Abstract
Rationale:
Currently, the treatment of hepatocellular carcinoma (HCC) associated with portal vein tumor thrombosis (PVTT) is a challenge. Percutaneous puncture endovascular placement of iodine-125 (125I) seeds strand and stent is reported to treat HCC with tumor thrombus effectively. However, it is proved to be only suitable for the main portal vein (MPV) thrombus.
Patient concerns:
A 42-year-old male patient was referred to our institution after experiencing right upper abdominal distention without abdominal pain, nausea, or vomiting for 2 weeks. The patient had a history of hepatitis B virus infection over a 20 year period.
Diagnosis:
After a full evaluation, the patient was diagnosed with primary HCC with a tumor thrombus in both main and branch portal veins.
Interventions:
We used a Y-configuration stent combined with 125I seeds strand to treat the tumor thrombus in both main and branch portal veins.
Outcomes:
The patient's liver function and the stent patency period were improved. More importantly, the patient had an acceptable survival time.
Lessons:
A Y-configuration stent makes it possible to treat tumor thrombosis in portal vein branches (PVBs). However, the long-term curative effects of Y-configuration stents need to be verified.
Keywords: hepatocellular carcinoma, portal vein branches, tumor thrombosis, Y-configuration stent
1. Introduction
Hepatocellular carcinoma (HCC) is the 6th most common cancer worldwide.[1] According to recent clinical data, portal vein tumor thrombosis (PVTT) is commonly associated with HCC in 31% to 34% of HCC patients.[2] Invasion of the portal vein by tumors can result in the metatasis, deterioration of liver function, and portal vein hypertension leading to intractable ascites, variceal rupture, hepatic encephalopathy, or death.[3] Therefore, PVTT is regarded as a crucial independent prognostic factor in HCC patients. HCC patients with PVTT are classified as being at an advanced stage according to the Barcelona-Clinic Liver Cancer staging classification.[4] Until now, the treatment of advanced HCC associated with PVTT is a challenge, and there is no consensus on the treatment of these patients.[5] As a 1st-line drug treatment, sorafenib only marginally prolongs patient survival. This low efficacy combined with the high cost of sorafenib makes it unsuitable as a standard therapy for advanced HCC.[6] Recently, brachytherapy with interstitial implantation of iodine-125 (125I) seeds strand, combined with intraportal vein stent placement for the treatment of PVTT, has shown promising results.[7] However, this treatment has only been proven to be effective for HCC with tumor thrombi of the main portal vein (MPV). To our knowledge, the use of a Y-configuration stent combined with 125I seeds strand for the treatment of HCC with tumor thrombi in a branch of the portal vein has never been reported before. In this study, we report the 1st case of HCC with a tumor thrombus in the portal vein branches (PVBs) treated with a Y-configuration stent combined with 125I seeds strand.
2. Case description
This study was approved by the Second Xiangya Hospital of Central South University review board, and the informed patient consent was obtained.
A 42-year-old male patient was referred to our institution after experiencing right upper abdominal distention without abdominal pain, nausea, or vomiting for 2 weeks. The patient had a history of hepatitis B virus infection for a period of 20 years. Laboratory findings were: AFP 775.90 ng/mL (normal value [NV] 0–7.00 ng/mL), ALT 79.9 U/L [NV 9.0–50.0 U/L], AST 85.3 U/L [NV 15.0–40.0 U/L], TBIL 18.9 μmol/L [NV 5.1–17.1 μmol/L], ALB 35.0 g/L [NV 40.0–55.0 g/L], and PT 12.50 seconds [NV 10.00–14.00 seconds]). The patient had Child–Pugh class A liver function. Abdominal contrast-enhanced magnetic resonance imaging (MRI) (Fig. 1) showed a 5.1 cm × 5.9 cm irregular and lobulated mass with ill-defined margins in the left lobe of the liver. The mass showed hypointensity in a T1-weighted image and slight hyperintensity in a T2-weighted image. After the injection of gadolinium-diethylenetriaminepentacetate, the tumor showed enhancement on the arterial phase and a washed-out appearance in the portovenous and delayed phases. An endovascular filling defect