Supplemental Digital Content is available in the text
Keywords: GEO and TCGA, meta-analysis, methylation, promoter, RASSF1A
Abstract
Background:
DNA promoter methylation can suppresses gene expression and shows an important role in the biological functions of Ras association domain family 1A (RASSF1A). Many studies have performed to elucidate the role of RASSF1A promoter methylation in thyroid carcinoma, while the results were conflicting and heterogeneous. Here, we analyzed the data of databases to determine the relationship between RASSF1A promoter methylation and thyroid carcinoma.
Methods:
We used the data from 14 cancer–normal studies and Gene Expression Omnibus (GEO) database to analyze RASSF1A promoter methylation in thyroid carcinoma susceptibility. The data from the Cancer Genome Atlas project (TCGA) database was used to analyze the relationship between RASSF1A promoter methylation and thyroid carcinoma susceptibility, clinical characteristics, prognosis. Odds ratios were estimated for thyroid carcinoma susceptibility and hazard ratios were estimated for thyroid carcinoma prognosis. The heterogeneity between studies of meta-analysis was explored using H, I2 values, and meta-regression. We adopted quality criteria to classify the studies of meta-analysis. Subgroup analyses were done for thyroid carcinoma susceptibility according to ethnicity, methods, and primers.
Results:
Result of meta-analysis indicated that RASSF1A promoter methylation is associated with higher susceptibility to thyroid carcinoma with small heterogeneity. Similarly, the result from GEO database also showed that a significant association between RASSF1A gene promoter methylation and thyroid carcinoma susceptibility. For the results of TCGA database, we found that RASSF1A promoter methylation is associated with susceptibility and poor disease-free survival (DFS) of thyroid carcinoma. In addition, we also found a close association between RASSF1A promoter methylation and patient tumor stage and age, but not in patients of different genders.
Conclusions:
The methylation status of RASSF1A promoter is strongly associated with thyroid carcinoma susceptibility and DFS. The RASSF1A promoter methylation test can be applied in the clinical diagnosis of thyroid carcinoma.
1. Introduction
Thyroid carcinoma, including papillary thyroid carcinoma, medullary thyroid carcinoma, and follicular thyroid carcinoma, is the most common endocrine malignant neoplasm worldwide.[1] In the United States, thyroid carcinoma accounts for 1.7% of all malignancies, corresponding to 2.6% of cancers in females and 0.85% of cancers in males, whereas in Japan the woman-to-man ratio may be 13.[2] Furthermore, many studies have demonstrated that the incidence of thyroid carcinoma is increasing for reasons remain unclear, but in part, could be related to epigenetic events.[3–5] Previous researches have shown that recurrence is a common event in thyroid carcinoma patients (15–30% of patients), especially in early-stage.[6,7] Therefore, it is very important to identify thyroid carcinoma patients at early recurrence so that more aggressive therapy and monitoring can be realized. As a common and important mechanisms for tumor suppressor gene inactivation in cancer, epigenetic alterations, such as aberrant promoter methylation, can yield powerful biomarkers for early detection of thyroid carcinoma.[8,9] Several revolutionary steps have been made to promote application of DNA methylation biomarkers in cancer screening.[10] Therefore, aberrant promoter methylation may be a powerful tool for thyroid carcinoma diagnosis.
RASS1FA is an important tumor suppressor protein in cells. It contain a Ras association domain, which can bind RAS proteins and may alter their function.[11–14] In doing so, Ras association domain family 1A (RASSF1A) affects multiple cellular processes.[15,16] However, several studies found that RASSF1A promoter region contains a CpG island A (737 bp) and its expression was decreased by its promoter methylation.[11,16,17] Two studies found that the frequency of RASSF1A promoter methylation in thyroid cancer was about 30% to 70%,[18–21] whereas other studies found the inverted.[22,23] Therefore, there lack a unified view of the methylation of RASSF1A promoter in thyroid carcinoma.
In this study, we used the data from 14 cancer–normal studies, Gene Expression Omnibus (GEO) and the Cancer Genome Atlas project (TCGA) databases to analyze the methylation of RASSF1A promoter in thyroid carcinoma susceptibility. Meanwhile, data from TCGA database were also used to analyze the methylation of RASSF1A promoter in thyroid carcinoma clinical characteristics (age status, genders, and pathologic tumor stages) and prognosis.
2. Materials and methods
2.1. Ethics statement
This study was approved by the First People's Hospital of Yunnan Province Ethics Committee. This study does not involve patients, so ethical approval was not required.
2.2. Search strategy, selection of studies, and data extraction
This pooled study involved searching a range of computerized databases, including Chinese National Knowledge Infrastructure, PubMed, Web of Science, and Google Scholar, for articles published in English or Chinese up to April 2017. The study used a subject and text word strategy with (RASSF1 or Ras association domain family member 1 A or RASSF1A or REH3P21 or RDA32 or NORE2A) AND (methylation or hypermethylation or epigenetics) AND (thyroid cancer or cancerous goiter or carcinoma of thyroid or thyroid carcinoma or papillary thyroid carcinoma or papillary thyroid cancer or medullary thyroid carcinoma or follicular thyroid carcinoma) as the primary search terms. Additional studies were identified by hand searching references in original articles and review articles.
