Abstract
We aimed to compare impulse oscillation system (IOS) and traditional pulmonary function tests (PFTs) for the assessment of the severity of chronic obstructive pulmonary disease (COPD), and to assess the use of IOS parameters to identify patients who were forced expiratory volume in 1 second (FEV1)%pred < 50%.
Patients with COPD (n = 215) were enrolled at the Ninth Hospital of Xi’an Affiliated Hospital of Xi’an Jiaotong University between October 2014 and September 2016. All patients were assessed by traditional PFT and IOS. Diagnostic performance of IOS parameters to determine indication for patients of FEV1%pred < 50% was assessed on receiver-operating characteristics (ROC) curve analysis.
Out of 215 patients, 18, 83, 78, and 36 patients were classified as grade 1, 2, 3, and 4, respectively, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) severity grading. On Spearman correlation analysis, FEV1%pred, MMEF 75%–25%, and residual volume/total lung capacity (RV/TLC) correlated with total respiratory impedance (Z5)%pred, resistance at 5 Hz (R5)-resistance at 20 Hz (R20), R5-R20% R5, R5, R5%pred, frequency response (Fres), reactance area (Ax), and reactance at 5 Hz (X5). On ROC curve analysis, the area under the curve (AUC) of X5 absolute value, Fres, Ax, Z5%pred, R5-R20, and R5-R20% R5 were 0.748, 0.755, 0.760, 0.705, 0.715, and 0.735, respectively, for COPD patients who required inhalational glucocorticoid therapy.
IOS parameters showed a good correlation with traditional pulmonary function parameters; reactance parameters showed a stronger correlation than that of the resistance parameters. IOS can be used as an alternative method for pulmonary function assessment in patients with COPD with FEV1%pred < 50% who need inhalational glucocorticoid therapy.
Clinical trial registration number: ChiCTR-OCH-14004904.
Keywords: chronic obstructive pulmonary disease, dyspnea, glucocorticoids, impulse oscillation system, pulmonary function test
1. Introduction
Chronic obstructive pulmonary disease (COPD) represents one of the leading causes of morbidity and mortality worldwide. According to the World Health Organization, an estimated 3 million deaths are attributable to COPD every year across the world.[1] In China, COPD is a major contributor to the overall morbidity and mortality burden owing to the relatively high prevalence of smoking and rising environmental pollution.[2,3] Therefore, accurate diagnosis of COPD and monitoring of response to treatment is of particular importance.
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations, pulmonary function tests (PFTs) are the gold standard for diagnosis of COPD, and the ratio of forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70% after bronchodilator inhalation is an essential criterion for the diagnosis of COPD.[4] The 2016 GOLD update, reemphasized inhalational corticosteroid therapy for COPD patients with forced expiratory volume in 1 second of the predicted value (FEV1%pred) <50%, that is, GOLD grade 3, 4.[5] According to the GOLD update in 2017, FEV1 is used for grading of disease severity but is not a variable used to guide treatment, which separates spirometric grades from the “ABCD” groups followed by clinical symptoms and acute exacerbation risk assessment. However, it also notes that the clinical criteria for escalation and deescalation of therapeutic strategies have not been systematically tested. Therefore, spirometry in conjunction with patient symptoms and exacerbation history continue to be the key criteria for determining the therapeutic approach. Groups C and D may require treatment with inhaled glucocorticoids. Roflumilast is recommended for group D patients who have chronic bronchitis and FEV1 < 50%pred. Therefore, the ability to distinguish patients with FEV1 < 50%pred is of much clinical importance.[6] However, clinical application of PFTs has several limitations. The traditional PFTs, especially the measurement of FVC, are particularly challenging for elderly patients, those who have cognitive impairment, poor motor coordination, and breathing difficulties.[7] Similar concerns were voiced by Janssens et al[8] in their research on respiratory resistance assessment in elderly individuals. The traditional pulmonary function parameter FEV1 is an important parameter for the diagnosis of COPD and evaluation of airflow limitation. However, it is not sensitive to peripheral airway obstruction and may not accurately reflect the pathological changes in their entirety.[9] The COPD Gene study[10] also suggested that traditional PFTs may be inadequate to detect COPD pathological damage and smoking-related lung disease. Therefore, more reliable methods for airway evaluation are required for patients with COPD.
Impedance comprises resistance and reactance. Impulse oscillation system (IOS) is a new technique to measure airway resistance and reactance. It is a type of forced oscillation,[11,12] which is transmitted along the bronchial tree by oscillating sound signals of various frequencies, typically 5 and 20 Hz. It provides a measure of the total airway resistance (resistance at 5 Hz [R5]), the proximal airway resistance (resistance at 20 Hz [R20]), and the peripheral airway resistance (R5-R20). (R5-R20)%R5 indicates the proportion of peripheral airway resistance to total airway resistance.[13] Reactance at 5 Hz (X5) relates to the physical properties of the lung parenchyma and its ability to expand and facilitate alveolar filling. Frequency response (Fres) is the point at which reactance is zero (when forces of inertia and capacitance are equal). The reactance area (Ax) is the sum of all the frequency values from X5 to the Fresonant frequency, that is, it quantifies the respiratory reactance between 5 Hz and Fres. Fres and Ax are now considered sensitive indicators of reactive airflow limitation.[13] IOS parameters are more sensitive to bronchial provocation test, and bronchodilation test as compared to the traditional PFTs.[14–16] However, the relationship between IOS index and traditional PFTs, and the diagnostic role of IOS parameters in COPD patients on inhaled glucocorticoid or roflumilast therapy is not well-characterized.
The aim of the present study was to investigate the association between IOS parameters and those of traditional PFTs in patients with COPD. Further, we sought to explore the diagnostic efficacy of IOS parameters to distinguish the special COPD group of FEV1%pred < 50%.
2. Materials and methods
2.1. Study design
Patients with COPD who were admitted to the Ninth Hospital of Xi’an Affiliated Hospital of Xi’an Jiaotong University between October 2014 and September 2016, and who met the GOLD diagnostic criteria (FEV1/FVC < 70% after bronchodilator inhalation) were included in this study.[5] All patients had risk factors for COPD and had respiratory symptoms such as cough and expectoration, except those attributed to other diseases. Depending on the severity of airflow obstruction, that is, based on FEV1%Pred, patients were divided into groups: GOLD grade 1 FEV1%pred ≥ 80%; grade 2 50% ≤ FEV1%pred <80%; grade 3 30% ≤ FEV1%pred <50%; and grade 4 FEV1%pred < 30%.
Exclusion criteria: age <40 years; pregnant women; patients with concomitant lung diseases such as lung cancer, pneumonia, active pulmonary tuberculosis, pulmonary embolism, and interstitial lung disease; history of lung surgery; patients unable to undergo PFT; asthma; and severe heart, liver, and kidney dysfunction. A total of 286 patients with COPD were enrolled in the study; 41 failed to complete PFTs, and 30 patients withdrew consent to participate in the study. Only 215 patients were eventually included in the survey. Patient selection criteria are illustrated in Fig. 1.
Figure 1.
