controls. Results are displayed as Tukey box-and-whisker plots showing the median, IQR and 1.5 times the IQR. Dots indicate values that differ from the median by more than 1.5 times the IQR. **p<0.01; ***p<0.001. CMV, cytomegalovirus; EBV-VCA, EBV viral capsid antigen; FDR, first-degree relative; HSV, herpes simplex virus; ISR, international standardised ratio.
Filipino patients with SLE who are seropositive for HSV-1 or HSV-2 have more lupus-associated autoantibodies
To investigate whether exposure to EBV, CMV, HSV-1 and HSV-2 in Filipino patients with SLE might influence their disease course, we compared ACR classification criteria and autoantibody positivity between seropositive patients and seronegative patients. In this cohort of patients with SLE, EBV-VCA seropositivity and CMV seropositivity showed no association with the total number of positive ACR criteria, the presence of individual ACR criteria or the total number of positive autoantibodies detected in a multiplexed bead-based assay (p>0.05; see online supplementary tables 1–3).
lupus-2017-000214supp001.docx (21.8KB, docx)
HSV-1 and HSV-2 seropositivity showed limited associations with clinical features of SLE. Patients seropositive for HSV-1 met significantly more ACR criteria than HSV-1 seronegative patients (6 vs 5; p<0.05; see online supplementary table 1). Compared with HSV-1 negative patients, HSV-1 positive patients also had more lupus-associated autoantibodies (median of 6 vs 3; p<0.05; see online supplementary table 3), with significantly increased prevalence of anti-dsDNA and anti-La/SSB (figure 3A).
Figure 3.
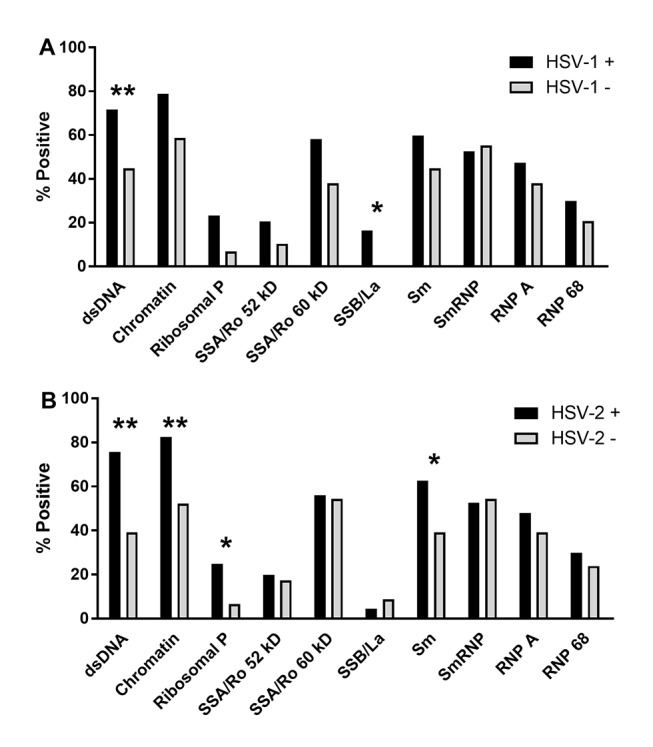
Lupus-associated autoantibodies are more prevalent in Filipino patients with SLE who are seropositive for (A) HSV-1 or (B) HSV-2. Bars indicate the per cent of virus-positive (black; n=204 HSV-1+, 185 HSV-2+) or virus-negative (grey; n=29 HSV-1–, 48 HSV-2–) patients with SLE who were positive for lupus-associated autoantibodies, as determined by multiplexed BioPlex assay. *p<0.05; **p<0.01. HSV, herpes simplex virus.
Similarly, HSV-2+ patients met significantly more ACR criteria compared with HSV-2− patients (6 vs 5; p<0.01; see online supplementary table 1), and were more likely to meet the ACR immunologic criterion (OR 3.08 (95% CI 1.52, 6.2); adjusted p=0.03; see online supplementary table 2). HSV-2+ patients had a median of 6 positive autoantibodies, compared with 4 for HSV-2− patients (p<0.05; see online supplementary table 3), with significantly higher prevalence of dsDNA, chromatin, ribosomal P and Sm specificities (figure 3B). Together, these results suggest that HSV-1 and HSV-2 infections are associated with the acquisition of lupus-associated autoantibodies.