Two independent reviewers (JY and HN) screened the titles and abstracts to identify relevant studies. The following types of studies were excluded: animal or cell experiments, case reports, meta-analyses or reviews, studies of non-normal–cancer studies or studies with insufficient data or those proving inaccessible after making contact with the authors. The remaining articles were further examined to see if they satisfy the following criteria: the patients had to be diagnosed with thyroid cancer (papillary thyroid carcinoma or medullary thyroid carcinoma or follicular thyroid carcinoma); the studies should be contain RASSF1A promoter methylation data; the studies should be included cancer samples (blood/tissue) and normal samples (healthy blood/tissue, or adjacent cancer normal tissue, or noncancer samples). Decisions were made and any disagreements regarding decisions were resolved by discussion with YH and KY. Studies which met the prespecified selection standards were summarized in data extraction forms. The following information was extracted from the studies: first author's last name, year of publication, original country of patients, age (mean or median), proportion of TNM stage, gender proportion (male/female), methylation detection methods and primers, the number of RASSF1A promoter methylations in individual cases and normal controls in individuals, and more. Ethnicity was categorized as “Caucasian,” “Asian,” or “mixed population” when a study did not state which ethnic groups were included.
2.3. Meta-analysis and SROC analysis
The data we acquired were analyzed and visualized mainly using R (R version 3.3.2) software. The strength of RASSF1A promoter methylation in thyroid carcinoma susceptibility was measured by a pooled odds ratio (OR) with a 95% confidence interval (CI) and P value. When the pooled OR with a threshold of P < .05, the significant difference was made. Heterogeneity was tested using the I2 and H statistic. If H > 1.5, I2 > 50%, and P ≤ .05, there was a strong heterogeneity between studies and a random-effects model was taken.[24–26] Otherwise, a fixed-effects model was used when H < 1.2, I2 ≤ 50%, and P > .05.[27] Tau-squared (τ2) can determine how much heterogeneity was explained by subgroup differences. Sensitivity analyses were performed to assess the contributions of single studies to the final results. Generally, Begg test and Egger test were used to assess funnel plot asymmetry related to reporting publication bias.[28,29] When Z < 1.96 and P > .05 by Begg test or P > .05 by Egger test, we considered that publication bias did not existed. If bias exists, we use a conventional meta-trim method to re-estimate the effect size. A summary receiver operating characteristic (SROC) analysis was applied to test the diagnostic value of meta-analysis.[30,31] The SROC curve shows the performance of the diagnostic ability of RASSF1A promoter methylation to thyroid carcinoma susceptibility. The exact area under the curve (AUC) for the SROC function was used to assess the accuracy of the test.[30]
2.4. The extraction and analysis of GEO and TCGA data
DNA methylation information for thyroid carcinoma was collected from the GEO (Illumina Infinium Human Methylation 27 [HM27] Bead Array platform) (GSE51090) and TCGA (Illumina Infinium Human Methylation 450 [HM450] Bead Array platform) databases. There were 25,978 probes in HM27 and 485,577 probes in HM450. The methylation status of each probe was defined according to the beta-value (beta-value = intensity of the methylated allele/[intensity of the methylated allele + intensity of the unmethylated allele]). When a beta-value is greater than the empirical threshold of 0.3, the probe will be considered methylated.[32,33]
According to the UCSC database-Table Browser-assembly-Mar.2006 (NCBI 36/hg18), it is known that RASSF1A translation start site (TSS) locates at chr3: 50353240. We also found that RASSF1A promoter region contains 2 CpG islands: 1 includes 139 CpG sites (locates a chr3: 50349269–50350633), 1 contains 84 CpG sites (locates at chr3: 50352808–50353544). Therefore, 35 probes (cg26357744, cg06063729, cg14884256, cg07344955, cg11035216, cg10152523, cg13497155, cg19152024, cg21522636, cg27149285, cg06375085, cg26093954, cg22796393, cg15043975, cg08078366, cg06821120, cg21418575, cg02930432, cg09386807, cg00743929, cg20826201, cg23147362, cg06117233, cg07130266, cg24859722, cg13872831, cg00777121, cg04743654, cg12966367, cg08047457, cg25747192, cg21554552, cg27569446, cg25486143, cg06172942) in HM450 and 9 probes (cg06063729, cg06821120, cg06980053, cg11035216, cg15043975, cg26357744, cg00777121, cg08047457, cg21554552) in HM27 were taken as the object of our study.
The strength of RASSF1A promoter methylation in thyroid carcinoma susceptibility and clinical characteristics (age status, gender, and pathologic tumor stage) was measured by Chi-squared test. A P value < .05 was considered significant. Overall survival (OS) and disease-free survival (DFS) curves were calculated using the Kaplan–Meier method and compared by log-rank testing. The receiver operating characteristics (ROC) curve of both specificity and sensitivity of the sets was also constructed.