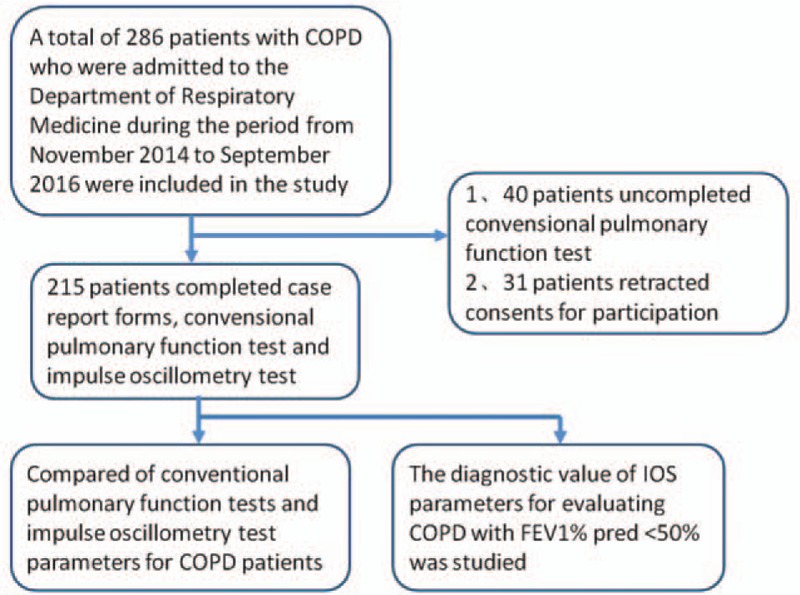
Flow chart showing patient selection.
The study employed the “digital lung” respiratory disease evaluation system and diagnostic criteria (201402013) approved by the China Clinical Research Experimental Center (approval number ChiCTR-OCH-14004904). The study protocol was approved by the Ethics Committee at The Ninth Hospital of Xi’an Affiliated Hospital of Xi’an Jiaotong University. Written informed consent was obtained from all patients.
2.2. Data collection
Data on baseline demographic variables were collected, including sex, age, smoking status, place of residence, health education about COPD, and hospitalization for acute exacerbation in the previous 12 months. To minimize recall bias, data on prior hospitalization were obtained from electronic medical records and confirmed by patients. Modified Medical Research Council (mMRC) dyspnea score was assessed and blood samples were collected for white blood cell counts, neutrophil counts, platelet counts, C-reactive protein, and fibrinogen levels.
2.3. Pulmonary function tests
PFTs were performed with Jaeger Masterscreen pulmonary function instrument in strict accordance with the American Thoracic Society/European Society of Respiratory Diseases guidelines.[17] Each subject completed at least 3 qualified lung function measurements, and the best results were selected based on the subject's performance and shape of the curve.[13]
The subjects were sequentially examined using IOS, spirometric flow-volume loop measurement, single breath transfer factor for carbon monoxide, and bronchodilation test. Parameters of traditional PFTs (FEV1/FVC; FEV1%pred; ratio of carbon monoxide diffusion capacity to alveolar ventilation; residual volume/total lung capacity [RV/TLC]; and maximal mid-expiratory flow rate [MMEF 75%–25%]) and parameters of IOS (total respiratory impedance [Z5]%pred, R5, R20, R5-R20, (R5-R20)%R5, and reactance parameter X5, Fres, and Ax) were recorded.
2.3.1. Impulse oscillation (Masterscreen IOS, Erich Jaeger, Hoechberg, Germany)
The procedure was conducted with subjects in sitting position with an airway clipped to the nose clip.[18] Subjects were required to keep the airway open with their teeth clenched on the mouthpiece, the tongue positioned in the mouth of the device and lips pursed around the device, so that the breathing channel remained patent and to prevent any air leak. Both hands were used to suppress the cheek in order to minimize the vibration of the cheeks. To increase the oral compliance, subjects were asked to breathe calmly. Start recording with 45 second once the patient was breathing evenly, and release the seizure device after stopping.
2.3.2. Spirometric flow-volume loop determination (Masterscreen CareFusion, Berlin, Germany)
The test was started 15 minutes after inhalation of salbutamol 200 μg. The subject was asked to breathe steadily (tidal breathing); at the end of the expiration up to the residual gas position, that is, platform, the subjects performed forced inhalation up to the maximum lung volume. The breath was required to be explosive, have a significant peak, and to be uninterrupted during the whole process. After testing 3 times, the best results were recorded provided the quality control criteria were met.
2.3.3. Single breath transfer factor for carbon monoxide
Using method of single breath transfer factor for carbon monoxide, calm breathing was performed for 3 to 4 cycles; after the baseline waveform of breathing was stable, the breath was completely exhaled to the residual volume, and then a volume of test gas equivalent to the total lung volume was inhaled (suction time was controlled to around 2.5 and 4 seconds in patients with airway obstruction, and the gas volume was required to be less than 85% vital capacity). After holding the breath for 10 seconds, the gas was exhaled at an average speed up to the position of residual gas; the mouth tube was then released after next inhalation, and the KCO and RV/TLC were recorded on the computer.
2.4. Statistical methods
Statistical analysis was performed with Statistical Package for the Social Sciences version 19 (SPSS Inc., Chicago, IL). Data pertaining to continuous variables are expressed as a mean ± standard deviation. Multigroup comparisons were performed using one-way ANOVA with LSD comparison between 2 groups. Correlation analysis was performed with Spearman test. Receiver-operating characteristics (ROC) curve analysis was performed, and Youden index was determined to determine the cut-off values for IOS parameters for the prediction of FEV1%pred < 50%. P < .05 was considered statistically significant. Partial trend graphs were prepared with the statistical software GraphPad Prism 5 (GraphPad Software, Inc., California San Diego, CA.
3. Results
3.1. Demographic and clinical data
Mean age of 215 COPD patients was 67.69 (±9.95) years. Men accounted for 94% (n = 202) of the patients. Mean body mass index (BMI) was 23.25 (±3.86) kg/m2. Patients with smoking history accounted for 80.5% of the patients, while current smokers accounted for 38.1% of the patients. The mean number of cigarette packs smoked per year was 43.58 (±35.40). mMRC was 1.70 (±1.11). Mean number of episodes of acute exacerbations in the past 12 months was 0.78 (±1.22). Mean white blood cell count was 7.36 (±3.34) 109/L; the percentage of neutrophils was 70.37 (±13.31)%; plasma fibrinogen level was 4.30 (±3.15) g/L, and C-reactive protein level was 26.31 (±39.62) mg/L. The demographic and clinical characteristics of patients disaggregated by COPD grade are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics of patients with COPD.
