Abstract
Objective
The variety of disease phenotypes among patients with SLE challenges the identification of new biomarkers reflecting disease activity and/or organ damage. Osteopontin (OPN) is an extracellular matrix protein with immunomodulating properties. Although raised levels have been reported, the pathogenic implications and clinical utility of OPN as a biomarker in SLE are far from clear. Thus, the aim of this study was to characterise OPN in SLE.
Methods
Sera from 240 well-characterised adult SLE cases classified according to the American College of Rheumatology (ACR) and/or the Systemic Lupus International Collaborating Clinics (SLICC) criteria, and 240 population-based controls were immunoassayed for OPN. The SLE Disease Activity Index 2000 (SLEDAI-2K) was used to evaluate disease activity and the SLICC/ACR Damage Index (SDI) to detect damage accrual.
Results
Serum OPN levels were in average raised fourfold in SLE cases compared with the controls (p<0.0001). OPN correlated with SLEDAI-2K, especially in patients with a disease duration of <12 months (r=0.666, p=0.028). OPN was highly associated with SDI (p<0.0001), especially in the renal (p<0.0001), cardiovascular (p<0.0001) and malignancy (p=0.012) domains. Finally, OPN associated with coherent antiphospholipid syndrome (APS; p=0.009), and both clinical and laboratory criteria of APS had significant positive impact on OPN levels.
Conclusions
In this cross-sectional study, circulating OPN correlates with disease activity in recent-onset SLE, reflects global organ damage and associates with APS. Longitudinal studies to dissect whether serum OPN also precedes and predicts future organ damage are most warranted.
Keywords: systemic lupus erythematosus, antiphospholipid syndrome, organ damage, disease activity, biomarker
Introduction
Osteopontin (OPN) was first identified as a protein involved in bone remodelling, but later also shown to have important immunological roles.1 The protein is produced by various cells including B cells and T cells, dendritic cells, macrophages, neutrophils, bone cells and neurons, and it is upregulated in response to injury and inflammation.1
In SLE, activation of the type I interferon (IFN) system is typical, and many patients therefore display raised circulating levels of IFN-α, and/or express IFN-inducible genes, that is,‘the type I IFN signature’.2 The main IFN-α producing cells are the plasmacytoid dendritic cells (pDC),3 which respond to viral nucleic acids via endosomal Toll-like receptors (TLRs) 7 and 9 by massive IFN-α production. Intracellular expression of OPN in pDC is required for TLR9-dependent expression of IFN-α,4 and overexpression of OPN in lupus-prone mice induces B cell activation and subsequent antibody production, for example, anti-double-stranded (ds) DNA,5 6 possibly implying an important mechanistic role of OPN in SLE pathogenesis. In line with this, raised OPN levels have been reported in SLE relative to healthy controls.7
SLE is a complex autoimmune condition which can affect almost any organ system and is frequently associated with antiphospholipid syndrome (APS).8 9 Over time, antiphospholipid antibodies occur in at least 30%–40% of patients with SLE and at least 20%–30% of these patients develop clinical APS.9 10
The variety of disease phenotype combinations among patients with SLE challenges the hunt for new and reliable biomarkers that adequately reflect disease activity and/or organ damage. The erythrocyte sedimentation rate (ESR), circulating cell counts, complement proteins and autoantibodies (eg, antibodies targeting dsDNA and complement protein C1q) are used to monitor global disease activity.11 12 However, the anti-dsDNA and anti-C1q antibodies are primarily associated with raised disease activity in cases with renal lupus.13 14
Whereas a recent study suggested that OPN identifies SLE cases at risk of developing organ damage,15 others have found associations with renal disease16 as well as global disease activity.17 However, the implications for OPN in autoimmunity and its utility as a biomarker in SLE are far from clear. Thus, the aim of the present study was to evaluate OPN as a marker of disease activity and/or organ damage in SLE.
Materials and methods
Patients with SLE and control subjects
Two hundred and forty adult SLE cases (208 women, 32 men; mean age 49 years; range 18–88 years) were included. All patients took part in the prospective structured follow-up programme ‘KLURING’ (Swedish acronym for Clinical LUpus Register in Northeast Gothia) at the Rheumatology outpatient clinic, Linköping University Hospital, Sweden, previously described in detail.18 19 Of the 240 cases, 202 (84%) met at least four of the 1982 American College of Rheumatology classification criteria (ACR-82).20 Another 38 patients (16%) fulfilled solely the 2012 Systemic Lupus International Collaborating Clinics (SLICC-12) classification criteria;21 198 patients (83%) met both ACR-82 and SLICC-12. The patients were recruited consecutively. Most were prevalent cases (199 patients, 83%), but 41 patients (17%) had recent-onset disease (ie, disease duration <12 months) at the time of sampling. The mean disease duration was 10 years (range 0–45 years). For assessment of accumulated damage, the SLICC/ACR Damage Index (SDI) was used.22 The damage was required to have been persistent for at least 6 months, and the cumulative damage from 12 organ systems was recorded. The SLE Disease Activity Index 2000 (SLEDAI-2K)23 was recorded at each visit, and acquired organ damage according to the SDI22 was registered at baseline and then annually after inclusion in KLURING. The Sydney Consensus Conference criteria24 were used to classify APS. According to these criteria, APS is present if at least one of the clinical criteria (ie, thrombosis or pregnancy morbidity) and one of the laboratory criteria (ie, a positive lupus anticoagulant test and/or presence of anticardiolipin or anti-β2-glycoprotein-I antibodies) are met.24 Further characteristics of the patients are summarised in table 1.