3. Results
3.1. Study characteristics
The literature search yielded about 60 published articles using the above keywords. After excluding those articles according to the prespecified exclusion criteria, 14 studies were used to report a relationship between the RASSF1A promoter methylation and thyroid carcinoma[8,18–21,34–42] (Fig. 1). In total, 652 thyroid carcinoma tissues and 325 normal counterpart tissues were collected (Table 1). The frequency of RASSF1A promoter methylation was 49.23% in the thyroid carcinoma samples and 9.54% in the normal control samples. Among the 14 studies, the patients of 5 articles were from the United States, 5 studies were belong to China, and 4 studies were from Germany, Italy, Sweden, and Iran, respectively (Table 1). Here, the United States was categorized as “mixed population”; China and Iran were categorized as “Asian”; and Germany, Italy, and Sweden were as “Caucasian.” For the experimental methods to explore RASSF1A promoter methylation status, 9 of 14 inclusions used methylation-specific polymerase chain reaction (MSP), 2 used quantitative MSP (QMSP), while 1 used combined bisulfite restriction analysis (COBRA) and 2 used MethylScreen technology (Table 1). Three kinds of methylation detection primers or probes were found to be utilized for the 14 studies. The information of the 3 sets of primers is listed in Table 2.
Figure 1.
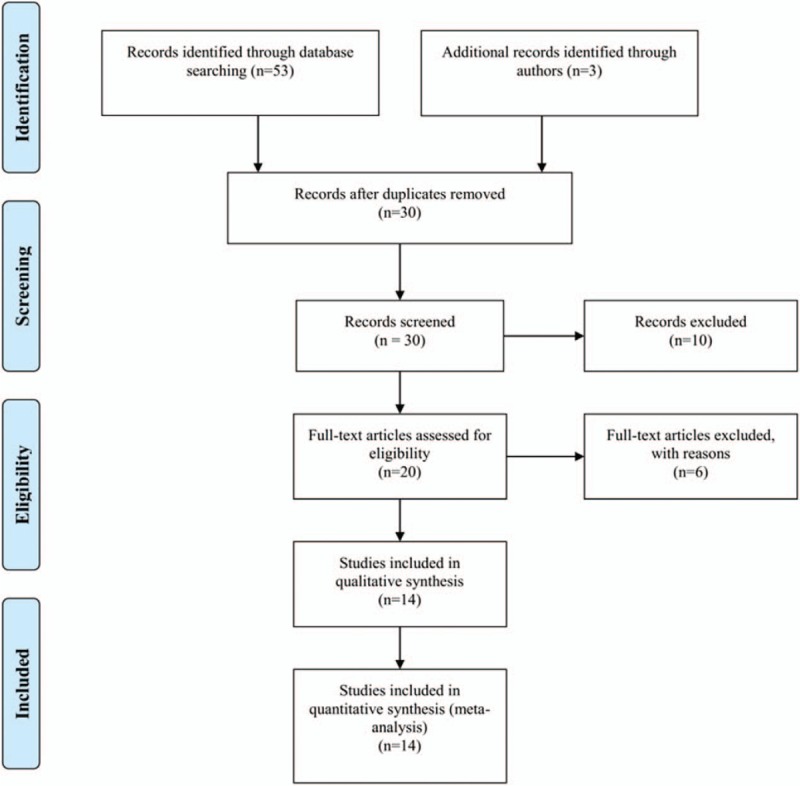
Flow chart shows study selection procedure and the distribution of the number of topic-related articles in the electronic database.
Table 1.
Characteristics of eligible studies considered in the report.
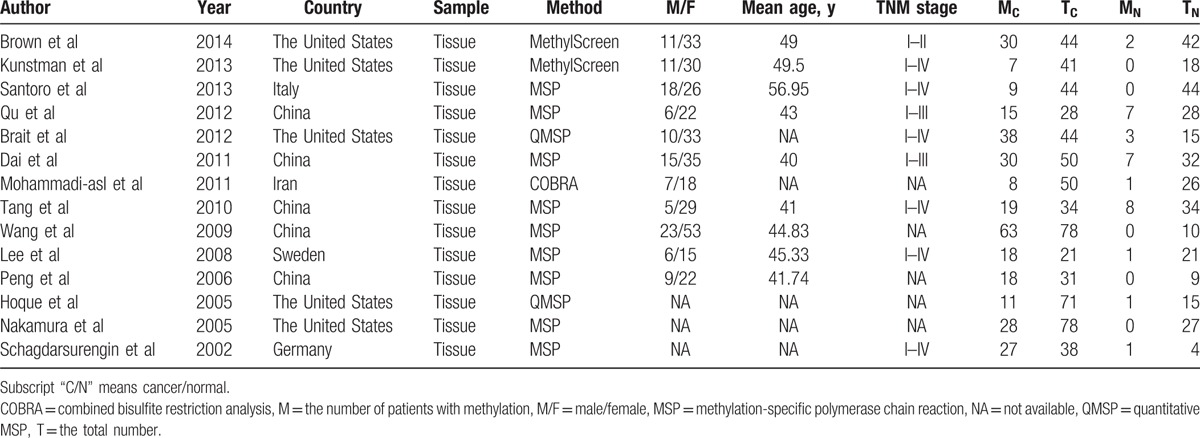
Table 2.
