Abstract
Rationale:
Pregnancy and lactation-associated osteoporosis (PLO) is very rare, but it can cause severe vertebral compression fractures with disabling back pain. Although it is a rare, PLO must be kept in mind in the differential diagnosis in patients presenting with low back pain during or after pregnancy.
Patient concerns:
A 23-year-old woman who suffered from lumbago and fractures in the vertebral column 1 month after delivery.
Diagnosis:
Pregnancy and lactation-associated osteoporosis.
Interventions:
Combination therapy of calcium and vitamin D were used for treatment, as well as regular follow-up.
Outcomes:
The patient's back pain had decreased significantly, the bone metabolic index, bone mineral density (BMD) had improved and she did not experience any recurrence.
Lessons:
Early diagnosis and treatment of calcium and vitamin D, as well as the efficacy of PLO intervention monitoring and evaluation are critical for the success of treatment.
Keywords: pregnancy and lactation-associated osteoporosis, vertebral fracture, vitamin D
1. Introduction
Pregnancy and lactation-associated osteoporosis (PLO) is a rare form of osteoporosis. Approximately 100 cases of pregnancy- and lactation-associated osteoporosis have been described between 1955 and 2006.[1] The incidence of PLO is 0.4 in 100,000 women. It is considered that the number of undiagnosed patients is even higher.[2] PLO led to multiple fragility compression fractures in the vertebra. To analyze the diagnosis and treatment of this case can help clinicians to pay attention to the disease and the evaluation of osteoporosis.
2. Case presentation
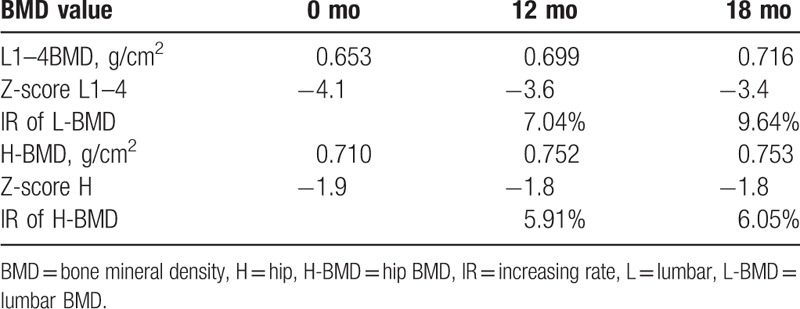
The patient was 23 years old at symptom onset. Her height decreases about 4 cm during pregnancy. Two months after delivering her first child, she began experiencing severe lower back pain without any apparent cause. She went to a local clinic for treatment, but the pain did not lessen. Her medical history was not remarkable for chronic disease, drug use, smoking, or alcohol use. During pregnancy, her fasting blood glucose was increased (specific unknown), and it was back to normal after pregnancy without treatment. And the patient took long-term vegetarian diet, lack of sunshine, and exercise. She breastfed for a month and menstruation has returned to normal. Physical examinations demonstrated mild anemia countenance and tenderness of her back. Her hemoglobin (HGB) was 105 g/L (115–150), and fasting blood-glucose (FBG) was 6.62 mmol/L. However, other laboratory examinations including serum BAP, serum PTH, and serum Ca were within normal ranges. Anemia-related metabolites: Vitamin B12 >2000 pg/mL (normal range 187.0–883.0) and serum iron 22.77 μmol/l (9.0–27.0). Iron staining of bone marrow smear suggested iron deficiency. She was diagnosed hypoferric anemia by Department of Hematology. The result of oral glucose tolerance test (OGTT) was impaired glucose tolerance (IGT). Gastroscopy and colonoscopy were normal. Bone scintigraphy with 99mTc–MDP showed T6–8, and L3 radioactive concentration increased focal. Magnetic resonance imaging (MRI) showed multiple vertebral fractures in various degrees (Fig. 1). The Z-score of Lumbar1–4 was -4.1. The patient was diagnosed with pregnancy and lactation-associated osteoporosis (PLO), IGT, and hypoferric anemia. We advised against breast-feeding and prescribed a daily intake of iron supplements, calcitonin, vitamin D (800 u/day), and calcium carbonate (1200 mg/day). With 3 month's treatment of stopping breastfeeding, and supplement of vitamin D (800 u/day) and calcium (1200 mg/day), the clinical symptoms of this patient gradually improved; however, her 25- (OH) -D3 level was not increased as expected. After a careful inquiry of the history, we found that this patient was still lack of sunlight and activity, and reduced the drug dose by herself. So, 25- (OH) - D3 levels did not increase significantly associated with decreased patient compliance. As a result, we strengthen patient education and regular follow-up every 3 months, 25 (OH) D3 level gradually increased.18 months after treatment, it turned to normal. One year after consultation, her back pain had decreased significantly, the bone metabolic index (Table 1), bone mineral density (BMD) had improved (Table 2), and she did not experience any recurrence.