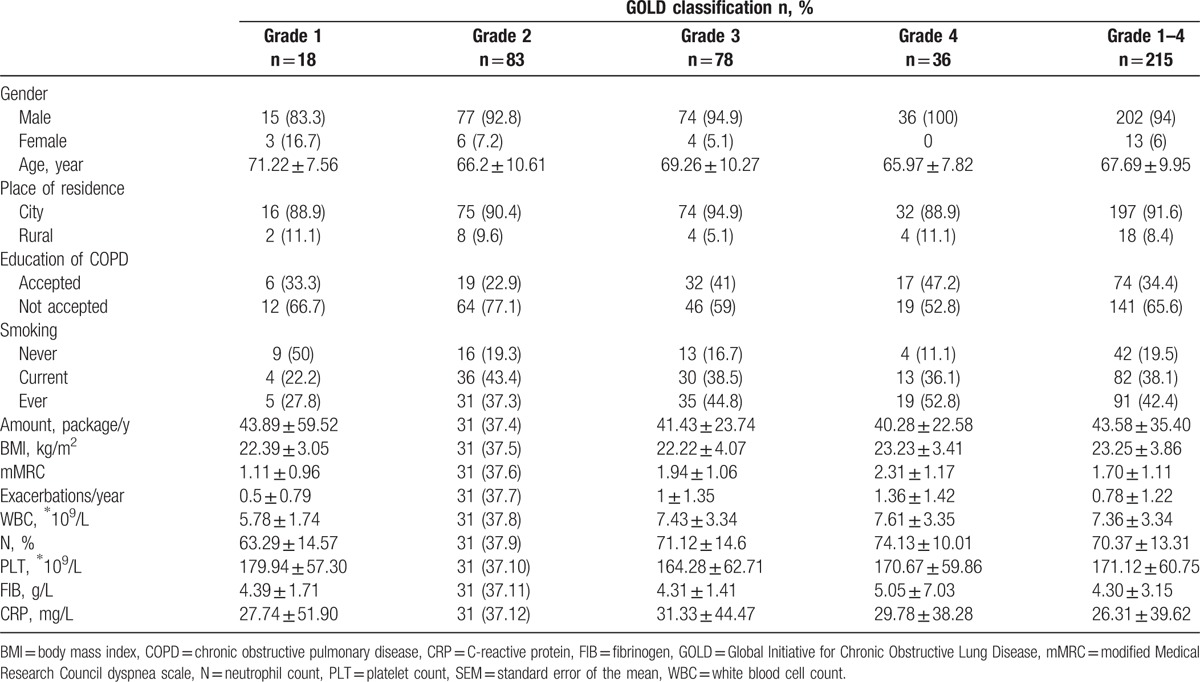
Univariate analysis showed no significant difference between patients with different grades of COPD with respect to age, smoking status, white blood cell count, platelet count, fibrinogen, and C-reactive protein (P > .05). There were statistically significant differences between COPD grades with respect to the number of acute exacerbations in the preceding 12 months, BMI, neutrophil ratio, and mMRC (P < .05; Table 2). There was a significant difference only with respect to BMI between patients with grades 2 and 1 (P < .05); there were significant differences in the number of acute exacerbations in the past 12 months, BMI, neutrophil ratio, and mMRC between grade 3 and 4 (P < .05) and between grade 1 and 2 (P < .05).
Table 2.
Traditional pulmonary function tests and IOS parameters of patients with COPD disaggregated by severity grade and one-way analysis of variance.
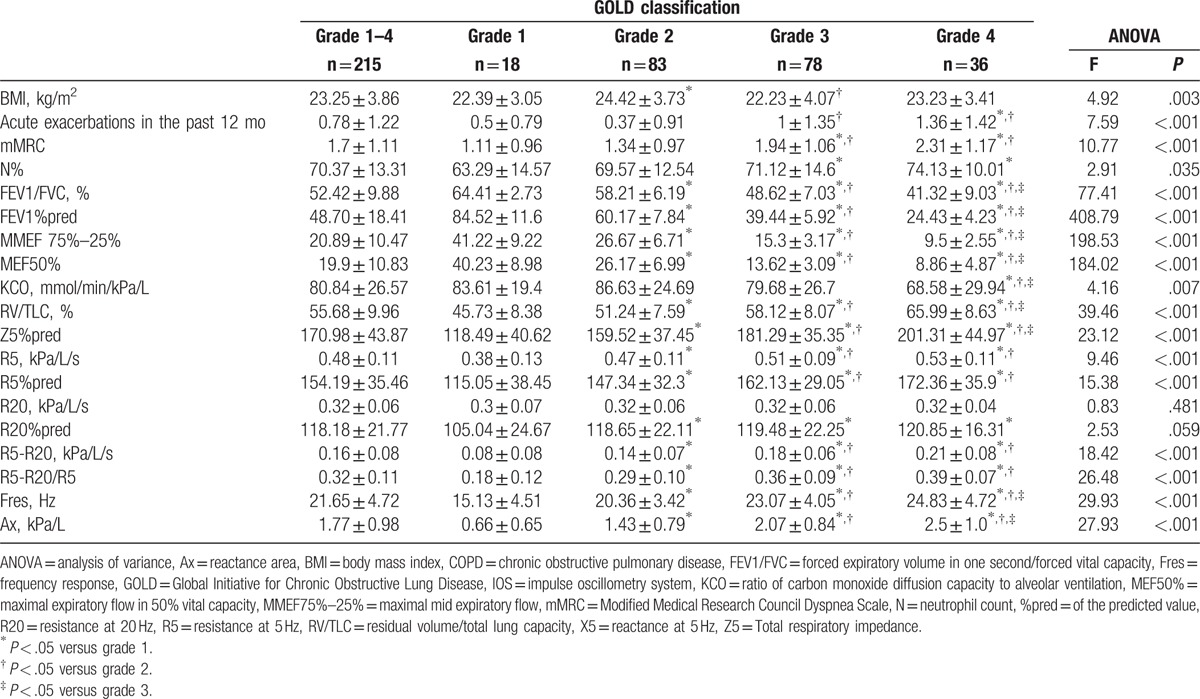
3.2. Traditional pulmonary function and IOS parameters for airflow limitation at COPD grades 1 to 4
After inhalation of salbutamol, reactive airflow limited parameters, including FEV1, FEV1%pred, FEV1/FVC, MMEF 75%–25%, reacting diffusion function parameters KCO, reacting gas retention trapping parameters RV/TLC, and IOS resistance parameters Z5%pred, R5, R5%pred, R20%pred, R5-R20, (R5-R20)%R5 and reactance parameters X5, Fres, and Ax were significantly different between patients with various COPD grades (P < .05). However, no significant difference was observed with respect to R20 and R20%pred between the various COPD grades (P > .05). With the increase in GOLD pulmonary function grade, IOS resistance parameters and reactance parameters Fres, and Ax were increased along with a decrease in X5 (Table 2 and Fig. 2).
Figure 2.
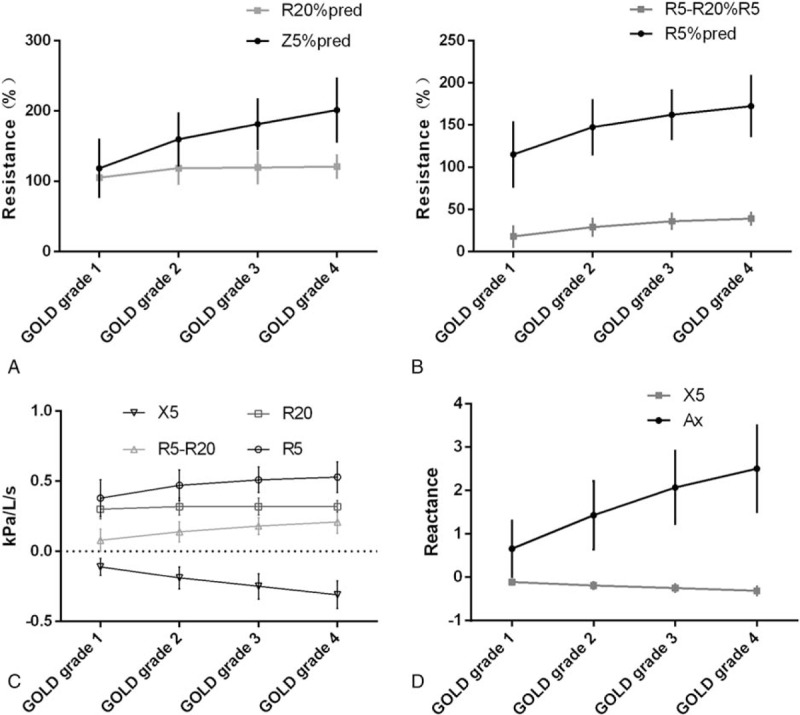
Trends of IOS parameters in GOLD pulmonary function classification. (A) Z5%pred and R20%pred; (B) R5%pred and (R5-R20)% R5 ∗ 100; (C) R5, R20, R5-R20, X5; and (D) Ax and Fres.