Table 1.
Characteristics of the patients with SLE, n=240
| Mean (range) or % | |
| Age (years) | 49 (18–88) |
| Women | 86.7% |
| Caucasian ethnicity | 90.4% |
| Disease duration (Years) | 10 (0–45) |
| Prednisolone dosage (mg) | 5.8 (0–60) |
| SLEDAI-2K (score) | 2.9 (0–24) |
| Patients meeting SLICC-12 (%) | 236 (98.3) |
| Patients meeting ACR-82 (%) | 202 (84.0) |
| Fulfilled ACR-82 criteria (n) | 4.7 (3–9) |
| Meeting APS criteria (%) | 17.9% |
| SDI (score) | 1.1 (0–9) |
| ACR-82 criteria | n (%) |
| 1. Malar rash | 104 (43.3) |
| 2. Discoid rash | 39 (16.3) |
| 3. Photosensitivity | 121 (50.4) |
| 4. Oral ulcers | 27 (11.3) |
| 5. Arthritis | 182 (75.8) |
| 6. Serositis | 92 (38.3) |
| 7. Renal disorder | 60 (25.0) |
| 8. Neurological disorder | 12 (5.0) |
| 9. Haematological disorder | 137 (57.1) |
| 10. Immunological disorder | 120 (50.0) |
| 11. IF-ANA | 237 (98.8) |
| SDI ≥1 | n (%) |
| Ocular | 19 (7.9) |
| Neuropsychiatric | 42 (17.5) |
| Renal | 12 (5.0) |
| Pulmonary | 9 (3.8) |
| Cardiovascular | 33 (13.8) |
| Peripheral vascular | 18 (7.5) |
| Gastrointestinal | 5 (2.1) |
| Musculoskeletal | 32 (13.3) |
| Skin | 9 (3.8) |
| Premature gonadal failure | 0 |
| Diabetes mellitus | 10 (4.2) |
| Malignancy | 8 (3.3) |
ACR-82, 1982 American College of Rheumatology; APS, antiphospholipid syndrome; IF-ANA, immunofluorescence microscopy antinuclear antibodies; SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SLEDAI-2K, SLE Disease Activity Index 2000; SLICC-12, 2012 Systemic Lupus International Collaborating Clinics.
Peripheral venous blood was drawn from each individual at baseline. Serum was prepared and stored at −70°C until analysed. In addition, 14 of the 240 included patients were selected for consecutive analyses (5–10 visits per patient), with serial serum samples drawn. These patients were chosen due to fluctuations in disease activity (ie, SLEDAI-2K peak score of at least 4 points) over time.
At all patient visits, routine laboratory analyses (leucocytes, erythrocytes, platelets, urinalysis, plasma creatinine, glomerular filtration rate (GFR), C-reactive protein, ESR, classical complement function and complement proteins/fragment C3, C3d and C4) were performed at the Clinical Chemistry Unit, Linköping University Hospital, or at Uppsala Akademiska Hospital, Sweden. The lupus anticoagulant test was also performed at the Clinical Chemistry Unit, by the dilute Russell's viper venom test and data were retrieved from medical records.
Sera from 240 population-based individuals (220 women, 20 men; mean age 40 years; range 18–73 years) included in the EIRA cohort (Swedish acronym for Epidemiological Investigation of Rheumatoid Arthritis)25 served as controls for the OPN analyses.
OPN immunoassay
A serum- and plasma-validated ELISA kit was used to analyse OPN levels in SLE and control sera (Quantikine, R&D Systems, Minnesota, USA), and analyses were performed according to the manufacturer's instructions. Briefly, serum (diluted 1:25) was added to ELISA plates, precoated with monoclonal antibodies directed against human OPN. After incubation and washing of the wells, a horseradish peroxide conjugated polyclonal OPN specific antibody was added and the plate was incubated followed by washing and addition of tetramethylbenzidine substrate. The enzymatic reaction was stopped by adding 2 N sulfuric acid and read at 450 nm (plate reader Sunrise, Tecan, Männedorf, Switzerland; software Magellan V.7.1, Tecan).
