Supplemental Digital Content is available in the text
Keywords: bladder cancer, diabetes mellitus, meta-analysis
Abstract
Background:
Epidemiologic studies have reported inconsistent results regarding the relationship between diabetes mellitus (DM) and the incidence of bladder cancer. This comprehensive systematic review and meta-analysis explored and evaluated this relationship in participants with different characteristics.
Methods:
Studies indexed in the PubMed, Embase, and the Cochrane Library databases that compared bladder cancer incidence mortality between DM and non-DM participants were included in the present study. The relative risks (RRs) of a random-effects model were used to assess these associations.
Results:
The final analysis included 21 cohort studies, involving a total of 13,505,643 participants. Overall, DM was associated with an increased risk of bladder cancer or cancer mortality when compared with non-DM participants (RR: 1.23; 95% confidence interval [CI]: 1.12–1.35; P < .001). Furthermore, DM had a harmful impact on subsequent bladder cancer risk in men compared with those without DM (RR: 1.23; 95% CI: 1.06–1.42; P = .005), whereas no significant relationship was observed between DM and bladder cancer in women (RR: 1.24; 95% CI: 0.95–1.61; P = .119). There was no significant gender difference for this relationship (ratio of RR: 0.99; 95% CI: 0.73–1.34; P = .958). In addition, cancer incidence (RR: 1.21; 95% CI: 1.09–1.35; P < .001) and cancer mortality (RR: 1.25; 1.17–1.35; P < .001) both increased in DM patients. Finally, smoking status and follow-up duration might also affect this relationship in men and women.
Conclusions:
The findings of this study indicated that DM was associated with elevated bladder cancer or cancer mortality risk, especially in men. This relationship in women requires further exploration.
1. Introduction
Bladder cancer is the 10th most common cancer worldwide and remains a major public health problem, with approximately 73,510 cases and 14,880 deaths in 2012.[1] The established causal risk factors for bladder cancer include gender, age, smoking, early menopause, and occupational exposure.[2–6] Diabetes mellitus (DM) is associated with elevated risks of cancer at different sites, including biliary tract,[7] lung,[8] hepatocellular,[9] colorectal,[10] ovarian,[11] prostate,[12] breast,[13] renal,[14] esophageal,[15] gastric,[16] non-Hodgkin lymphoma, leukemia, and myeloma,[17] pancreatic,[18] and endometrial[19] cancers. Further, although numerous meta-analyses have evaluated the association of DM with the risk of bladder cancer,[20–25] inconsistent results have been reported and require further verification.
Fang et al[20] reported the positive association between DM and risk of bladder cancer, which was significant only in women. The study conducted by Zhu et al[21] indicated that men with DM have a modestly increased risk of bladder cancer, while women with DM did not. Yang et al[22] also reported DM to be a risk factor for bladder cancer, with no gender differences in this relationship. Xu et al[23] performed a meta-analysis of 15 cohort studies and observed a positive association between DM and the risk of bladder cancer but did not report a gender difference. Zhu et al suggested that DM patients have an increased bladder cancer incidence and mortality. Furthermore, the incidence of bladder cancer was significantly increased only in men, while bladder cancer mortality was significantly increased in both men and women.[24] Finally, Larsson et al[25] concluded that DM patients may have a modestly increased risk of bladder cancer in their assessment of case–control or cohort studies. However, whether the association between DM and the risk of bladder cancer differs according to different patient characteristics remains unclear. This meta-analysis further examined whether diabetes is a risk factor for bladder cancer in specific subpopulations.
2. Methods
2.1. Data sources, search strategy, and selection criteria
This review was conducted and reported according to the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement (Checklist S1).[26] Ethics approval was not necessary for this study, as only deidentified pooled data from individual studies were analyzed. Cohort studies that explored the relationship between DM and bladder cancer were eligible for inclusion in the present study, with no restrictions on the language and publication status. Electronic databases including PubMed, Embase, and the Cochrane Library were systematically searched using the following text word or Medical Subject Heading terms: (“diabetes mellitus” OR “diabetes” OR “DM” OR “impaired glucose”) AND (“bladder” OR “urothelium” OR “transitional cell” OR “urothelial”) AND (“neoplasm” OR “tumor” OR “cancer” OR “carcinoma” OR “malignant”) AND (“cohort” OR “epidemiologic”) through October 10, 2017. The details of search strategies are presented in Supplementary Material 1. In order to identify unpublished studies or updated information that could provide useful data, we contacted corresponding authors to obtain potential relevant data. The reference lists of the included studies or relevant reviews were also manually screened in order to identify additional relevant studies.
The inclusion criteria of this study are as follows: cohort design; comparison of the incidence or mortality of bladder cancer in DM and non-DM individuals; and reported odds ratios (ORs), hazard ratios (HRs), or relative risks (RRs) with corresponding confidence intervals (CIs). The exclusion criteria included case–control, cross-sectional, or cohort studies of diabetic patients that provided data as standard incidence ratios; patients with previous with bladder cancer; and unavailable data, whether through lack of calculations or translation. The search strategies and study selection were conducted independently by 2 authors; any inconsistencies were settled by the corresponding author, who made a final decision by referring to the original articles.