involving MPV and PVBs was observed. Consequently, we made a diagnosis of primary HCC with a portal vein tumor thrombus. The patient had no intervention contraindications. Access to the posterior segment of the portal vein was obtained using ultrasound and fluoroscopic guidance. A portal venogram showed complete occlusion of the MPV trunk and left intrahepatic portal vein with extensive collaterals, as well as partial occlusion of the right intrahepatic portal vein (RIPV) (Fig. 2A). Three self-expandable Protégé GPS stents (14 mm × 8 mm, 14 mm × 6 mm, and 10 mm × 4 mm) (ev3, Minnesota), combined with a 125I seed strand loaded with 15 radioactive seeds, were placed in the obstructed MPV and the posterior segment of the RIPV (Fig. 2B). The 125I seed strand was fixed between the stents and the obstructed portal vein wall. However, follow-up portography showed acute intrastent thrombosis (Fig. 2C). After 14 hours of catheter-directed thrombolysis, blood flow in the MPV was restored (Fig. 2D). The patient was readmitted 2 weeks after the 1st intervention. Laboratory tests showed improved liver function (ALT 53.4 U/L, AST 45.8 U/L, TBIL 8.1 μ/L, ALB 33.5 g/L, and PT 11.7 seconds). Transarterial chemoembolization (TACE) was performed using 5-fluorouracil (5-FU) (1000 mg/m2), lobaplatin (40 mg/m2), and pirarubicin (THP) (40 mg/m2) after the posterior segment portal vein stent placement. No additional TACE performed during the follow-up period. One week after TACE, indirect photography showed satisfactory patency of the MPV and the posterior segment of the RIPV. However, the anterior segment of the RIPV was evaluated and a 4.5 cm portal vein tumor thrombus (measured using the Omi flush catheter [AngioDynamics, New York]) was observed. A self-expandable Protégé GPS stent (12 mm × 60 mm) (ev3) with 12 radioactive 125I seed strand was implanted. The distal segment of the 2nd stent was placed in the right anterior branch of the portal vein, whereas the proximal segment of the stent was situated within the previously placed stent, thereby forming a Y-configuration. This Y-configuration stent makes it possible to treat tumor thrombi in the MPV and RIPV. Follow-up portography demonstrated satisfactory patency of the MPV and RIPV (Fig. 2E). The patient had no serious complications related to the stent and 125I seed strand implantation. After 2 days, loose 125I seeds were implanted into the tumor at intervals of 1 cm using a gun under computed tomography (CT) guidance. Patency of the stent, as well as tumor response, was evaluated by abdominal contrast-enhanced CT. After 4 months, abdominal contrast-enhanced CT images revealed a significant tumor size reduction without the occurrence of new lesions (Fig. 3). The stents with implanted 125I seed strands in the MPV and RIPV performed well (Fig. 3). However, laboratory tests indicated decreased liver function (ALT 540.8 U/L, AST 583.5 U/L, TBIL 38.0 μmol/L, ALB 26.3 g/L, and PT 15.20 seconds). The patient with Child–Pugh class C liver function was discharged from hospital and followed up on a monthly basis. He was referred to our department 5 months after the last intervention. Laboratory tests showed improved liver function compared to the previous tests (ALT 115.9 U/L, AST 154.1 U/L, TBIL 72.1 μmol/L, ALB 28.3 g/L, and PT 19.20 seconds). An abdominal contrast-enhanced CT revealed a slightly reduced tumor; however, liver ascites had increased (Fig. 4). The stents with implanted 125I seed strands in the MPV and RIPV still performed well at the time of follow-up (Fig. 4).
Figure 1.
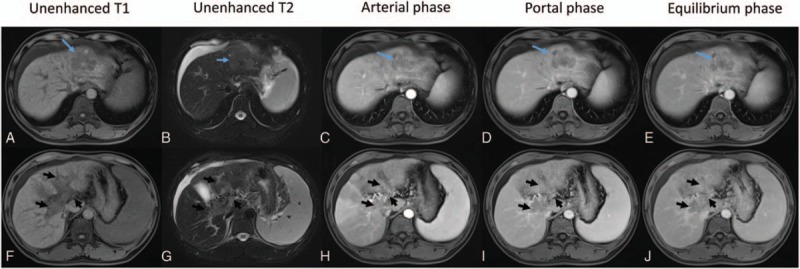
Abdominal contrast-enhanced MRI. (A–E) Showing an irregular and lobulated tumor in the left lobe of the liver. The mass (blue arrows) showed hypointensity in a T1-weighted image and slight hyperintensity in a T2-weighted image. After the injection of Gd-DTPA, the tumor showed enhancement on arterial phase images and a washed-out appearance on the portal venous and equilibrium phase images. (F–J) An endovascular filling defect involving MPV and PVBs was observed (black arrows). We were able to observe enhancement in the filling defect. Gd-DTPA = gadolinium-diethylenetriaminepentacetate, MPV = main portal vein, MRI = magnetic resonance imaging, PVB = portal vein branch.
