Abstract
Some patients with hepatitis C virus (HCV) infections who fail to achieve sustained virological responses (SVRs) after interferon (IFN) therapy do not develop hepatocellular carcinoma (HCC). Risk stratification of these patients may help identify those who would benefit most from treatment with direct-acting antivirals (DAAs).
A total of 552 HCV-infected patients with non-SVR status were enrolled. Laboratory data before and after IFN treatment were analyzed to determine the relationship of changes in serum markers with development of HCC during the 7-year study period.
HCC developed in 93 patients. The risk factors for HCC were pre-existing liver cirrhosis, low hemoglobin level at baseline, low pretreatment platelet count, high post-treatment alpha-fetoprotein (AFP) level (≥15 ng/mL), and high post-treatment Fibrosis 4 (FIB4) index (>3.25). For patients without pre-existing cirrhosis, those with high post-treatment AFP level and FIB4 index had the highest risk of HCC (1 year: 6.7%; 3 years: 10.9%; 5 years: 29.7%), followed by those with high post-treatment AFP level and low post-treatment FIB4 index (5 years: 25%), and those with low post-treatment AFP level and high post-treatment FIB4 index (1 year: 3.7%; 3 years: 5.2%; 5 years: 10.6%). The risk was even lower for patients with low post-treatment AFP level and FIB4 index (1 year: 0%; 3 years: 0.4%; 5 years: 2.5%). None of the patients with FIB4 indexes consistently below 1.45 developed HCC.
The combined use of post-treatment AFP level and FIB4 index was useful for risk stratification of HCV-infected patients with non-SVR status after IFN therapy. These data may help clinicians to identify patients who most urgently need DAA treatment.
Keywords: direct-acting antiviral, hepatitis C virus, hepatocellular carcinoma, interferon, sustained virological response
1. Introduction
Direct-acting antivirals (DAAs) are important new interferon (IFN)-free therapies that are more likely to provide sustained virological responses (SVRs) than IFN therapies. However, the high prices of DAAs make it infeasible to treat all hepatitis C virus (HCV)-infected patients with these drugs, even those who do not achieve SVRs following IFN therapy. Thus, a treatment strategy based on risk stratification may help to identify patients most in need of DAAs, before HCV-induced liver damage becomes irreversible. The key is to evaluate the stage of liver fibrosis in all HCV-infected patients, rather than to simply focus on those without SVRs. Achievement of an SVR after antiviral therapy is associated with a reduced risk of hepatocellular carcinoma (HCC).[1–3] However, this risk remains elevated in patients with SVRs, especially those with advanced fibrosis or cirrhosis.[2,4,5] In fact, about 8.7% to 12% of patients experience fibrosis after virus eradication,[5,6] and some patients who did not achieve SVRs remain free of HCC for long periods.[7]
Many biomarkers and derived indexes correlate with the stage of liver fibrosis, including liver functions, alpha-fetoprotein (AFP),[8] platelet count,[9,10] Aminotransferase (AST) to Platelet Ratio Index (APRI),[11] and the Fibrosis 4 (FIB4) index.[12] The levels of these indicators, either at baseline[8,14–16] or after treatment,[17–19] have been used to predict the risk of HCC. Our previous study of hepatitis C (HPC) patients with SVRs determined the risk of HCC according to changes in several biomarkers.[4] We found that the risk of HCC was highest in SVR patients with high levels of AFP (≥15 ng/mL) and APRI (≥0.7) before and after treatment, moderate in those whose AFP and APRI levels declined to the normal range after treatment, and lowest in those who had normal AFP and APRI levels before and after treatment. However, it is also important to determine the risk of HCC in patients without SVRs after antiviral therapy to identify patients who would most benefit from treatment with a DAA.
Thus, we conducted a retrospective cohort study of the influence of changes in biomarkers (liver functions, AFP, platelet count, APRI, and FIB4 index) on the development of HCC in a cohort of HCV-infected patients who had non-SVR status after IFN therapy.
2. Patients and methods
From January 2006 to February 2013, 2405 HCV-infected patients who were treated with IFN or pegylated IFN (peg-IFN) plus ribavirin at our institution were enrolled. All patients had HCV antibodies, detectable HCV RNA in serum, and elevated alanine aminotransferase (ALT) levels for more than 6 months before enrollment. Patients with decompensated liver disease, hepatitis due to hepatitis B virus infection, autoimmune hepatitis, or hepatitis due to alcohol abuse were excluded. After exclusion of patients who had HCC before treatment or within 6 months after the end of therapy (n = 159), patients lost to follow-up (n = 339), and those without complete laboratory data (n = 4), 552 of the 1903 (29%) patients with non-SVR status were assessed for analysis. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (104-4253B).
