Abstract
Programmed cell death 2 (Pdcd2) is a highly conserved protein of undefined function, and is widely expressed in embryonic and adult tissues. The observations that knockout of Pdcd2 in the mouse is embryonic lethal at preimplantation stages, and that in Drosophila, Zfrp8, the ortholog of Pdcd2, is required for normal lymph gland development suggest that Pdcd2 is important for regulating hematopoietic development. Through genetic and functional studies, we investigated pdcd2 function during the zebrafish ontogeny. Knockdown of pdcd2 expression in zebrafish embryos resulted in defects in embryonic hematopoietic development. Loss of pdcd2 function caused increased expression of progenitor markers, and accumulation of erythroid progenitors during primitive hematopoiesis. Additionally, hematopoietic stem cells (HSCs) failed to appear in the aorta-gonad mesonephros, and were not able to terminally differentiate or reconstitute hematopoiesis. Pdcd2 effects on HSC emergence were cell autonomous and P53-independent, and loss of pdcd2 function was associated with mitotic defects and apoptosis. Restoration of runx1 function(s) and modulation of apoptosis through the inhibition of Jak/Stat signaling rescued the hematopoietic and erythroid defects resulting from pdcd2 knockdown. Our studies suggest that pdcd2 plays a critical role in regulating the transcriptional hierarchy controlling hematopoietic lineage determination. Furthermore, the effects of pdcd2 in regulating mitotic cell death may contribute to its role(s) in directing hematopoietic differentiation during development.
Introduction
In all vertebrates from zebrafish to human, hematopoiesis is a dynamic process that occurs in successive stages and at distinct anatomic sites [1]. The successive hematopoietic waves are directed by morphogenetic movements, and by expression of transcription factors that regulate lineage commitment and cellular reprogramming [2]. In mammals, primitive hematopoiesis is characterized by generation of primitive macrophages and erythroid progenitors in the extraembryonic yolk sac. In zebrafish, this first hematopoietic wave occurs in 2 distinct regions in the anterior lateral mesoderm (ALM) and posterior lateral mesoderm (PLM) [2]. The bilateral stripes of cells in the PLM later converge at midline to form the intermediate cell mass (ICM), the zebrafish equivalent to mammalian yolk sac blood islands at the 20-somite stage or ∼19-hour postfertilization (hpf) [2]. The specification of PLM-derived cells into hematopoietic lineages marked by scl, gata2, and lmo2, and endothelial lineages marked by flk1 and fli1 occurs before the morphogenetic movement of the PLM to form the ICM [3,4]. PLM-derived cells later differentiate into proerythroblasts and endothelial cells in the trunk vasculature. Within the ICM, proerythroblasts that are gata1+ enter the circulation between 24–26 hpf, and subsequently mature into primitive erythrocytes.
A transient wave between primitive and definitive hematopoiesis is reported to occur between 1 and 2 days postfertilization (dpf) in the zebrafish posterior blood island, and is characterized by the appearance of bipotential erythromyeloid progenitors (EMP) [5]. In zebrafish as well as in the mouse, the close association between hematopoietic cells and the developing endothelium suggests that both lineages arise from either a mesodermal progenitor(s) with endothelial and hematopoietic potential called the hemangioblast [2], or from the hemogenic endothelium. Using single-cell labeling and live-cell imaging, hemangioblasts have been reported in zebrafish [6], and differentiation of hematopoietic cells from the hemogenic endothelium was demonstrated [7–9]. The 2 views are not mutually exclusive and it has been proposed that flk1+ hemangioblasts first generate the hemogenic endothelium in a step that depends on scl expression, and then produce definitive hematopoietic cells through runx1 expression [10].
Multipotent definitive hematopoietic stem cells (HSCs) that express runx1 and c-myb [2], and are capable of self-renewal and generation of all definitive blood lineages first emerge from a region between the dorsal aorta and the posterior cardinal vein equivalent to the aorta-gonad mesonephros (AGM) region in mammals [11,12]. From 48 hpf, c-myb+ cells are found in the posterior region in the tail called the caudal hematopoietic tissue (CHT) [13,14], a site equivalent to mammalian fetal liver. These c-myb+ cells later appear in the thymus from 3 dpf and in the kidney marrow from 4 dpf [13,14], the sites for adult hematopoiesis in larval and adult zebrafish.
Runx1 is a key regulator of definitive hematopoiesis in all vertebrates, including zebrafish [11,12]. Runx1 expression modulates both the efficiency of HSC emergence from the hemogenic endothelium as well as the survival of these HSCs [8,15]. The formation of HSCs expressing runx1 and cmyb is known to require blood flow [16]. Further, hematopoietic differentiation is regulated by equilibrium between downstream transcription factors, such as gata1 and pu.1 that regulate myeloid versus erythroid progenitor cell fate [17], where gata1 promotes erythroid differentiation [18], while pu.1 marks early myelopoiesis and promotes myeloid differentiation [19].
Clearly, many important regulators of hematopoiesis and HSC function remain to be described. Programmed cell death 2 (Pdcd2) was initially identified among genes upregulated in rat thymocytes triggered to undergo apoptosis by irradiation or dexamethasone treatment [20]. Studies in Drosophila suggested that pdcd2 plays an important role in hematopoiesis [21]. Mutations in the Drosophila ortholog of pdcd2, zfrp8, resulted in hyperplasia of the fly lymph gland, and zfrp8 was subsequently shown to be essential for maintaining the stem cell population, but dispensable for their daughter progenitor cells [21,22]. Interestingly, transcriptional profiling of various stem cells identified Pdcd2 among genes enriched in mouse embryonic, neural, and HSCs [23]. Pdcd2 is highly expressed in mouse blastocysts [24], and is required for ESC maintenance [25]. PDCD2 is also enriched in human ESCs compared to their differentiated lineages [26]. Human PDCD2 is located on chromosome 6q27 in a region involved in both translocations and deletions in leukemias and lymphomas [27]. This involvement suggests that PDCD2 is a potential tumor suppressor gene [28] that might be required for myeloid and/or lymphoid cell fate.
Little is known of the function of PDCD2, although it has been shown to physically interact with the host cell factor-1 (hcf1) that controls cell cycle progression, and to bind to N-CoR through its MYND domain implying a possible role in transcriptional regulation [29]. We hypothesized that pdcd2 is required for normal hematopoiesis in zebrafish. We demonstrate that loss of pdcd2 perturbs embryonic hematopoietic development, and this phenotype was associated with cell cycle defects and apoptosis, demonstrating a critical role for pdcd2 in regulating hematopoietic development.
Materials and Methods
Fish maintenance
The transgenic zebrafish Fli1:EGFP and gata1:dsRed, and the P53 mutant zebrafish lines were purchased from the zebrafish international resource center (ZIRC) (University of Oregon). Transgenic CD41:EGFP zebrafish were previously described [30]. Zebrafish were maintained at 28.5°C in a recirculating aquaculture system at the zebrafish facility of Cancer Institute of New Jersey according to guidelines established at UMDNJ.
mRNA synthesis, morpholinos, and microinjection
Capped pdcd2 mRNA was synthesized using the mMessage mMachine kit (Ambion). RNA synthesis for in situ probes was performed using either digoxigenin- or Fluorescein-UTP (Roche). Morpholino oligonucleotides (MO; Gene Tools LLC) were ATG-MO (5′-TGTATTTGCAGCCATCTGTCAAACT-3′), which targets the 5′UTR of pdcd2, and 5ssMO (5′-TAACATCTGTTTTGCATCACCTGGA-3′), which targets splicing of intron 5. Control MO had 5 mismatched nucleotides to the ATG-MO displayed in lower cases (5′-TGTAaTTcCAGCg- ATCTGTgAAtCT-3′). Each MO was injected into 1–2 cell embryos after titration at 5.5, 2, and 5.5 ng/embryo, respectively. A P53 MO (5′-GCGCCATTGCTTTGCAAGAATTG-3′) was used at 4.5 ng/embryo.
Whole-mount in situ hybridization, histology, and immunohistochemistry
RNA whole-mount in situ hybridization (WISH) for zebrafish pdcd2, scl, c-myb, pu.1, gata1, runx1, lmo2, rag1, hbae1, mpo, l-plastin, and lck were performed as described [31]. Immunohistochemistry (IHC) for human, mouse, and zebrafish PDCD2 was performed as described [32] using a polyclonal anti-PDCD2 antibody (1:500; Proteintech). Mouse embryo collections, mouse Pdcd2 IHC, and 2,7-diaminofluorene (DAF) labeling for zebrafish were performed as described (Supplementary Data are available online at www.liebertpub.com/scd). Phospho-histone H3 (p-H3) was detected using a polyclonal anti-p-H3 antibody (1:750; Millipore), the Alexa-555 secondary antibody (Invitrogen), and DAPI counterstaining. Periodic Acid Schiff (PAS) staining (Sigma) and Sudan Black labeling were performed as described [33]. Apoptosis assays and BrdU labeling were performed as described [34].
Flow cytometry, fluorescent activated cell sorting, and cytology
Embryos were prepared for cytometry as described ([35], and Supplementary Data). Cell sorting of gata1:DsRed embryos was performed using a BD cell sorter, and transplantation was performed as described [16]. For cytology, DsRed+ cells were spun for 3 min at 300 rpm using a cytospin3 centrifuge (Shandon), and slides were stained with May-Grunwald Giemsa.
Generation of transgenic pdcd2 reporter zebrafish
Transgenesis was achieved by injection of pdcd2 (−1.6 kb):AcGFP in one-cell stage embryos as described ([32], and Supplementary Data). Multiple founder lines were established based on enhanced green fluorescent protein (EGFP) expression. RNA preparations for RT-PCR were done using the RNAqueous kit (Ambion). Oligo-dT primed cDNA from embryonic stages and tissues were used for PCR using pdcd2-, runx1-, and β-actin-specific primers (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd).