Anticardiolipin and anti-β2-glycoprotein-I antibody assays
Anticardiolipin and anti-β2-glycoprotein-I antibodies (IgM and IgG) were analysed at the Clinical Immunology Unit at Linköping University Hospital using a fluoroenzyme-immunoassay (Phadia-250 instrument, Thermo-Fisher Scientific Phadia AB, Uppsala, Sweden). As defined by the Sydney criteria,24 we used the ≥99th centile of 507 control sera (75% women) for each antibody isotype to calculate an adequate cut-off level. Of these controls, 212 were healthy blood donors (mean age 44 years) and 295 were controls from the general population without any history of thrombosis or obstetric morbidity (mean age 48 years).
Statistics
Independent samples t-test was used to evaluate differences in OPN levels between patients with SLE and controls. Correlation analyses between OPN and disease activity variables were performed, and significant associations were further analysed in a univariate general linear model to adjust for age, sex, corticosteroid medication and disease duration. Relations between disease activity and organ damage, respectively, with OPN were assessed using stepwise linear regression model including SLEDAI-2K, SDI, age, sex, corticosteroids and disease duration with OPN as the response variable. Univariate general linear models with adjustment for age, sex, ongoing corticosteroid medication and disease duration was also used to evaluate the impact of disease activity, organ damage and APS on OPN levels. One-way ANOVA with Tukey's post hoc test was used to assess statistical differences between nephritis groups, between patients with extensive, moderate and no damage, and between SDI increase groups. p Values below 0.05 were considered statistically significant. Statistical analyses were performed with SPSS Statistics V.22 (IBM, Armonk, New York, USA) or GraphPad Prism, V.5.04 (GraphPad Software, La Jolla, California, USA).
Results
Serum OPN is increased in SLE
Levels of OPN were markedly higher among patients with SLE (mean 40.6±41.1 ng/mL) compared with the population-based controls (mean 10.1±12.3 ng/mL, p<0.0001; figure 1). There were no statistically significant differences between men (mean 48.6±29.3 ng/mL) and women (mean 39.9±30.6 ng/mL) among the patients, nor among the controls (mean for men 12.6±15.7 ng/mL, mean for women 9.7±11.9 ng/mL).
Figure 1.
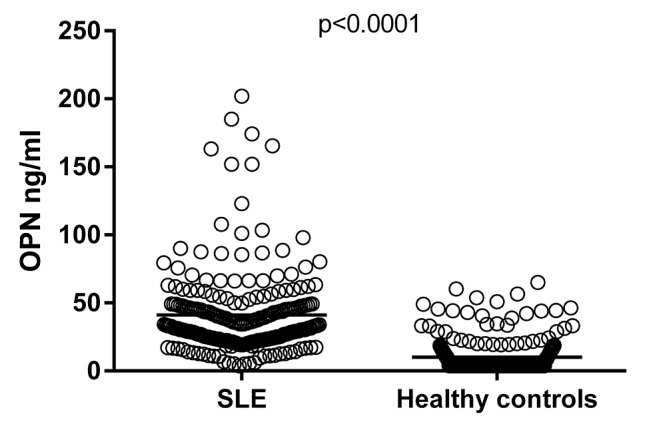
Serum osteopontin (OPN) levels in population-based controls and in cases with SLE. Serum levels of OPN, determined by ELISA, were significantly higher among patients with SLE (mean 40.6 ng/mL) compared with controls (mean 10.1 ng/mL).
OPN and disease activity
Cross-sectional correlation analyses between OPN and disease activity variables were performed, and significant associations were further analysed in a univariate general linear model to adjust for age, sex, corticosteroid medication and disease duration. Significant positive associations with OPN were found for ESR (p=0.001) and creatinine (p<0.0001), while a negative connection was found for haemoglobin (p<0.0001). However, we did not find any associations between OPN and GFR or the levels of complement C3, C3d or C4, or classical complement function. A weak positive correlation was found between OPN and SLEDAI-2K (r=0.211, p=0.039) when adjusting for age, sex, corticosteroids and disease duration. Furthermore, in patients with recent-onset disease (n=41) a stronger correlation was found between OPN and SLEDAI-2K (r=0.666, p=0.028) when adjusting for age, sex, corticosteroids and disease duration. Patients with ongoing nephritis at sampling had higher levels of OPN compared with patients with a history of nephritis (p=0.008), and with patients without a history of nephritis (p<0.0001) (figure 2). OPN levels were also analysed in the consecutive samples from 14 cases (see online supplementary figure 1). To evaluate if OPN reflects disease activity over time, we compared OPN levels between the time point of highest disease activity and lowest disease activity, respectively. Despite the visual impressions of a moderate compliance with disease activity, no significant differences were observed.
Figure 2.