Three kinds of primers of the present 14 studies.

DNA methylation information for thyroid carcinoma was collected from GEO and TCGA databases including methylation 27 K (HM27) and 450 K (HM450) datasets. We analyzed 9 different probes from HM27 dataset and 35 different probes from HM450 dataset overlap the RASSF1A promoter region (Fig. 2A). We collected 83 primary thyroid cancer samples and 8 adjacent normal samples from GEO database (GSE51090). In the data from TCGA database, we collected 507 thyroid cancer samples and 56 adjacent normal tissue samples (Table S1). Among the 507 patients, the patient's age ranged from 15 to 89 years, the mean age was 47.16 ± 15.59. In addition, 134 males and 364 females (Table S1) among these patients, the American Joint Committee on Cancer (AJCC) pathologic tumor stage ranged from I to IV (Tables 3 and S1). We chose 486 patients to analyze the methylation of RASSF1A gene promoter in thyroid cancer DFS, and 498 patients were for thyroid cancer OS.
Figure 2.
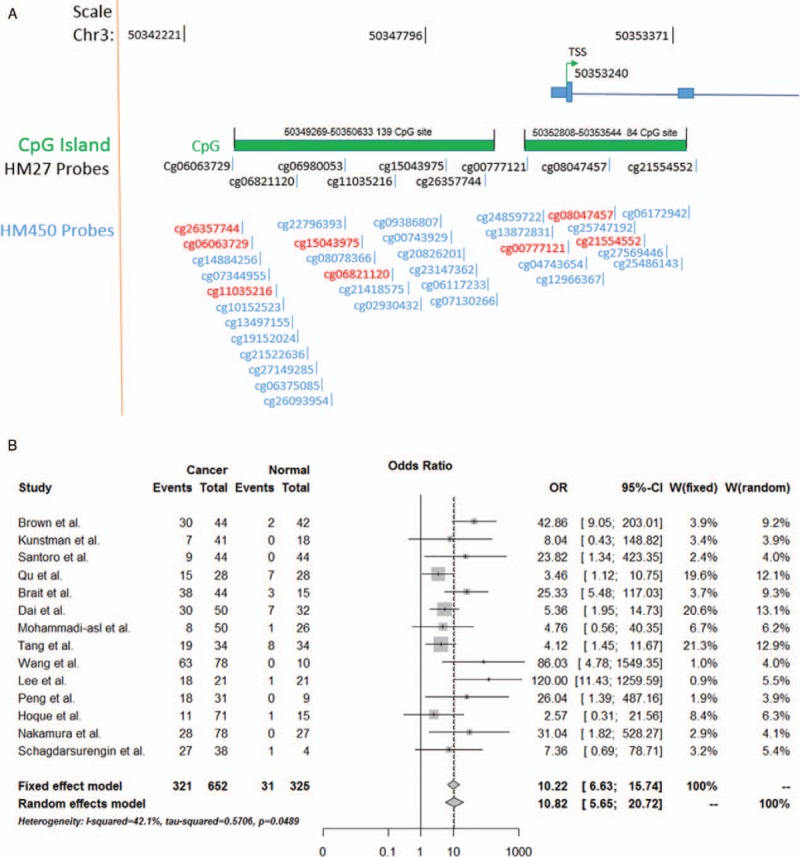
(A) DNA methylation probes matching RASSF1A promoter region CpG islands. (B) Meta-analysis for the methylation of RASSF1A promoter in thyroid carcinoma susceptibility based on random-effects model and fixed-effects model.
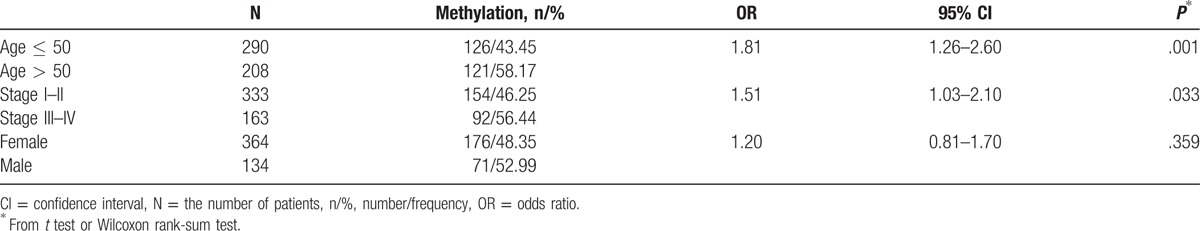
Table 3.
The methylation of RASSF1A gene promoter in thyroid carcinoma clinical characteristics.