Results
Identification of the zebrafish pdcd2 gene and putative regulatory elements
We found the zebrafish pdcd2 gene [zgc:63685, designated as ENSDARG00000007892 in Ensembl (zv8)] to be composed of 6 exons spanning 3,766 bp encoding a 358 aa protein (∼47 kDa), which shares significant homology, a central MYND, and a C-terminal PDCD2 domain with all pdcd2 orthologs (Supplementary Fig. S1A, D). Phylogenetic analysis using the PDCD2 domain suggests that pdcd2 is conserved among all eukaryotes (Supplementary Fig. S1A–D). Zebrafish pdcd2 is located on chromosome 13, and despite a smaller zebrafish chromosome size, both pdcd2 and the syntenic neighboring gene tbp are conserved (Supplementary Fig. S1C). To identify the zebrafish pdcd2 promoter and subsequently characterize the in vivo expression of pdcd2, we performed in silico promoter and transcription factor-binding site (TFBS) analyses. A minimal 1.6-Kb core promoter region was identified, and included a proximal 800-bp region containing over 500 TFBS, including factors regulating hematopoiesis, such as runx1, gata factors, c-myb, ap1, c/ebp, scl/tal1, p53, lmo2, ets factors, and stat (data not shown). We utilized this zebrafish pdcd2 promoter to drive EGFP expression in a transgenic reporter line, and the expression of EGFP in these transgenic embryos was detected ubiquitously throughout embryonic development (Supplementary Fig. S2A, B). Importantly, EGFP was detected in circulating blood cells in the stable transgenic pdcd2 (−1.6 kb):EGFP embryos at ∼26 hpf (Supplementary Movie S1).
Expression of pdcd2 in human, mouse, and zebrafish during development
As noted above, it has been suggested that PDCD2 plays a role in development and hematopoiesis. We therefore investigated pdcd2 expression in human, mouse, and zebrafish tissues using IHC and RNA WISH. Pdcd2 expression is ubiquitous and expressed in hematopoietic tissues in normal human bone marrow (BM) (Fig. 1A, B), mouse fetal liver (Fig. 1C–E), and zebrafish embryonic stages (Fig. 1F–H). Using WISH of developing zebrafish embryos, pdcd2 mRNA was found to be maternally expressed at the 4-cell stage (Supplementary Fig. S3A), and widely distributed during gastrulation and somitogenesis (Supplementary Fig. S3B–F). In zebrafish embryos, pdcd2 mRNA was detected by RT-PCR during the first 5 days of embryonic development (Supplementary Fig. S3G). Strong pdcd2 expression was detected in the ICM (Fig. 1G), and at the AGM region, the site of HSC formation at later stages (Fig. 1H). In adult fish, pdcd2 expression was also detectable in all tissues examined except muscle, with a substantial enrichment in kidney marrow (Supplementary Fig. S3H). Collectively, pdcd2 expression analyses in mammals and zebrafish suggest that while pdcd2 is expressed ubiquitously, it is highly enriched in both neural and hematopoietic tissues.
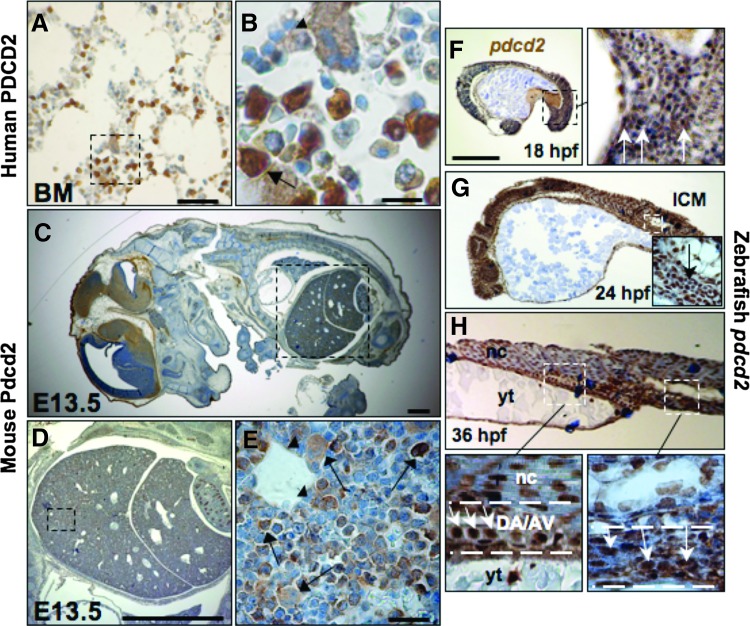
FIG. 1.
PDCD2 expression in human, mouse, and zebrafish tissues. IHC of PDCD2 expression in human BM (A, B), mouse fetal liver (C–E), and zebrafish embryos (F–H). (B) is a higher magnification of outlined area in (A). Notice the dense nuclear and cytoplasmic staining in progenitor-like cells (arrow) compared with a lighter staining in surrounding differentiated cells, and in a megakaryocyte (arrowhead). (C) IHC of Pdcd2 expression in a sagital section of an E13.5 mouse embryo showing the fetal liver (outlined area) in (C), and higher magnifications in (D) and (E). Strong Pdcd2 expression is seen in hematopoietic and dividing cells (arrows in E), but not in cells lining the liver sinusoids (arrowhead in E). Pdcd2 protein expression by IHC of zebrafish embryos with strong ubiquitous expression in early embryonic stages at 18 hpf (F), 24 hpf (G), and 36 hpf (H). Insets are magnifications of the outlined areas in G and H). Arrows point to hematopoietic cells in the ICM and AGM regions. Images are lateral view, anterior to the left, and dorsal upward. Scale bars are 100 μm in (A, F, and G), 20 μm in (B, E, and H), and 500 μm in (C, D, and F). Regions outlined include the AGM, and the tail region that would latter contain the CHT/PBI. nc, notochord; yt, yolk tube; DA/AV, dorsal aorta and axial vein region; AGM, aorta-gonad mesonephros; BM, bone marrow; CHT, caudal hematopoietic tissue; hpf, hour postfertilization; ICM, intermediate cell mass; IHC, immunohistochemistry; Pdcd2, programmed cell death 2. Color images available online at www.liebertpub.com/scd
Pdcd2 knockdown during zebrafish embryonic development
We assessed zebrafish development after pdcd2 site-directed knockdown using pdcd2-specific MO. We used mismatched MOs as controls, and 2 pdcd2-specific MOs: ATGMO, which targets the translational start codon, and 5ssMO, which represses the splicing of intron 5 of pdcd2 (Supplementary Fig. S4A).
Western blotting using the anti-pdcd2 antibody and RT-PCR analyses revealed reductions in the pdcd2 protein, or aberrant pdcd2 splicing in ATGMO- or 5ssMO-injected embryos, respectively (Supplementary Fig. S4B, C). Given the widespread pdcd2 expression, we anticipated that pdcd2 knockdown would produce severe developmental defects. However, pdcd2 ATGMO-injected embryos had no apparent defects up to late somitogenesis. Starting at ∼18 hpf and up to 48 hpf, we noted that ATGMO-injected embryos displayed smaller eyes, blocky rather than chevron-shaped somites, dilated brain ventricles, oblong-shaped yolk with shortened yolk tube, and curly and thickened tails compared to control embryos (Fig. 2A–D). None of the mismatch MO-injected embryos displayed this phenotype. Injections of the pdcd2 splice 5ssMO reproduced these defects at significantly lower doses (2 ng for 5ssMO vs. 5.5 ng for ATGMO). These phenotypes were seen in ∼90% of ATGMO- or 5ssMO-injected embryos. Given that 5ssMO was most efficient in repressing pdcd2 expression (Supplementary Fig. S4C), we used the 5ssMO in most of our studies.
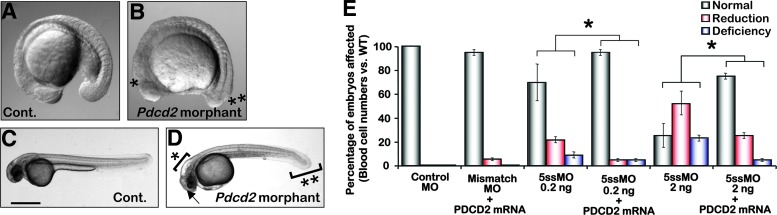
FIG. 2.
Developmental defects in primitive hematopoiesis in pdcd2 morphants. (A–D) Bright field images of control (A, C) and pdcd2 morpholino injected embryos (B, D) at 18 hpf (A, B) and 48 hpf (C, D). Pdcd2 morphants displayed significantly smaller eyes (arrow), a slightly shorter axis, dilated brain ventricles (*) with brain necrosis, and curly and thickened tails at both 18 and 48 hpf (**) (D). The yolk appeared oblong in shape due to accumulation of hematopoietic cells at the lower anterior part of the yolk sac. These phenotypes were seen in nearly 90% of >5,000 ATGMO- or 5ssMO-injected pdcd2 morphant embryos. (E) Scoring system based on the numbers of blood cells released to the circulation after injection of control-mismatch MO, or pdcd2 5ssMO alone or combined with pdcd2 mRNA to rescue the phenotype. Embryos were classified into 3 classes based on their flowing blood cell numbers: (i) indistinguishable from the control (normal) as indicated by the gray bar, (ii) cell number reduction (<30 cells transiting the axial or intersegmental vessels) (reduction) compared to control as indicated by the red bar, and (iii) severely deficient or almost no flowing blood cells (<10 cells transiting the axial or intersegmental vessels) (deficiency) compared to control as indicated by the blue bar (Bars—s.e.m., *three 5ssMO doses are significantly different than control, mRNA rescue of 5ssMO effects were significantly different than 5ssMO alone, P<0.01). (F–K) Representative fluorescent DsRed images of control MO- (cont.), 5ssMO-, or ATGMO-injected gata1:DsRed embryos at 24 or 48 hpf showing failure of hematopoietic cell release. Primitive erythrocytes have cleared the ICM and entered the major vessels (outlined rectangle) and yolk circulation (outlined circle) in 24 hpf control embryos (F), while in pdcd2 morphant embryos (G, H), cells appear to remain in the ICM region. Gata1-DsRed+ cells are distributed throughout the circulatory system at 48 hpf in control embryos (I) partitioned between the yolk's duct of Cuvier (I′) and the vasculature (e.g., trunk vessels in I′′). DsRed+ cells in pdcd2 morphant embryos (J, K) accumulate at the duct of Cuvier (J′, K′) and fail to continuously release into the interstitial and major trunk vessels (J′′, K′′). Data represent 6 independent experiments utilizing ∼100 embryos/variable, and phenotypes were seen in nearly 90% of control MO-, ATGMO-, or 5ssMO-injected pdcd2 morphant embryos. MO, morpholino oligonucleotides; s.e.m., standard error of the mean. Color images available online at www.liebertpub.com/scd
Loss of pdcd2 impairs primitive erythropoiesis
In zebrafish embryos, primitive erythroid cells undergo synchronized intravasation and release from vessel walls into the circulation at 23–26 hpf [36]. Pdcd2 ATGMO- and 5ssMO-injected embryos (pdcd2 morphants) exhibited a dose-dependent failure in this process and only small numbers of erythrocytes were observed in circulation even at 30 hpf (n≥500 embryos). We attempted to establish a dose–response curve to pdcd2 knockdown by examining the numbers of erythrocytes in circulation through different components of the embryonic vasculature at 48 hpf (Fig. 2E). We observed a dose-dependent effect of pdcd2 knockdown in pdcd2 morphant embryos with high doses, resulting in complete absence of circulating erythrocytes in both axial and intersegmental vessels. Importantly, these effects were only seen in pdcd2 ATGMO- and 5ssMO-injected, but not in mismatch MO-injected embryos, and were rescued by the injection pdcd2 mRNA (Fig. 2E), suggesting that the hematopoietic phenotype is specific to pdcd2 knockdown. Circulation through intersegmental vessels appeared to be most sensitive to pdcd2 knockdown with subtle patterning defects in the number and position of intersegmental vessels. The release of primitive erythrocytes into the circulation must be tightly coordinated with vascular organization and contractions of the primitive heart tube [37,38]. Thus, the hematopoietic circulatory defects in pdcd2 morphants might be attributable to defects in cardiac or vascular development, or due to the presence of defective primitive hematopoietic cells.