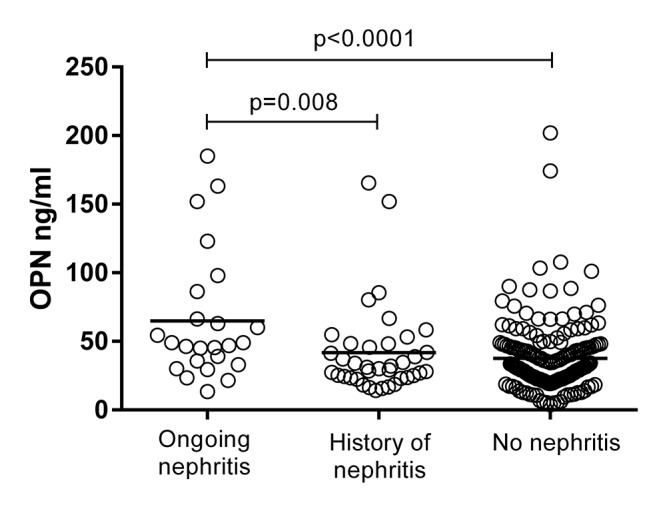
Serum osteopontin (OPN) levels in SLE cases with nephritis. Higher OPN levels were found among patients with ongoing nephritis (mean 65.0 ng/mL), compared with patients with a history of nephritis (mean 41.8 ng/mL) and patients without a history of nephritis (mean 37.7 ng/mL).
lupus-2017-000225supp001.jpg (878.2KB, jpg)
OPN reflects global organ damage
The mean SDI score was 1.1, while the median value was 0 (range 0–9; table 1). The proportion of patients with organ damage is presented in table 1. A correlation was identified between OPN and SDI (r=0.374, p<0.0001). A univariate general linear model was used to evaluate the impact of organ damage on OPN levels, and be able to adjust for age, sex, corticosteroids and disease duration. The relation between OPN and global organ damage (SDI) is shown in figure 3A. OPN was strongly associated with SDI (p<0.0001) (table 2) and patients with extensive damage (ie, SDI≥3) displayed increased levels of OPN (mean 68.4±44.9 ng/mL) compared with patients with moderate damage (ie, SDI 1–2, mean 36.0±25.0 ng/mL, p<0.0001) and no damage (ie, SDI=0, mean 35.6±22.9 ng/mL, p<0.0001) (figure 3B). Furthermore, separating SDI into different organ systems revealed a significant positive impact on OPN levels for the renal (p<0.0001), cardiovascular (p<0.0001) and malignancy (p=0.012) domains (table 2).
Figure 3.
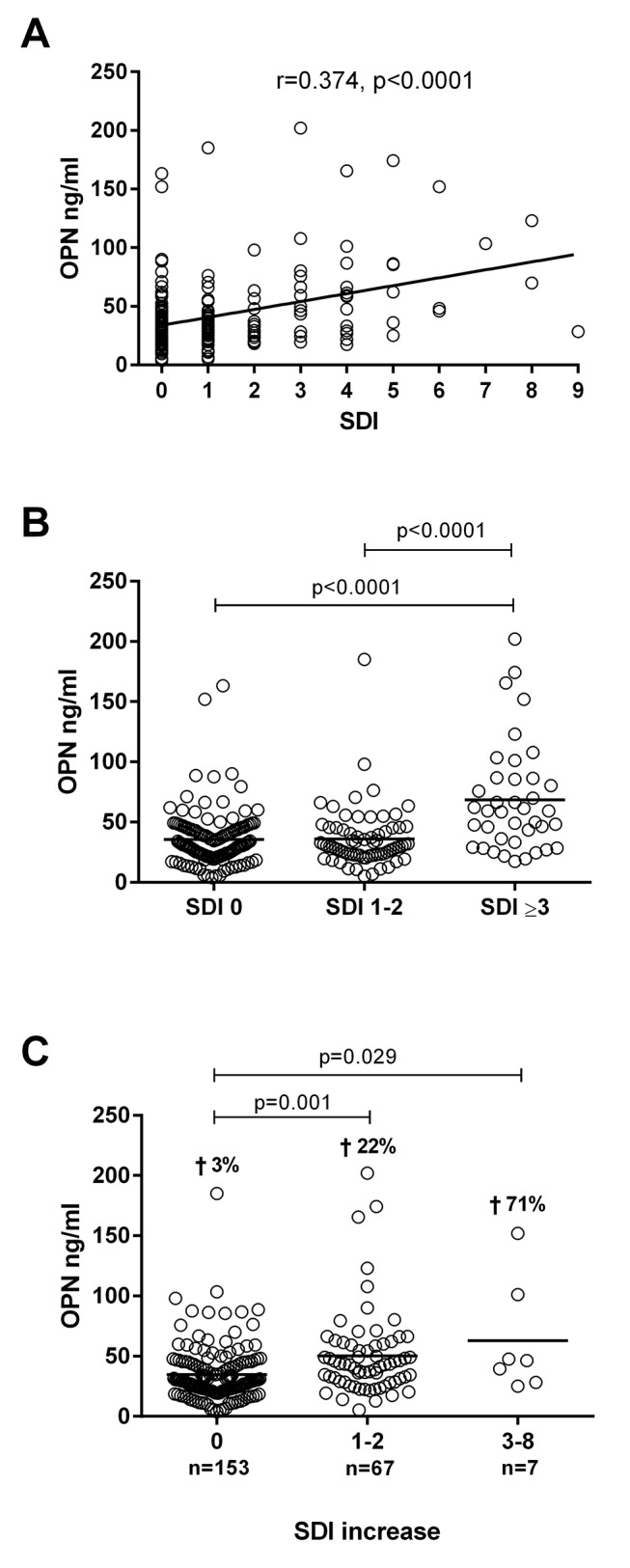
Association between serum osteopontin (OPN) and damage accrual. (A) Correlation between serum levels of OPN and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Correlation coefficient and p value are not adjusted for sex, age, corticosteroids and disease duration. (B) Patients with extensive damage (ie, SDI≥3) displayed increased levels of OPN (mean 68.4 ng/mL) compared with patients with moderate damage (ie, SDI 1–2; mean 36.0 ng/mL) and no damage (ie, SDI=0; mean 35.6 ng/mL). (C) Patients with highly elevated SDI (ie, SDI increase 3–8; mean 62.9 ng/mL) and moderately elevated SDI (ie, SDI increase 1–2; mean 50.4 ng/mL) had significantly higher OPN levels compared with patients with no SDI increase (mean 34.8 ng/mL). Crosses indicate the percentage of deceased patients for each SDI category.