2.1. Treatment and HCC surveillance
The HCV therapy consisted of subcutaneous peg-IFN alfa-2a (Pegasys, F Hoffmann-La Roche, Basel, Switzerland; 180 mg/wk), peg-IFN alfa-2b (Peg-Intron, Schering-Plough, Kenilworth, NJ; 1–1.5 mg/kg per wk), or IFN alfa-2b (Intron-A, Schering-Plough; 3 or 5 million units, thrice weekly). In addition, oral ribavirin (Rebetol, Schering-Plough) was administered daily. The daily dose of ribavirin was 1000 mg for patients who weighed <75 kg, and 1200 mg for patients who weighed more than 75 kg; the ribavirin dose was reduced if there was a decline in hemoglobin. The duration of therapy was 24 weeks for those with HCV genotype 2, and 48 weeks for those with HCV genotype 1. Serum HCV RNA was measured at the onset and conclusion of therapy, and at 24 weeks after the conclusion of therapy. An SVR was defined as no detectable serum HCV RNA at week 24 after the end of treatment; the detection of HCV RNA at this time was defined as a non-SVR. After completion of antiviral therapy, all patients were regularly followed every 3 to 6 months. The duration of HCC follow-up was defined from the date 24 weeks after the end of treatment to the date of HCC development, or the date of the last follow-up. The final observations were on July 31, 2014. The follow-up studies included clinical assessment, biochemical tests, and HCC surveillance, using serum AFP and ultrasonography (US). Detection or suspicion of a new space-occupying lesion by US was followed by computed tomography and/or magnetic resonance imaging, selective hepatic angiography, or fine needle aspiration. Diagnosis of HCC was according to the 2005 guidelines of the American Association for the Study of Liver Disease.[20] Aside from histological evidence, HCC was also diagnosed if there was 1 typical HCC feature on a dynamic image and/or AFP above 200 ng/mL with a tumor diameter more than 2 cm in a cirrhotic liver, or if there were 2 typical HCC features on a dynamic image and a tumor diameter of 1 and 2 cm in a cirrhotic liver. The diagnosis of liver cirrhosis was made according to clinical, laboratory, abdominal US, and/or histological findings.[21]
2.2. Histological evaluation
Not all patients received liver biopsies before the start of antiviral therapy, especially those enrolled after 2012, because some were concerned about potential complications. Therefore we used the APRI and FIB4 index for staging of liver fibrosis before and after IFN therapy. An APRI of 0.7 and above[11] and an FIB4 index above 3.25 were used as indicators of significant fibrosis.[12]
2.3. Laboratory investigations
The presence of antibodies against HCV was assessed using a 3rd-generation enzyme-linked immunosorbent array (Ax SYM HCV 3.0, Abbott Laboratories, Chicago, IL). Qualitative detection of HCV RNA was performed by a standardized qualitative reverse transcription-polymerase chain reaction assay (Amplicor, Roche Diagnostics, Branchburg, NJ), using biotinylated primers for the 5′ noncoding region. The detection limit of this assay was 100 copies/mL (50 IU/mL). Serum HCV RNA levels were determined by a branched-DNA (b-DNA) signal amplification assay (VERSANT HCV RNA 3.0. Assay, Bayer Diagnostics, Emeryville, CA). This assay was a sandwich nucleic acid hybridization procedure with a detection limit of 3400 copies/mL. Genotyping of HCV was performed using a reverse hybridization assay (Inno-LiPATM HCV II; Innogenetics NV, Ghent, Belgium) with HCV-Amplicor products. Identification of the interleukin 28 (IL28) B-associated variant single nucleotide polymorphism (SNP) rs12979860 was performed by polymerase chain reaction and fluorescence monitoring, and each was classified as the C/C, C/T, or T/T allele.
Hematological and biochemical data were collected before IFN therapy. Post-treatment hematological and biochemical data were collected at least 1 year after IFN therapy and on the date of the last follow-up, if possible. AFP data were obtained more than 6 months prior to HCC onset to exclude HCC-induced elevation of AFP.