Figure 2.
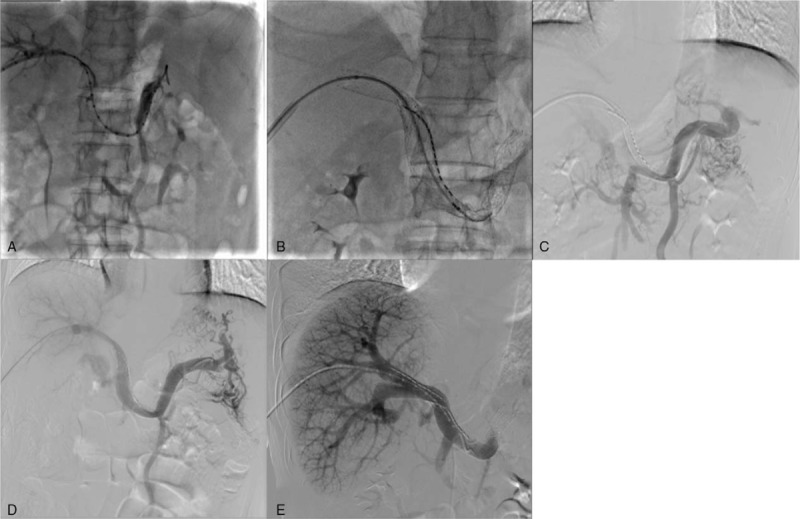
(A) Portal venogram showing complete occlusion of the MPV trunk and LIPV with extensive collaterals, as well as partial occlusion of the RIPV. (B) Three self-expandable stents and 125I seed strand loaded with 15 radioactive seeds were placed in the obstructed MPV and the posterior segment of the RIPV. (C) Follow-up portography showed acute intrastent thrombosis. (D) After 14 hours of catheter-directed thrombolysis, blood flow in the MPV was restored. (E) Follow-up portography showed satisfactory patency of the MPV and RIPV. LIPV = left intrahepatic portal vein, MPV = main portal vein, RIPV = right intrahepatic portal vein.
Figure 3.
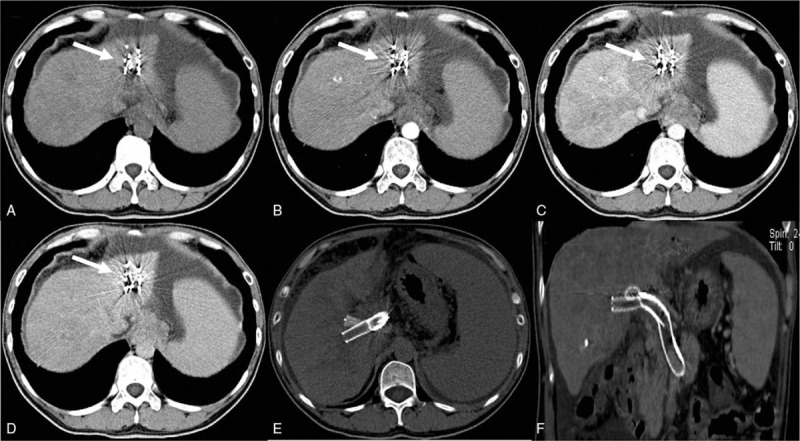
Abdominal contrast-enhanced computed tomography (CT) at 4 months after the last intervention. (A–D) Reveals a significant reduction in the size of the mass (white arrows). (E–F) Shows that the Y-configuration stent with implanted 125I seed strands performed well.
Figure 4.