Table 2.
The impact of damage accrual and clinical events related to APS on OPN levels
| Variable | B | p-value |
| SDI / SDI domain | ||
| Global SLICC/ACR DI | 6.5 | <0.0001 |
| Renal | 18.8 | <0.0001 |
| Cardiovascular | 12.3 | <0.0001 |
| Malignancy | 18.1 | 0.012 |
| Clinical APS related events | ||
| Valvular surgery | 38.8 | <0.0001 |
| Valvular heart disease | 26.1 | <0.0001 |
| Myocardial infarction | 17.4 | 0.019 |
| Ischaemic stroke | 14.1 | 0.026 |
| Arterial embolism | 12.6 | 0.031 |
| Any arterial event | 11.3 | 0.044 |
| Pulmonary embolism | 16.9 | 0.053 |
All univariate general linear models are adjusted for sex, age, corticosteroids and disease duration.
APS, antiphospholipid syndrome; OPN, osteopontin; SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SLICC/ACR DI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Disease Index.
Raised OPN precedes damage accrual
To investigate a possible predictive value of OPN, the change in SDI between study inclusion and 2–6 years after inclusion was calculated. Significantly higher OPN levels were found among patients with highly elevated SDI (ie, SDI increase between 3 to 8, p=0.029), and patients with moderately elevated SDI (ie, SDI increase 1–2, p=0.001), compared with patients without SDI increase (figure 3C). In addition, higher death rates were found among patients in the two groups with SDI increase. Relations between disease activity and organ damage, respectively, with OPN were assessed using stepwise linear regression model including SLEDAI-2K, SDI, age, sex, corticosteroids and disease duration with OPN as the response variable. This model retained SDI (p<0.0001), but not SLEDAI-2K.
OPN is associated with APS
A univariate general linear model was used to evaluate the impact of APS associated clinical and laboratory manifestations on OPN levels. Analysing the different disease manifestations revealed positive significant impact of APS on OPN levels (p=0.009; figure 4). When dissecting APS with regard to clinical manifestations related to APS, we found arterial event (p=0.044), myocardial infarction (p=0.019), ischaemic stroke (p=0.026), arterial emboli (p=0.031), valvular heart disease (p<0.0001) and valvular surgery (p<0.0001) to have positive significant impact on OPN levels (table 2). A borderline significance was observed for pulmonary embolism (p=0.053). Regarding laboratory items included in the APS criteria (ie, the lupus anticoagulant, anticardiolipin and anti-β2-glycoprotein-I antibodies), we found associations with a positive lupus anticoagulant test (p=0.033) and IgM anticardiolipin antibodies (p=0.027). However, no differences were found between triple positive (lupus anticoagulant and IgG/IgM anticardiolipin and IgG/IgM anti-β2-glycoprotein-I antibodies) patients compared with those that were not.
Figure 4.
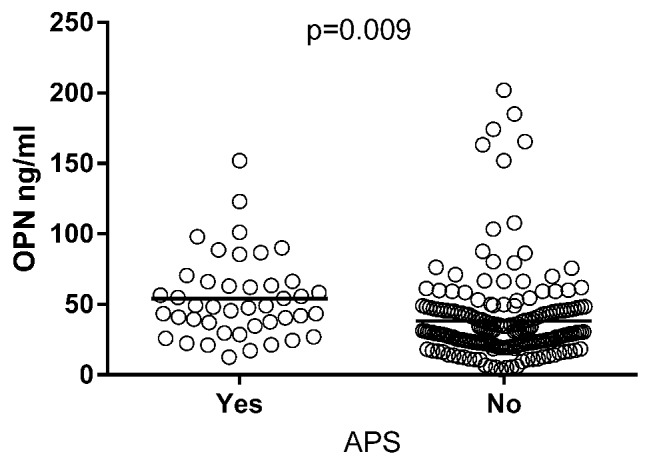
Serum osteopontin (OPN) levels in SLE cases with antiphospholipid syndrome (APS). Patients classified with APS showed higher levels of OPN (mean 54.1 ng/mL) compared with patients without APS (mean 38.2 ng/mL). The p value is adjusted for sex, age, corticosteroids and disease duration.