Circulating cells during early embryogenesis are comprised mostly of primitive erythrocytes and a few primitive macrophages [2]. Thus, we investigated the effects of pdcd2 knockdown in gata1:DsRed transgenic fish [35] with DsRed-labeled erythrocytes. The initial defects were morphologically visible at onset of circulation when DsRed+ cells from gata1:DsRed pdcd2 morphants showed delayed egress from the ICM (Fig. 2F–H). At a time when control embryos exhibited release of circulatory erythrocytes (from 26 hpf onward), very few erythrocytes were observed to be actively circulating in pdcd2 morphant embryos (Supplementary Movies S2 and S3). Exit of most erythrocytes from the ICM had occurred in >90% of the embryos by 36 hpf, and gata1:DsRed+ cells were observed to accumulate in the yolk vascular sinuses thereafter (n=34; data not shown). By 48 hpf, control embryos exhibited robust numbers of circulating primitive erythrocytes at the duct of Cuvier and trunk vessels, while gata1:DsRed+ cells from pdcd2 morphants remained pooled at the yolk vascular sinuses, and only occasional DsRed+ cells were observed in the trunk vessels and within the interstitial space coincident with the former ICM (Fig. 2J, K, Supplementary Movies S4 and S5). Thus, pdcd2 knockdown results in aberrant erythroid cell movement with limited numbers of cells intravasating into the circulation without the synchrony apparent in control embryos (Compare Supplementary Movie S5 to control in Supplementary Movie S4). Moreover, once within the vasculature, pdcd2 morphant erythrocytes fail to circulate freely, and the small number of circulating erythrocytes present at 48 hpf occasionally traversed the trunk vasculature.
We sought to determine whether the effects upon erythroid cell movements were a secondary consequence of an underlying cardiac or vascular defect. Both light microscopic analyses and microangiography indicate that heart function is not grossly perturbed (Fig. 3A, B). Pdcd2 morphants displayed regular cardiac development and contractions of the primitive heart tubes. Heart rates were 98±4 beats/min in pdcd2 morphants compared to 100±3 beats/min in control MO-injected embryos, and 99±4 beats/min in WT embryos at 32 hpf, which are comparable to previously reported values [37]. Additionally, microangiography with fluoresceinated carboxylated latex beads [39] indicated that pdcd2 morphants had patent vasculature that exhibits plasma flow (compare Fig. 3A–A′ and 3B–B′).
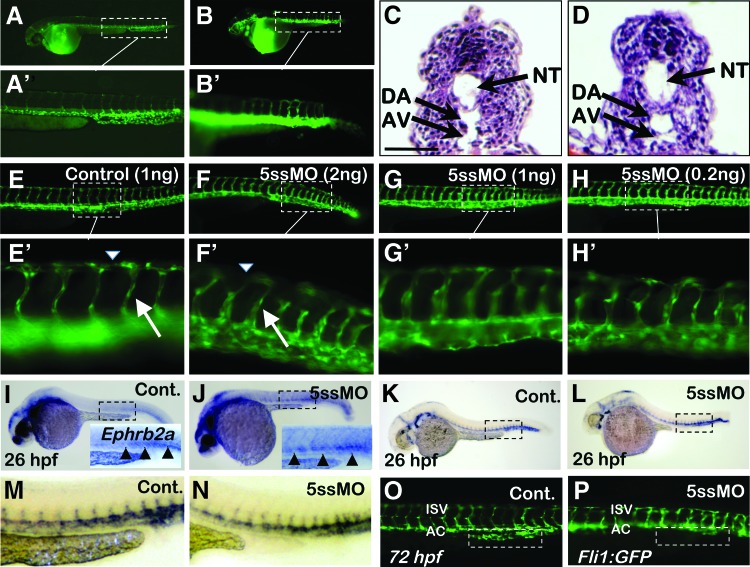
FIG. 3.
Analysis of vasculogenesis in pdcd2 morphant embryos. Microangiography of control (A, A′) and 5ssMO-injected wild-type embryos (B, B′) injected with fluorescein-labeled beads at 48 hpf in the anterior yolk sinus. Images collected within 30 min of injection indicate that the major vessels as well as the smaller ancillary vessels of the trunk are patent and permit blood flow. (C, D) H&E histological sections of the mid-trunk region of control MO-injected (C) and 5ssMO-injected (D) zebrafish embryos. The sections demonstrate the presence of comparable endothelial cell thickness and lumen size of major trunk vessels (black arrows). NT, neural tube; DA, dorsal aorta; AV, axial vein. (E–H) Analysis of dose-dependent effects of pdcd2 knockdown on vascular development in fli1:EGFP embryos shown at 48 hpf. Injection of control or 5ssMO pdcd2 MO had no discernable effect on the presence of major vessels (DA and AV) in the trunk or head (E–H, E′–H′ and data not shown). Intersegmental vessels were present at intersomitic boundaries and appear to be slightly thicker in pdcd2 morphants than in controls (arrows). This may be a consequence of the deformed nature of the somites discussed earlier (Fig. 2B). Gaps in the dorsal axial vein (DLAV) represent regions adjacent to the posterior tail somites that were irregular at higher morpholino inputs (arrowheads). (I, J) WISH of mature endothelial cell marker; ephrin (ephrb2a) in 5ssMO-injected embryos (J) compared to controls (I) at 26 hpf (controls, n=26/26 (100%) showed the displayed phenotype; 5ssMO, n=24/25, 96% showed the displayed phenotype). Arterial specification occurred, and levels of ephrb2a+ cells (arrowheads in inset) were comparable between control and 5ssMO-treated embryos. (K–N) Expression of the generalized endothelial cell marker, flk1, was comparable at 26 hpf between control (K, M) and pdcd2 knockdown embryos (L, N) (control; n=36 (97%) showed the displayed phenotype, 5ssMO; n=47, 89% showed the displayed phenotype). (O, P) Formation of the caudal venous plexus (dashed boxes O, control and P, 5ssMO) in pdcd2 morphants at 72 hpf was defective as visualized in Fli1:EGFP. ISV, intersegmental vessels; AC, axial circulation; EGFP, enhanced green fluorescent protein; WISH, whole-mount in situ hybridization. Color images available online at www.liebertpub.com/scd
To further assess any potential fine perturbations in vascular development, we examined the labeled vasculature in the Fli1:EGFP transgenic embryos with reduced pdcd2 function. Observations of embryos spanning the developmental period during which the embryonic vasculature is established indicated that the major axial vessels in the trunk appear to develop normally (Fig. 3C, D). The number of EGFP+ cells detected in morphant embryos was similar to that observed in controls, indicating that the expression of EGFP from the fli1 promoter was unaffected by pdcd2 knockdown (compare Fig. 3E–H). Similar to the effect of pdcd2 knockdown on erythrocytes, Fli1:EGFP morphants displayed an accumulation of circulatory cells at the yolk's duct of Cuvier (data not shown). Endothelial expression of EGFP cells in Fli1:EGFP morphants indicates that the major vessels of the trunk, including the dorsal aorta and caudal vein, were established at about the same developmental timing as seen in control embryos (Fig. 3E–H and data not shown). Arterial specification occurred, and mature endothelial cells expressing ephrinb2a (ephrb2a) were not affected in pdcd2 morphants (Fig. 3I, J). Further, the expression of the VEGF receptor flk1 in endothelial sites was comparable between pdcd2 morphants and controls (Fig. 3K–N). Intersegmental vessels and the dorsal axial vein appeared to sprout and form fairly normally. Pdcd2 morphants displayed array of intersegmental vessels that are lacking the chevron-shaped path exhibited by control intersegmental vessels. However, this is likely a reflection of the blocky nature of somite boundaries in morphants that delineates the path that these vessels follow, and it is unlikely a consequence of any intrinsic angiogenic defect (see Fig. 3A–H). One notable difference in morphant embryos was the formation of the caudal venous plexus. Formation of this anastomosing network of vasculature is reported to begin at ∼32 hpf, and is believed to serve as a niche for both hematopoietic precursor and stem cells [40]. Pdcd2 morphants displayed a defect in the formation of this vascular network that became more apparent at later stages and may be a consequence of the inability of both hematopoietic precursor and phagocytic cells to efficiently colonize and remodel this site (Fig. 3O, P). Collectively, the analyses of endothelial markers coupled with microangiography illustrate that knockdown of pdcd2 does not appear to cause a primary defect in vasculogenesis, and that patent vessels capable of serving as a conduit for blood cell circulation are formed. Moreover, the pdcd2 morphant phenotype appears to be distinct from that of silent heart mutants with defects in cardiac development and blood flow, where large numbers of blood cells are released and pooled in major vessels [16].