3.2. The methylation of RASSF1A promoter in thyroid carcinoma susceptibility and clinical characteristics
Based on the meta-analysis, the OR for RASSF1A promoter methylation in cancer samples compared with that in normal controls were 10.22 (95% CI = [6.63; 15.74], z = 10.54, P < .0001) in fixed-effects model and 10.82 (95% CI = [5.65; 20.72], z = 7.1861, P < .0001) in random-effects model pooled, demonstrating a statistically significant increasing in likelihood of methylation in thyroid cancer samples comparing to normal controls (Fig. 2B). For heterogeneity of the meta-analysis, the H = 1.31, I2 = 42.1% (0%; 69.1%), P = .0489, suggesting a significant heterogeneity between the 14 studies. Therefore, meta-regression by random effect was taken. Meta-regression reveals that the primer sets are an important heterogeneity source. It explains 100% of overall heterogeneity, as the subgroup analyses demonstrate the same result (Fig. S1A). However, other factors, such as ethnicity and detection methods, fail to explain heterogeneity (Fig. S1B and C). We then performed bias analysis and sensitivity analysis of the 14 articles. The visual assessment of the Begg test (Z = 1.5876, P = .1124) and Egger test (t = 2.2957, df = 12, P = .04051) did not reveal any evidence of obvious asymmetry of funnel plot (Fig. S2A). Therefore, there does not appear to be any publication bias in the 14 studies. Sensitivity analyses were conducted to determine the effect of omitting a single study on the overall effect, the overall ORs were between 8.99 (95% CI = [4.93;16.40]) and 12.58 (95% CI = [6.36; 24.9]) in the random-effects method, which suggested that combined OR was consistent and reliable (Fig. S2B).
Using data obtained from GEO and TCGA databases, we were able to compare the frequency of RASSF1A promoter methylation in thyroid carcinoma samples and normal control samples. Among the 83 thyroid carcinoma samples and 8 normal control samples from GEO database, there was 70 (84.34%) patients has RASSF1A gene promoter methylation and none in normal control. We then analyzed the methylation status of RASSF1A promoter in TCGA database, and found a higher frequency methylation in thyroid carcinoma samples (49.51%) than normal control (1.79%). Therefore, as similar to the meta-analysis result, a significant difference was found in RASSF1A promoter methylation of thyroid carcinoma samples and normal control by GEO (OR = 6.39) and TCGA databases (OR = 53.93) (P < .0001; Fig. 3A and B).
Figure 3.
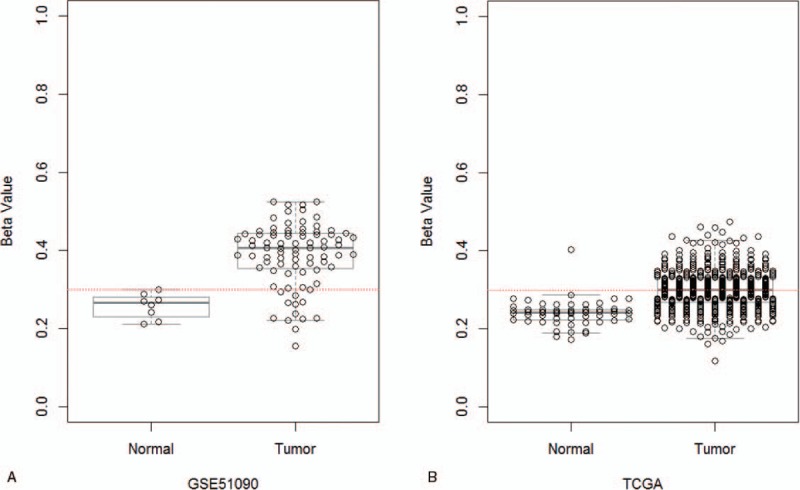
The relationship between RASSF1A promoter methylation and thyroid carcinoma susceptibility using the GEO and TCGA databases. (A) The relationship between RASSF1A promoter methylation and thyroid carcinoma susceptibility using the GEO database. The P = 3.88 × 10−8 by t test. (B) The relationship between RASSF1A promoter methylation and thyroid carcinoma susceptibility using TCGA database. The P = 2.01 × 10−20 by t test. Red-dotted line indicates beta-value = 0.3. GEO = Gene Expression Omnibus, TCGA = the Cancer Genome Atlas project.
SROC analysis was assessed in the meta-analysis of diagnostic tests. The pooled sensitivity, specificity, and AUC of the RASSF1A promoter methylation test in the meta-analysis were 0.51 (0.36–0.67), 0.94 (0.86–0.97), and 0.87 (0.83–0.89), respectively, which revealed that this meta-analytic method represents a good quantitative approach to summarize the performances of diagnostic tests (Fig. S3A). We also used ROC curve as the diagnostic tests for the RASSF1A promoter methylation in GEO and TCGA databases. The AUC was 0.922 for GEO data and 0.739 for TCGA data, suggesting a fair ability for thyroid carcinoma diagnosis by these 2 databases (Fig. S3B and C).
DNA methylation is thought to be linked to certain clinical characteristics, such as age status and pathologic tumor stage. Therefore, using TCGA data, we conducted further analysis based on age status, gender status, and pathologic tumor stage. Significant differences were found between the OR = 1.81 (P = .001) of the younger (age ≤ 50) and older (age > 50) (Table 3). The patients with stage III to IV had a significantly bigger OR = 1.506 than that stage I to II patients (P = .033) (Table 3), suggesting that advanced thyroid carcinoma has a high frequency of RASSF1A gene promoter methylation. However, there is nonsignificantly between the OR = 1.204 (P = .359) of males and females in thyroid carcinoma patients (Table 3).