Discussion
The heterogeneity of SLE motivates the search for more informative biomarkers which mirror general disease activity and/or organ damage. OPN has recently been argued to identify cases prone to develop organ damage,15 and was previously shown to associate with disease activity17 as well as with renal lupus.16 The aim of the present study was to evaluate OPN in this context. The results indicate that OPN levels reflect irreversible global organ damage and, particularly, damage within the renal, cardiovascular and malignancy domains of SDI. Furthermore, the level of OPN was also associated with several clinical events of APS (primarily on the arterial side), which also constitute parts of SDI.
In line with Lee et al, 7 we detected elevated levels of OPN in patients with SLE compared with controls. Correlation analysis showed an association between OPN and disease activity (ie, SLEDAI-2K) and, looking separately at patients with recent-onset disease, the correlation between SLEDAI-2K and OPN was even more convincing. Furthermore, we found that OPN and global organ damage (SDI) were highly positively associated. Rullo et al reported that increased circulating OPN levels preceded increased cumulative disease activity and organ damage in patients with SLE, especially in paediatric SLE.15 The stepwise linear regression model in the present study, including both SLEDAI-2K and SDI with OPN as the response variable, retained SDI but not SLEDAI-2K in the model. The analysis of the longitudinal variations of OPN and SLEDAI-2K showed no distinct association with disease activity. Our cross-sectional analyses imply that OPN is a marker of disease activity among patients with recent-onset disease, whereas later on, in established disease, it serves as a marker of organ damage. The SDI increase calculated from SDI values at inclusion and 2–6 years after inclusion showed significantly higher OPN levels among patients with moderately or highly elevated SDI after study inclusion, as compared with patients without SDI increase. This implies that OPN is a marker of future organ damage. It is known that the SDI value is a good predictor of survival as well as of mortality.26 27 In line with this, we found higher death rates among patients in the two groups with SDI increase. However, we certainly acknowledge that the analyses of OPN versus future SDI increase may be biased both by the fact that pre-existing organ damage per se predicts further subsequent organ damage,27–29 and the limited follow-up time (2–6 years).
Another limitation of our study is the low number (17%) of cases with recent-onset disease. Longitudinal studies in cohorts with recent-onset SLE are highly warranted to further investigate if OPN precedes organ damage and thus acts as a predictor.
When SDI was separated into the different organ systems, we found a significant positive impact on OPN levels for the renal, cardiovascular and malignancy SDI domains. High levels of OPN have earlier been found to associate with renal impairment in SLE,16 17 and it has been hypothesised that OPN plays a part in a vicious circle of inflammatory damage in the kidneys, leading to persistent proteinuria and interstitial fibrosis.30 31 Furthermore, in OPN knockout mice less infiltration of macrophages and reduced fibrosis was seen,32 just as treatment with anti-OPN in nephritic rats reduces albuminuria and invasion of macrophages.33
We also investigated the association of OPN with different clinical presentations. Patients with nephritis at sampling had significantly higher levels of OPN. Patients meeting classification criteria for APS also displayed increased levels of OPN. Dissection of APS into associated clinical manifestations revealed that several events had positive significant impact on OPN levels. In contrast to Quaglia et al, who did not find any APS associations,16 we identified associations regarding OPN and a positive lupus anticoagulant test, as well as with the occurrence of IgM anticardiolipin antibodies. OPN levels have previously been linked to manifestations on the arterial side, such as the severity of coronary atherosclerosis, increased risk for major adverse cardiac events and peripheral arterial disease.34 35 However, the role of OPN in cardiovascular disease is not fully clear. Some studies have suggested that OPN is an enhancer of atherosclerosis due to its proinflammatory property.34 36 On the other hand, OPN may also exert potentially protective vascular effects. Increased expression of OPN has been hypothesised to play a protective role in postmyocardial infarction by recruiting macrophages and neutrophils to clean up debris from dead cells.37 In addition, OPN is also able to modulate collagen deposition and fibrosis.38
OPN is likely to play a critical role in chronic inflammation and, in SLE, potentially due to insufficient waste disposal. Overexpression of OPN in lupus prone mice induces B cell activation and subsequent production of anti-dsDNA antibodies,5 6 and intracellular expression of OPN in pDC is required for TLR9-dependent expression of IFN-α.4 The antibodies may form immune complexes that deposit in tissue and cause inflammation in situ. Furthermore, OPN induces migration, activation and macrophage cytokine production.39 40 Defective clearance of apoptotic cells is a central feature of the SLE pathogenesis and OPN has been shown to inhibit apoptosis.6 41 In this way a vicious circle of impaired clearance, autoantigen exposure, autoantibody production, chronic inflammation and tissue damage may be fuelled and refuelled.