Figure 1.
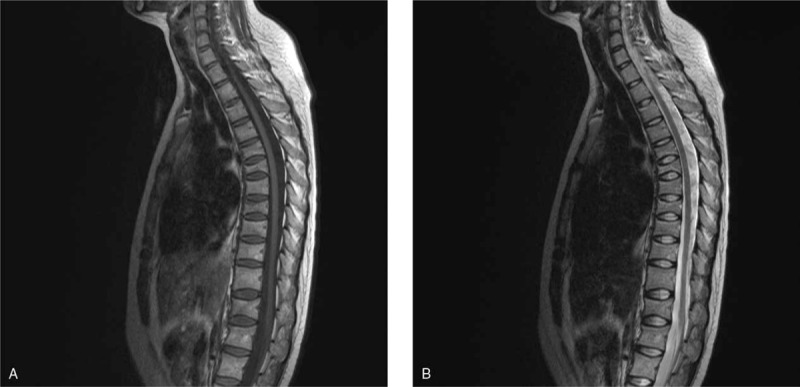
Lumbar MRI de T1 (A) and T2 (B); loss of height in multiple vertebral bodies in various degrees and a biconcave appearance in vertebral bodies.
Table 1.
The bone metabolic index before and after treatment.
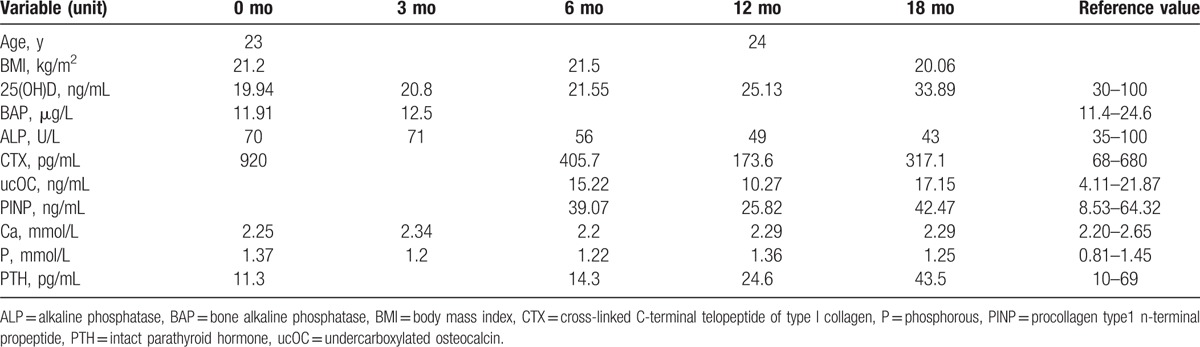
Table 2.
Changes in BMD values.
3. Discussion
Osteoporosis is a common disease in people aged 50 years and over, but it can also strike the younger age groups. Pregnancy and lactation-associated osteoporosis, which was first described as a clinical syndrome by Nordin and Roper,[3] is a relatively rare disease in young women. It results in severe low back pain in the last trimester of pregnancy and in the postpartum period, decreases in height, and fragility fractures, particularly in the vertebra.[4,5] Although its etiology is unclear, the presence of PLO in first degree relatives, low BMI, physical inactivity, poor nutrition, insufficient calcium intake, and smoking have been determined as risk factors.[1,6] The patient was a normal menstruation young woman with unexplained pain, brittle fracture, and BMD decreased. In this case, we first considered the metabolic bone diseases, which had to be carefully identified with osteoporosis and other metabolic bone diseases. According to the clinical, biochemical, and imaging features, we excluded the osteogenesis imperfect, which was easy to cause fracture and the bone density decrease, and osteomalacia at first. And then we focused on the identification of secondary osteoporosis. Through detailed history taking, signs, blood and urine routine, biochemical test (such as calcium, phosphorus, alkaline phosphatase), thoracic and lumbar lateral phase, bone changed-over markers, hormone levels (including PTH), 25-(OH)-D3, function tests, imaging, bone density, pathology and other comprehensive examination, as well as multidisciplinary consultation, we excluded drug exclusion and other causes of osteoporosis one by one. At last, the diagnosis of primary osteoporosis was established. Due to the long-term vegetarian diet, special living habits such as lack of sunlight and exercise, and the onset of lactation in pregnancy, we made a diagnosis of PLO finally. After 6 months treatment of stopping breast-feeding, avoiding loading, receiving treatment of calcium, active vitamin D, calcitonin, and nutritional support, this patient significantly reduced pain, increased activity, and no fractures happened again.