Filipino patients with SLE have higher rates of EBV-EA seropositivity compared with FDRs or controls
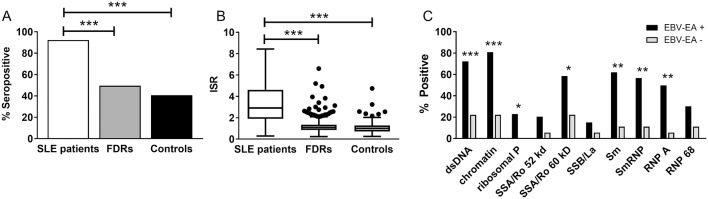
While the seroprevalence of EBV-VCA was only marginally higher in Filipino patients with SLE compared with controls, patients with SLE had a dramatically higher rate of seropositivity for anti-EBV-EA, a marker for viral reactivation, compared with FDRs (OR 12.04 (95% CI 7.42, 20.74), p<0.001) or unrelated controls (OR 17.1 (95% CI 10.1, 30.6), p<0.001; figure 4A). In addition, the concentration of anti-EBV-EA antibodies was significantly higher in patients with SLE compared with FDRs or controls (figure 4B). Therefore, despite the similar prevalence of original EBV infection in Filipino patients with SLE, FDRs and healthy controls, EBV reactivation may be more common in patients with SLE.43
Figure 4.
EBV-EA seropositivity in Filipino patients with SLE is associated with increased autoantibody positivity. (A) Seropositivity for EBV-EA is increased in patients with SLE (white bars) compared with unaffected FDRs (grey) or controls (black). (B) Anti-EBV-EA concentrations are greater in patients with SLE compared with FDRs or controls. Tukey box-and-whisker plots show the median, IQR and 1.5 times the IQR of the ISR values. Dots indicate values that differ from the median by more than 1.5 times the IQR. (C) The per cent of individuals positive for lupus-associated autoantibodies, as determined by multiplexed BioPlex assay, is shown for EBV-EA-positive (black; n=215) and EBV-EA-negative (grey; n=18) patients with SLE. *p<0.05; **p<0.01; ***p<0.001. EBV-EA, EBV early antigen; FDR, first-degree relative; ISR, international standardised ratio.
EBV-EA seropositivity was not associated with ACR criteria (see online supplementary tables 1 and 2), but strongly correlated with the presence of several lupus-associated autoantibodies in patients with SLE. EBV-EA− patients with SLE had a median of 0 positive autoantibodies, compared with 6 in EBV-EA+ patients with SLE (p<0.001; see online supplementary table 3). The rates of positivity for antibodies against 60 kD Ro/SSA (adjusted p<0.05), Sm (adjusted p<0.01), SmRNP (adjusted p<0.01) and RNP A (adjusted p<0.01) were more than fivefold higher in EBV-EA+ patients with SLE compared with EBV-EA− patients with SLE, and the rates of positivity for anti-dsDNA (adjusted p<0.001), antichromatin (adjusted p<0.001) and antiribosomal P (adjusted p<0.05) were also significantly increased in EBV-EA+ patients compared with EBV-EA− patients (figure 4C).
Sera of Filipino patients with SLE show altered reactivity against EBNA-1
Because EBNA-1 antibody responses have previously been associated with the development of lupus autoantibodies, we evaluated the anti-EBNA-1 response in Filipino patients with SLE. Anti-EBNA-1 seropositivity and serum concentrations were similar between patients with SLE, FDRs and controls (figure 5A,B). Epitope mapping showed similarities and differences in the specificity of the anti-EBNA-1 response between Filipino patients with SLE (figure 5C), FDRs (figure 5D) and unrelated healthy controls (figure 5E) with high serum reactivity against EBNA-1 (n=5 each). Sera from patients with SLE, FDRs and controls recognised several of the same EBNA-1 epitopes, with notable binding of the highly repetitive glycine and alanine domain (figure 5C,D; peptides 90–328). In this analysis, the sera of patients with SLE did not show increased binding to the two GAGGGAG-containing peptides compared with FDRs or healthy controls (peptides 311–312; recognised by one patient with SLE, two FDRs and three healthy controls).