Comparison between groups is shown in Fig. 3. There were significant differences with respect to the other lung function parameters, except R20 and KCO, between grades 2 and 1, and between grade 3 and grades 1, 2. All lung function parameters in patients with grade 4 COPD (including KCO) were significantly different from those in patients with grade 1 and 2 COPD. The differences in the traditional parameters of lung function between grade 4 and 3 were statistically significant (P < .05), but the reactance parameters (X5, Fres, Ax, and Z5%pred) and viscous resistance parameters (R5, R5%pred, R20, R20%pred, R5-R20, [R5-R20]%R5) in IOS were not significantly different (P > .05).
Figure 3.
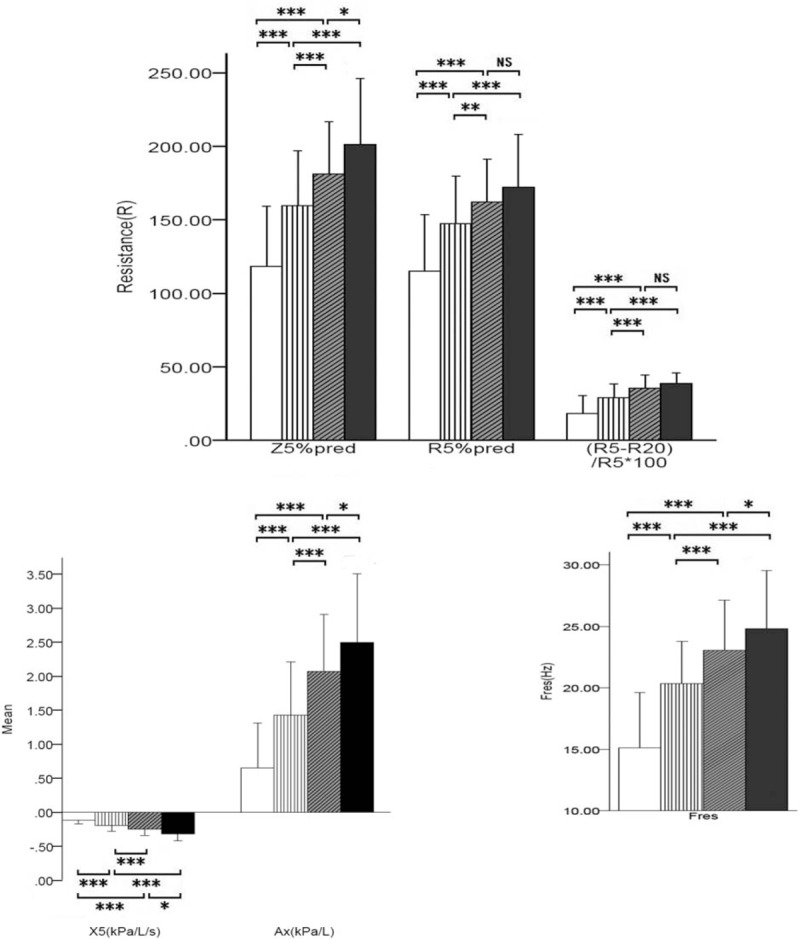
Trends of resistance and reactance parameters in GOLD pulmonary function classification. (A) Resistance parameters Z5%pred, R5%pred, and (R5-R20)% R5 ∗ 100; (B) reactance parameters X5 and Ax; and (C) reactance parameters Fres. Ax = reactance area, Fres = frequency response, GOLD = Global Initiative for Chronic Obstructive Lung Disease, %pred = of the predicted value, R20 = resistance at 20 Hz, R5 = resistance at 5 Hz, X5 = reactance at 5 Hz, Z5 = total respiratory impedance.
3.3. Correlation between traditional pulmonary function and IOS parameters for assessment of COPD airflow limitation, gas trapping, and small airway dysfunction
A moderate negative correlation was observed between FEV1%pred and Z5%pred, R5-R20, (R5-R20)%R5, Fres, Ax (Spearman correlation coefficient r: −0.435, −0.425, −0.474, −0.5, and −0.521, respectively, P < .01). FEV1%pred showed a moderately positive correlation with X5 (r = 0.54, P < .01). There was a weak negative correlation with R5 and R5%pred (r = −0.35 and −0.292, respectively, P < .01). R20, R20%pred showed no correlation with FEV1%pred (P > .05) (Table 3 and Fig. 4). FEV1/FVC, which reflects airflow limitation, was consistent with IOS parameters, but the correlation was weaker than that with FEV1%pred. The small airway dysfunction index MMEF 75%–25% showed a moderate negative correlation with Z5%pred, R5-R20, (R5-R20)%R5, Fres, and Ax (Spearman correlation coefficient r = −0.439, −0.452, −0.489, −0.510, and −0.540, respectively, P < .01). X5 showed a moderately positive correlation with MMEF 75%–25% (r = 0.569, P < .01). R5 and R5%pred showed a weak negative correlation with MMEF 75%–25% (r = −0.323, −0.354, respectively, P < .01). R20, R20%pred showed no such correlation with it, P > .05 (Table 3 and Fig. 5). Another parameter, maximal expiratory flow in 50% vital capacity (MEF50%pred), showed a correlation with IOS parameters which were consistent with MMEF 75%–25%.
Table 3.
Results of Spearman correlation analysis showing correlation between traditional pulmonary function parameters and IOS parameters.
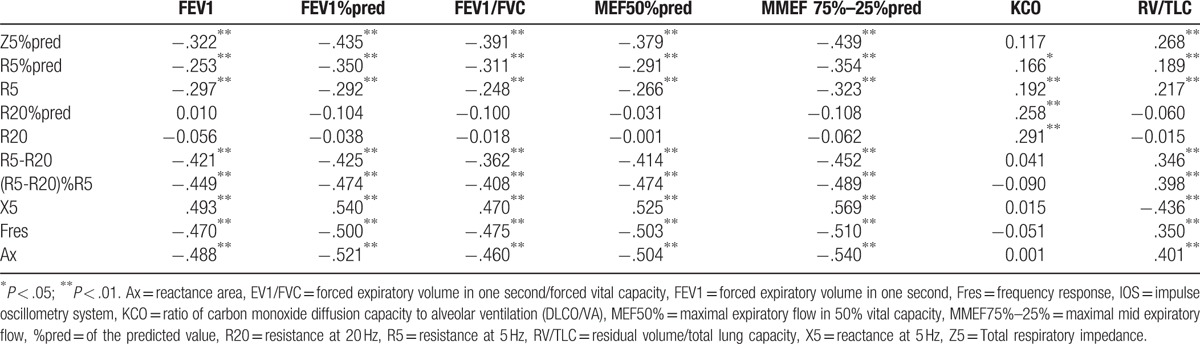
Figure 4.