2.2. Data collection and quality assessment
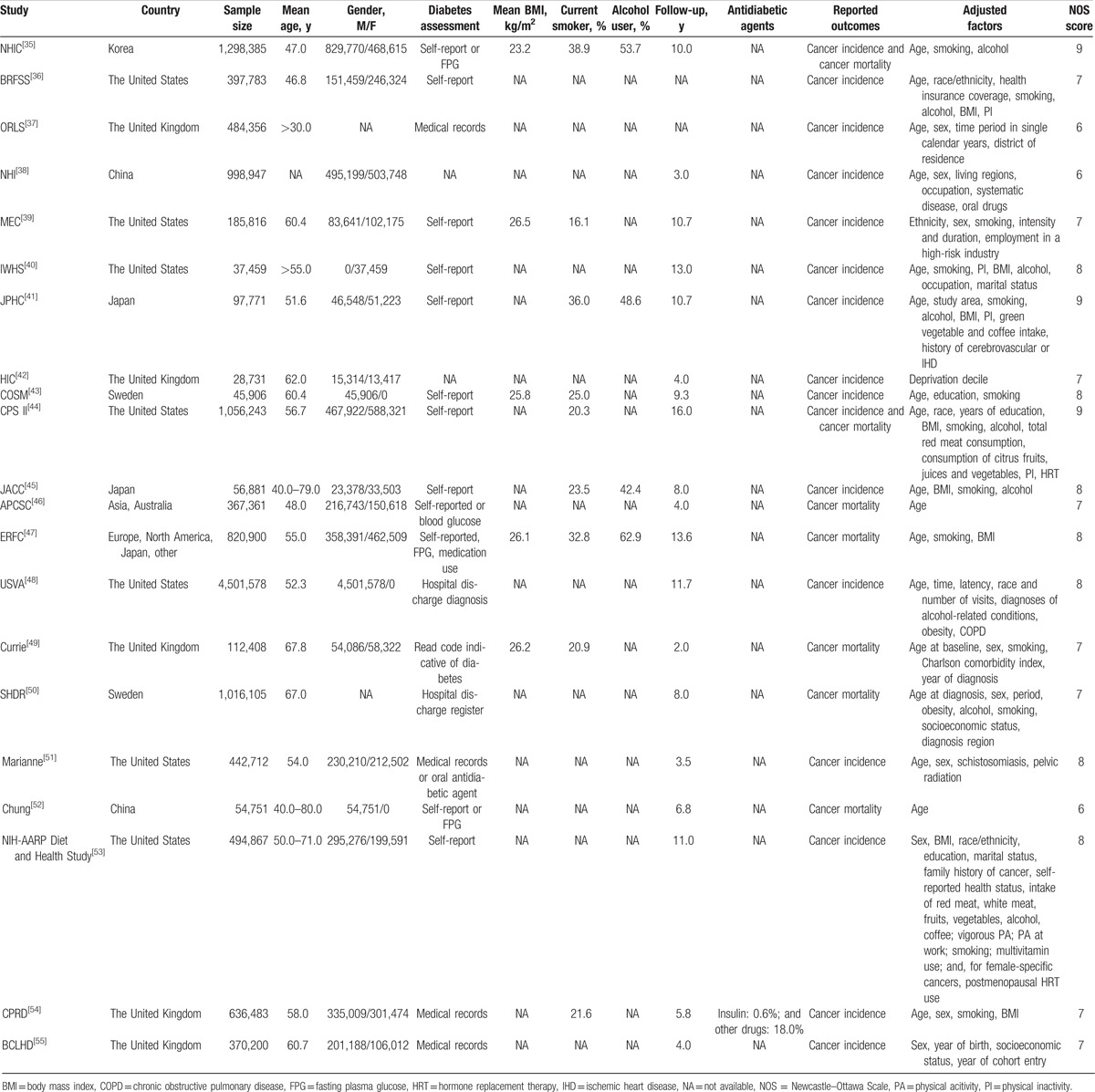
Two authors independently extracted the baseline characteristics and data of the included studies, including the first author or study group name, country, sample size, mean age, number of men and women separately, diabetes assessment, mean body mass index (BMI), percentage of current smokers, percentage of alcohol users, follow-up duration, reported outcomes, adjusted factors, and effect estimate of the relationship between DM and bladder cancer. If direct reports of the effect estimate and corresponding 95% CI were not available, the estimated value was derived indirectly from other data. The Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of the included studies based on selection (4 items), comparability (1 item), and outcome (3 items).[27] A “star system” (range, 0–9) was developed for assessment (Table 1).
Table 1.
Baseline characteristics of studies included.
2.3. Statistical analysis
The RRs with 95% CIs were defined as the effect estimates for the relationship between DM and the risk of bladder cancer. The HR was considered equivalent to the RR in cohort studies and OR was assumed to be an accurate estimate of RR due to the low incidence of bladder cancer in DM patients. The maximum adjusted RR was used to avoid bias caused by adjusted factors. A fixed-effects model was used for pooled RR and 95% CI in single studies if the effect estimates were reported separately according to different characteristics.[28] The pooled RRs and 95% CIs for the DM versus non-DM and the risk of bladder cancer were calculated by using a random-effects model.[29] Heterogeneity was assessed by the I2 and Q statistics, with P < .10 indicative of significant heterogeneity.[30,31] Subgroup analysis was performed to evaluate the effects of country (Western vs. Eastern), mean age (≥60 vs. <60 years), follow-up duration (≥10.0 vs. <10.0 years), reported outcomes (cancer incidence vs. cancer mortality), adjusted BMI (yes vs. no), and adjusted smoking (yes vs. no). Sensitivity analyses were performed by removing each individual study from the overall analysis to assess the influence of a single study in the meta-analysis.[32] We assessed publication bias visually with funnel plots and statistically using Begg and Egger tests.[33,34] All P values were 2-sided, with a significant level of .05. Statistical analyses were performed using STATA (version 10.0; Stata Corporation, College Station, TX).