2.4. Statistical analysis
Continuous variables are expressed as means ± standard deviations and categorical variables as frequencies. Kaplan–Meier curves of the cumulative incidence of HCC were compared using the log-rank test. Factors associated with HCC risk were determined by a Cox proportional hazard model. The association of HCC with age, sex, obesity, diabetes mellitus (DM), liver cirrhosis before IFN treatment, hemoglobin, total bilirubin, ALT, virological response, pre- and post-treatment platelet counts, AFP, APRI, and FIB4 index was determined. Risk factors identified as significant by simple Cox regression were further analyzed by multiple Cox regression for determination of adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs). All tests were 2 tailed, and a P below .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
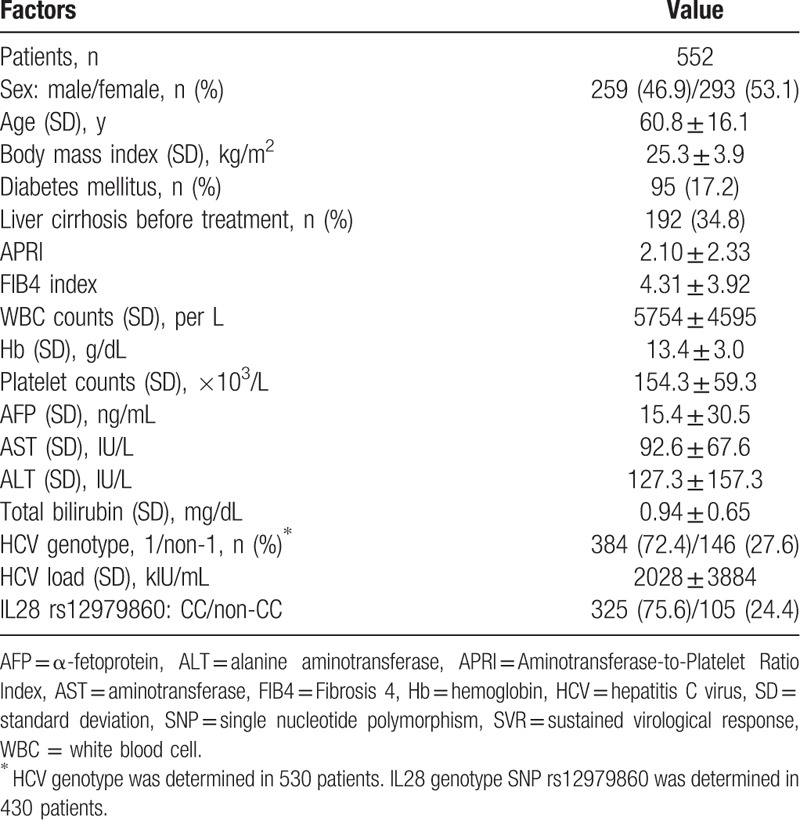
Table 1 shows the baseline demographic, virological, and clinical characteristics of the 552 patients. Before treatment, 192 patients (34.8%) had liver cirrhosis and 95 (17.2%) had DM. The IL28 genotype SNP rs12979860 was present in 430 patients, and 325 of them (75.6%) had the CC allele. At enrollment, the mean APRI was 2.10 ± 2.33 and the mean FIB4 index was 4.31 ± 3.92. Overall, HCC developed in 93 patients during follow-up; 21 patients within the 1st year, 52 patients at the 3-year follow-up, and 80 patients at the 5-year follow-up. The 1-, 3-, and 5-year cumulative incidence rates of HCC were 3.8%, 9.6%, and 17.3%, respectively.
Table 1.
Baseline characteristics of non-SVR patients at the time of enrollment.
3.2. Risk factors for HCC
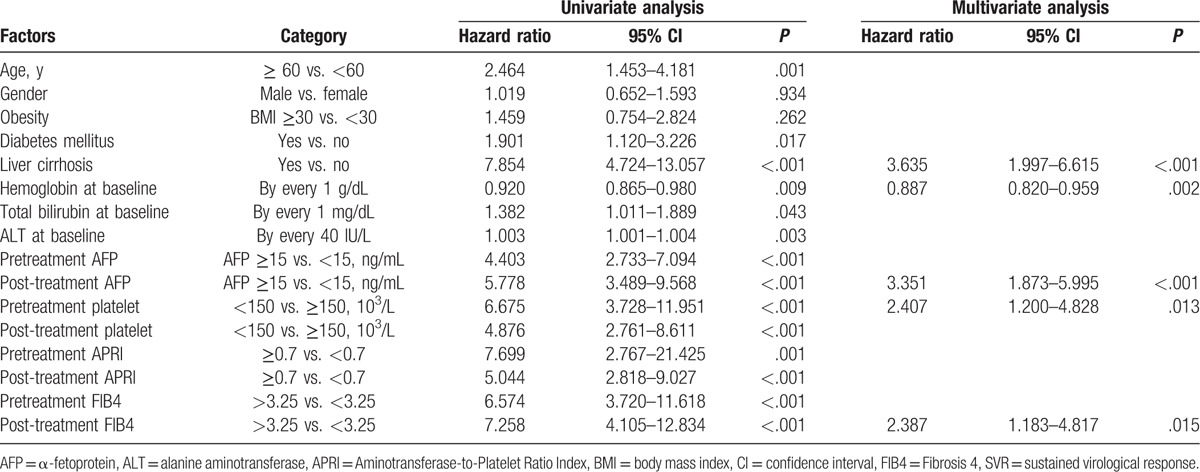
Table 2 shows the results of univariate and multivariate analyses of factors associated with HCC. The univariate analysis indicates that old age (≥60 years), obesity (body mass index ≥ 30), DM, liver cirrhosis, low hemoglobin (each 1 g/dL), high bilirubin (each 1 mg/dL), high ALT (each 40 IU/L), high pre- and post-treatment AFP (≥15 ng/mL), low pre- and post-treatment platelet level (<150 × 103/μL), high pre- and post-treatment APRI (≥0.7), and high pre- and post-treatment FIB4 index (>3.25) were significantly associated with HCC. Multivariate analysis indicates that liver cirrhosis (aHR: 3.635, 95% CI: 4.724–13.057, P < .001), hemoglobin level (aHR: 0.887, 95% CI: 0.820–0.959, P = .002), pretreatment platelet count (aHR: 2.407, 95% CI: 1.200–4.828, P = .013), post-treatment AFP (aHR: 3.351, 95% CI: 1.873–5.995, P < .001), and post-treatment FIB4 index (HR: 2.387, 95% CI: 1.183–4.817, P = .015) were significantly related to HCC. The APRI was not a significant indicator of risk.