3.3. The methylation of RASSF1A promoter in thyroid carcinoma prognosis
The methylation of the RASSF1A promoter in cancers prognosis has been research by several studies.[43–45] However, the role of the RASSF1A promoter methylation in the prognosis of thyroid carcinoma was not known. Here, the data extracted from TCGA project were conducted to evaluate the relationship between the RASSF1A promoter methylation and prognosis as defined by OS and DFS in all thyroid carcinoma patients (Table S1). The hazard ratio (HR) of 486 thyroid carcinoma patients analyzed for DFS was 2.63 (95% CI = [1.28; 5.38], P = .0116). Among these 486 thyroid carcinoma patients, 30 patients were recurrence and 21 with RASSF1A promoter methylation (Table S1). Therefore, this result demonstrates that thyroid carcinoma patients with the RASSF1A promoter methylation have higher chance of recurrence after surgery or other treatment (such as chemotherapy and combined treatment) than that unmethylation patients (Fig. 4A). In addition, the HR was found to be 1.26 for TCGA data (95% CI = [0.45; 3.62], P = .6635) (Fig. 4B) for OS when we used 498 thyroid carcinoma patients analyzed by the Kaplan–Meier method, which suggests that with the RASSF1A promoter methylation is not associated with the OS of thyroid carcinoma patients.
Figure 4.
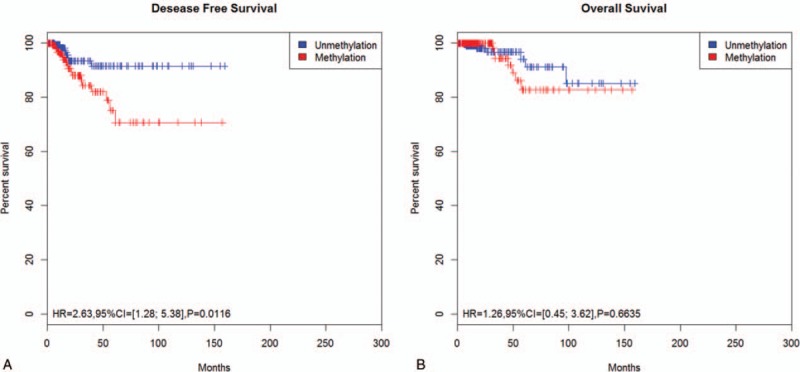
Association of thyroid carcinoma patient survival and RASSF1A promoter methylation status by the Kaplan–Meier method. (A) Disease-free survival curves by methylation status of RASSF1A promoter. The number of censored cases methylation and unmethylation was 244 and 242, respectively. (B) The Kaplan–Meier survival analysis of overall survival showing the association between thyroid carcinoma and RASSF1A methylation status. The number of censored cases methylation and unmethylation was 251 and 247, respectively.
4. Discussion
RASSF1A has been reported as an important tumor suppressor in numerics for cancers, such as breast[46] and lung cancers.[47] Although, in thyroid carcinoma, several studies found that the frequency of RASSF1A gene methylation in cancer was significant higher than normal control, 2 studies found the frequency of RASSF1A gene methylation in cancer samples was not or lower than in benign samples.[22,23] Considering this opposite view in the relationship between RASSF1A promoter methylation and thyroid carcinoma, we carried out this study, containing meta-analysis and bioinformatics analysis, to evaluate the relationship between RASSF1A promoter methylation and thyroid carcinoma.
In this study, higher methylation of the RASSF1A promoter has been found to frequently occur in thyroid carcinoma samples than normal control samples. Meanwhile, higher frequency of RASSF1A promoter methylation was also found in older and advanced stage patients, suggesting that RASSF1A promoter methylation may be an early event in thyroid tumorigenesis and carcinoma development. The HR for DFS was 2.63 (95% CI = [1.28; 5.38], P = .0116), which suggests that RASSF1A promoter methylation is associated with the DFS of thyroid carcinoma patients. Previous researches have shown that recurrence is a common event in thyroid carcinoma patients,[6,7] so the methylation of RASSF1A promoter in thyroid carcinoma has a higher probability of recurrence.
There was some heterogeneity in the present meta-analysis, and primer sets were the most important heterogeneity sources from meta-regression analysis. In addition, the opposite result with us by previous research due to it used the benign thyroid tumor samples as the control.[22] Therefore, considered the pooled sensitivity (0.51), specificity (0.94), and AUC (0.87) of the RASSF1A methylation test in the present meta-analysis, RASSF1A methylation status may be a good biomarker in thyroid carcinoma diagnosis. Many studies have shown that aberrant methylation of the promoter genes plays a potential role in the formation and progression of thyroid cancer.[48,49] Meanwhile, in the absence of genome-wide methylation changes case, the promoter methylation of RASSF1A gene may serve as an important methylation event with a potential driving effect on the early stages of thyroid neoplasia formation. Aberrant methylation of the tumor suppressor gene promoter, containing RASSF1A promoter, may lead to a further abnormal change of genome methylation in the late stages of thyroid cancer.[50,51] Therefore, any single tumor suppressor gene promoter methylation change in thyroid cancer may occur as a random event on the abnormal regulation of genome-wide epigenetics.