Figure 5.
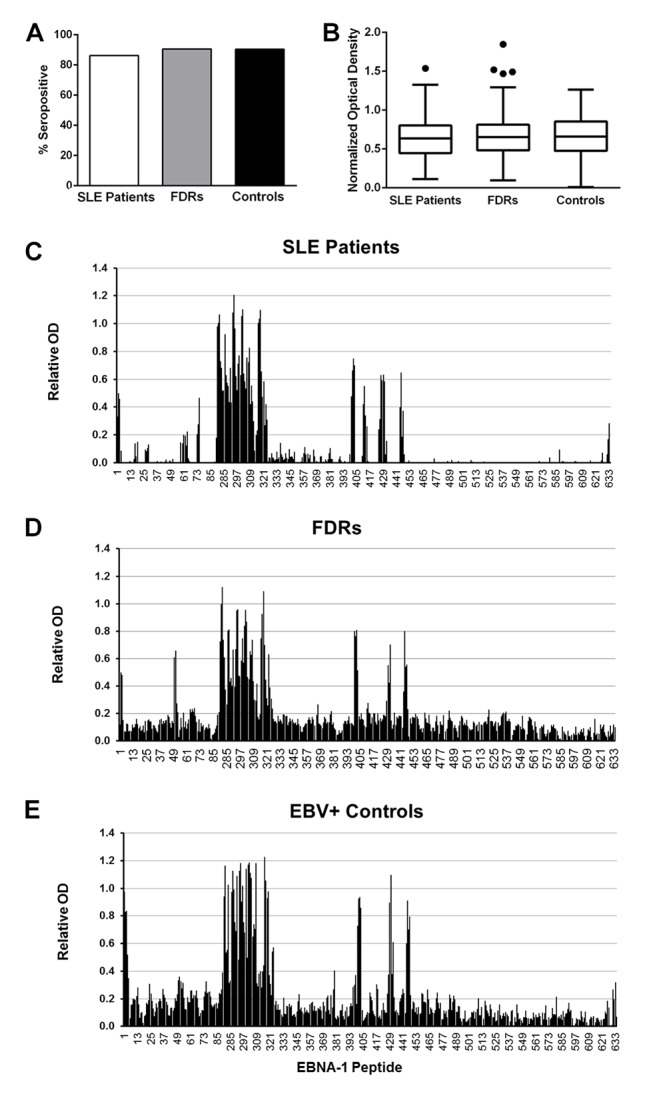
Filipino patients with SLE have an aberrant humoral response to EBNA-1. (A) Seropositivity for EBNA-1 is similar in patients with SLE (white bars), unaffected FDRs (grey) and controls (black) (p>0.05). (B) Anti-EBNA-1 concentrations are similar in patients with SLE, FDRs and controls (p>0.05). Tukey box-and-whisker plots show the median, IQR and 1.5 times the IQR of the OD, normalised by subtracting the mean OD of EBV-VCA-negative healthy controls. Dots indicate values that differ from the median by more than 1.5 times the IQR. (C–E) Epitope specificity of anti-EBNA-1 responses in serum varies between patients with SLE (C), FDRs (D) and healthy, unrelated controls (E). Binding to individual, overlapping peptides of EBNA-1 (along the X-axis) is shown as the mean normalised OD of patient or control serum binding minus that of EBV-VCA-negative healthy, unrelated controls. EBNA, EBV nuclear antigen; EBV, Epstein-Barr virus; EBV-VCA, EBV viral capsid antigen; FDR, first-degree relative; OD, optical density.