Table 2.
Factors associated with hepatocellular carcinoma for patients with non-SVR (n = 552).
3.3. Association of HCC with changes in platelets, AFP, APRI, and FIB4 index after IFN therapy
We also analyzed the effect of changes in the levels of different markers on HCC development (Fig. 1). There was a significantly higher incidence of HCC in non-SVR patients who had high pre- or post-treatment levels of AFP. As with the SVR patients in our previous study,[4] the post-treatment AFP level had a stronger impact on survival. Notably, the risk of HCC development did not increase for patients whose AFP levels were normal after treatment. Patients who had low pre- or post-treatment platelet counts (<150 × 103/μL) had a significantly higher incidence of HCC than those with normal platelet counts. A low baseline platelet count was associated with the greatest risk of HCC after antiviral therapy. There was also a significantly higher incidence of HCC in patients with pre- or post-treatment APRI of 0.7 and above and FIB4 index above 3.25.
Figure 1.
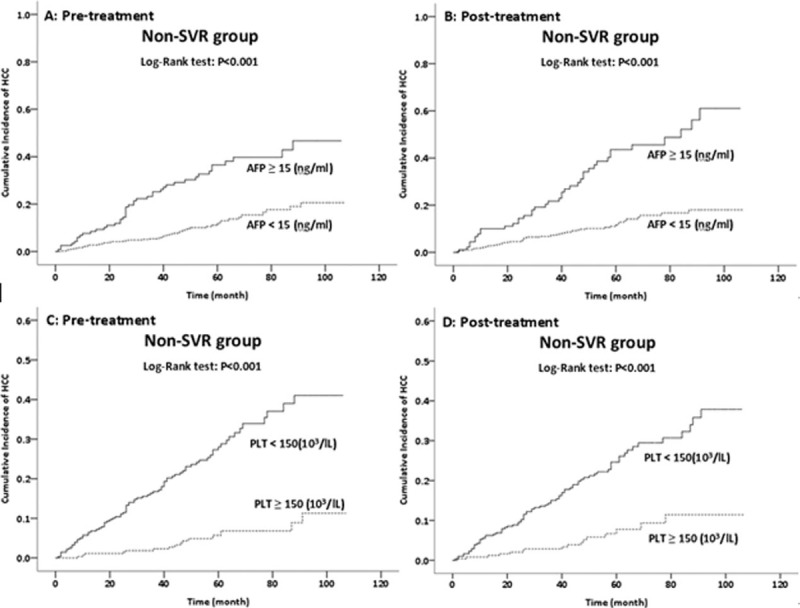
Effect of pretreatment and post-treatment AFP level (A and B) and pretreatment and post-treatment platelet count (C and D) on HCC development in HPC patients without SVRs after IFN therapy (n = 552). AFP = alpha-fetoprotein, HCC = hepatocellular carcinoma, HPC = hepatitis C, IFN = interferon, SVRs = sustained virological responses.
We also analyzed the association of changes in serum AFP level, platelet count, APRI, and FIB4 index, with HCC (Fig. 2). Non-SVR patients with high pre- and post-treatment AFP levels had highest risk of HCC, those with low pretreatment levels but high post-treatment levels and those with high pretreatment levels but low post-treatment levels had intermediate risk, and those with low pre- and post-treatment levels had the lowest risk. Overall, the post-treatment AFP level was a better predictor of HCC than the pretreatment level (Table 2; Fig. 3).
Figure 2.