Pdcd2 has a cell autonomous function(s) required for erythroid maturation and HSC formation
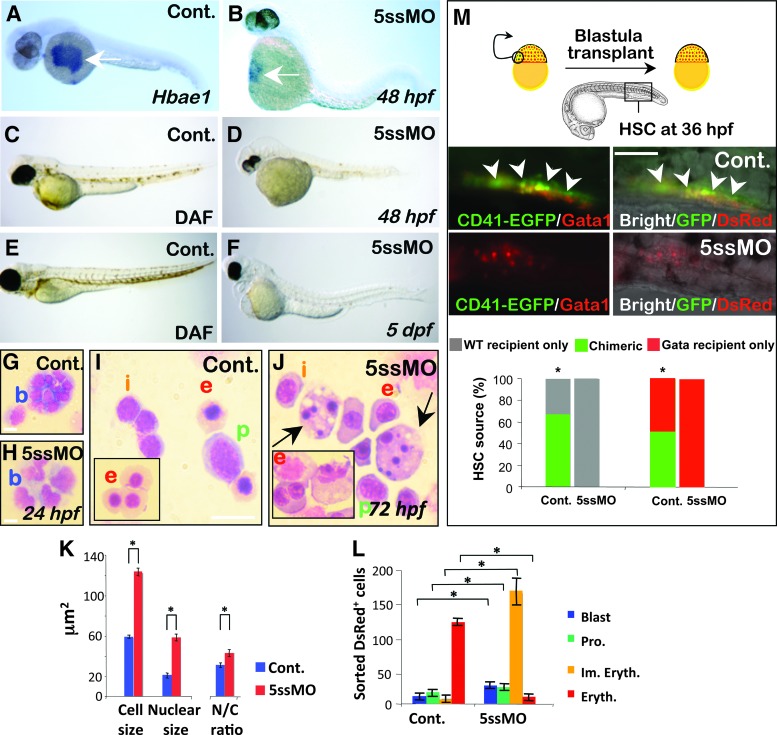
The defects in the release of erythroid cells were mirrored by defects in the expression of erythrocyte-specific markers: pdcd2 morphants had reduced expression of hemoglobin alpha embryonic-1 marker (hbae1) (Fig. 4A, B), and lacked hemoglobin-stained erythrocytes as evidenced by the lack of DAF-stained cells at both 48 and 120 hpf (Fig. 4C–F). To study the morphological changes associated with loss of pdcd2 function(s) in erythrocytes, we examined cytospin preparations from sorted DsRed+ cells from gata1:DsRed pdcd2 morphants. Morphological examination revealed that pdcd2 morphant cells have significantly larger polychromatophilic proerythroblasts with open chromatin at 24 hpf (Fig. 4G, H). At 72 hpf, these cells frequently appeared as large multinucleated cells with highly condensed nuclear chromatin and vacuolated cytoplasm that may represent apoptotic cells (arrows in Fig. 4J). Many of the pdcd2 morphant cells failed to differentiate into mature primitive erythrocytes (inset in Fig. 4J). Consistent with their classification as precursor erythroblasts, they were significantly larger in size, and had higher nuclear/cytoplasmic ratios compared to control cells (Fig. 4K). Additionally, at 72 hpf, gata1:DsRed pdcd2 morphants had increased numbers of polychromatophilic erythroblasts and proerythroblasts than controls (Fig. 4L). Together, these data suggest that embryos with reduced pdcd2 function accumulate hematopoietic precursors, and that pdcd2 is required for completion of the erythroid differentiation program.
FIG. 4.
Impaired erythropoiesis and HSC development in pdcd2 morphants. (A, B) WISH of the erythroid marker hbae1 in control (A) and 5ssMO-injected (B) 48 hpf embryos (arrow) (Control, n=85/91, 93%; 5ssMO, n=64/66, 97%). (C–F) Reduced DAF staining and hemoglobin containing erythrocytes in pdcd2 morphants compared to controls at 48 dpf (C vs. D) and 5 dpf (E vs. F). (G–J) Morphology of clusters of DsRed+ cells sorted from pdcd2 morphants and control embryos at 24 hpf (G, H) and 72 hpf (I, J). Polychromatophilic erythroblasts (b) from controls (G), or pdcd2 morphants (H) at 24 hpf, and Proerythrocytes (p), immature erythrocytes (i), and mature erythrocytes (e in insert) from controls (I) and pdcd2 morphants (J) are shown. (K) Average cell sizes, nuclear sizes (mean±s.e.m), and nuclear/cytoplasmic ratio (N/C) of sorted DsRed+ cells at 24 hpf from control MO or pdcd2 gata1:DsRed morphants. (L) Mean erythroid DsRed+ cells (±s.e.m) sorted from control and morphant embryos at 72 hpf. Data were determined in 3 independent experiments (*P<0.001). (M) Top, schematic illustration of the blastula transplantation strategy used to investigate whether pdcd2 hematopoietic effects are cell-autonomous. Cells from control MO- or 5ssMO-injected CD41-EGFP transgenic donor embryos were transplanted at the blastula stage of either WT or 5ssMO-injected gata1-DsRed recipients. Donor HSCs contribution (arrows) at the PBI/CHT region was detected from control MO-injected cells in gata1-DsRed recipients, but none of 5ssMO-treated donor cells contributed to recipient HSC formation (n=12 embryos/transplant). Bottom, HSC chimerism determined according to percentage of embryos from either control MO- or 5ssMO–injected CD41- EGFP transplanted donor cells that contributed to either WT or gata1-DsRed recipient HSC formation in 3 independent experiments (*P<0.001, Fisher's exact test). DAF, 2,7-diaminofluorene; dpf, days postfertilization; HSC, hematopoietic stem cell; WT, wild type. Color images available online at www.liebertpub.com/scd
To test whether pdcd2 effects on erythroid development are cell-autonomous, DsRed+ cells were sorted from mismatch- or 5ssMO-injected gata1-DsRed embryos at the 30-somite stage, and were transplanted into the dorsal aortic region of age-matched nonfluorescent wild-type hosts. From 20 recipient embryos transplanted each with 1–20 DsRed+ cells, 66% of cells from mismatch MO-treated embryos were released into the embryonic circulation, and contributed to the chimeric host erythropoiesis. In contrast, only 37% of transplanted cells from 5ssMO-treated embryos were released into the circulation, and the remaining cells accumulated at the yolk, and failed to contribute to host erythropoiesis (Fisher's exact test, P<0.001, n≥20). This observation suggests that intrinsic functional defects within the erythroid lineage preclude the movements as well as the morphological and biochemical changes normally associated with erythroid differentiation.
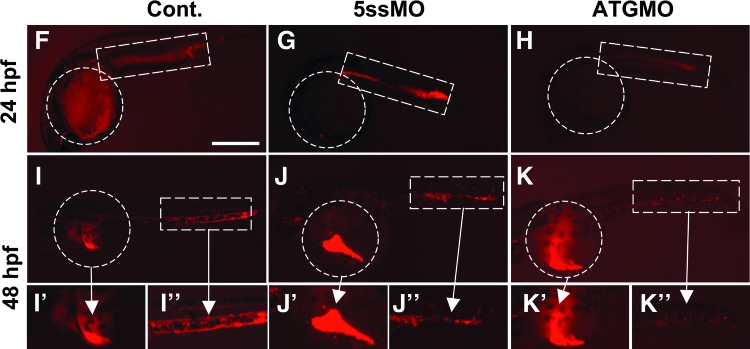
We next sought to determine whether pdcd2's effects on HSC emergence were cell-autonomous. We utilized a CD41:EGFP line, which labels both thrombocytes and HSCs. To investigate whether thrombocytes and HSCs are formed in pdcd2 morphants, we injected the 5ssMO into the CD41:EGFP embryos. In this transgenic line, HSCs are GFPlow, and appear first in the AGM at 33 hpf, while thrombocytes are GFPhigh and are detected first in the CHT at 48 hpf [30]. Consistent with the effects of pdcd2 knockdown on the HSC markers, c-myb and runx1 (see below), CD41:EGFP pdcd2 morphants exhibited a failure to populate the AGM with any CD41:EGFP+ HSCs at 36 hpf (Supplementary Fig. S5A, B). Transplantation of cells at the blastula/early gastrula boundary destined to form blood cell progenitors from CD41-EGFP embryos treated with a control MO into either WT or gata1-DsRed 5ssMO-treated embryos revealed that cells from control MO-treated embryos populate the PBI/CHT with donor-derived GFPlow HSCs or GFPhigh thrombocytes in pdcd2 morphant recipient embryos (Supplementary Fig. S5C–E). However, blastula cells from pdcd2 morphants failed to populate recipient PBI/CHT with HSCs at 36 hpf or thrombocytes at 48 hpf either in WT or in gata1:DsRed recipients (Fig. 4M), therefore suggesting that pdcd2 effects on HSC emergence and/or the appearance of thrombocytes are cell-autonomous.