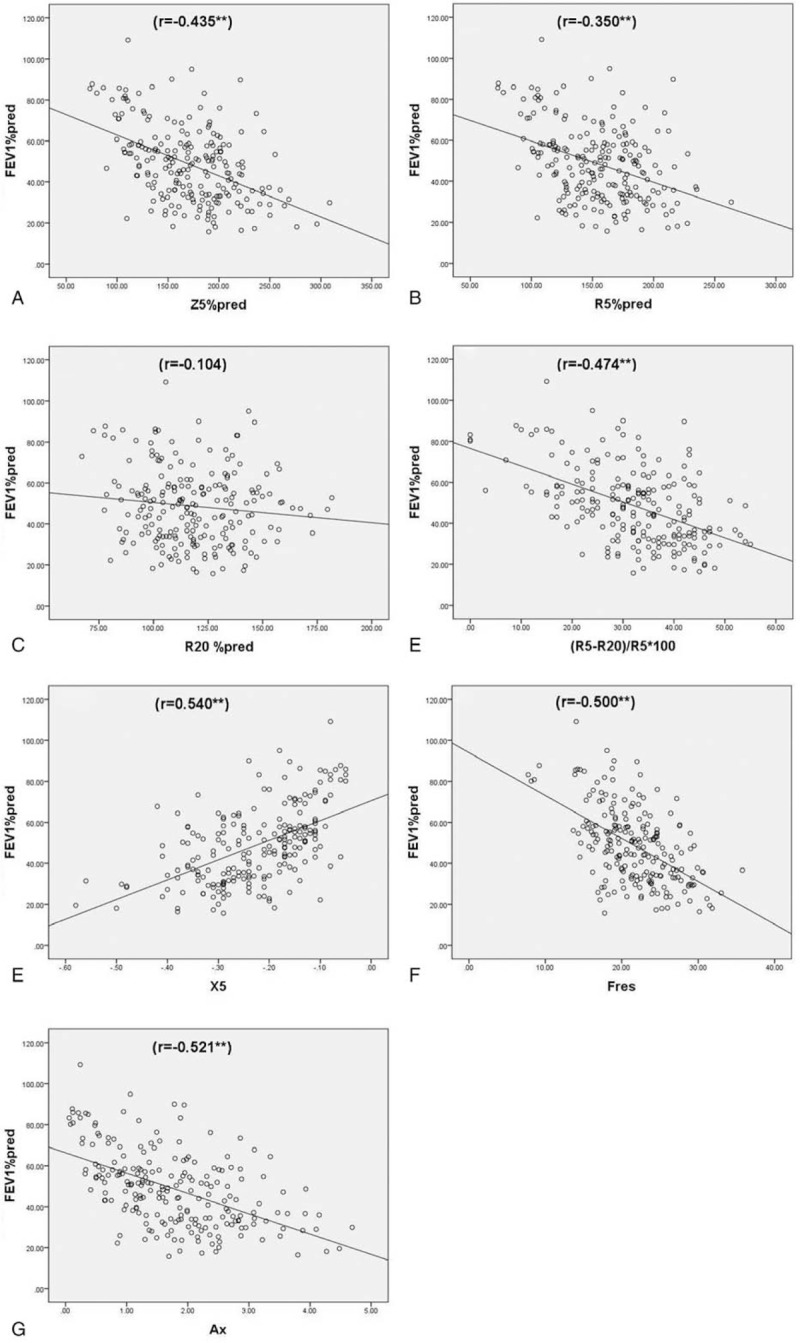
Spearman correlation analysis of FEV1%pred and IOS parameters. (A) Z5%pred (r = −0.435∗∗); (B) R5%pred (r = −0.350∗∗); (C) R20%pred r = −0.104; (D) (R5-R20)%R5 (r = −0.474∗∗); G = Ax (r = −0.521∗∗); (E) X5 (r = 0.540∗∗); (F) Fres (r = −0.474∗∗). Ax = reactance area, FEV1 = forced expiratory volume in one second, Fres = frequency response, IOS = impulse oscillometry system, %pred = of the predicted value, R20 = resistance at 20 Hz, R5 = resistance at 5 Hz, X5 = reactance at 5 Hz, Z5 = total respiratory impedance.
Figure 5.
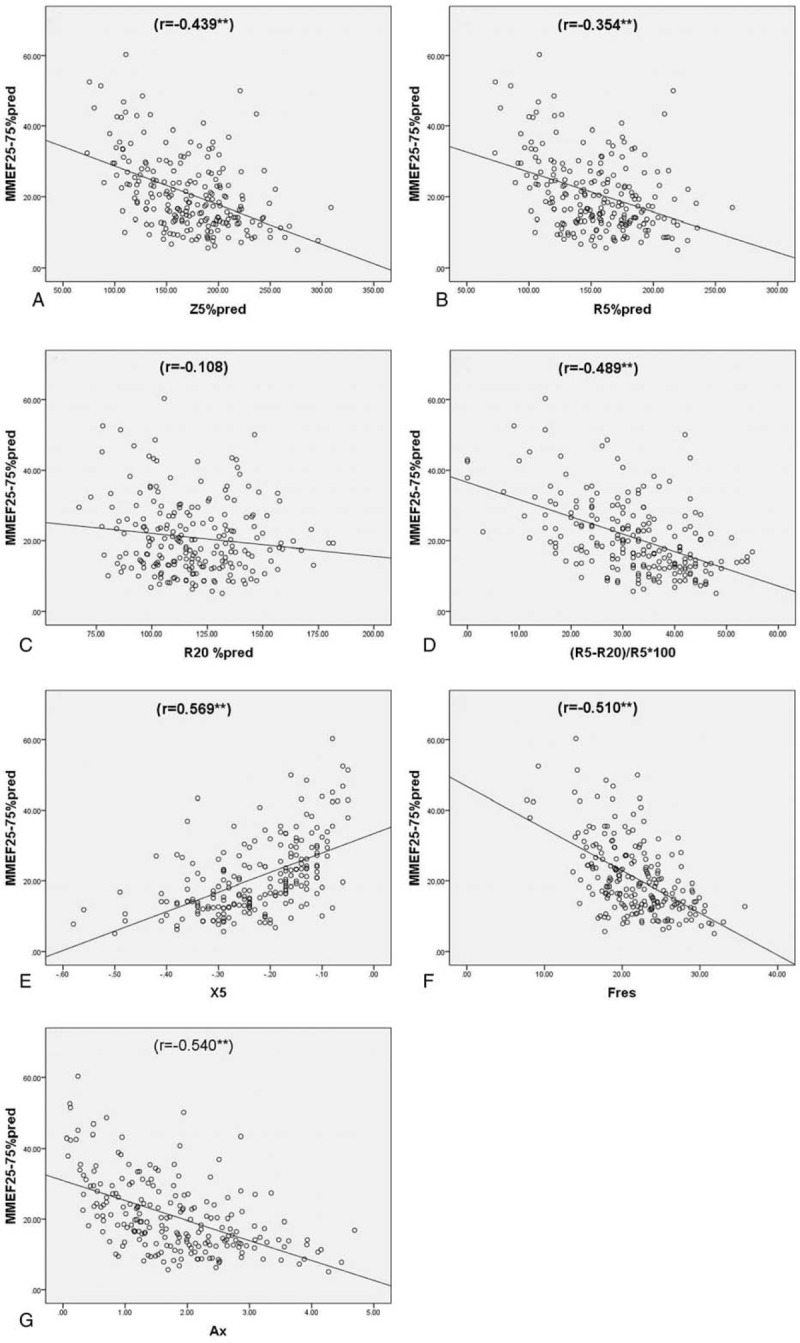
Spearman correlation analysis of MMEF75%–25%pred and IOS parameters. (A) Z5%pred (r = −0.439∗∗); (B) R5%pred (r = −0.354∗∗); (C) R20%pred (r = −0.108); (D) (R5-R20)%R5∗100 (r = −0.489∗∗); (E) X5 (r = 0.569∗∗); (F) Fres (r = −0.510∗∗); (G) Ax (r = −0.540∗∗). Ax = reactance area, Fres = frequency response, IOS = impulse oscillometry system, MMEF75%–25% = maximal mid expiratory flow, %pred = of the predicted value, R20 = resistance at 20 Hz, R5 = resistance at 5 Hz, X5 = reactance at 5 Hz, Z5 = total respiratory impedance.