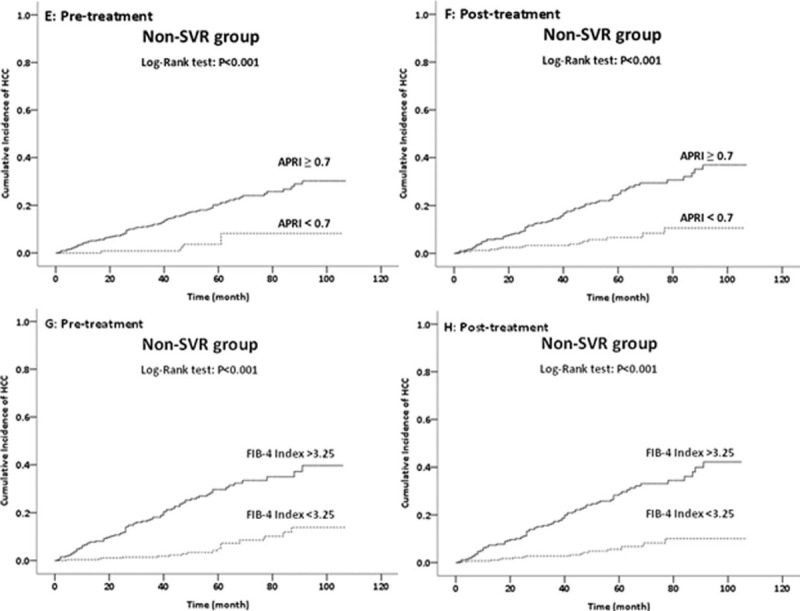
Effect of pretreatment and post-treatment APRI (E and F) and pretreatment and post-treatment FIB4 index (G and H) on HCC development in HPC patients without SVRs after IFN therapy (n = 552). APRI = Aminotransferase-to-Platelet Ratio Index, FIB4 = Fibrosis 4, HCC = hepatocellular carcinoma, HPC = hepatitis C, IFN = interferon, SVRs = sustained virological responses.
Figure 3.
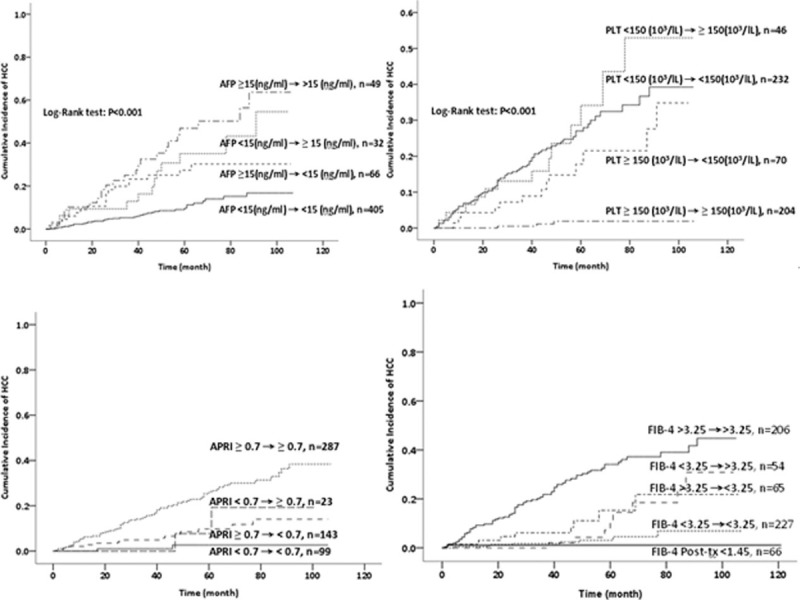
Effect of changes in pretreatment and post-treatment AFP level (A), platelet count (B), APRI (C), and FIB4 index (D) on HCC development in HPC patients without SVRs after IFN therapy (n = 552). AFP = alpha-fetoprotein, APRI = Aminotransferase-to-Platelet Ratio Index, FIB4 = Fibrosis 4, HCC = hepatocellular carcinoma, HPC = hepatitis C, IFN = interferon, SVRs = sustained virological responses.
Non-SVR patients with low pre- and post-treatment platelet counts and low pretreatment but normal post-treatment counts had highest risk of HCC, followed by those with normal pretreatment counts but low post-treatment counts and those with normal pre- and post-treatment counts. Overall, the pretreatment platelet count was a better predictor of HCC than the post-treatment count (Table 2).
Non-SVR patients with high pre- and post-treatment APRI had highest risk of HCC, followed those with low pretreatment but high post-treatment APRI; the risks were comparable for the other 2 groups. In addition, non-SVR patients with high pre- and post-treatment FIB4 indexes had the highest risk of HCC, followed by those with low pretreatment but high post-treatment indexes, high pretreatment but low post-treatment indexes, and low pre- and post-treatment indexes. Notably, none of the non-SVR patients with consistently low FIB4 indexes (<1.45; indicative of mild fibrosis) developed HCC.