Pdcd2 is required for terminal differentiation of multiple hematopoietic lineages
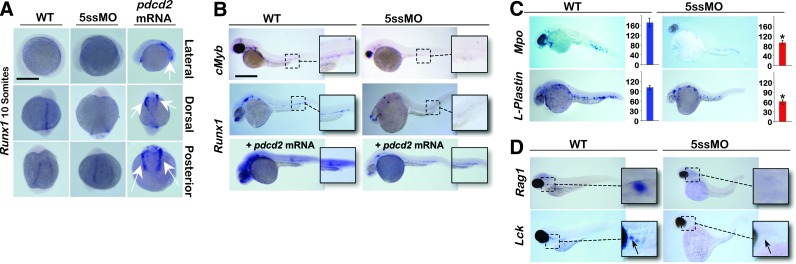
We used WISH to assess hematopoietic specification through the expression of transcription factors that constitute the regulatory transcriptional hierarchy in pdcd2 morphants. Knockdown of pdcd2 had no significant effect on the expression of either the paraxial mesoderm marker myod (Data not shown), the mesoderm marker ntl (Supplementary Fig. S6A, B), or the hematopoietic embryonic marker cdx4 (Supplementary Fig. S6C, D), suggesting that loss of pdcd2 has no clear effects on mesodermal differentiation and patterning. We then examined the effects of pdcd2 knockdown on the expression of markers of the hemangioblast (scl/tal1), or HSCs (runx1 and c-myb) (Fig. 5A, B). The expression of scl indicating hemangioblast formation [4] in both the ALM and PLM was stronger in pdcd2 morphants than in controls (Supplementary Fig. S6G). The upregulation of scl upon pdcd2 knockdown was further confirmed using quantitative real-time (RT)-PCR (Q-PCR) (Supplementary Fig. S6H). In contrast, pdcd2 morphants demonstrated a reduction in c-myb expression (Fig. 5B), and a near complete loss of runx1-expressing cells in the ventral wall of the zebrafish dorsal aorta (Fig. 5B), suggesting failure of HSC initiation. On the contrary, the expression of lmo2, an early hematopoietic and vascular endothelial cell marker, appeared to be relatively normal in comparison to control embryos (Supplementary Fig. S6E, F). The relationship between pdcd2 and runx1 became apparent from experiments increasing the dosage of pdcd2 through mRNA injection. Injection of 350 pg of pdcd2 mRNA at the 1-cell stage caused upregulation of runx1 in the PLM at the 10 somite stage and throughout the embryo at 30 hpf (Fig. 5A, B). Thus, there appears to be a correlation between pdcd2 expression and runx1 levels and/or Runx1 expressing cells. These data suggest that HSC emergence was disrupted by pdcd2 deficiency, and that this effect may be, at least in part, a consequence of pdcd2's effects on runx1 expression.
FIG. 5.
Pdcd2 knockdown modulates the expression of hematopoietic transcription factors. (A) WISH of runx1 at the 10-somite stage (13.5 hpf) in WT, pdcd2 5ssMO-injected embryos, or pdcd2 mRNA-injected embryos. Note the upregulation of runx1 with mRNA injection (arrows). (B) WISH of cmyb and runx1 at 30 hpf demonstrating loss of HSCs in pdcd2 morphants and upregulation of runx1 transcript with the pdcd2 mRNA (cmyb control, n=111/113, 98%; cmyb 5ssMO, n=165/180, 92%; runx1 control, n=117/123, 95%; and runx1 5ssMO, n=84/93, 90%). (C) WISH of mpo, and l-plastin at 30 hpf. (mpo control, n=40/42, 95%; mpo 5ssMO, n=38/41, 93%; l-plastin control, n=65/68, 96%; and l-plastin 5ssMO, n=67/78, 86%). The mean numbers (±s.e.m) of mpo+ and l-plastin+ cells/embryo are significantly reduced in pdcd2 morphants (red bars) compared to WT (blue bars) (*P<0.001). (D) WISH of lymphoid progenitor marker rag1 at 96 hpf, and mature T-cell marker lck at 5 dpf, respectively (rag1 control, n=28/38, 74%; rag1 5ssMO, n=49/51, 96%; lck control, n=53/56, 95%; and lck 5ssMO, n=46/46, 100%). Arrows point to the thymus. Scale bar, 500 μm. mpo, myeloperoxidase. Color images available online at www.liebertpub.com/scd
The effects of pdcd2 on the expression of scl, runx1, and c-myb would likely result in changes in expression of their downstream targets and/or cell fate decisions. We assessed these changes using WISH with lineage-specific hematopoietic makers. It is important to note that the effects on markers of myeloid differentiation at 30 hpf might represent an aggregate effect of pdcd2 knockdown on both primitive and definitive hematopoiesis, with a likely contribution from short-term progenitors (EMPs) [5]. There was a significant reduction in hematopoietic cell maturation (Fig. 5C) with reduced expression of both myeloid-specific myeloperoxidase (mpo) (184±12 mpo+ cells/control vs. 102±8 mpo+ cells/pdcd2 morphant), and macrophage/myeloid-specific marker l-plastin (75±8 l-plastin+ cells/control vs. 38±11 l-plastin+ cells/pdcd2 morphant) indicating a failure of early embryonic myelopoiesis. We have demonstrated that pdcd2 morphants display a strong reduction in hbae1 expression (Fig. 2C, D). Furthermore, the expression of erythroid and myeloid cells from both the primitive and definitive hematopoietic waves (hbae1, mpo, plastin) as well as definitive cell types, such as common lymphoid cell progenitors (rag1) and T-cells (lck), were markedly reduced in pdcd2 morphants (Fig. 5D).
Additionally, cells expressing the erythroid marker gata1 failed to appear at the yolk and collected at the PBI/CHT (Supplementary Fig. S7A), while cells expressing the early myeloid progenitor marker pu.1 were reduced at 30 hpf, and pu.1+ cells failed to appear in the PBI/CHT in pdcd2 morphants (Supplementary Fig. S7B). These data were supported by diminished Sudan Black staining of more mature neutrophils in the PBI/CHT of pdcd2 morphants at 48 hpf (Supplementary Fig. S7B) (73±4 Sudan black+ cells/control embryo vs. 37±5 Sudan black+ cells/pdcd2 morphant). Pu.1 expression was partially rescued with the forced expression of the zebrafish pdcd2 mRNA, suggesting that pdcd2 morphant phenotypes were due to loss of pdcd2 function (Supplementary Fig. S7B). Collectively, these data support a model, where pdcd2 effects on bipotent EMPs [5] are essential for maintenance, but not the initiation of these progenitors, while loss of pdcd2 function(s) interferes with the initiation and/or maintenance of HSCs.
Loss of pdcd2 results in mitotic defects and ensuing P53-independent apoptosis
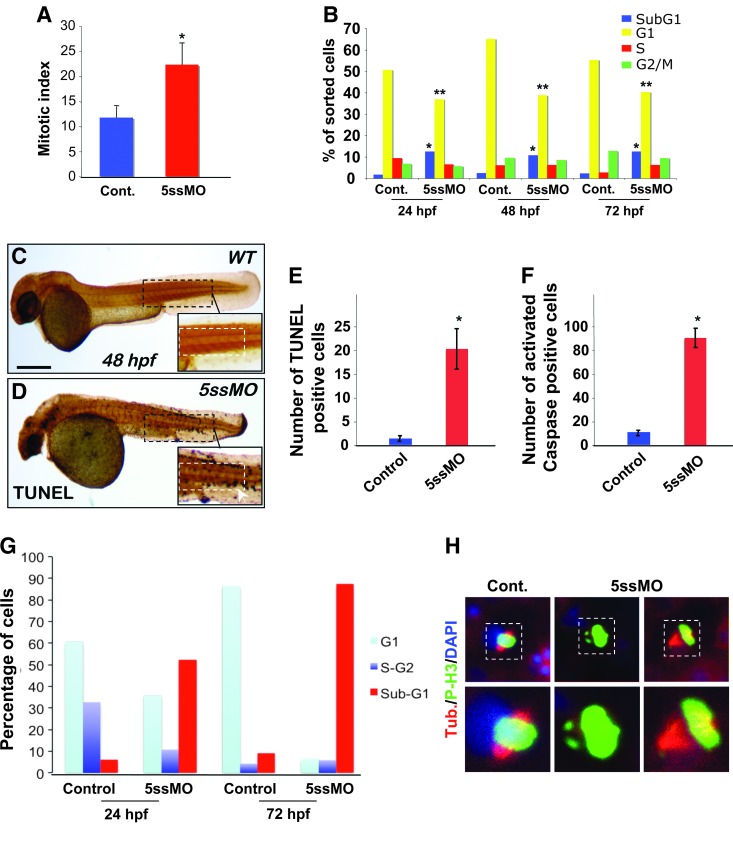
Given our observations with markers for hematopoietic development, we reasoned that the defect in hematopoietic cell maturation in pdcd2 morphants might be attributed to aberrant progenitor maintenance and/or proliferation, apoptotic loss of differentiated cell types, or a combination of both of these processes. To help clarify the effects of pdcd2 loss, we first assessed the mitotic index of cells derived from morphant embryos using a mitosis-specific p-H3 antibody. We found a significant increase in the mitotic index in cells of the trunk and tail of the embryo proper at 24 hpf (Fig. 6A and Supplementary Fig. S8A–F). This difference could be attributed to an increase in S-phase entry/progression, or delayed mitotic exit. BrdU labeling demonstrated similar levels of S-phase occupancy between morpholino and control embryos at 30 hpf, and examination of DNA content of cells from controls and pdcd2 morphants by flow cytometry revealed no difference in S-phase progression (Supplementary Fig. S8I). Cells from control embryos showed typical distribution of normal cycling cells at 24 hpf (G1=64%, S=31%, and G2/M 5%). In contrast, pdcd2 morphant cells had a significant increase in the sub-G1 population and a significant decrease in G1 subsets of cells consistent with cell death likely due to apoptosis (Fig. 6B). This effect extends out to 72 hpf, the latest time point tested. Consistent with this observation, we found that the number of TUNEL+ cells in pdcd2 morphants were significantly higher than controls at both 18 and 24 hpf (n=120; effect seen in 95%±3% of 5ssMO-injected embryos, P<0.001) (Fig. 6C–G). The apoptotic effect observed appears to occur via a caspase-dependent cascade as quantification of activated caspase-3-positive cells in 30 hpf embryo trunks showed a 10-fold increase in the number present in morphant embryos relative to controls (Fig. 6F). While the apoptotic effect was clearly not restricted to regions of the embryo undergoing active hematopoiesis (e.g., PBI), we noted from examination of cytological preparations from TUNEL-labeled embryos that regions known to be active sites of hematopoiesis, such as the PBI, contained a larger number of apoptotic cells (Fig. 6C–E). Moreover, our cytospin preparations of gata1:DsRed erythroid cells identified cells that appeared apoptotic (Fig. 4J). Therefore, we attempted to determine whether the effect(s) of pdcd2 knockdown upon erythropoiesis might occur through an apoptotic mechanism. To this end, we sorted cells from gata1:DsRed embryos injected with pdcd2 and control morpholinos at 24 and 72 hpf, and determined the DNA content profile of DsRed+ erythrocytes. As suggested from the previous cytology, pdcd2 loss was associated with increased levels of apoptosis in the erythroid lineage with a large increase of sub-G1 cells in pdcd2 knockdown embryos relative to controls (Fig. 6G).
FIG. 6.