5. Conclusion
In conclusion, this quantitative assessment provides a strong evidence that the methylation status of the RASSF1A promoter is strongly associated with thyroid cancer susceptibility and patient prognosis. Meanwhile, RASSF1A promoter methylation is strongly associated with an advanced stage and older patients. Therefore, methylation of the RASSF1A promoter can be a promising diagnostic assay for the clinical diagnosis of thyroid cancer.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AUC = area under the curve, DFS = disease-free survival, GEO = Gene Expression Omnibus, HR = hazard ratio, OR = odds ratio, OS = overall survival, RASSF1A = Ras association domain family 1A, SROC = summary receiver operating characteristics, TCGA = the cancer genome atlas project.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Kilfoy BA, Zheng T, Holford TR, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 2009;20:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wartofsky L. Increasing world incidence of thyroid cancer: increased detection or higher radiation exposure? Hormones (Athens) 2010;9:103–8. [DOI] [PubMed] [Google Scholar]
- [3].Verdelli C, Forno I, Vaira V, et al. Epigenetic alterations in human parathyroid tumors. Endocrine 2015;49:324–32. [DOI] [PubMed] [Google Scholar]
- [4].Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 2011;7:569–80. [DOI] [PubMed] [Google Scholar]
- [5].Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn 2008;8:83–95. [DOI] [PubMed] [Google Scholar]
- [6].Showalter TN, Siegel BA, Moley JF, et al. Prognostic factors in patients with well-differentiated thyroid cancer presenting with pulmonary metastasis. Cancer Biother Radiopharm 2008;23:655–9. [DOI] [PubMed] [Google Scholar]
- [7].LiVolsi VA, Fadda G, Baloch ZW. Prognostic factors in well-differentiated thyroid cancer. Rays 2000;25:163–75. [PubMed] [Google Scholar]
- [8].Brown TC, Juhlin CC, Healy JM, et al. Frequent silencing of RASSF1A via promoter methylation in follicular thyroid hyperplasia: a potential early epigenetic susceptibility event in thyroid carcinogenesis. JAMA Surg 2014;149:1146–52. [DOI] [PubMed] [Google Scholar]
- [9].Dumitrescu RG. Epigenetic markers of early tumor development. Methods Mol Biol 2012;863:3–14. [DOI] [PubMed] [Google Scholar]
- [10].Dietrich D, Kneip C, Raji O, et al. Performance evaluation of the DNA methylation biomarker SHOX2 for the aid in diagnosis of lung cancer based on the analysis of bronchial aspirates. Int J Oncol 2012;40:825–32. [DOI] [PubMed] [Google Scholar]
- [11].Burbee DG, Forgacs E, Zochbauer-Muller S, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J Natl Cancer Inst 2001;93:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dammann R, Li C, Yoon JH, et al. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet 2000;25:315–9. [DOI] [PubMed] [Google Scholar]
- [13].Guo C, Tommasi S, Liu L, et al. RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr Biol 2007;17:700–5. [DOI] [PubMed] [Google Scholar]
- [14].Song MS, Song SJ, Ayad NG, et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol 2004;6:129–37. [DOI] [PubMed] [Google Scholar]
- [15].Donninger H, Vos MD, Clark GJ. The RASSF1A tumor suppressor. J Cell Sci 2007;120:3163–72. [DOI] [PubMed] [Google Scholar]
- [16].Hesson LB, Cooper WN, Latif F. The role of RASSF1A methylation in cancer. Dis Markers 2007;23:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pfeifer GP, Yoon JH, Liu L, et al. Methylation of the RASSF1A gene in human cancers. Biol Chem 2002;383:907–14. [DOI] [PubMed] [Google Scholar]
- [18].Schagdarsurengin U, Gimm O, Hoang-Vu C, et al. Frequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinoma. Cancer Res 2002;62:3698–701. [PubMed] [Google Scholar]
- [19].Santoro A, Pannone G, Carosi MA, et al. BRAF mutation and RASSF1A expression in thyroid carcinoma of southern Italy. J Cell Biochem 2013;114:1174–82. [DOI] [PubMed] [Google Scholar]
- [20].Kunstman JW, Korah R, Healy JM, et al. Quantitative assessment of RASSF1A methylation as a putative molecular marker in papillary thyroid carcinoma. Surgery 2013;154:1255–61. [DOI] [PubMed] [Google Scholar]
- [21].Hoque MO, Rosenbaum E, Westra WH, et al. Quantitative assessment of promoter methylation profiles in thyroid neoplasms. J Clin Endocrinol Metab 2005;90:4011–8. [DOI] [PubMed] [Google Scholar]
- [22].Zhang B, Liu S, Zhang Z, et al. Analysis of BRAF(V600E) mutation and DNA methylation improves the diagnostics of thyroid fine needle aspiration biopsies. Diagn Pathol 2014;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schagdarsurengin U, Gimm O, Dralle H, et al. CpG island methylation of tumor-related promoters occurs preferentially in undifferentiated carcinoma. Thyroid 2006;16:633–42. [DOI] [PubMed] [Google Scholar]