3.4. Use of post-treatment AFP and FIB4 index to predict HCC
We performed separate analyses of patients with or without pre-existing liver cirrhosis (Fig. 4). Patients with pre-existing cirrhosis had a significantly higher risk of HCC than those without cirrhosis (35.9% [69/192] vs. 6.7% [24/360], P < .001). For patients without pre-existing cirrhosis, those with high post-treatment AFP level and FIB4 index had the highest risk of HCC (1 year: 6.7%; 3 years: 10.9%; 5 years: 29.7%), followed by those with high post-treatment AFP level and low post-treatment FIB4 index (5 years: 25%), and those with low post-treatment AFP level and high post-treatment FIB4 index (1 year: 3.7%; 3 years: 5.2%; 5 years: 10.6%). The risk was lowest for patients with low post-treatment AFP levels and FIB4 indexes (1 year: 0%; 3 years: 0.4%; 5 years: 2.5%).
Figure 4.
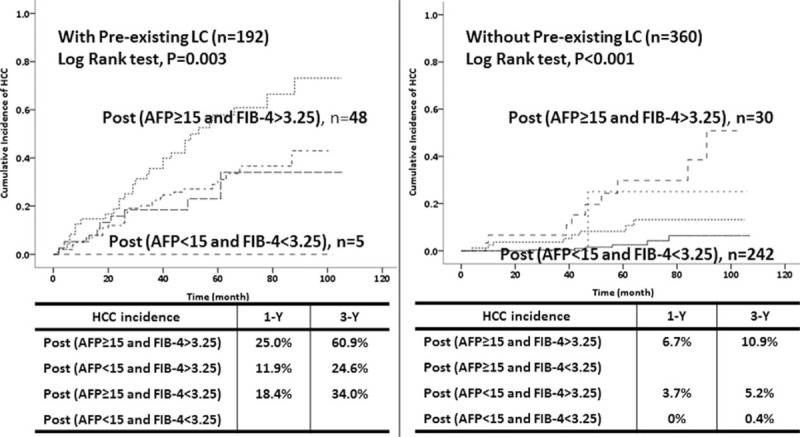
Effect of the combined use of post-treatment AFP level and FIB4 index on development of HCC in patients without SVRs after IFN therapy in those with pre-existing liver cirrhosis (A) and without pre-existing liver cirrhosis (B) (n = 552). AFP = alpha-fetoprotein, FIB4 = Fibrosis 4, HCC = hepatocellular carcinoma, IFN = interferon, SVRs = sustained virological responses.
4. Discussion
HCV-infected patients have a decreased risk of HCC after IFN treatment, especially if they achieve SVRs.[3,13,14] Many previous studies reported that certain biomarkers (AFP, platelet count, APRI, and FIB4 index)—either at baseline, after treatment, or changes over time—can predict HCC in patients after IFN therapy.[4,8,15–19] Viral eradication is believed to be the major factor protecting against development of HCC. With the increasing use of DAAs for treatment of HCV infections, most patients can achieve SVR status. In contrast to the well-established impact of IFN antiviral therapy on decreasing the risk of HCC, there are controversial results regarding the effect of DAA treatment on HCC development. In particular, some patients with prior HCC had an unexpectedly high rate of early tumor recurrence after DAA treatment,[22,23] possibly due to facilitation of metastatic clones by disruption of immune surveillance. However, these data should be viewed cautiously, because of the small number of patients and the short follow-up period. Recent studies reported a reduced incidence of HCC among HCV-infected patients who achieved SVRs after DAA treatment.[24,25] However, it is not yet known whether DAA treatment decreases the probability of HCC as successfully as IFN treatment. Large-scale prospective cohort studies of this topic are needed.
DAA therapies provide more effective eradication of HCV than the traditional IFN therapy. However, the high prices of DAAs make full health insurance coverage for all HCV-infected patients impracticable. Thus, many insurance companies in the United States only approve DAAs for the sickest patients. Fox et al[26] recommended monitoring of noninvasive clinical markers of disease progression to guide the timing of antiviral therapy. The results of the present study show that changes in serum markers over time can predict HCC development in patients with non-SVR status after IFN therapy. More specifically, a low pretreatment platelet count (<150 × 103/μL), high post-treatment AFP level (≥15 ng/mL), and high post-treatment FIB4 index (>3.25) were significantly associated with HCC development. As for the role of APRI, non-SVR patients had AST levels insignificantly decreased (pretreatment: 92.62 U/L, post-treatment: 89.9 U/L, P > .05, data not shown). Therefore, APRI and platelet may therefore have similar predictive power because AST component is not significantly changed. Furthermore, non-SVR patients who maintained normal platelet counts, AFP levels, and low FIB4 indexes after antiviral treatment had lower risk of HCC. Notably, there were no HCC cases among non-SVR patients with FIB4 indexes below 1.45 before and after treatment, suggesting that this group of patients does not require DAA treatment.