3. Results
3.1. Study selection
We identified 406 records in the initial search; after discarding 325 duplicates and irrelevant studies, 81 full-text records were assessed. We further excluded studies that enrolled single-arm DM patients, patients with a history of bladder cancer, or studies which focused on antidiabetic drugs (n = 22). A total of 59 studies were included in the qualitative analysis. We removed 34 studies with duplicate cohorts and 4 studies that did not report an effect estimate. Finally, 21 studies were included in the meta-analysis.[35–55] A manual search of the reference lists of these studies did not yield any new eligible studies. The study selection process is shown in Fig. 1.
Figure 1.
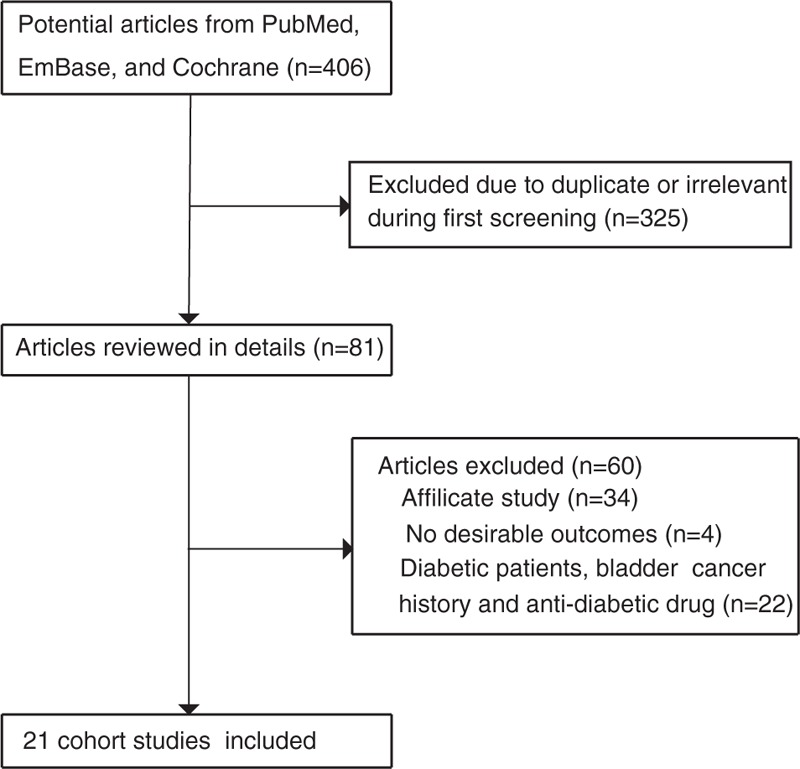
Flowchart of the study selection process.
3.2. Characteristics of the included studies
Table 1 summarizes the baseline characteristics of the included studies. The sample size ranged from 28,731 to 4,501,578 individuals, for a total of 13,505,643 participants. Seven studies were conducted in the United States, 7 in Europe, 5 in Asia, and 2 were international multicenter studies. The follow-up duration ranged from 2.0 to 16.0 years. Sixteen studies reported the relationship between DM and the incidence of bladder cancer, while 7 studies reported the relationship between DM and the risk of bladder cancer mortality. Study quality was evaluated by using the NOS and studies with scores ≥7 were considered to be high quality. Three studies had a score of 9, 7 studies had a score of 8, 8 studies had a score of 7, and 3 studies had a score of 6.
3.3. Bladder cancer or cancer mortality in the total cohort
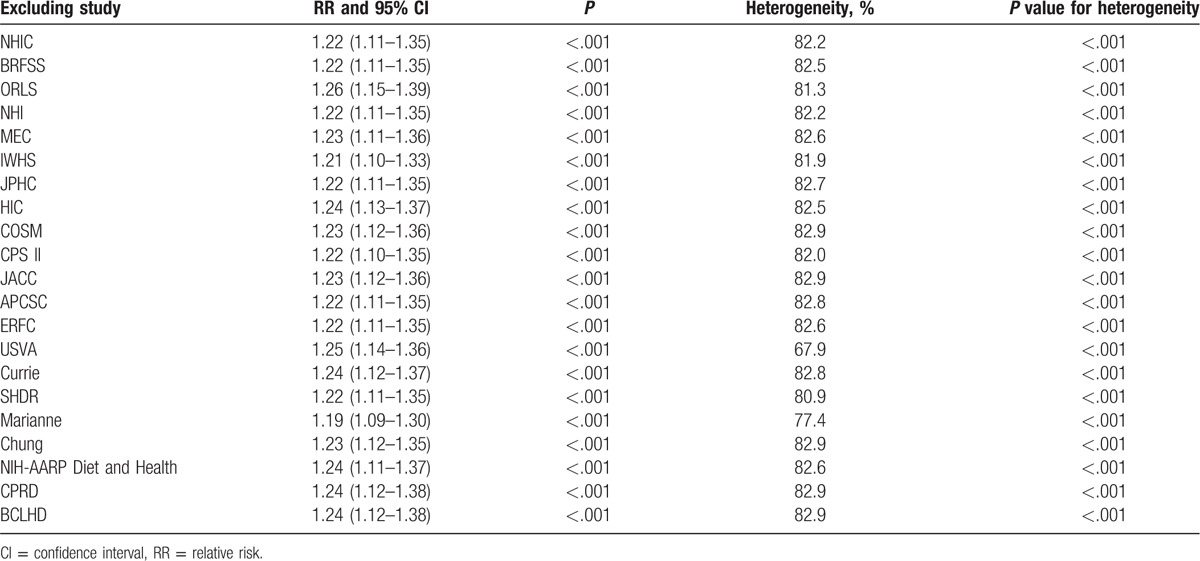
A total of 21 studies reported an association between DM and the risk of bladder cancer or cancer mortality. The pooled RR showed that DM was associated with an increased risk of bladder cancer or cancer mortality when compared with participants without DM (RR: 1.23; 95% CI: 1.12–1.35; P < .001; Fig. 2). Furthermore, significant heterogeneity was detected across the included studies (I2 = 82.0%; P < .001). A sensitivity analysis was performed, in which the result was not affected (Table 2).