To our knowledge this is the first study reporting a relationship between OPN and APS in SLE. In primarily established cases of SLE, OPN appears to reflect damage accrual and cardiovascular damage. The association with APS may predominantly relate to the damage occurring in connection with arterial events. To conclude, circulating OPN associates with APS and appears to be a marker of disease severity. Longitudinal studies are warranted to further investigate whether or not OPN precedes organ damage and thus acts as a predictor.
Acknowledgments
The authors thank Marianne Peterson for biobank administration, Lars Valter for advice on statistical analyses and Jan Ernerudh for providing control subjects. The authors also thank Elisabet Svenungsson and Kerstin Elvin for help with the antiphospholipid antibody assays. The authors also thank Leonid Padyukov and the EIRA Study personnel for providing information and sera from population-based control subjects for their study.
Footnotes
Contributors: LW performed the OPN ELISA, contributed to study design, statistical analysis, interpretation of data and manuscript writing. MF contributed to APS data and manuscript drafting. HE contributed to interpretation of data and manuscript drafting. TS contributed to the original idea and study design, interpretation of data and manuscript writing. JW contributed to the original idea and study design, interpretation of data and manuscript writing. CS contributed to the original idea and study design, patient characterisation, interpretation of data and manuscript writing.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Regional Ethics Review Board in Linköping, Sweden. Decision No. M75-08/2008.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1. Kaleta B. Role of osteopontin in systemic lupus erythematosus. Arch Immunol Ther Exp 2014;62:475–82. doi:10.1007/s00005-014-0294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rönnblom L, Alm GV, Eloranta ML. The type I interferon system in the development of lupus. Semin Immunol 2011;23:113–21. doi:10.1016/j.smim.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 3. Rönnblom L, Alm GV. The natural interferon-alpha producing cells in systemic lupus erythematosus. Hum Immunol 2002;63:1181–93. doi:10.1016/S0198-8859(02)00757-7 [DOI] [PubMed] [Google Scholar]
- 4. Shinohara ML, Lu L, Bu J, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol 2006;7:498–506. doi:10.1038/ni1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iizuka J, Katagiri Y, Tada N, et al. Introduction of an osteopontin gene confers the increase in B1 cell population and the production of anti-DNA autoantibodies. Lab Invest 1998;78:1523–33. [PubMed] [Google Scholar]
- 6. Sakamoto K, Fukushima Y, Ito K, et al. Osteopontin in spontaneous germinal Centers inhibits apoptotic cell Engulfment and promotes Anti-Nuclear antibody production in Lupus-Prone mice. J Immunol 2016;197:2177–86. doi:10.4049/jimmunol.1600987 [DOI] [PubMed] [Google Scholar]
- 7. Lee YH, Song GG. Correlation between circulating osteopontin level in systemic lupus erythematosus and disease activity and associations between osteopontin polymorphisms and disease susceptibility: a meta-analysis. Lupus 2016;26:132–8. doi:10.1177/0961203316655214 [DOI] [PubMed] [Google Scholar]
- 8. Bengtsson AA, Rönnblom L. Systemic lupus erythematosus: still a challenge for physicians. J Intern Med 2017;281:52–64. doi:10.1111/joim.12529 [DOI] [PubMed] [Google Scholar]
- 9. Pons-Estel GJ, Andreoli L, Scanzi F, et al. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J Autoimmun 2017;76:10–20. doi:10.1016/j.jaut.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 10. Ünlü O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol 2016;3:75–84. doi:10.5152/eurjrheum.2015.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohan C, Assassi S. Biomarkers in rheumatic diseases: how can they facilitate diagnosis and assessment of disease activity? BMJ 2015;351:h5079 doi:10.1136/bmj.h5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enocsson H, Sjöwall C, Wirestam L, et al. Four Anti-dsDNA antibody assays in relation to systemic lupus erythematosus disease specificity and activity. J Rheumatol 2015;42:817–25. doi:10.3899/jrheum.140677 [DOI] [PubMed] [Google Scholar]
- 13. Sjöwall C, Olin AI, Skogh T, et al. C-reactive protein, immunoglobulin G and complement co-localize in renal immune deposits of proliferative lupus nephritis. Autoimmunity 2013;46:205–14. doi:10.3109/08916934.2013.764992 [DOI] [PubMed] [Google Scholar]
- 14. Orbai AM, Truedsson L, Sturfelt G, et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus 2015;24:42–9. doi:10.1177/0961203314547791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rullo OJ, Woo JM, Parsa MF, et al. Plasma levels of osteopontin identify patients at risk for organ damage in systemic lupus erythematosus. Arthritis Res Ther 2013;15:R18 doi:10.1186/ar4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quaglia M, Chiocchetti A, Cena T, et al. Osteopontin circulating levels correlate with renal involvement in systemic lupus erythematosus and are lower in ACE inhibitor-treated patients. Clin Rheumatol 2014;33:1263–71. doi:10.1007/s10067-014-2665-4 [DOI] [PubMed] [Google Scholar]