The FIB4 index considers age, AST, ALT, and platelet count, and is a better predictor of outcome than the APRI in HCV-infected patients.[27] More specifically, the FIB4 index is an accurate indicator of fibrosis and prediction of 5-year survival in HCV-infected patients.[12,28] Changes in the FIB4 index are also useful for real-time estimation of progression of liver fibrosis.[29] Therefore, Fox et al[26] suggested use of the FIB4 index to prioritize patients for DAA treatment. Apart from the FIB4 index, the post-treatment AFP level may also help to predict prognosis. Previous studies reported that AFP might enhance tumor cell growth and the proliferation of human hepatoma, and that IFN therapy, irrespective of viral eradication, reduced this risk.[30–33] Thus, non-SVR patients might have a reduced risk of HCC after IFN treatment due to their lower AFP levels. Therefore, the combined use of post-treatment AFP level and FIB4 index may provide a more precise prediction of the risk of HCC.
Before evaluation of HPC patients, clinicians should test for the presence of a pre-existing liver cirrhosis, because it is a well-established risk factor for development of HCC. The role of other factors in risk stratification should also be addressed. In the present study, we found that non-SVR patients with liver cirrhosis had a significantly higher risk of HCC than those without cirrhosis (HR: 3.635, 95% CI: 4.724–13.057, P < .001). Thus, HPC patients with pre-existing liver cirrhosis and non-SVR status after AFP treatment should be given priority for DAA treatment. The current study also provides important new information, in that the combined use of post-treatment AFP level and post-treatment FIB4 index is useful for risk stratification of patients with non-SVR status. Thus, for non-SVR patients without pre-existing liver cirrhosis, those with high post-treatment AFP level (≥15 ng/mL) and high FIB4 index (>3.25) had the highest risk of HCC; the risk was lowest for those with persistently low post-treatment AFP level (<15 ng/mL) and low post-treatment FIB4 index (<3.25). The results were similar to those for patients with pre-existing liver cirrhosis. Clinicians should therefore consider our results when seeking to prioritize HPC patients for DAA treatment.
There were some limitations in this study. First, the enrolled patients received different IFN-based antiviral regimens (peg-IFN alfa-2a, peg-IFN alfa-2b, or IFN alfa-2b). Second, some patients did not receive liver biopsies before or after treatment. In addition, apart from the noninvasive biomarkers we examined, transient elastography may be used to evaluate liver fibrosis. Thus, some studies reported use of liver stiffness measurement (LSM) to predict HCC development in HPC patients.[34,35] However, little is known about the prognostic value of LSM after antiviral therapy. Narita et al[36] reported that LSM could be used to stratify HCV-infected patients receiving IFN therapy according to the risk of HCC, but this previous study was limited by the small number of patients and the short follow-up period. A long-term study of the prognostic value of LSM is not yet available. Instead of LSM, we used changes in serum markers to evaluate changes in fibrosis from before treatment to after treatment.
In conclusion, the combined use of post-treatment AFP level and post-treatment FIB4 index are useful for risk stratification of HPC patients with non-SVR status after IFN therapy. Clinicians should consider this strategy when prioritizing HPC patients for urgent DAA treatment.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, AST = aminotransferase, aHRs = adjusted hazard ratios, ALT = alanine aminotransferase, APRI = AST to Platelet Ratio Index, CI = confidence interval, DAA = direct-acting antiviral, DM = diabetes mellitus, FIB4 = Fibrosis 4, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HPC = hepatitis C, IFN = interferon, IL28 = interleukin 28, LSM = liver stiffness measurement, peg-IFN = pegylated IFN, SVRs = sustained virological responses.
Conception and design: T-HH and C-KW. Manuscript writing: C-MH. Collection and assembly of data: C-MH, T-HH, K-CC, P-LT, S-NL, C-HC, J-HW, C-ML, M-CT, M-TL, Y-HY, C-HH, C-LC, and C-KW. Data analysis and interpretation: C-KW, C-MH, and T-HH. Final approval of manuscript: all authors.
This study was supported by grant CMRPG8E1642 from Chang Gung Memorial Hospital.
The authors have no conflicts of interest to disclose.