An epitope spanning EBNA-1410-420 (peptides 410–413; GEADYFEYHQE) was identified in patients with SLE (figure 5C,D). Peptides within this epitope were recognised by four of the five sera of patients with SLE, with two patients showing binding to all four peptides within the epitope and moderate to strong binding to at least one peptide. FDRs had an intermediate response, with weak binding to all four peptides in one FDR, to peptides 401–412 in another FDR and to either 411 or 413 in two additional FDRs. Binding to peptides within the EBNA-1410-420 epitope appeared weaker and more limited among healthy controls.
Discussion
Herpes viruses, particularly EBV, are associated with various aspects of SLE aetiology and pathogenesis, yet this is the first study of the relationship between herpes viruses and SLE in the Filipino population. Seropositivity rates and concentrations of antiviral antibodies in patients with SLE, FDRs and unrelated controls, along with a broad analysis of autoantibodies associated with herpes virus seropositivity, suggest that EBV reactivation and exposure to other herpes viruses may influence SLE development in the Filipino population.
More than 95% of Filipino patients with SLE, FDRs and controls in this cohort are seropositive for anti-EBV-VCA, while previous results show an EBV seroprevalence of 82%.44 However, only patients with SLE demonstrate a dramatically increased seroprevalence and higher concentrations of anti-EBV-EA, suggestive of increased EBV reactivation. This is consistent with the decreased EBV-specific cytotoxic T cell responses45 46 and reduced control of viral replication in patients with SLE.28 47 Therefore, intrinsic immune differences may contribute to increased EBV reactivation in patients with SLE. In addition, previous studies have shown that anti-EBV-EA responses are common in patients with SLE26 27 48 and have broader specificity in patients with SLE than in patients with mixed connective tissue disease.49
In the current cohort, patients with SLE who are seropositive for EBV-EA have increased prevalence of several lupus-associated autoantibodies, including anti-dsDNA, anti-Ro, anti-Sm and anti-SmRNP. Each of these autoantibody specificities has previously been associated with increased type I interferon activity.50–52 Similarly, anti-EBV-EA responses in patients with SLE were recently correlated with autoantibodies against extractable nuclear antigens and with plasma galectin-3 binding protein, a marker of type I interferon responses.53 In addition to stimulating type I interferon that may support autoantibody production,54 55 EBV infection increases exposure to potentially cross-reactive viral antigens, such as EBNA-1.14 38 56
The specificity of the EBNA-1 response in Filipino patients with SLE showed similarities and differences to those of FDRs and controls. Overall, the EBNA-1 response was more focused in Filipino adult patients with SLE compared with a previous US-based cohort of paediatric patients with SLE, while Filipino FDRs and EBV-positive controls had more diverse EBNA-1 responses compared with a US-based cohort of unrelated paediatric controls.38 In addition, these sera of Filipino patients with SLE recognised an epitope that has not been reported in patients with SLE (EBNA-1410-420; GEADYFEYHQE). Together, these lines of evidence suggest that EBV may contribute to SLE pathogenesis in the Filipino population, although the mechanisms of this association may vary depending on genetics, epigenetic factors, unique environmental influences or other factors.
Like EBV-EA seropositivity, HSV-1 and HSV-2 seropositivities are associated with an increase in lupus-associated autoantibodies in Filipino patients with SLE. The seroprevalence of HSV-2 among controls in this study is higher than the seroprevalence of 9.2% previously reported for middle-aged Filipino women,57 potentially due to the smaller number of individuals studied here or differences in the assays used. Nonetheless, patients with SLE have increased seroprevalence of HSV-2 and concentrations of anti-HSV-1 and anti-HSV-2 compared with FDRs or controls, suggesting a correlation between SLE and HSV-1/2 infection in the Filipino population. It is possible that lupus increases susceptibility to HSV-1/2 infection, given that HSV-1 and HSV-2 seropositivities were enriched in patients with more ACR criteria and more autoantibodies. This is also consistent with the association between HSV-2 and the ACR immunologic criterion. However, additional studies are needed to address this question in more depth.