Mitotic defects in pdcd2 morphants are due to defective spindle assembly. (A) Quantification of IHC mitotic cells in control and pdcd2 knockdown embryos using p-H3 antibodies (see representative images; Supplementary Fig. S8A–F). Mitotic index calculated as p-H3+ cells over DAPI+ cells in control and pdcd2 morphants at 24 hpf. (*P<0.05). (B) Summary of propidium iodide DNA content profiles for both control and pdcd2 knockdown (5ssMO) as determined by flow cytometry or whole dissociated embryos (for a representative profile, see Supplementary Fig. S8I). Pdcd2 morphants have significantly fewer cells in G1 and increase in the number of sub-G1 cells presumed to be apoptotic based on further analyses. *Indicates an increase, **indicates a decrease compared to control. *, **P<0.01. (C, D) Whole mount TUNEL labeling of 48 hpf embryos. Dashed boxes highlight the region encompassing the CHT that is actively undergoing hematopoiesis at this time point. (E) Scoring of the mean number of TUNEL+ cells in 48 hpf embryos (*P<0.001). (F) Scoring of the mean number of activated caspase+ cells in 48 hpf embryos (*P<0.001). (G) Cell cycle stage analysis (24 and 72 hpf) based on DNA content profiles for sorted gata1:DsRed positive cells labeled with DAPI. Control and Pdcd2 knockdown gata1:DsRed embryos were dissociated and labeled with DAPI and subjected to fluorescent activated cell sorting analysis to determine cell cycle stage. At both 24 and 72 hpf, there was an increase in apoptotic erythroid cells (gata1+). (H) Immunofluorescence images of asynchronous cells from control, and 5ssMO-injected 26 hpf embryos stained with b-tubulin (red), p-H3 (green), and DAPI (blue). Lower panels are magnifications of the outlined area. Note the 2 examples of chromosomes with apparent monotelic attachment in cells from pdcd2 morphants. Scale bars are 100 μm in (C, D) and 10 μm in (H). p-H3, phospho-histone H3. Color images available online at www.liebertpub.com/scd
Given the absence of evidence for increased proliferation, we deduced that the increase in mitotic index observed in pdcd2 morphants might be a function of failed mitoses or a delay in mitotic exit. Therefore, we stained pdcd2 morphants embryos with antibodies to p-H3 and β-tubulin to determine if the spindle structure was affected. Interestingly, pdcd2 morphants often displayed defective spindle formation most likely derived from an inability to properly position chromosomes at the metaphase plate (19% pdcd2 morphant vs. 3% control; P<0.005; Fig. 6H). The presence of monotelic chromosome attachment is predictive of mitotic delay by virtue of the spindle assembly checkpoint [41], and the degree to which such defective figures are present in pdcd2 morphant embryos provides a likely explanation for the increase in mitotic index.
Since p53 is involved in the regulation of apoptosis in hematopoietic progenitors, and extensive cell death could occur due to p53 activation as an off-target effect of morpholino injection [42], we investigated a possible role for p53 in the hematopoietic defects seen in pdcd2 morphants. We coinjected the pdcd2 and p53 MOs, and we still observed a significant reduction in the numbers of runx1+ cells in the ICM at 30 hpf (Supplementary Fig. S9D) that could be restored by coinjection of the pdcd2 RNA (data not shown, and Supplementary Fig. S9E). These data suggest that pdcd2 effects are specific, and that p53 loss cannot fully restore the impairment of HSC formation. This was further confirmed by knockdown of pdcd2 in p53 homozygous mutant embryos. Injection of pdcd2 ATGMO or 5ssMO in p53−/− embryos led to impaired release of erythrocytes into the circulation, and a significant reduction in runx1 expressing cells in the ICM at 30 hpf similar to that observed in p53wt/wt embryos (Data not shown) further substantiating the evidence that pdcd2 effects on runx1 expressing HSCs and erythroid maturation are specific and largely p53-independent.
Restoration of runx1 expression and inhibition of apoptosis reverse the effects of pdcd2 loss on erythropoiesis
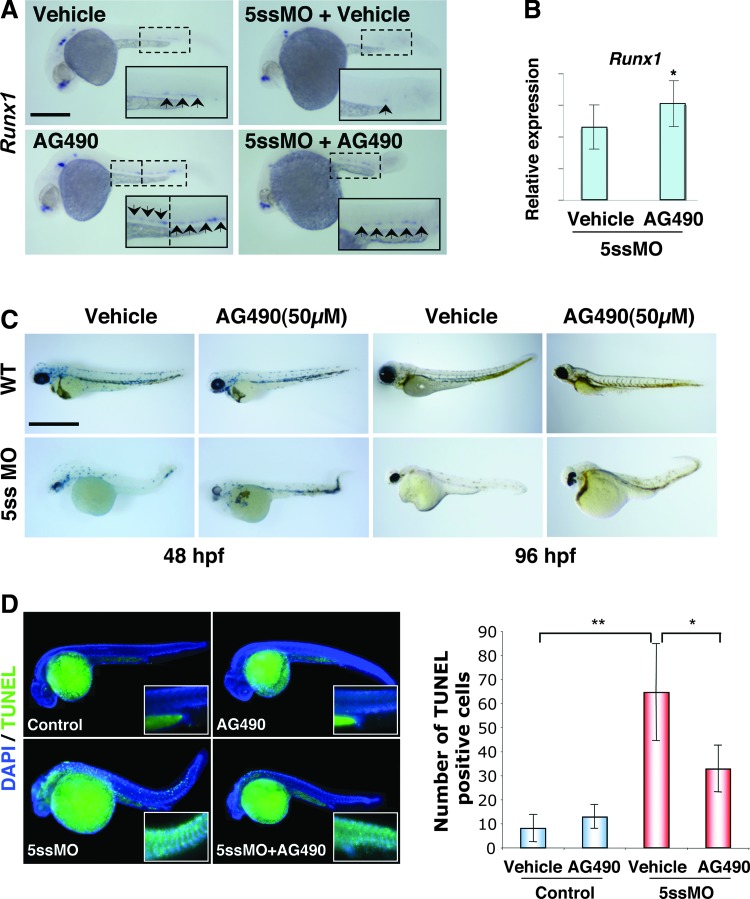
Jak2/Stat signaling transduces signals of hematopoietic cytokines to enhance erythroid cell proliferation. Treatment of zebrafish embryos with the jak inhibitor AG490 reduced phosphorylated stat5 [43]. Reduction in phosphorylated Stat5 might result in upregulation of gene expression of Stat5-target genes that include runx1. Moreover, STAT5 co-immunoprecipitates with RUNX1, and inhibits RUNX1 nuclear localization and transcriptional activity [44]. Thus, we investigated if pharmacological inhibition of stat5 activity might restore runx1 function(s) in pdcd2 morphants. We first determined whether AG490 treatment resulted in increased runx1 transcript levels in 30 hpf embryos. Examination of runx1 transcripts by WISH supports this observation with the increase being most visible in the anterior aspect of the AGM (inset AG490, Fig. 7A). We then asked whether treatment with AG490 would de-repress the attenuated expression of runx1 observed in pdcd2 knockdown embryos. First, we examined the expression of runx1 by WISH and observed that runx1 transcripts are present throughout the AGM in pdcd2 morphant embryos treated with AG490 (Fig. 7A). We dissected trunks and tails of embryos to minimize the contribution of neurally expressed runx1 and examined runx1 transcript levels. Q-PCR analyses revealed a significant increase in runx1 expression in dissected trunks of AG490-treated morphant embryos (Fig. 7B). These data suggest that pdcd2 effects possibly involve runx1-mediated maintenance of HSCs.
FIG. 7.
Upregulation of runx1 and modulation of apoptosis through inhibition of Jak reverses the effects of pdcd2 knockdown on erythropoiesis. (A) WISH of runx1 expressing cells in the arterial and AGM regions of treated embryos at 30 hpf. Treatment with AG490 resulted in increased runx1 expression in the arterial and AGM regions (upward arrowheads in the AG490 panel), and possibly expansion of the anterior myelopoietic compartment (downward arrowheads). Note the dramatic rescue of runx1 expression in the ICM with the combination of AG490 and 5ssMO compared to the combination of vehicle and 5ssMO on top. (Vehicle n=35 (71%); AG490 n=28 (86%); 5ssMO n=34 (91%); and 5ssMO+ AG490 n=36 (14%) showed the displayed phenotypes). (B) Q-PCR analyses of runx-1 expression relative to β-actin in the AGM region of pdcd2 morphants after AG490 treatment (mean±s.e.m, *P<0.05). Scale bar, 500 μm. (C) DAF staining of hemoglobinated cells in WT, or 5ssMO-injected embryos at 48, or 96 hpf treated with either vehicle, 50 μM AG490, pdcd2 5ssMO, or combinations of 5ssMO and AG490. Numbers and percentages of embryos representing the demonstrated DAF+ staining are for WT with vehicle at either 48 or 96 hpf, n=23 (100%), WT with AG490 at 48 hpf, n=18 (100%), and at 96 hpf, n=31 (100%). For 5ssMO treated with vehicle at 48 hpf, n=20 (40%), and at 96 hpf, n=24 (21%), and 5ssMO treated with AG490 at 48 hpf, n=22 (68%), and at 96 hpf, n=14 (86%). The effects of combined 5ssMO+ AG490 treatments were significant when compared to individual treatment (P<0.01). (D) Left, representative images from the 4 groups of TUNEL-stained 30-hpf embryos. Insets demonstrate the AGM/PBI region where hematopoietic cells were counted. Right, quantification of the antiapoptotic effects of Jak inhibition in pdcd2 morphant embryos. Sixteen embryos were analyzed for each treatment from the AGM/PBI region highlighted in (A) in 3 experiments with 3 investigators blinded to treatment performing the counts for TUNEL-positive cells. Data for each embryo are derived from a series of Z sections spanning the depth of the embryo, and then numbers of apoptotic cells were averaged from counts of 3 investigators. (*P<0.01; **P<10−4; bars s.e.m.). Scale bar, 500 μm. Color images available online at www.liebertpub.com/scd
We then attempted to determine whether the effects of pdcd2 knockdown on erythroid differentiation were modulated by de-repression of runx1. We utilized DAF reactivity as a measure for the presence of mature hemoglobinated erythrocytes. Treatment of control embryos with 50 μM AG490 resulted in no apparent effect on the number of DAF+ erythrocytes at either 48 or 96 hpf. However, similar treatment of pdcd2 morphant embryos revealed a dramatic increase in the number of mature erythrocytes relative to vehicle-treated embryos. These results suggest that the de-repression of runx1 afforded by the inhibition of Jak/Stat activity relieves pdcd2's effects on erythroid differentiation block.