The parameters RV/TLC which reflect gas trapping showed a weak correlation with the resistance parameters, Z5%pred, R5, R5%pred, R5-R20, and (R5-R20)%R5 (r = 0.268, 0.217, 0.189, 0.346, and 0.398, respectively, P < .01). The reactance parameter, Fres, showed a weak correlation (r = 0.350, P < .01), and X5 and Ax showed a moderate correlation with RV/TLC (r = −0.436 and 0.401, respectively, P < .01); no such correlation was observed between RV/TLC and R20, R20%, P > .05 (Table 3 and Fig. 6). The reactance parameters were found to be superior to the resistance parameters, whether reactive typical pathological changes of airflow limitation, small airway dysfunction, or gas trapping. Reactance parameter X5 showed the best correlation with traditional lung function, followed by Fres and Ax. Of all the resistance parameters, resistance parameter (R5-R20)%R5, which reflects peripheral airway resistance showed the best correlation with traditional pulmonary function.
Figure 6.
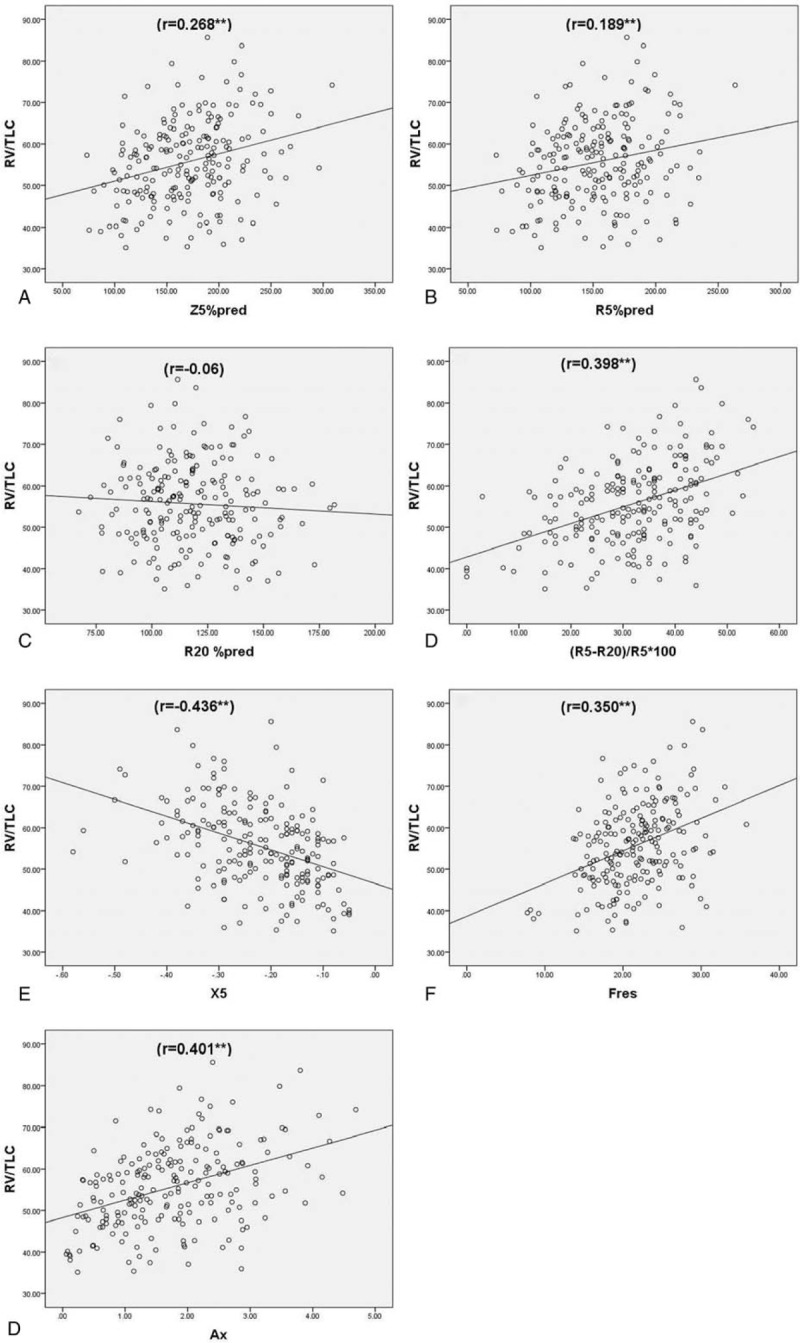
Spearman correlation analysis of RV/TLC and IOS parameters, (A) Z5%pred (r = 0.268∗∗); (B) R5%pred (r = 0.189∗∗); (C) R20%pred (r = −0.060); (D) (R5-R20)%R5∗100 (r = 0.398∗∗); (E) X5 (r = −0.436∗∗); (F) Fres (r = 0.350∗∗); (G) Ax (r = 0.401∗∗). Ax = reactance area, Fres = frequency response, IOS = impulse oscillometry system, %pred = of the predicted value, R20 = resistance at 20 Hz, R5 = resistance at 5 Hz, RV/TLC = residual volume/total lung capacity, X5 = reactance at 5 Hz, Z5 = total respiratory impedance.
3.4. IOS parameters in subjects with COPD who do not tolerate pulmonary function tests and who may require inhaled corticosteroids or roflumilast
From the above pairwise comparisons, we observed statistically significant differences in IOS parameters between COPD grade 2 and 3 (P < .05). Therefore, ROC curves were prepared considering FEV1%pred < 50% as the gold standard, to explore the diagnostic efficacy and reference values of IOS parameters (Table 4 and Fig. 7). The area under the curve (AUC) for the reactance parameters, X5 absolute value, Fres, and Ax were 0.748, 0.755, and 0.760, respectively, and the Youden indices were 0.437, 0.382, and 0.4, respectively (Table 4 and Fig. 7B). The AUC for the resistance parameters Z5%pred, R5-R20, and R5-R20% R5 were 0.705, 0.715, and 0.735, respectively. The Youden indices were 0.307, 0.328, and 0.357, respectively. The AUC for R5 and R5%pred were 0.646, 0.655 and the Youden indices were 0.286, 0.293 (Table 4 and Fig. 7A). The results show that the reactance parameters were better than the resistance parameters. On combining the reactive parameter X5 absolute value with the best diagnostic performance and the resistance parameter R5-R20/R5, or on combining the 3 reactance parameters X5 absolute value, Fres, Ax, and resistance parameters X5-X20, the diagnostic efficacy was similar to that of the reactance parameters alone (Table 4 and Fig. 7B). The AUC were 0.76 and 0.782, respectively, and the Youden indices were 0.449 and 0.425, respectively. To distinguish between inhaled and noninhaled corticosteroids in COPD population, the reactance indices X5, Fres, and Ax can be referenced as −0.185 kPa/L/s, 22.445 Hz, and 1.495 kPa/L, respectively. The cut-off values of resistance parameters Z5%pred, R5, R5%pred, R5-R20, and (R5-R20)%R5 were 143.05%, 0.395 kPa/L/s, 122.3%, 0.125 kPa/L/s, 0.355, respectively.
Table 4.
Diagnostic performance∗ of IOS parameters to assess the need for inhalational glucocorticoid therapy in COPD patients.