Figure 2.
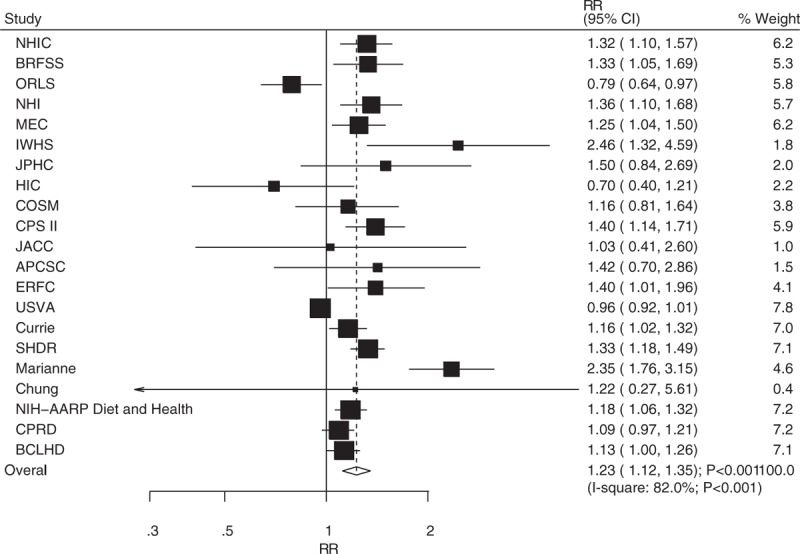
Association between diabetes mellitus and the risk of bladder cancer or cancer mortality.
Table 2.
Sensitivity analysis for total cohort.
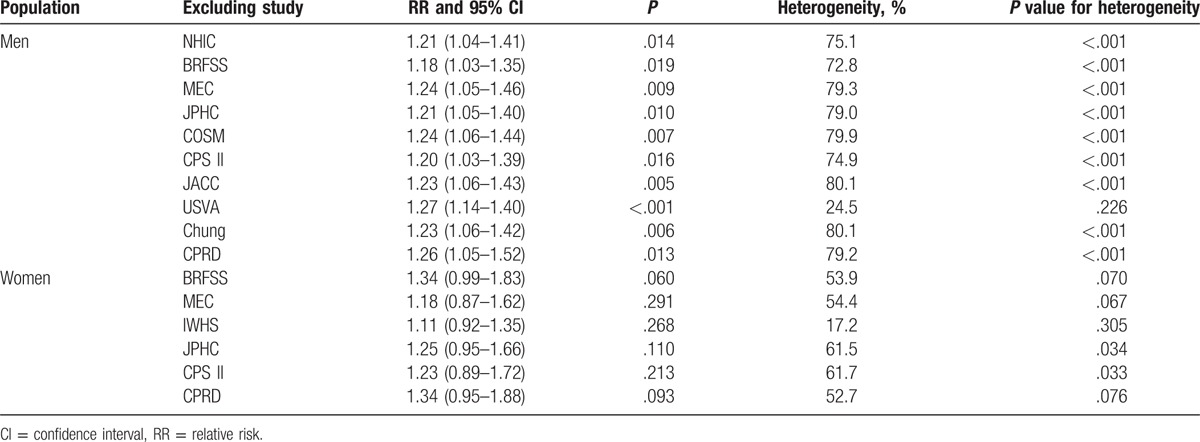
3.4. Bladder cancer or cancer mortality in men and women
The breakdown of the number of studies available for bladder cancer or cancer mortality included 10 and 6 studies in men and women, respectively. The pooled analysis results for men indicated that the comparison of DM versus non-DM individuals showed a harmful effect (RR: 1.23; 95% CI: 1.06–1.42; P = .005; Fig. 3), whereas there was no significant difference in women (RR: 1.24; 95% CI: 0.95–1.61; P = .119; Fig. 4). Heterogeneity was observed in the magnitude of the relationship across the included studies for both men (I2 = 77.7%; P < .001) and women (I2 = 53.5%; P = .057). Sensitivity analyses for men and women separately did not alter the conclusions (Table 3). Furthermore, there was no significant gender difference in this relationship (ratio of RR: 0.99; 95% CI: 0.73–1.34; P = .958).
Figure 3.