The ability of jak/stat inhibition to rescue the effects of pdcd2 knockdown may be attributed to downregulation of scl and/or other progenitor genes, modulation of apoptosis, in addition to increased runx1 expression after AG490 treatment (Fig. 7A, B). An increase in scl expression has been previously reported for embryos with reduced runx1 function [12], we found similar effects on scl expression in pdcd2 morphants that could be reversed by injection of the pdcd2 mRNA (Fig. S6H). Previous analysis of runx1 morphants indicated defects both in definitive HSC formation, and an accumulation of immature hematopoietic progenitors coincident with a block to erythroid differentiation [12]. We reasoned that Jak/Stat inhibition might also have distinct effects on modulating apoptosis. Therefore, we examined apoptosis through TUNEL labeling in both control and pdcd2 morphant embryos in the presence of the Jak inhibitor. We assessed apoptosis in 30 hpf embryos within a region encompassing the AGM, including the posterior blood island. The analysis of these embryos indicated a significant decrease in the number of apoptotic cells in pdcd2 morphant embryos when treated with Jak inhibitor versus vehicle controls, suggesting that inhibition of apoptosis is, at least in part, responsible for the rescue observed in AG490-treated embryos (Fig. 7D). Notably, in the absence of pdcd2 knockdown, AG490 treatment caused a slight, but not a significant increase in TUNEL-positive cells in the AGM/PBI region of control embryos. Thus, we conclude that loss of pdcd2 function(s) during zebrafish hematopoietic development results in erythroid differentiation arrest, likely at the proerythroblast stages, and failure of HSC emergence. These effects could be attributed to modulated runx1 expression and/or function (s), mitotic defects, and p53-independent caspase-dependent apoptosis.
Discussion
Hematopoietic progenitors have to rapidly integrate extrinsic and intrinsic cues that activate or repress transcription factors controlling cell fate. Pdcd2 knockdown led to ineffective hematopoiesis, pooling of hematopoietic progenitor cells that failed to release into circulation over the yolk, impaired hematopoietic cell differentiation, mitotic defects, and apoptosis. The resulting pancytopenia was associated with upregulation of scl during primitive hematopoiesis, and downregulation of the HSC markers, runx1 and cmyb. These results are consistent with requirement of pdcd2 function(s) for maintenance, but not for initiation of primitive hematopoietic progenitors. Moreover, loss of pdcd2 function(s) interferes with initiation and/or maintenance of HSCs. There are several possible mechanisms for the pdcd2 morphant phenotype. One is that reduced pdcd2 levels in the ICM affected hematopoietic differentiation through regulating the expression of runx1, scl, their downstream targets, soluble factor(s), or other essential hematopoietic gene(s) that were not examined in this study. Scl is essential for EMP lineage commitment, but not for HSC maintenance in the adult human BM [45]. In mouse studies, Scl and Runx1 were shown to be necessary for the initiation, but dispensable for the maintenance of HSCs [46]. The lack of hematopoietic clusters in the AGM of Runx1−/− mice suggests that Runx1 regulates hematopoietic endothelial cell transition in the dorsal aorta. Indeed, knockdown of runx1 by morpholino in zebrafish decreases the hematopoietic endothelial transition and induces apoptosis in these cells [8]. Therefore, failure of progenitor cell circulatory release in pdcd2 morphants may occur due to prolongation of an uncommitted progenitor state from persistent scl expression, or due to loss of runx1 expression in the ICM. These 2 possibilities are interdependent as runx1 knockdown embryos exhibited persistent scl expression in zebrafish [12]. Mouse yolk sac erythrocyte progenitors were shown to clump at the yolk, while other cells circulate freely [38], suggesting a change of adhesive properties of primitive blood cells as they are released to the circulation. Similarly, zebrafish blood cells were recently shown to detach from the endothelial wall by cell-autonomous proteolytic degradation of extracellular adhesion molecules [36]. These coordinated events would likely require that blood cells be released into circulation only when they reach a specific differentiation state, or that an inhibitory mechanism to hematopoietic cell intravasation is reversed. Whether pdcd2 function(s) is involved in this regulatory process remains to be investigated.
Transplantation studies suggest that pdcd2 acts predominantly in a cell autonomous fashion to initiate and maintain a definitive HSC pool in the CHT/PBI. Moreover, pdcd2 deficiency leads to abnormal HSC differentiation and apoptosis that occurs mostly independent of direct p53 activity. Studies in rat embryonic fibroblasts determined that induction of the pdcd2 homologue Rp8 is independent of p53-mediated apoptosis [47]. Flies with mutations in the pdcd2 homologue, zfrp8, displayed lymph gland hyperplasia with defects in hemocyte differentiation. Hyperplasia of hemocytes that are confined within the drosophila lymph gland appears similar to the accumulation of cells over the yolk of zebrafish pdcd2 morphants. However, distinct signaling pathways and stages of differentiation during the multiple waves of hematopoiesis in vertebrates might explain the differences between mutant fly and zebrafish phenotypes. Circulating cells during fly larval hematopoiesis are mostly plasmatocytes that are destined to become macrophage-like cells, and there are no cellular equivalents to red blood cells or lymphocytes [48]. The posterior signaling center (PSC) in fly lymph gland maintains blood precursors under control of signals, such as the runx1-homologue Lozenge [49]. Regulation of HSCs in zfrp8 mutants is dependent on these PSC signals [22]. Since zebrafish pdcd2 morphants showed increased expression of scl and decreased expression of runx1, it appears that a conserved cross talk between pdcd2 and scl and/or runx1 might regulate HSC maturation.
Jak/stat signaling is activated during intermediate stages of lineage commitment of developing zebrafish erythroid progenitors [50]. Erythropoietin (epo)-mediated activation of stat/5, PI3K signaling, and bcl-xl response leads to modulation of apoptosis [51] through regulation of STAT-target genes encoding apoptosis inhibitors (e.g., Bcl-xL, Mcl-1, and survivin) and cell cycle regulators (e.g., cyclins D1/D2 and c-Myc) [52]. Loss of zebrafish stat5 blocked the expansion of erythrocytes by exogenous epo, demonstrating a conserved role for stat5 in zebrafish erythropoiesis [53]. Treatment with AG490, a soluble jak2 inhibitor, reduced phosphorylated stat5 [43], which is essential for bcl-xl induction and survival [54], and restored runx1 expression in rescued pdcd2 morphants. While inhibition of STAT3/5 signaling almost always results in growth inhibition and induction of apoptosis in tumor cells, disruption of Stat3 signaling in normal fibroblasts causes growth arrest, but not apoptosis [55,56], suggesting that normal cells, unlike tumor cells, might not depend on stat signaling to sustain their survival. Thus, restoration of runx1 expression, and inhibition of stat/5/bcl-xl-mediated apoptotic pathways during primitive erythropoiesis are 2 potential mechanisms by which the effects of pdcd2 were reversed with AG490 treatment. It will be critical to identify pdcd2 interacting partners and determine the mechanisms of cross talk between pdcd2, runx1, and Jak/Stat signaling.
Hematopoiesis migrates successively from the yolk sac and fetal liver or placenta in mammals, or from CHT/PBI in zebrafish, to the bone or kidney marrow. The properties of yolk or CHT/PBI and marrow HSCs reflect diverse environments and intrinsic HSC features at each stage. For instance, fetal liver HSCs have active cell cycle, whereas adult marrow HSCs are largely quiescent. Pdcd2 knockdown in embryos induced mitotic defects with a higher mitotic index. The spindle assembly checkpoint guards against chromosome mis-segregation by delaying cell cycle progression through mitosis, and defects in the mitotic checkpoint generate aneuploidy and might facilitate tumorigenesis [57]. Cells from pdcd2 morphants appeared to progress through the cell cycle, although with an aberrant mitosis and with frequent monotelic chromosome orientation. Progression from metaphase to anaphase with monotelic orientation produces chronically activated checkpoints that provoke cell death and subsequent apoptosis through an undefined pathway [57]. Whether similar mitotic defects played a role in the inviability of pdcd2 ESCs in mice [25] will be important to investigate.
Deregulated pdcd2 expression has been implicated in human leukemias and lymphomas [27]. Our studies suggest that pdcd2 maintains progenitor cells through interactions with runx1 and other cell fate-determining factors. Clearly, further studies will be necessary to more precisely determine the functions of pdcd2 during hematopoietic cell differentiation and survival. Loss of pdcd2 function(s) in zebrafish perturbs HSC differentiation due to mitotic defects during cell cycle progression and p53-independent apoptosis. These observations, the findings that PDCD2 is strongly expressed in adult hematopoietic tissues, and our recent study demonstrating the requirement of human PDCD2 in erythroid progenitor cell differentiation [58] suggest that PDCD2 controls transcriptional networks regulating hematopoietic and other progenitor cell development, and therefore would likely participate widely in the oncogenic process. The challenge for future studies will be to elucidate the basic mechanisms of pdcd2 function, including the identification of direct targets and partners that allow pdcd2 to regulate HSC fate, to determine how pdcd2 repression or activation is differentially achieved in different cells, and to understand how pdcd2 might participate in tumor development.
Supplementary Material
Acknowledgments
We thank Leonard Zon for the Casper zebrafish, Robert Handin for the CD41-EGFP transgenic zebrafish, Raman Sood for multiple ISH probes, Jesús Torres-Vázquez for the ephrin probe, Neil Campbell for technical assistance, and Ruth Steward and Jennifer Rhodes for critical reading of the article.
This work was supported by funds from the Cancer Institute of New Jersey (H.E.S), and support of the Robert Wood Johnson Foundation for the Child Health Institute of New Jersey (A.B.R).