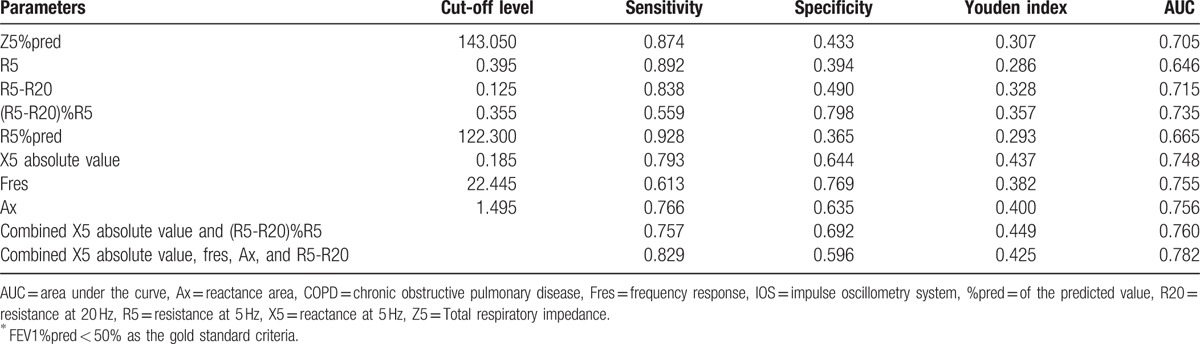
Figure 7.
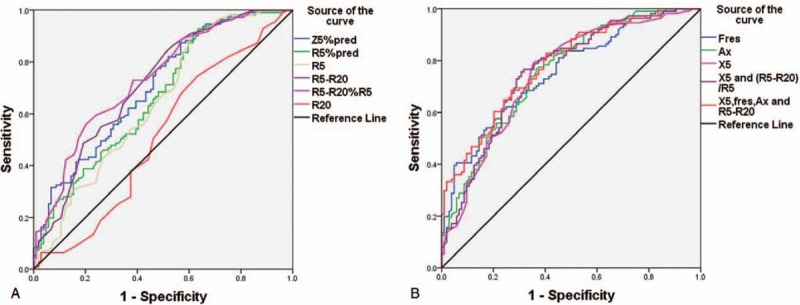
ROC curves of IOS parameters and combination of parameters to diagnose FEV1%pred <50% of the COPD population. COPD = chronic obstructive pulmonary disease, FEV1 = forced expiratory volume in one second, IOS = impulse oscillometry system, %pred = of the predicted value, ROC = receiver-operating characteristic.
4. Discussion
In recent years, IOS has gradually been accepted by respiratory physicians owing to its simple operation, convenience, and objective, credible, and reproducible results. In the present study, we observed a significant correlation between IOS parameters and traditional parameters of pulmonary function. IOS can act as an alternative and as an add-on to the traditional PFTs to help clinicians better understand the heterogeneity of individual patients with COPD. IOS parameters may be able to predict patients with severe COPD (GOLD 3, 4) that are more prone to frequent exacerbations and, hence, require inhaled corticosteroid or roflumilast therapy. However, it may be a little strong to say that IOS can be used as an alternative method for COPD patient with FEV1 < 50%pred.
Among the many blood inflammatory markers, only neutrophil ratio showed a close association with GOLD grade. The other inflammatory markers such as WBC count, platelet count, and C-reactive protein level did not closely mirror the changes in GOLD grade. The nature of COPD is inflammation, but there are no reliable inflammatory markers that can be used to guide clinical diagnosis and treatment,[19] which is a hot spot in COPD research. Our findings also validate this conclusion. The recent updates to GOLD guidelines pay increasing attention to the number of acute exacerbations and symptom scores in the preceding year. The 2017 guidelines even promote this as a basis for the choice of medication.[6] Our results showed that the acute exacerbations in the past 12 months, BMI, and mMRC changed along with the GOLD grade, which indicates that the deterioration in lung function is always accompanied by increased frequency of acute exacerbations, aggravation of dyspnea, decreased BMI, and poor prognosis, all of which require intensive treatment.
COPD is a heterogeneous disease[20] with much variability with respect to pathological changes and impairment of lung function. The traditional pulmonary function parameters to some extent reflect the pathological changes of COPD: small airway dysfunction (MMEF75%–25%); airflow limitation (FEV1, FEV1/FVC); gas retention (RV/TLC); and pulmonary parenchymal destruction (KCO). However, these may not be sensitive enough to take into account the full picture of the disease. Meanwhile, the traditional PFTs are not always feasible in elderly patients, those with cognitive impairment, poor motor coordination, and difficulty in breathing.[7,8,18,21] Especially flow capacity curve cannot be effectively completed. Along with more severe lung function impairment, the difficulty in breathing is more obvious, and the results of the PFT tend to vary widely. This may be the reason that the individual monitoring and treatment guidance is not accurate although traditional PFTs are invaluable for the diagnosis, evaluation, and treatment of patients with COPD. Some authors have reported a good correlation between IOS and traditional pulmonary function parameters for assessment of COPD.[22–25] Therefore, IOS can serve as a valuable adjunct to traditional PFTs. But there are different views. Crim et al's[26] ECLIPSE study on COPD and healthy people suggested that although the mean resistance increased with the severity of GOLD deterioration, the actual correlation is weak, and the IOS parameters used to assess disease pathology progression were limited. Our results showed a correlation of IOS parameters of total airway resistance, peripheral airway resistance, and peripheral elastic resistance with FEV1%pred, MMEF 75%–25%, RV/TLC, but not with KCO. Moreover, the proximal airway resistance index R20 did not correlate with the traditional pulmonary function parameters. These results indicate that just like traditional PFT parameters, the IOS parameters could also reflect the pathological changes of airway obstruction, gas trapping, and decrease in compliance in COPD patients to a certain extent. It can also reflect the airway obstruction abnormalities which cannot be detected with traditional lung function. For example, Kanda et al[27] found abnormal IOS parameters in asthmatic patients who had normal FEV1/FVC. Therefore, we believe that IOS cannot only supplement the traditional lung function tests for the assessment of COPD pathology and pathophysiological changes, but also provides additional information for a comprehensive assessment. In the present study, IOS resistance parameters, including Z5%pred, R5, R5%pred, R5-R20, and (R5-R20)%R5 showed a gradual increase with increase in GOLD grade; however, R20 and R20%pred did not show such a trend, which suggests that the increase in total resistance is exclusively due to the increase in resistance in the peripheral airways. Likewise, the reactance indices X5, Fres, and Ax, which reflect the peripheral airway, lung tissue, and thoracic elastic resistance, also showed a trend of gradual change with the COPD grade, which was more evident than that of change in resistance. Shintarou et al[27] reported significantly higher X5 negative values in COPD patients with severe lung dysfunction, which reflects the small airway collapse. To some degree, IOS can reflect the pathological changes, especially in the peripheral airways. Therefore, it can supplement the pathophysiological changes represented by FEV1 and can clearly reflect the lesion site.
Previous studies[22–25,28] have demonstrated a good correlation of IOS parameters with traditional pulmonary functional parameters in elderly patients. As an alternative, IOS has the advantage of ease of operation. However, its ability to effectively identify COPD patients of FEV1%pred < 50% is not clear. We used ROC curves to investigate the diagnostic value and threshold levels of IOS parameters. It was seen that the reactance parameters X5 absolute values, Fres, Ax, and resistance parameters Z5%pred, R5-R20, and (R5-R20)%R5 had diagnostic significance, and that the diagnostic relevance of reactance parameters was superior to that of resistance parameters. We also investigated the diagnostic performance of combined use of reactance and resistance parameters. We respectively combined the absolute value of the reactance parameter X5 and resistance parameters (R5-R20)%R5, and then the 3 reactance parameters X5 absolute value, Fres, Ax, and resistance parameters X5-X20. The diagnostic performance was similar to that of reactance parameter alone, with a slight increase. Therefore, IOS can replace the traditional PFTs to identify COPD patients with FEV1%pred < 50% for targeted therapy, who may not tolerate the traditional PFTs. The reactance indices X5, Fres, and Ax can be referred to as −0.185, 22.445, and 1.495, respectively, and the resistance indices Z5%pred, R5, R5%pred, R5-R20, and (R5-R20)%R5 are 143.05, 0.395, 122.3, 0.125, and 0.355, respectively. It should be noted that both the Z5%pred and the R5%pred are less than 150% of the reference values commonly used in clinical practice. Gong et al[29] pointed out that the threshold 120% of R5%pred in Chinese patients with early COPD was associated with optimal efficacy. Whether the reference value of 150% as the diagnostic threshold is appropriate for Chinese patients with COPD needs more research. Another study[18] also investigated the forced oscillation parameters to distinguish FEV1%pred < 50% in elderly patients with COPD; the results were similar to our results, although FOT and IOS were not exactly the same.