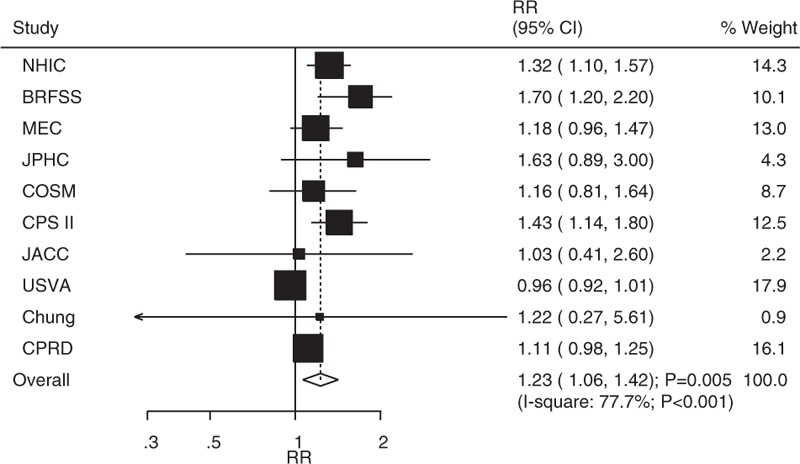
Association between diabetes mellitus and the risk of bladder cancer or cancer mortality in men.
Figure 4.
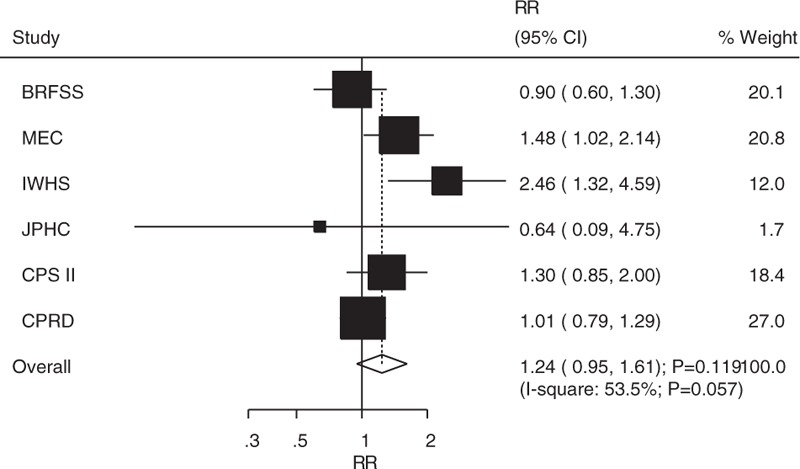
Association between diabetes mellitus and the risk of bladder cancer or cancer mortality in women.
Table 3.
Sensitivity analysis for men and women.
3.5. Subgroup analysis of the total cohorts of men and women
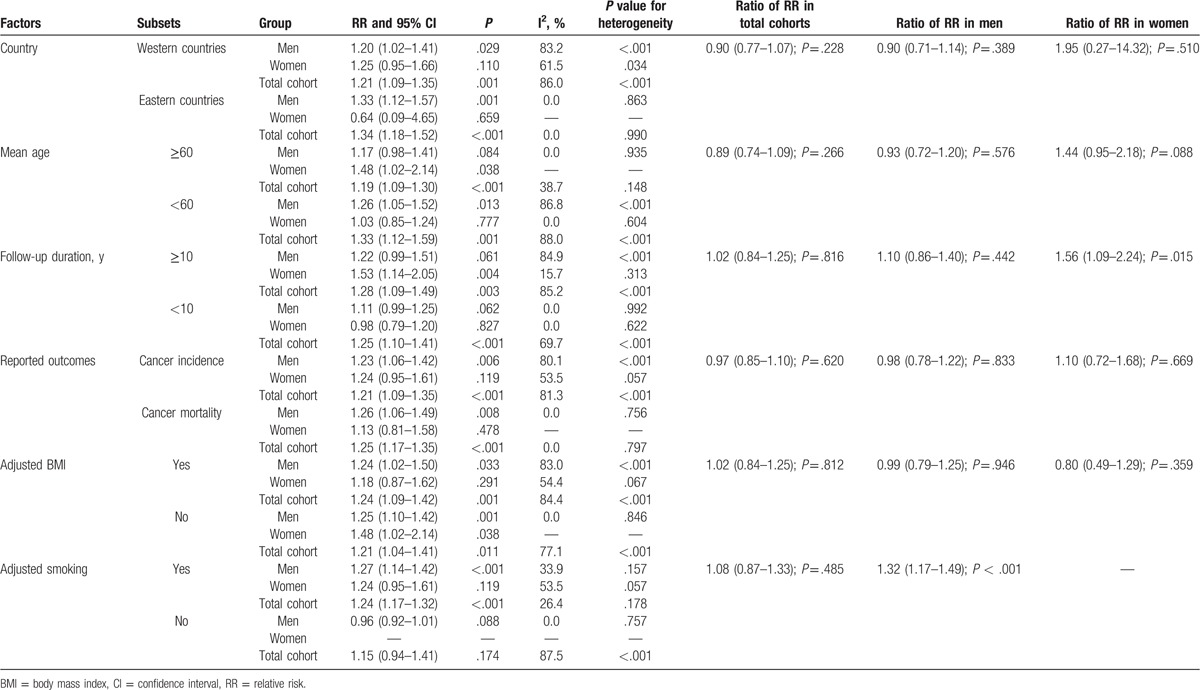
Substantial heterogeneity was detected in the total cohort of men and women. Therefore, we performed subgroup analyses of the association between DM with the risk of bladder cancer in the total cohort and men and women in order to minimize heterogeneity and evaluate this relationship in specific subpopulations. The results are presented in Table 4. First, stratified analysis in the total cohort was consistent with the overall analysis except if the study did not adjust for smoking and there were no significant differences between subgroups in total cohorts based on predefined factors; second, DM was not associated with bladder cancer in men if the mean age was ≥60.0 years, the study did not adjust for smoking, and neglected to include the follow-up duration. Furthermore, there was a significant difference between subgroups based on adjusting for smoking (ratio of RR: 1.32; 95% CI: 1.17–1.49; P < .001), whereas there were no significant differences based on other factors. Third, DM was associated with increased risk of bladder cancer in women ≥60.0 years, a follow-up duration >10.0 years, and in studies that did not adjust for BMI. Furthermore, there was a significant difference between subgroups based on follow-up duration (ratio of RR: 1.56; 95% CI: 1.09–2.24; P = .015). No significant differences were observed in the other stratified analyses.