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Medvinsky A. Rybtsov S. Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 2.Orkin SH. Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gering M. Rodaway AR. Gottgens B. Patient RK. Green AR. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 1998;17:4029–4045. doi: 10.1093/emboj/17.14.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dooley KA. Davidson AJ. Zon LI. Zebrafish scl functions independently in hematopoietic and endothelial development. Dev Biol. 2005;277:522–536. doi: 10.1016/j.ydbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand JY. Kim AD. Violette EP. Stachura DL. Cisson JL. Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogeli KM. Jin SW. Martin GR. Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–339. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 7.Bertrand JY. Chi NC. Santoso B. Teng S. Stainier DY. Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kissa K. Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 9.Boisset JC. van Cappellen W. Andrieu-Soler C. Galjart N. Dzierzak E. Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 10.Lancrin C. Sroczynska P. Stephenson C. Allen T. Kouskoff V. Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns CE. DeBlasio T. Zhou Y. Zhang J. Zon L. Nimer SD. Isolation and characterization of runxa and runxb, zebrafish members of the runt family of transcriptional regulators. Exp Hematol. 2002;30:1381–1389. doi: 10.1016/s0301-472x(02)00955-4. [DOI] [PubMed] [Google Scholar]
- 12.Kalev-Zylinska ML. Horsfield JA. Flores MV. Postlethwait JH. Vitas MR. Baas AM. Crosier PS. Crosier KE. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- 13.Murayama E. Kissa K. Zapata A. Mordelet E. Briolat V. Lin HF. Handin RI. Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Jin H. Xu J. Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood. 2007;109:5208–5214. doi: 10.1182/blood-2007-01-069005. [DOI] [PubMed] [Google Scholar]
- 15.Lam EY. Hall CJ. Crosier PS. Crosier KE. Flores MV. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010;116:909–914. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- 16.North TE. Goessling W. Peeters M. Li P. Ceol C. Lord AM. Weber GJ. Harris J. Cutting CC et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes J. Hagen A. Hsu K. Deng M. Liu TX. Look AT. Kanki JP. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev Cell. 2005;8:97–108. doi: 10.1016/j.devcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Galloway JL. Wingert RA. Thisse C. Thisse B. Zon LI. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev Cell. 2005;8:109–116. doi: 10.1016/j.devcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Berman JN. Kanki JP. Look AT. Zebrafish as a model for myelopoiesis during embryogenesis. Exp Hematol. 2005;33:997–1006. doi: 10.1016/j.exphem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Owens GP. Hahn WE. Cohen JJ. Identification of mRNAs associated with programmed cell death in immature thymocytes. Mol Cell Biol. 1991;11:4177–4188. doi: 10.1128/mcb.11.8.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minakhina S. Druzhinina M. Steward R. Zfrp8, the Drosophila ortholog of PDCD2, functions in lymph gland development and controls cell proliferation. Development. 2007;134:2387–2396. doi: 10.1242/dev.003616. [DOI] [PubMed] [Google Scholar]
- 22.Minakhina S. Steward R. Hematopoietic stem cells in Drosophila. Development. 2010;137:27–31. doi: 10.1242/dev.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramalho-Santos M. Yoon S. Matsuzaki Y. Mulligan RC. Melton DA. “Stemness”.: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 24.Su AI. Wiltshire T. Batalov S. Lapp H. Ching KA. Block D. Zhang J. Soden R. Hayakawa M et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mu W. Munroe RJ. Barker AK. Schimenti JC. PDCD2 is essential for inner cell mass development and embryonic stem cell maintenance. Dev Biol. 2010;347:279–288. doi: 10.1016/j.ydbio.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skottman H. Mikkola M. Lundin K. Olsson C. Stromberg AM. Tuuri T. Otonkoski T. Hovatta O. Lahesmaa R. Gene expression signatures of seven individual human embryonic stem cell lines. Stem Cells. 2005;23:1343–1356. doi: 10.1634/stemcells.2004-0341. [DOI] [PubMed] [Google Scholar]
- 27.Merup M. Moreno TC. Heyman M. Ronnberg K. Grander D. Detlofsson R. Rasool O. Liu Y. Soderhall S et al. 6q deletions in acute lymphoblastic leukemia and non-Hodgkin's lymphomas. Blood. 1998;91:3397–3400. [PubMed] [Google Scholar]
- 28.Steinemann D. Gesk S. Zhang Y. Harder L. Pilarsky C. Hinzmann B. Martin-Subero JI. Calasanz MJ. Mungall A et al. Identification of candidate tumor-suppressor genes in 6q27 by combined deletion mapping and electronic expression profiling in lymphoid neoplasms. Genes Chromosomes Cancer. 2003;37:421–426. doi: 10.1002/gcc.10231. [DOI] [PubMed] [Google Scholar]
- 29.Scarr RB. Sharp PA. PDCD2 is a negative regulator of HCF-1 (C1) Oncogene. 2002;21:5245–5254. doi: 10.1038/sj.onc.1205647. [DOI] [PubMed] [Google Scholar]
- 30.Lin HF. Traver D. Zhu H. Dooley K. Paw BH. Zon LI. Handin RI. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106:3803–3810. doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thisse C. Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 32.Sabaawy HE. Azuma M. Embree LJ. Tsai HJ. Starost MF. Hickstein DD. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2006;103:15166–15171. doi: 10.1073/pnas.0603349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Guyader D. Redd MJ. Colucci-Guyon E. Murayama E. Kissa K. Briolat V. Mordelet E. Zapata A. Shinomiya H. Herbomel P. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- 34.Shepard JL. Stern HM. Pfaff KL. Amatruda JF. Analysis of the cell cycle in zebrafish embryos. Methods Cell Biol. 2004;76:109–125. doi: 10.1016/s0091-679x(04)76007-0. [DOI] [PubMed] [Google Scholar]
- 35.Traver D. Paw BH. Poss KD. Penberthy WT. Lin S. Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 36.Iida A. Sakaguchi K. Sato K. Sakurai H. Nishimura D. Iwaki A. Takeuchi M. Kobayashi M. Misaki K et al. Metalloprotease-dependent onset of blood circulation in zebrafish. Curr Biol. 2010;20:1110–1116. doi: 10.1016/j.cub.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 37.Baker K. Warren KS. Yellen G. Fishman MC. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci U S A. 1997;94:4554–4559. doi: 10.1073/pnas.94.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucitti JL. Jones EA. Huang C. Chen J. Fraser SE. Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein BM. Stemple DL. Driever W. Fishman MC. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med. 1995;1:1143–1147. doi: 10.1038/nm1195-1143. [DOI] [PubMed] [Google Scholar]
- 40.Herbomel P. Thisse B. Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 41.Musacchio A. Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 42.Robu ME. Larson JD. Nasevicius A. Beiraghi S. Brenner C. Farber SA. Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma AC. Ward AC. Liang R. Leung AY. The role of jak2a in zebrafish hematopoiesis. Blood. 2007;110:1824–1830. doi: 10.1182/blood-2007-03-078287. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa S. Satake M. Ikuta K. Physical and functional interactions between STAT5 and Runx transcription factors. J Biochem. 2008;143:695–709. doi: 10.1093/jb/mvn022. [DOI] [PubMed] [Google Scholar]
- 45.Reynaud D. Ravet E. Titeux M. Mazurier F. Renia L. Dubart-Kupperschmitt A. Romeo PH. Pflumio F. SCL/TAL1 expression level regulates human hematopoietic stem cell self-renewal and engraftment. Blood. 2005;106:2318–2328. doi: 10.1182/blood-2005-02-0557. [DOI] [PubMed] [Google Scholar]
- 46.Ichikawa M. Asai T. Saito T. Seo S. Yamazaki I. Yamagata T. Mitani K. Chiba S. Ogawa S. Kurokawa M. Hirai H. AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat Med. 2004;10:299–304. doi: 10.1038/nm997. [DOI] [PubMed] [Google Scholar]
- 47.Guenal I. Mignotte B. Studies of specific gene induction during apoptosis of cell lines conditionally immortalized by SV40. FEBS Lett. 1995;374:384–386. doi: 10.1016/0014-5793(95)01157-a. [DOI] [PubMed] [Google Scholar]
- 48.Williams MJ. Drosophila hemopoiesis and cellular immunity. J Immunol. 2007;178:4711–4716. doi: 10.4049/jimmunol.178.8.4711. [DOI] [PubMed] [Google Scholar]
- 49.Daga A. Karlovich CA. Dumstrei K. Banerjee U. Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes Dev. 1996;10:1194–1205. doi: 10.1101/gad.10.10.1194. [DOI] [PubMed] [Google Scholar]
- 50.Oates AC. Brownlie A. Pratt SJ. Irvine DV. Liao EC. Paw BH. Dorian KJ. Johnson SL. Postlethwait JH. Zon LI. Wilks AF. Gene duplication of zebrafish JAK2 homologs is accompanied by divergent embryonic expression patterns: only jak2a is expressed during erythropoiesis. Blood. 1999;94:2622–2636. [PubMed] [Google Scholar]
- 51.Bao H. Jacobs-Helber SM. Lawson AE. Penta K. Wickrema A. Sawyer ST. Protein kinase B (c-Akt), phosphatidylinositol 3-kinase, and STAT5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells) Blood. 1999;93:3757–3773. [PubMed] [Google Scholar]
- 52.Buettner R. Mora LB. Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 53.Paffett-Lugassy N. Hsia N. Fraenkel PG. Paw B. Leshinsky I. Barut B. Bahary N. Caro J. Handin R. Zon LI. Functional conservation of erythropoietin signaling in zebrafish. Blood. 2007;110:2718–2726. doi: 10.1182/blood-2006-04-016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Socolovsky M. Fallon AE. Wang S. Brugnara C. Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 55.Bowman T. Broome MA. Sinibaldi D. Wharton W. Pledger WJ. Sedivy JM. Irby R. Yeatman T. Courtneidge SA. Jove R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niu G. Heller R. Catlett-Falcone R. Coppola D. Jaroszeski M. Dalton W. Jove R. Yu H. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 1999;59:5059–5063. [PubMed] [Google Scholar]
- 57.Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–924. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- 58.Kokorina N. Granier CJ. Zakharkin SO. Davis S. Rabson AB. Sabaawy HE. PDCD2 knockdown inhibits erythroid but not megakaryocytic lineage differentiation of human hematopoietic stem/progenitor cells. Exp Hematol (in press) 2012 doi: 10.1016/j.exphem.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.