Despite similar rates of anti-CMV seropositivity, anti-CMV concentrations are significantly higher in patients with SLE compared with FDRs, consistent with previous studies in Chinese patients with SLE.29 30 Therefore, SLE in the Filipino population may be associated with altered humoral responses to HSV-1 and CMV, rather than infection per se. Anti-CMV has been associated with anti-Sm and anti-RNP in patients with SLE,58 yet CMV seropositivity is not associated with an increase in anti-Sm, anti-RNP or total positive autoantibodies among patients with SLE in this cohort (data not shown). It is unclear if the altered humoral responses to CMV contribute to disease in patients with SLE, or if these differences are simply a result of general immune dysregulation. However, immunisation with CMV proteins has been shown to exacerbate humoral autoimmunity and renal disease in a lupus-prone mouse model.30
Based on study design and sample availability, weaknesses are present in this study. The only available unrelated controls were from medical personnel rather than unrelated healthy friends or household contacts of patients with SLE, which may skew infection exposures. In addition, the average age of FDRs was higher than the average age of patients with SLE or controls because the FDRs included both siblings and parents of patients with SLE. Because the seroprevalences of many viruses increase with age, it is possible that this may obscure differences between patients with SLE and FDRs or produce artefactual differences between FDRs and controls. However, similar results were obtained for seroprevalence and autoantibody concentrations whether comparing patients with SLE and controls with all FDRs or with siblings only (data not shown). DNA or PBMCs were also not available in this study, thereby limiting the ability to measure expression of lytic viral genes. Finally, future studies would be strengthened by the analysis of gene–environment interactions that may influence the relationship between virus seropositivity, autoantibody positivity and SLE classification.
In conclusion, this study provides the first evidence that EBV, HSV-1 and HSV-2 seroprevalences are associated with SLE classification and lupus-associated autoantibodies in the Filipino population. These results provide a foundation for future studies to delineate the causal and mechanistic relationship between herpes viruses and SLE pathogenesis in the Filipino population.
Acknowledgments
We would like to thank the individuals who participated in this study, as well as Beverly Hurt and Jourdan R Anderson for technical support, and Meghan Liles for editorial assistance.
Footnotes
Contributors: EV, JBH, JMG, SVN and JAJ designed the study. EV, MHW, MLI, LH, RBT, NJG, JMR, JBH, JMG, SVN and JAJ acquired data. EV, HC, RLB, BFB, JMR, JMG, SVN and JAJ analysed and/or interpreted data. All authors assisted with the development of the manuscript and gave final approval of the version to be published. JAJ had final responsibility for the decision to submit for publication.
Funding: The authors acknowledge the following grant support: P30 AR053483, U01 AI101934, U19 AI082714, U54 GM104938, P30 GM103510, U01 AI130830, R01 AI024717, U01 HG008666, P30 AR070549.
Competing interests: None declared.
Patient consent: The results presented are aggregated results, with no identifying information. All study participants provided appropriate written, informed consent prior to beginning study-specific procedures.
Ethics approval: The Institutional Review Boards of the Oklahoma Medical Research Foundation, University of Santo Tomas Hospital and Cedars-Sinai Medical Center.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All relevant data for this study are being published. Please contact the corresponding author with any additional requests, which will be processed through the Oklahoma Rheumatic Disease Research Cores Center.