Silva et al[7] reported that the patient had other risk factors of fracture, such as low calcium intake, and a treatment with thyroid hormones for weight loss. In the other case, they did not find any risk factors.[7] The medical history of some patients reported to have PLO in the literature was remarkable for secondary causes of osteoporosis such as oligomenorrhoea, infertility therapy with clomiphene, gluten enteropathy, and heparin administration.[8,9] The current case did not have any condition that could result in secondary osteoporosis. The identified risk factors were physical inactivity and insufficient calcium, vitamin D intake. But so far, the etiology and pathophysiology of this condition is unknown. There are several hypotheses regarding causes of PLO: increase of parathyroid hormone-related peptide (PTH-rP) secretion by lactation, increase of calcium supply to fetal bone and breast-milk, decrease of estrogen after delivery, osteopenia existing before pregnancy, and heritable factors.[5,7,10,11]
There is no mutually agreed opinion or guideline in the treatment of this condition. The treatment options are limited to those practiced in the reported cases. The cessation of breastfeeding is recommended in most cases.[4,7,11] Other options include calcium and vitamin D,[4] bisphosphonates,[1] teriparatide,[12,13] strontium ranelate,[14] and kyphoplasty to treat postpartum vertebral fractures.[15] The current case was administered with calcium, vitamin D supplementation, and pain therapy. In the literature, Stathopoulos et al[16] identified a total of 78 cases involving fetuses whose mothers had been exposed to bisphosphonates before conception or during pregnancy, along with 7 cases of bisphosphonates exposure before or during lactation. The vast majority of mothers and infants did not demonstrate serious adverse effects. However, there were cases of shortened gestational age, low neonatal birth weight, and transient hypocalcaemia of the newborns, while the very few reported cases of spontaneous abortions and congenital anomalies probably resulted from maternal underlying diseases and concomitant medication.[16] Winarno et al[12] presented successful treatment of 1–34 parathyroid hormone (PTH) after failure of bisphosphonate therapy in a complex case of pregnancy-associated osteoporosis and multiple fractures.[12] After 18 months treatment of PTH1–34, the patient's BMD had further improvement. And she did not suffer from de novo fractures. Tsuchie et al[17] demonstrate for the first time therapeutic effects of vitamin K2 (menatetrenone) on PLO with multiple vertebral fractures in 4 cases. Nakamura et al[18] also report 2 such cases occurring in the early postpartum period that led to multiple fragility compression fractures. Combination therapy of vitamin D and vitamin K enabled a marked gradual increase in BMD.[18] However, the need for such treatments is uncertain given that a progressive increase in bone mass subsequently occurs in most women who present with a fracture during pregnancy or lactation.[19] Although bisphosphonates is recommended in the treatment of PLO by most of the literatures, however, there is no indication of bisphosphonates in Chinese premenopausal women. This patient eventually refused to use the drug after we fully informed.
It is very important to monitor and evaluate the efficacy of the treatment, which determined the success or failure of the treatment of PLO. In our case, with regular follow-up, education, and supervision, this patient could finally reach normal level of 25 (OH) D3. At follow-up, we should pay attention to the evaluation of the new fracture in clinical and image. Due to vertebral fracture is different with hip and forearm fracture, which often happens with no clinical symptoms and a clear history of falls, it is easily to be missed in diagnosis. Therefore, vertebral imaging is an effective method to discover new fractures during follow-up.
In conclusion, PLO is a rare clinical condition and it must be kept in mind in the differential diagnosis in patients presenting with low back pain during or after pregnancy. Although not specified as a diagnostic criterion, the exclusion of other reasons for osteoporosis and progressive clinical course are necessary and helpful in the diagnostic process. At the same time, the monitoring and evaluation of the efficacy of PLO intervention is also very important.
4. Conclusion
The fractures related to PLO may be an important cause of disability in the long term. Early diagnosis and treatment of calcium and vitamin D, and regular follow-up of these cases are particularly important in the prevention of fractures and increasing the quality of life of the patients.
Footnotes
Abbreviations: ALP = alkaline phosphatase, BAP = bone alkaline phosphatase, BMD = Bone mineral density, BMI = body mass index, CTX = cross-linked C-terminal telopeptide of type I collagen, FBG = fasting blood-glucose, H-BMD = hip BMD, HGB = hemoglobin, IGT = impaired glucose tolerance, IR = increasing rate, L-BMD = lumbar BMD, OGTT = oral glucose tolerance test, P = phosphorous, PINP = procollagen type1 n-terminal propeptide, PLO = pregnancy and lactation-associated osteoporosis, PTH = parathyroid hormone, PTH-rP = parathyroid hormone-related peptide, ucOC = undercarboxylated osteocalcin.