Table 4.
Subgroup analysis of relative risk for bladder cancer in men, women, and total cohort.
3.6. Publication bias
Review of the funnel plot could not rule out potential publication bias for the relationship between DM and bladder cancer risk in the total cohort (Fig. 5). Furthermore, although the Begg test results showed no evidence of publication bias (P = .928), and the Egger test showed significant publication bias (P = .009). After adjusting for publication bias using the trim and fill method, we noted that DM was associated with greater risk of bladder cancer or cancer mortality (RR: 1.16; 95% CI: 1.06–1.28; P = .002).[56]
Figure 5.
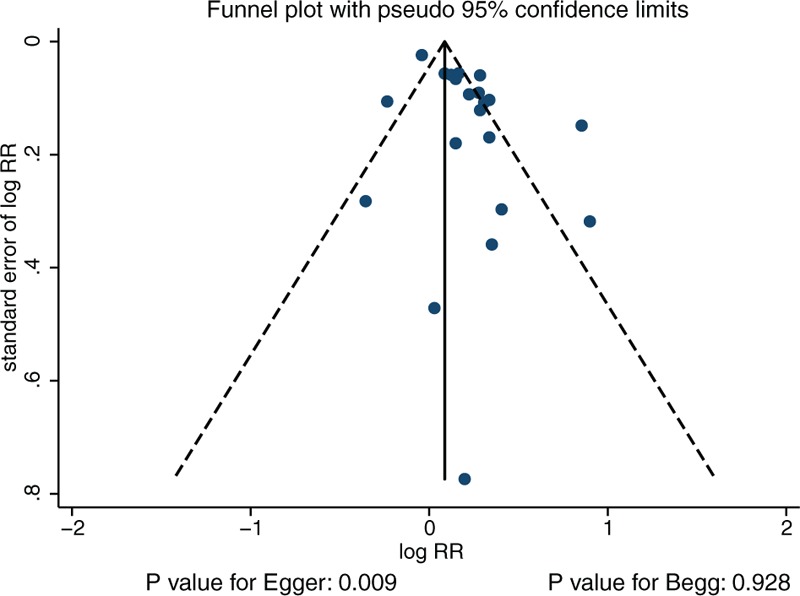
Funnel plot.
4. Discussion
This meta-analysis used data from cohort studies to explore the correlations between DM and the risk of bladder cancer. This large quantitative study included 13,505,643 individuals from 21 cohort studies with a broad range of baseline characteristics. The findings from this study indicated that DM patients had an increased risk of bladder cancer or cancer mortality. Although DM play a harmful effect on bladder cancer in men but not in women, but the gender difference for the relationship between DM and bladder cancer was not associated with statistically significant. Subgroup analysis revealed a positive relationship in both cancer incidence and cancer mortality. Furthermore, adjustment for smoking status played an important role in this relationship in men, whereas follow-up duration may affect this relationship in women.
The methodological assessment of the included studies was based on the representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, demonstration that the outcomes were not present at the start of the study, comparability on the basis of the design or analysis, assessment of outcome, adequate follow-up duration, and adequate follow-up rate. Although most of the studies included in this meta-analysis provided clear information on these items, ascertainment of exposure, follow-up duration, and different adjusted factors might have contributed to the heterogeneity among the included studies. Furthermore, the number of studies on this relationship in specific populations might have affected the pooled outcomes. Therefore, the relationship between DM and bladder cancer in specific subpopulations should be interpreted with caution.
Previous meta-analyses combined case–control and cohort studies in order to evaluate the association of DM and the risk of bladder cancer[20–22,25]; although stratified analyses were conducted based on several important confounders, the results of the subgroup analyses might unreliable due to different study designs across the stratified analysis. Furthermore, the included cohort studies of diabetic patients used the total population as the control, which might have affected the accuracy of the selection of the nonexposed cohort.[21,23–25] In addition, the relationship in men and women was not performed, and the ratio of RR between subgroups remains unknown. Finally, the effect estimates of bladder cancer and cancer mortality were summarized separately due to the lower number of cases of cancer mortality, which might have biased the final conclusion. The important strengths of our study include the comprehensive inclusion of cohort studies with large sample sizes; most of the cohorts were prospective designs, population-based, and with long follow-up durations. In addition, the heterogeneity was evaluated in multiple ways and detailed stratified analyses were also conducted.
Previous studies have illustrated the burden of DM as an important cause of premature illness and death due to cancer.[7–25] This study showed that DM was associated with an increased risk of bladder cancer or cancer mortality. However, significant heterogeneity was detected across the included studies; to minimize the consequences of heterogeneity, we conducted a sequential exclusion of each study from the pooled analysis. The findings remained stable after each individual study was sequentially excluded, which supported the relationship between DM and bladder cancer.