References
- 1. Rullo OJ, Tsao BP. Recent insights into the genetic basis of systemic lupus erythematosus. Ann Rheum Dis 2013;72(Suppl 2):ii56–ii61. doi:10.1136/annrheumdis-2012-202351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Costenbader KH, Gay S, Alarcón-Riquelme ME, et al. . Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev 2012;11:604–9. doi:10.1016/j.autrev.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 3. Alarcón GS, McGwin G, Petri M, et al. . Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus 2002;11:95–101. doi:10.1191/9612332lu155oa [DOI] [PubMed] [Google Scholar]
- 4. Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2002;16:847–58. doi:10.1053/berh.2002.0259 [DOI] [PubMed] [Google Scholar]
- 5. Serdula MK, Rhoads GG. Frequency of systemic lupus erythematosus in different ethnic groups in Hawaii. Arthritis Rheum 1979;22:328–33. doi:10.1002/art.1780220403 [DOI] [PubMed] [Google Scholar]
- 6. Fernando MM, Freudenberg J, Lee A, et al. . Transancestral mapping of the MHC region in systemic lupus erythematosus identifies new independent and interacting loci at MSH5, HLA-DPB1 and HLA-G. Ann Rheum Dis 2012;71:777–84. doi:10.1136/annrheumdis-2011-200808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navarra SV, Ishimori MI, Uy EA, Ea U, et al. . Studies of Filipino patients with systemic lupus erythematosus: autoantibody profile of first-degree relatives. Lupus 2011;20:537–43. doi:10.1177/0961203310385164 [DOI] [PubMed] [Google Scholar]
- 8. Gerona JG, Navarra SV. Salmonella infections in patients with systemic lupus erythematosus: a case series. Int J Rheum Dis 2009;12:319–23. doi:10.1111/j.1756-185X.2009.01440.x [DOI] [PubMed] [Google Scholar]
- 9. Vargas PJ, King G, Navarra SV. Central nervous system infections in Filipino patients with systemic lupus erythematosus. Int J Rheum Dis 2009;12:234–8. doi:10.1111/j.1756-185X.2009.01416.x [DOI] [PubMed] [Google Scholar]
- 10. Victorio-Navarra ST, Dy EE, Arroyo CG, et al. . Tuberculosis among Filipino patients with systemic lupus erythematosus. Semin Arthritis Rheum 1996;26:628–34. doi:10.1016/S0049-0172(96)80013-8 [DOI] [PubMed] [Google Scholar]
- 11. Francis L, Perl A. Infection in systemic lupus erythematosus: friend or foe? Int J Clin Rheumtol 2010;5:59–74. doi:10.2217/ijr.09.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James JA, Lupus RJM, Barr E. Curr Opin Rheumatol 2012;24:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Draborg A, Izarzugaza JM, Houen G. How compelling are the data for Epstein-Barr virus being a trigger for systemic lupus and other autoimmune diseases? Curr Opin Rheumatol 2016;28:398–404. doi:10.1097/BOR.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 14. McClain MT, Heinlen LD, Dennis GJ, et al. . Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med 2005;11:85–9. doi:10.1038/nm1167 [DOI] [PubMed] [Google Scholar]
- 15. Poole BD, Scofield RH, Harley JB, et al. . Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity 2006;39:63–70. doi:10.1080/08916930500484849 [DOI] [PubMed] [Google Scholar]
- 16. Iwakiri D, Zhou L, Samanta M, et al. . Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J Exp Med 2009;206:2091–9. doi:10.1084/jem.20081761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. James JA, Gross T, Scofield RH, et al. . Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: sm B/B'-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med 1995;181:453–61. doi:10.1084/jem.181.2.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poole BD, Schneider RI, Guthridge JM, et al. . Early targets of nuclear RNP humoral autoimmunity in human systemic lupus erythematosus. Arthritis Rheum 2009;60:848–59. doi:10.1002/art.24306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lossius A, Johansen JN, Torkildsen Ø, et al. . Epstein-Barr virus in systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis—association and causation. Viruses 2012;4:3701–30. doi:10.3390/v4123701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reisman D, Sugden B. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol 1986;6:3838–46. doi:10.1128/MCB.6.11.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yates JL, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 1985;313:812–5. doi:10.1038/313812a0 [DOI] [PubMed] [Google Scholar]