- [24].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [26].Huizenga HM, Visser I, Dolan CV. Testing overall and moderator effects in random effects meta-regression. Br J Math Stat Psychol 2011;64:1–9. [DOI] [PubMed] [Google Scholar]
- [27].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [28].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ Open 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [30].Midgette AS, Stukel TA, Littenberg B. A meta-analytic method for summarizing diagnostic test performances: receiver-operating-characteristic-summary point estimates. Med Decis Making 1993;13:253–7. [DOI] [PubMed] [Google Scholar]
- [31].Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg 2005;79:16–20. [DOI] [PubMed] [Google Scholar]
- [32].Sproul D, Nestor C, Culley J, et al. Transcriptionally repressed genes become aberrantly methylated and distinguish tumors of different lineages in breast cancer. Proc Natl Acad Sci USA 2011;108:4364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marzese DM, Scolyer RA, Huynh JL, et al. Epigenome-wide DNA methylation landscape of melanoma progression to brain metastasis reveals aberrations on homeobox D cluster associated with prognosis. Hum Mol Genet 2014;23:226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qu F, Xue WJ. RASSF1A methylation and its clinical roles in papillary thyroid carcinoma. J Nantong Univ (Med Sci) 2012;32:490–2. [Google Scholar]
- [35].Brait M, Loyo M, Rosenbaum E, et al. Correlation between BRAF mutation and promoter methylation of TIMP3, RARbeta2 and RASSF1A in thyroid cancer. Epigenetics 2012;7:710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dai YL, Cai DH, Chen H, et al. The methylation of TSHR and RASSFIA gene in thyroid carcinoma. Shaanxi Med J 2011;40:1446–9. [Google Scholar]
- [37].Mohammadi-asl J, Larijani B, Khorgami Z, et al. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARbeta2 genes in papillary thyroid carcinoma. Med Oncol 2011;28:1123–8. [DOI] [PubMed] [Google Scholar]
- [38].Tang JD, Su XL. Research of CpG island methylation status of NIS and RASSF1A gene promoters in papillary thyroid carcinomas. China J Mod Med 2010;20:3282–5. [Google Scholar]
- [39].Wang XH, Zhang GC, Liu YL, et al. Research of the RASSF1A promoter aberrant methylation in thyroid cancer patients. Shaanxi Med J 2009;38:790–2. [Google Scholar]
- [40].Lee JJ, Geli J, Larsson C, et al. Gene-specific promoter hypermethylation without global hypomethylation in follicular thyroid cancer. Int J Oncol 2008;33:861–9. [PubMed] [Google Scholar]
- [41].Peng ZL. The methylation of p16 and RASSFIA gene in thyroid carcinoma. Nan Hua Univ 2006;3:18–20. [Google Scholar]
- [42].Nakamura N, Carney JA, Jin L, et al. RASSF1A and NORE1A methylation and BRAFV600E mutations in thyroid tumors. Lab Invest 2005;85:1065–75. [DOI] [PubMed] [Google Scholar]
- [43].Wang J, Wang B, Chen X, et al. The prognostic value of RASSF1A promoter hypermethylation in non-small cell lung carcinoma: a systematic review and meta-analysis. Carcinogenesis 2011;32:411–6. [DOI] [PubMed] [Google Scholar]
- [44].Jiang Y, Cui L, Chen WD, et al. The prognostic role of RASSF1A promoter methylation in breast cancer: a meta-analysis of published data. PLoS ONE 2012;7:e36780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li YS, Xie Q, Yang DY, et al. Role of RASSF1A promoter methylation in the pathogenesis of hepatocellular carcinoma: a meta-analysis of 21 cohort studies. Mol Biol Rep 2014;41:3925–33. [DOI] [PubMed] [Google Scholar]
- [46].Karray-Chouayekh S, Trifa F, Khabir A, et al. Aberrant methylation of RASSF1A is associated with poor survival in Tunisian breast cancer patients. J Cancer Res Clin Oncol 2010;136:203–10. [DOI] [PubMed] [Google Scholar]
- [47].Helmbold P, Lahtz C, Herpel E, et al. Frequent hypermethylation of RASSF1A tumour suppressor gene promoter and presence of Merkel cell polyomavirus in small cell lung cancer. Eur J Cancer 2009;45:2207–11. [DOI] [PubMed] [Google Scholar]
- [48].Xing M. Gene methylation in thyroid tumorigenesis. Endocrinology 2007;148:948–53. [DOI] [PubMed] [Google Scholar]
- [49].Kondo T, Asa SL, Ezzat S. Epigenetic dysregulation in thyroid neoplasia. Endocrinol Metab Clin North Am 2008;37:389–400. [DOI] [PubMed] [Google Scholar]
- [50].Ellis RJ, Wang Y, Stevenson HS, et al. Genome-wide methylation patterns in papillary thyroid cancer are distinct based on histological subtype and tumor genotype. J Clin Endocrinol Metab 2014;99:E329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rodriguez-Rodero S, Fernandez AF, Fernandez-Morera JL, et al. DNA methylation signatures identify biologically distinct thyroid cancer subtypes. J Clin Endocrinol Metab 2013;98:2811–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