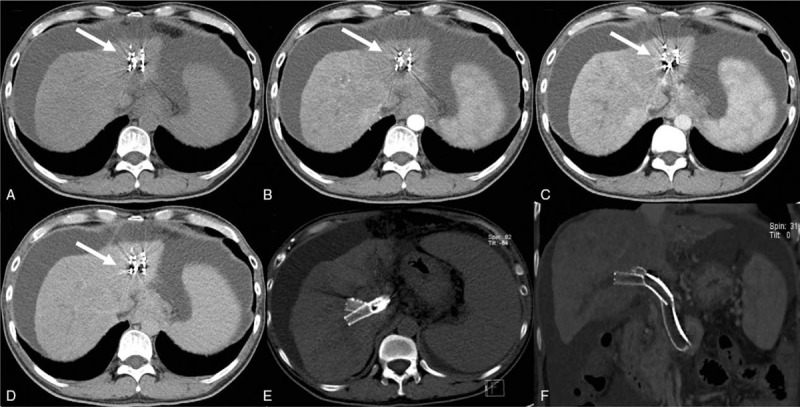
Abdominal contrast-enhanced computed tomography (CT) at 5 months after the last intervention. (A–D) Shows a somewhat reduced tumor (white arrows) with increased liver ascites compared with previous CT images. (E–F) Shows that the Y-configuration stent was still performing well.
3. Discussion
Considerable progress has been made in the treatment of HCC. However, HCC patients complicated by PVTT still have poor prognosis with a median survival of only 2.7 to 4.0 months.[8,9] No real progress has been made in the treatment of HCC with MPV invasion. Several therapies were applied, including sorafenib, TACE, 3-dimensional conformal radiotherapy, and transarterial radioembolization.[10] However, blood flow in an obstructed MPV cannot be restored immediately using these methods alone. Endovascular placement of 125I seeds strand and stent through a percutaneous puncture has been proven to be an effective treatment for HCC with tumor thrombi in the MPV.[7] It is well-documented that intraluminal brachytherapy can counteract the development of neointimal hyperplasia and reduce the incidence of restenosis after stent placement.[11] It enables prompt restoration of blood flow in the obstructed MPV, as well as effective reduction of high portal pressure caused by tumor thrombi. This may result in significantly improving liver function as well as the period of stent patency.[12] A Y-configuration stent makes it possible to treat tumor thrombosis in PVBs; however, the long-term curative effects of Y-configuration stents need to be confirmed.
Footnotes
Abbreviations: HCC = hepatocellular carcinoma, MPV = main portal vein, PVB = portal vein branch, PVTT = portal vein tumor thrombosis, RIPV = right intrahepatic portal vein, TACE = transarterial chemoembolization.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol 2010;7:448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kim JY, Chung SM, Choi BO, et al. Hepatocellular carcinoma with portal vein tumor thrombosis: improved treatment outcomes with external beam radiation therapy. Hepatol Res 2011;41:813–24. [DOI] [PubMed] [Google Scholar]
- [3].Pirisi M, Avellini C, Fabris C, et al. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol 1998;124:397–400. [DOI] [PubMed] [Google Scholar]
- [4].Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Poddar N, Avezbakiyev B, He Z, et al. Hepatocellular carcinoma presenting as an incidental isolated malignant portal vein thrombosis. J Gastrointest Cancer 2012;43:486–9. [DOI] [PubMed] [Google Scholar]
- [6].Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [7].Luo JJ, Zhang ZH, Liu QX, et al. Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int 2016;10:185–95. [DOI] [PubMed] [Google Scholar]
- [8].Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 2006;12:7561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Villa E, Moles A, Ferretti I, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology 2000;32:233–8. [DOI] [PubMed] [Google Scholar]
- [10].Han K, Kim JH, Ko GY, et al. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: a comprehensive review. World J Gastroenterol 2016;22:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chuan XL, Xu H, Bao SH, et al. Efficacy of therapy for hepatocellular carcinoma with portal vein tumor thrombus: chemoembolization and stent combined with iodine-125 seed. Cancer Biol Ther 2011;12:865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Luo JJ, Yan ZP, Liu QX, et al. Endovascular placement of Iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. J Vasc Interv Radiol 2011;22:479–89. [DOI] [PubMed] [Google Scholar]