- 22. Kaufman KM, Kirby MY, Harley JB, et al. . Peptide mimics of a major lupus epitope of SmB/B'. Ann N Y Acad Sci 2003;987:215–29. doi:10.1111/j.1749-6632.2003.tb06051.x [DOI] [PubMed] [Google Scholar]
- 23. James JA, Harley JB. Linear epitope mapping of an sm B/B' polypeptide. Journal of Immunology 1992;148:2074–9. [PubMed] [Google Scholar]
- 24. Poole BD, Gross T, Maier S, et al. . Lupus-like autoantibody development in rabbits and mice after immunization with EBNA-1 fragments. J Autoimmun 2008;31:362–71. doi:10.1016/j.jaut.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esen BA, Yılmaz G, Uzun S, et al. . Serologic response to Epstein-Barr virus antigens in patients with systemic lupus erythematosus: a controlled study. Rheumatol Int 2012;32:79–83. doi:10.1007/s00296-010-1573-4 [DOI] [PubMed] [Google Scholar]
- 26. Rasmussen NS, Draborg AH, Nielsen CT, et al. . Antibodies to early EBV, CMV, and HHV6 antigens in systemic lupus erythematosus patients. Scand J Rheumatol 2015;44:143–9. doi:10.3109/03009742.2014.973061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zandman-Goddard G, Berkun Y, Barzilai O, et al. . Exposure to Epstein-Barr virus infection is associated with mild systemic lupus erythematosus disease. Ann N Y Acad Sci 2009;1173:658–63. doi:10.1111/j.1749-6632.2009.04754.x [DOI] [PubMed] [Google Scholar]
- 28. Kang I, Quan T, Nolasco H, et al. . Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J Immunol 2004;172:1287–94. doi:10.4049/jimmunol.172.2.1287 [DOI] [PubMed] [Google Scholar]
- 29. Chen J, Zhang H, Chen P, et al. . Correlation between systemic lupus erythematosus and cytomegalovirus infection detected by different methods. Clin Rheumatol 2015;34:691–8. doi:10.1007/s10067-015-2868-3 [DOI] [PubMed] [Google Scholar]
- 30. Chang M, Pan MR, Chen DY, et al. . Human cytomegalovirus pp65 lower matrix protein: a humoral immunogen for systemic lupus erythematosus patients and autoantibody accelerator for NZB/W F1 mice. Clin Exp Immunol 2006;143:167–79. doi:10.1111/j.1365-2249.2005.02974.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halenius A, Hengel H. Human cytomegalovirus and autoimmune disease. Biomed Res Int 2014;2014:1–15. doi:10.1155/2014/472978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Draborg AH, Duus K, Houen G. Epstein-Barr virus and systemic lupus erythematosus. Clin Dev Immunol 2012;2012:1–10. doi:10.1155/2012/370516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poole BD, Templeton AK, Guthridge JM, et al. . Aberrant Epstein-Barr viral infection in systemic lupus erythematosus. Autoimmun Rev 2009;8:337–42. doi:10.1016/j.autrev.2008.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. James JA, Neas BR, Moser KL, et al. . Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum 2001;44:1122–6. doi:10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- 35. Tan EM, Cohen AS, Fries JF, et al. . The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. doi:10.1002/art.1780251101 [DOI] [PubMed] [Google Scholar]
- 36. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism 1997;40:1725 doi:10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 37. Karlson EW, Sanchez-Guerrero J, Wright EA, et al. . A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995;5:297–302. doi:10.1016/1047-2797(94)00096-C [DOI] [PubMed] [Google Scholar]
- 38. McClain MT, Poole BD, Bruner BF, et al. . An altered immune response to Epstein-Barr nuclear antigen 1 in pediatric systemic lupus erythematosus. Arthritis Rheum 2006;54:360–8. doi:10.1002/art.21682 [DOI] [PubMed] [Google Scholar]
- 39. Zandman-Goddard G, Shoenfeld Y, Molokhia M, et al. . Infections and SLE. systemic lupus erythematosus: genes versus environment in high risk populations. Autoimmunity 2005;38:473–85. [DOI] [PubMed] [Google Scholar]
- 40. Bruner BF, Guthridge JM, Lu R, et al. . Comparison of autoantibody specificities between traditional and bead-based assays in a large, diverse collection of patients with systemic lupus erythematosus and family members. Arthritis Rheum 2012;64:3677–86. doi:10.1002/art.34651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. James JA, Harley JB. Human lupus anti-spliceosome A protein autoantibodies bind contiguous surface structures and segregate into two sequential epitope binding patterns. J Immunol 1996;156:4018–26. [PubMed] [Google Scholar]