Some limitations of our study should be noted. First, we conducted a retrospective study but not a randomized controlled trial because of the various applications of IOS and traditional PFTs. Second, the present study did not provide diagnostic criteria for COPD based on IOS.
5. Conclusion
IOS parameters showed a good correlation with traditional pulmonary function parameters; reactance parameters showed a stronger correlation than that of the resistance parameters. IOS can be used as an alternative method for pulmonary function assessment in patients with COPD with FEV1%pred < 50% who need inhalational glucocorticoid therapy.
Acknowledgement
The authors thank the grant from Shaanxi Province social development science and technology research project (Grant No. 2016SF-151) and Xi’an Science and Technology Project (Grant No. 2016045SF/YX01) for the support.
Footnotes
Abbreviations: AUC = area under the curve, Ax = reactance area, BMI = body mass index, COPD = chronic obstructive pulmonary disease, FEV1 = forced expiratory volume in 1 second, Fres = frequency response, FVC = forced vital capacity, GOLD = Global Initiative for Chronic Obstructive Lung Disease, IOS = impulse oscillation system, MMEF 75%–25% = maximal mid expiratory flow, mMRC = Modified Medical Research Council Dyspnea Scale, PFT = pulmonary function test, %pred = of the predicted value, R20 = resistance at 20 Hz, R5 = resistance at 5 Hz, ROC = receiver-operating characteristics, RV/TLC = residual volume/total lung capacity, X5 = reactance at 5, Hz, Z5 = total respiratory impedance.
Authorship: XW, ZS, and YG conceived and designed the trial. XW, ZM, JR, JM, JL, and SX performed the trial. XW, ZS, and YG analyzed the data. XW and YG wrote the paper.
Funding/support: This research was supported by a grant from Shaanxi Province social development science and technology research project (Grant No. 2016SF-151) and by a grant from Xi’an Science and Technology Project (Grant No. 2016045SF/YX01).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to dosclose.
References
- [1].Diaz-Guzman E, Mannino DM. Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin Chest Med 2014;35:7–16. [DOI] [PubMed] [Google Scholar]
- [2].Yin P, Wang H, Vos T, et al. A subnational analysis of mortality and prevalence of COPD in China from 1990 to 2013: findings from the global burden of disease study 2013. Chest 2016;150:1269–80. [DOI] [PubMed] [Google Scholar]
- [3].Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax 2016;72:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 2005;36:2251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dettman SJ, Dowell RC, Choo D, et al. Long-term communication outcomes for children receiving cochlear implants younger than 12 months: a multicenter study. Otol Neurotol 2016;37:e82. [DOI] [PubMed] [Google Scholar]
- [6].Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: Gold executive summary. Archivos de bronconeumologia 2017;53:128–49. [DOI] [PubMed] [Google Scholar]
- [7].Nishi SP, Wang Y, Kuo YF, et al. Spirometry use among older adults with chronic obstructive pulmonary disease: 1999–2008. Ann Am Thorac Soc 2013;10:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Janssens JP, Nguyen MC, Hrrmann FR, et al. Diagnostic value of respiratory impedance measurements in elderly subjects. Respir Med Case Rep 2001;95:415–22. [DOI] [PubMed] [Google Scholar]
- [9].Jarenbäck L, Ankerst J, Bjermer L, et al. Flow-volume parameters in COPD related to extended measurements of lung volume, diffusion,and resistance. Pulm Med 2013;11:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 2015;175:1539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther 2001;14:341–50. [DOI] [PubMed] [Google Scholar]
- [12].Oostveen E, MacLeod DM, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003;22:1026–41. [DOI] [PubMed] [Google Scholar]
- [13].Piorunek T, Kostrzewska M, Cofta S, et al. Impulse oscillometry in the diagnosis of airway resistance in chronic obstructive pulmonary disease. Adv Exp Med Biol 2015;838:47–52. [DOI] [PubMed] [Google Scholar]
- [14].Kim HY, Shin YH, Jung DW, et al. Resistance and reactance in oscillation lung function reflect basal lung function and bronchial hyperresponsiveness respectively. Respirology 2009;14:1035–41. [DOI] [PubMed] [Google Scholar]
- [15].Mansur AH, Manney S, Ayres JG. Methacholine-induced asthma symptoms correlate with impulse oscillometry but not spirometry. Respir Med 2008;102:42–9. [DOI] [PubMed] [Google Scholar]
- [16].Li YY, Chen Y, Wang P. Application of impulse oscillometry and bronchial dilation test for analysis in patients with asthma and chronic obstructive pulmonary disease. Int J Clin Exp Med 2015;8:1271–5. [PMC free article] [PubMed] [Google Scholar]
- [17].Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- [18].Tse HN, Tseng CZS, Wong KY, et al. Accuracy of forced oscillation technique to assess lung function in geriatric COPD population. Int J Chron Obstruct Pulmon Dis 2016;11:1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mannino DM. Biomarkers in COPD: the search continues!. Eur Respir J 2015;45:872–4. [DOI] [PubMed] [Google Scholar]
- [20].Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management,and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–55. [DOI] [PubMed] [Google Scholar]
- [21].Fang X, Wang X, Bai C. Copd in china: The burden and importance of proper management. Chest 2011;139:920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jarenback L, Ankerst J, Bjermer L, et al. Acinar ventilation heterogeneity in COPD relates to diffusion capacity, resistance and reactance. Respir Med 2016;110:28–33. [DOI] [PubMed] [Google Scholar]
- [23].Nikkhah M, Amra B, Eshaghian A, et al. Comparison of impulse osillometry system and spirometry for diagnosis of obstructive lung disorders. Tanaffos 2011;10:19–25. [PMC free article] [PubMed] [Google Scholar]
- [24].Williamson PA, Clearie K, Menzies D, et al. Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Lung 2011;189:121–9. [DOI] [PubMed] [Google Scholar]
- [25].Kolsum U, Borrill Z, Roy K, et al. Impulse oscillometry in COPD: identification of measurements related to airway obstruction, airway conductance and lung volumes. Respir Med 2009;103:136–43. [DOI] [PubMed] [Google Scholar]
- [26].Crim C, Celli B, Edwards LD, et al. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med 2011;105:1069–78. [DOI] [PubMed] [Google Scholar]
- [27].Kanda S, Fujimoto K, Komatsu Y, et al. Evaluation of respiratory impedance in asthma and COPD by an impulse oscillation system. Intern Med 2010;49:23–30. [DOI] [PubMed] [Google Scholar]
- [28].Haruna A, Oga T, Muro S, et al. Relationship between peripheral airway function and patient-reported outcomes in COPD: a cross-sectional study. BMC Pulm Med 2010;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gong SG, Yang WL, Liu JM, et al. Change in pulmonary function in chronic obstructive pulmonary disease stage 0 patients. Int J Clin Exp Med 2015;8:21400–6. [PMC free article] [PubMed] [Google Scholar]