Several potential mechanisms may explain the increased bladder cancer burden in DM patients. First, hyperglycemia in these patients might lead to the dysregulation of energy balance, which affects intracellular metabolism and impair the immune system. These phenomena are associated with increased risks of cancer at different sites.[57] Second, insulin in commonly used by DM patients, which results in increased levels of insulin-like growth factor. These increased levels might stimulate cell proliferation and inhibit apoptosis.[57–60] Finally, DM is associated with a higher incidence of urinary tract infections,[61] which might affect the risk of bladder cancer.[38,62]
In the present study, DM was associated with the increased risk of bladder cancer only in men. One possible reason for this observation could be that the number of studies that included women was smaller than expected. Furthermore, the incidence of bladder cancer in women was lower than that in men,[1] which led to lower statistical power and broad CIs. In addition, adjustment for smoking status played an important role in this relationship in men but not in women. The reason could be the higher proportion of male smokers in which smoking had a significant effect on the risk of bladder cancer,[3] whereas this impact in women was small due to the relatively lower proportion of smokers in this population. Finally, follow-up duration affected the association of DM with bladder cancer in women. Most of the included studies that reported on women were conducted in the United States; the criteria for DM in the United States became more sensitive at a later period, which led to the increased inclusion of the earlier stages of DM. Finally, the symptoms of bladder cancer may be hidden and diagnosis might be delayed in women due to other gynecological diseases.
This meta-analysis has several limitations: different DM assessment approaches to indicate the severity of DM might bias this relationship; antihyperglycemic drugs may be associated with the risk of bladder cancer, but mostly were not adjusted for in most of the included studies;[63,64] the inherent bias included recall and selection in retrospective cohort studies, which could affect the incidence of bladder cancer; the ascertainment of the incidence of bladder cancer in different countries may be liable to bias; different adjusted factors across the included studies might affect the progression of bladder cancer; and the publication bias could be due to our searching strategy, missing the studies with negative results. As a result, the risk of bladder cancer in diabetes could be overestimated in our study.
In conclusion, our study indicated that DM has a harmful effect on the risk of bladder cancer or cancer mortality. The composite outcomes of bladder cancer incidence and cancer mortality were significantly increased in men but not in women. Smoking and follow-up duration might affect this relationship. Future large-scale cohort studies should explore this relationship in women.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DM = diabetes mellitus, HR = hazard ratio, NOS = Newcastle–Ottawa Scale, OR = odds ratio, RR = relative risk.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- [2].World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- [3].Freedman N, Silverman D, Hollenbeck A, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dietrich K, Demidenko E, Schned A, et al. Parity, early menopause and the incidence of bladder cancer in women: a case-control study and meta-analysis. Eur J Cancer 2011;47:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stern MC, Lin J, Figueroa JD, et al. Polymorphisms in DNA repair genes, smoking, and bladder cancer risk: findings from the international consortium of bladder cancer. Cancer Res 2009;69:6857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bachand A, Mundt KA, Mundt DJ, et al. Meta-analyses of occupational exposure as a painter and lung and bladder cancer morbidity and mortality 1950–2008. Crit Rev Toxicol 2010;40:101–25. [DOI] [PubMed] [Google Scholar]
- [7].Ren HB, Yu T, Liu C, et al. Diabetes mellitus and increased risk of biliary tract cancer: systematic review and meta-analysis. Cancer Causes Control 2011;22:837–47. [DOI] [PubMed] [Google Scholar]
- [8].Lee JY, Jeon I, Lee JM, et al. Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer 2013;49:2411–23. [DOI] [PubMed] [Google Scholar]
- [9].Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 2012;130:1639–48. [DOI] [PubMed] [Google Scholar]
- [10].Jiang Y, Ben Q, Shen H, et al. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 2011;26:863–76. [DOI] [PubMed] [Google Scholar]
- [11].Lee JY, InPyo Jeon J, Weon Kim, et al. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer 2013;23:402–12. [DOI] [PubMed] [Google Scholar]
- [12].Gang PJ, Mo L, Lu Y, et al. Diabetes mellitus and the risk of prostate cancer: an update and cumulative meta-analysis. Endocr Res 2015;40:54–61. [DOI] [PubMed] [Google Scholar]
- [13].Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007;121:856–62. [DOI] [PubMed] [Google Scholar]
- [14].Chen L, Li H, Gu L, et al. The impact of diabetes mellitus on renal cell carcinoma prognosis: a meta-analysis of cohort studies. Medicine 2015;94:e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang W, Ren H, Ben Q, et al. Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control 2012;23:263–72. [DOI] [PubMed] [Google Scholar]
- [16].Ge Z, Ben Q, Qian J, et al. Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol 2011;23:1127–35. [DOI] [PubMed] [Google Scholar]
- [17].Castillo JJ, Mull N, Reagan JL, et al. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood 2012;119:4845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Song S, Wang B, Zhang X, et al. Long-term diabetes mellitus is associated with an increased risk of pancreatic cancer: a meta-analysis. PLoS ONE 2015;10:e0134321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Friberg E, Orsini N, Mantzoros CS, et al. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007;50:1365–74. [DOI] [PubMed] [Google Scholar]