- 42. James JA, Harley JB. Peptide autoantigenicity of the small nuclear ribonucleoprotein C. Clin Exp Rheumatol 1995;13:299–305. [PubMed] [Google Scholar]
- 43. Huggins ML, Todd I, Powell RJ. Reactivation of Epstein-Barr virus in patients with systemic lupus erythematosus. Rheumatol Int 2005;25:183–7. doi:10.1007/s00296-003-0420-2 [DOI] [PubMed] [Google Scholar]
- 44. Evans AS, Espiritu-Campos L. Acute respiraotry diseases in students at the University of the Philippines, 1964-69. Bull World Health Organ 1971;45:103–12. [PMC free article] [PubMed] [Google Scholar]
- 45. Draborg AH, Jacobsen S, Westergaard M, et al. . Reduced response to Epstein-Barr virus antigens by T-cells in systemic lupus erythematosus patients. Lupus Sci Med 2014;1:e000015 doi:10.1136/lupus-2014-000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Larsen M, Sauce D, Deback C, et al. . Exhausted cytotoxic control of Epstein-Barr virus in human lupus. PLoS Pathog 2011;7:e1002328 doi:10.1371/journal.ppat.1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsokos GC, Magrath IT, Balow JE. Epstein-Barr virus induces normal B cell responses but defective suppressor T cell responses in patients with systemic lupus erythematosus. J Immunol 1983;131:1797–801. [PubMed] [Google Scholar]
- 48. Sculley DG, Sculley TB, Pope JH. Reactions of sera from patients with rheumatoid arthritis, systemic lupus erythematosus and infectious mononucleosis to Epstein-Barr virus-induced polypeptides. J Gen Virol 1986;67:2253–8. doi:10.1099/0022-1317-67-10-2253 [DOI] [PubMed] [Google Scholar]
- 49. Ngou J, Segondy M, Seigneurin JM, et al. . Antibody responses against polypeptide components of Epstein-Barr virus-induced early diffuse antigen in patients with connective tissue diseases. J Med Virol 1990;32:39–46. doi:10.1002/jmv.1890320107 [DOI] [PubMed] [Google Scholar]
- 50. Kennedy WP, Maciuca R, Wolslegel K, et al. . Association of the interferon signature metric with serological disease manifestations but not global activity scores in multiple cohorts of patients with SLE. Lupus Sci Med 2015;2:e000080 doi:10.1136/lupus-2014-000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weckerle CE, Franek BS, Kelly JA, et al. . Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum 2011;63:1044–53. doi:10.1002/art.30187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karageorgas TP, Tseronis DD, Mavragani CP. Activation of type I interferon pathway in systemic lupus erythematosus: association with distinct clinical phenotypes. J Biomed Biotechnol 2011;2011:1–13. doi:10.1155/2011/273907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rasmussen NS, Nielsen CT, Houen G, et al. . Humoral markers of active Epstein-Barr virus infection associate with anti-extractable nuclear antigen autoantibodies and plasma galectin-3 binding protein in systemic lupus erythematosus. Lupus 2016;25:1567–76. doi:10.1177/0961203316644334 [DOI] [PubMed] [Google Scholar]
- 54. Severa M, Giacomini E, Gafa V, et al. . EBV stimulates TLR- and autophagy-dependent pathways and impairs maturation in plasmacytoid dendritic cells: implications for viral immune escape. Eur J Immunol 2013;43:147–58. doi:10.1002/eji.201242552 [DOI] [PubMed] [Google Scholar]
- 55. Quan TE, Roman RM, Rudenga BJ, et al. . Epstein-Barr virus promotes interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum 2010;62:1693–701. doi:10.1002/art.27408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McClain MT, Harley JB, James JA. The role of Epstein-Barr virus in systemic lupus erythematosus. Front Biosci 2001;6:e137–47. doi:10.2741/A703 [DOI] [PubMed] [Google Scholar]
- 57. Smith JS, Herrero R, Muñoz N, et al. . Prevalence and risk factors for herpes simplex virus type 2 infection among middle-age women in Brazil and the Philippines. Sex Transm Dis 2001;28:187–94. doi:10.1097/00007435-200104000-00001 [DOI] [PubMed] [Google Scholar]
- 58. Newkirk MM, van Venrooij WJ, Marshall GS. Autoimmune response to U1 small nuclear ribonucleoprotein (U1 snRNP) associated with cytomegalovirus infection. Arthritis Res 2001;3:253–8. doi:10.1186/ar310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2017-000214supp001.docx (21.8KB, docx)