- 17. Wong CK, Lit LC, Tam LS, et al. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology 2005;44:602–6. doi:10.1093/rheumatology/keh558 [DOI] [PubMed] [Google Scholar]
- 18. Ighe A, Dahlström Ö, Skogh T, et al. Application of the 2012 Systemic Lupus International Collaborating Clinics classification criteria to patients in a regional Swedish systemic lupus erythematosus register. Arthritis Res Ther 2015;17:3 doi:10.1186/s13075-015-0521-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frodlund M, Dahlström O, Kastbom A, et al. Associations between antinuclear antibody staining patterns and clinical features of systemic lupus erythematosus: analysis of a regional Swedish register. BMJ Open 2013;3:e003608 doi:10.1136/bmjopen-2013-003608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. doi:10.1002/art.1780251101 [DOI] [PubMed] [Google Scholar]
- 21. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. doi:10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. doi:10.1002/art.1780390303 [DOI] [PubMed] [Google Scholar]
- 23. Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 24. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. doi:10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 25. Saevarsdottir S, Wedrén S, Seddighzadeh M, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the epidemiological investigation of rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum 2011;63:26–36. doi:10.1002/art.27758 [DOI] [PubMed] [Google Scholar]
- 26. Nived O, Jönsen A, Bengtsson AA, et al. High predictive value of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for survival in systemic lupus erythematosus. J Rheumatol 2002;29:1398–400. [PubMed] [Google Scholar]
- 27. Bruce IN, O'Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. doi:10.1136/annrheumdis-2013-205171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alarcon GS, Roseman JM, McGwin G, et al. Damage as a predictor of further damage. Rheumatology 2004;43:202–5. [DOI] [PubMed] [Google Scholar]
- 29. Legge A, Doucette S, Hanly JG. Predictors of organ damage progression and effect on Health-related Quality of Life in systemic lupus erythematosus. J Rheumatol 2016;43:1050–6. doi:10.3899/jrheum.150985 [DOI] [PubMed] [Google Scholar]
- 30. Kramer AB, Ricardo SD, Kelly DJ, et al. Modulation of osteopontin in proteinuria-induced renal interstitial fibrosis. J Pathol 2005;207:483–92. doi:10.1002/path.1856 [DOI] [PubMed] [Google Scholar]
- 31. Hsieh C, Chang A, Brandt D, et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res 2011;63:865–74. doi:10.1002/acr.20441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Persy VP, Verhulst A, Ysebaert DK, et al. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int 2003;63:543–53. doi:10.1046/j.1523-1755.2003.00767.x [DOI] [PubMed] [Google Scholar]
- 33. Panzer U, Thaiss F, Zahner G, et al. Monocyte chemoattractant protein-1 and osteopontin differentially regulate monocytes recruitment in experimental glomerulonephritis. Kidney Int 2001;59:1762–9. doi:10.1046/j.1523-1755.2001.0590051762.x [DOI] [PubMed] [Google Scholar]
- 34. Wolak T. Osteopontin - a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014;236:327–37. doi:10.1016/j.atherosclerosis.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 35. Koshikawa M, Aizawa K, Kasai H, et al. Elevated osteopontin levels in patients with peripheral arterial disease. Angiology 2009;60:42–5. doi:10.1177/0003319708314250 [DOI] [PubMed] [Google Scholar]
- 36. Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 2007;27:2302–9. doi:10.1161/ATVBAHA.107.144824 [DOI] [PubMed] [Google Scholar]
- 37. Singh M, Foster CR, Dalal S, et al. Role of osteopontin in heart failure associated with aging. Heart Fail Rev 2010;15:487–94. doi:10.1007/s10741-010-9158-6 [DOI] [PubMed] [Google Scholar]
- 38. Ashizawa N, Graf K, Do YS, Ys D, et al. Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J Clin Invest 1996;98:2218–27. doi:10.1172/JCI119031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber GF, Zawaideh S, Hikita S, et al. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol 2002;72:752–61. [PubMed] [Google Scholar]
- 40. Ma R, Jiang W, Li Z, et al. Intrarenal macrophage infiltration induced by T cells is associated with podocyte injury in lupus nephritis patients. Lupus 2016;25:1577–86. doi:10.1177/0961203316646861 [DOI] [PubMed] [Google Scholar]
- 41. Fan K, Dai J, Wang H, et al. Treatment of collagen-induced arthritis with an anti-osteopontin monoclonal antibody through promotion of apoptosis of both murine and human activated T cells. Arthritis Rheum 2008;58:2041–52. doi:10.1002/art.23490 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2017-000225supp001.jpg (878.2KB, jpg)