- [20].Fang H, Yao B, Yan Y, et al. Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis of observational studies. Diabetes Technol Ther 2013;15:914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhu Z, Wang X, Shen Z, et al. Risk of bladder cancer in patients with diabetes mellitus: an updated meta-analysis of 36 observational studies. BMC Cancer 2013;13:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang XQ, Xu C, Sun Y, et al. Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis. Asian Pac J Cancer Prev 2013;14:2583–9. [DOI] [PubMed] [Google Scholar]
- [23].Xu X, Wu J, Mao Y, et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS ONE 2013;8:e58079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhu Z, Zhang X, Shen Z, et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS ONE 2013;8:e56662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Larsson SC, Orsini N, Brismar K, et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006;49:2819–23. [DOI] [PubMed] [Google Scholar]
- [26].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wells G, Shea B, O’Connell D. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2009. [Google Scholar]
- [28].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [29].Ades AE, Lu G, Higgins JP. The interpretation of random-effects metaanalysis in decision models. Med Decis Making 2005;25:646–54. [DOI] [PubMed] [Google Scholar]
- [30].Deeks JJ, Higgins JPT, Altman DG. Higgins J, Green S. Analyzing data and undertaking meta-analyses. The Cochrane Collaboration, Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: 2008. [Google Scholar]
- [31].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull 1999;47:15–7. [Google Scholar]
- [33].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [35].Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194–202. [DOI] [PubMed] [Google Scholar]
- [36].Li C, Balluz LS, Ford ES, et al. Association between diagnosed diabetes and self-reported cancer among U.S. Adults: findings from the 2009 Behavioral Risk Factor Surveillance System. Diabetes Care 2011;34:1365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wotton CJ, Yeates DGR, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia 2011;54:527–34. [DOI] [PubMed] [Google Scholar]
- [38].Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia 2011;54:2009–15. [DOI] [PubMed] [Google Scholar]
- [39].Woolcotta CG, Maskarinecb G, Haiman CA, et al. Diabetes and urothelial cancer risk: the Multiethnic Cohort Study. Cancer Epidemiol 2011;35:551–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tripathi A, Folsom AR, Anderson KE. Risk factors for urinary bladder carcinoma in postmenopausal women: the Iowa Women's Health Study. Cancer 2002;95:2316–23. [DOI] [PubMed] [Google Scholar]
- [41].Inoue M, Iwasaki M, Otani T, et al. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 2006;166:1871–7. [DOI] [PubMed] [Google Scholar]
- [42].Ogunleye AA, Ogston SA, Morris AD, et al. A cohort study of the risk of cancer associated with type 2 diabetes. Brit J Cancer 2009;101:1199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Larsson SC, Andersson SO, Johansson JE, et al. Diabetes mellitus, body size and bladder cancer risk in a prospective study of Swedish men. Eur J Cancer 2008;44:2655–60. [DOI] [PubMed] [Google Scholar]
- [44].Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 2004;159:1160–7. [DOI] [PubMed] [Google Scholar]
- [45].Khan MMH, Mori M, Fujino Y, et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pacific J Cancer Prev 2006;7:253–9. [PubMed] [Google Scholar]
- [46].Lam EKK, Batty GD, Huxley RR, et al. Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol 2011;22:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Atchison EA, Gridley G, Carreon JD, et al. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer 2011;128:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Currie CJ, Gale EAM, Poole CD, et al. Mortality after incident cancer in people with and without type 2 diabetes. Diabetes Care 2012;35:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu X, Ji J, Sundquist K, et al. The impact of type 2 diabetes mellitus on cancer-specific survival: a follow-up study in Sweden. Cancer 2012;118:1353–61. [DOI] [PubMed] [Google Scholar]
- [51].Marianne UY, Susan AO, Ulka BC, et al. Incidence of cancer in a population-based cohort of patients with type 2 diabetes. Diabetes Metab Syndr 2009;3:12–6. [Google Scholar]
- [52].Chung H. Diabetes and risk of death from cancer of the prostate, kidney, and urinary bladder. Urology 2009;74:S36–7. [Google Scholar]
- [53].Lai GY, Park Y, Hartge P, et al. The association between self-reported diabetes and cancer incidence in the NIH-AARP Diet and Health Study. J Clin Endocrinol Metab 2013;98:E497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Goossens ME, Zeegers MP, Bazelier MT, et al. Risk of bladder cancer in patients with diabetes: a retrospective cohort study. BMJ Open 2015;5:e007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Colmers IN, Bowker SL, Majumdar SR, et al. Detection bias and overestimation of bladder cancer risk in type 2 diabetes: a matched cohort study. Diabetes Care 2013;36:3070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Duvall S, Tweedie R. A nonparametric “trim and fill” method for assessing publication bias in meta-analysis. J Am Stat Assoc 2000;95:89–98. [Google Scholar]
- [57].Vigneri P, Frasca F, Sciacca L, et al. Diabetes and cancer. Endocr Relat Cancer 2009;16:1103–23. [DOI] [PubMed] [Google Scholar]
- [58].Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin 2010;60:207–21. [DOI] [PubMed] [Google Scholar]
- [59].Dunn SE, Kari FW, French J, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res 1997;57:4667–72. [PubMed] [Google Scholar]
- [60].Zhao H, Grossman HB, Spitz M, et al. Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. J Urol 2003;169:714–7. [DOI] [PubMed] [Google Scholar]
- [61].Silverman DT, Devesa SS, Moore LE. Schottenfeld D, Fraumeni JFJ, et al. Bladder cancer. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2006. 1101–28. [Google Scholar]
- [62].MacKenzie T, Zens M, Ferrara A, et al. Diabetes and risk of bladder cancer: evidence from a case–control study in New England. Cancer 2011;117:1552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Turner RM, Kwok CS, Chen-Turner C, et al. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol 2014;78:258–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].He S, Tang YH, Zhao G, et al. Pioglitazone prescription increases risk of bladder cancer in patients with type 2 diabetes: an updated meta-analysis. Tumour Biol 2014;35:2095–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