Authorship: MZ and PC were responsible for data collection, patient follow-up and performed the statistical analysis. BL and TP participated in Literature review. JD performed the statistical analysis. JYC participated in the design of the study.
All data are fully available without restriction.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
We have got the Approval Letter from Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital ethics committee. The reference number is 2014 No. 43 by Ethics Committee of Sichuan Provincial People's Hospital.
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- [1].O'Sullivan SM, Grey AB, Singh R, et al. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int 2006;17:1008–12. [DOI] [PubMed] [Google Scholar]
- [2].Hellmeyer L, Hadji P, Ziller V, et al. Osteoporosis in pregnancy. Geburtshilfeund Frauenheilkunde 2004;64:38–45. [Google Scholar]
- [3].Nordin BE, Roper A. Post-pregnancy osteoporosis; a syndrome? Lancet 1955;268:431–4. [DOI] [PubMed] [Google Scholar]
- [4].Terzi R, Terzi H, Özer T, Kale A. A rare cause of postpartum low back pain: pregnancy- and lactation-associated osteoporosis. Biomed Res Int 2014;2014:287832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bonacker J, Janousek M, Kröber M. Pregnancy-associated osteoporosis with eight fractures in the vertebral column treated with kyphoplasty and bracing: a case report. Arch Orthop Trauma Surg 2014;134:173–9. [DOI] [PubMed] [Google Scholar]
- [6].Dunne F, Walters B, Marshall T. Heath D. A. Pregnancy associated osteoporosis. Clin Endocrinol 1993;39:487–90. [DOI] [PubMed] [Google Scholar]
- [7].Silva L, Sampaio L, Pinto J, et al. Osteoporotic fractures in pregnancy: conjunction of factors? Acta Reumatol Port 2009;34:641–5. [PubMed] [Google Scholar]
- [8].Phillips AJ, Ostlere SJ, Smith R. Pregnancy-associated osteoporosis: does the skeleton recover? Osteoporos Int 2000;11:449–54. [DOI] [PubMed] [Google Scholar]
- [9].Smith R, Phillips AJ. Osteoporosis during pregnancy and its management. Scand J Rheumatol 1998;107:66–7. [DOI] [PubMed] [Google Scholar]
- [10].Ofluoglu O, Ofluoglu D. A case report: pregnancy-induced severe osteoporosis with eight vertebral fractures. Rheumatol Int 2008;29:197–201. [DOI] [PubMed] [Google Scholar]
- [11].Ozturk C, Atamaz FC, Akkurt H, et al. Pregnancy-associated osteoporosis presenting severe vertebral fractures. J Obstet Gynaecol Res 2014;40:288–92. [DOI] [PubMed] [Google Scholar]
- [12].Winarno AS, Kyvernitakis I, Hadji P. Successful treatment of 1-34 parathyroid hormone (PTH) after failure of bisphosphonate therapy in a complex case of pregnancy associated osteoporosis and multiple fractures. Z Geburtshilfe Neonatol 2014;218:171–3. [DOI] [PubMed] [Google Scholar]
- [13].Lee SH, Hong MK, Park SW, et al. A case of teriparatide on pregnancy-induced osteoporosis. J Bone Metab 2013;20:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tanriover MD, Oz SG, Sozen T, et al. Pregnancy- and lactation-associated osteoporosis with severe vertebral deformities: can strontium ranelate be a new alternative for the treatment? Spine J 2009;9:e20–4. [DOI] [PubMed] [Google Scholar]
- [15].Bayram S, Ozturk C, Sivrioglu K, et al. Kyphoplasty for pregnancy-associated osteoporotic vertebral fractures. Joint Bone Spine 2006;73:564–6. [DOI] [PubMed] [Google Scholar]
- [16].Stathopoulos IP, Liakou CG, Katsalira A, et al. The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones (Athens) 2011;10:280–91. [DOI] [PubMed] [Google Scholar]
- [17].Tsuchie H, Miyakoshi N, Hongo M, et al. Amelioration of pregnancy-associated osteoporosis after treatment with vitamin K2: a report of four patients. Ups J Med Sci 2012;117:336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakamura Y, Kamimura M, Ikegami S, et al. A case series of pregnancy- and lactation-associated osteoporosis and a review of the literature. Ther Clin Risk Manag 2015;11:1361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kovacs CS. Osteoporosis presenting in pregnancy, puerperium, and lactation. Curr Opin Endocrinol Diabetes Obes 2014;21:468–75. [DOI] [PubMed] [Google Scholar]