References
- [1].Thevenot T, Regimbeau C, Ratziu V, et al. Metaanalysis of interferon randomized trials in the treatment of viral hepatitis C in naive patients: 1999 update. J Viral Hepat 2001;8:48–62. [DOI] [PubMed] [Google Scholar]
- [2].Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010;52:833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158:329–37. [DOI] [PubMed] [Google Scholar]
- [4].Wu CK, Chang KC, Hung CH, et al. Dynamic α-fetoprotein, platelets and AST-to-Platelet Ratio Index predict hepatocellular carcinoma in chronic hepatitis C patients with sustained virological response after antiviral therapy. J Antimicrob Chemother 2016;71:1943–7. [DOI] [PubMed] [Google Scholar]
- [5].Poynard T, Moussalli J, Munteanu M, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol 2013;59:675–83. [DOI] [PubMed] [Google Scholar]
- [6].Maylin S, Martinot-Peignoux M, Moucari R, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology 2008;135:821–9. [DOI] [PubMed] [Google Scholar]
- [7].Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med 1999;131:174–81. [DOI] [PubMed] [Google Scholar]
- [8].Tateyama M, Yatsuhashi H, Taura N, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol 2011;46:92–100. [DOI] [PubMed] [Google Scholar]
- [9].Adinolfi LE, Giordano MG, Andreana A, et al. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol 2001;113:590–5. [DOI] [PubMed] [Google Scholar]
- [10].Kee KM, Wang JH, Hung CH, et al. Improvement of thrombocytopenia in hepatitis C-related advanced fibrosis patients after sustained virological response. Dig Dis Sci 2013;58:556–61. [DOI] [PubMed] [Google Scholar]
- [11].Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-Platelet Ratio Index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726–36. [DOI] [PubMed] [Google Scholar]
- [12].Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. [DOI] [PubMed] [Google Scholar]
- [13].Hiramatsu N, Oze T, Takehara T. Suppression of hepatocellular carcinoma development in hepatitis C patients given interferon-based antiviral therapy. Hepatol Res 2015;45:152–61. [DOI] [PubMed] [Google Scholar]
- [14].Manthravadi S, Paleti S, Pandya P. Impact of sustained viral response postcurative therapy of hepatitis C-related hepatocellular carcinoma: a systematic review and meta-analysis. Int J Cancer 2017;140:1042–9. [DOI] [PubMed] [Google Scholar]
- [15].Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 1993;328:1797–801. [DOI] [PubMed] [Google Scholar]
- [16].Lu SN, Wang JH, Liu SL, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer 2006;107:2212–22. [DOI] [PubMed] [Google Scholar]
- [17].Okamura Y, Ashida R, Yamamoto Y, et al. FIB-4 index is a predictor of background liver fibrosis and long-term outcomes after curative resection of hepatocellular carcinoma. Ann Surg Oncol 2016;23(suppl 4):467–74. [DOI] [PubMed] [Google Scholar]
- [18].Asahina Y, Tsuchiya K, Nishimura T, et al. α-Fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology 2013;58:1253–62. [DOI] [PubMed] [Google Scholar]
- [19].Oze T, Hiramatsu N, Yakushijin T, et al. Post-treatment levels of α-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol 2014;12:1186–95. [DOI] [PubMed] [Google Scholar]
- [20].Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36. [DOI] [PubMed] [Google Scholar]
- [21].Hung CH, Lu SN, Wang JH, et al. Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus related cirrhosis. J Gastroenterol 2003;38:153–7. [DOI] [PubMed] [Google Scholar]
- [22].Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719–26. [DOI] [PubMed] [Google Scholar]
- [23].Strazzulla A, Iemmolo RMR, Carbone E, et al. The risk of hepatocellular carcinoma after directly acting antivirals for hepatitis C virus treatment in liver transplanted patients: is it real? Hepat Mon 2016;16:e41933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kobayashi M, Suzuki F, Fujiyama S, et al. Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J Med Virol 2017;89:476–83. [DOI] [PubMed] [Google Scholar]
- [25].Virlogeux V, Pradat P, Hartig-Lavie K, et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int 2017;37:1122–7. [DOI] [PubMed] [Google Scholar]
- [26].Fox DS, McCombs JS. Optimizing HCV treatment—moving beyond the cost conundrum. J Hepatol 2016;65:222–5. [DOI] [PubMed] [Google Scholar]
- [27].Houot M, Ngo Y, Munteanu M, et al. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment Pharmacol Ther 2016;43:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011;140:1970–9. [DOI] [PubMed] [Google Scholar]
- [29].Tamaki N, Kurosaki M, Tanaka K, et al. Noninvasive estimation of fibrosis progression overtime using the FIB-4 index in chronic hepatitis C. J Viral Hepat 2013;20:72–6. [DOI] [PubMed] [Google Scholar]
- [30].Wang XW, Xie H. Alpha-fetoprotein enhances the proliferation of human hepatoma cell in vitro. Life Sci 1999;64:17–23. [DOI] [PubMed] [Google Scholar]
- [31].Wang XW, Xu B. Stimulation of tumor cell growth by alpha-fetoprotein. Int J Cancer 1998;75:596–9. [DOI] [PubMed] [Google Scholar]
- [32].Hino K, Okita K. Interferon therapy as chemoprevention of hepatocarcinogenesis in patients with chronic hepatitis C. J Antimicrob Chemother 2004;53:19–22. [DOI] [PubMed] [Google Scholar]
- [33].Miki A, Yano Y, Kato H, et al. Anti-tumor effect of pegylated interferon in the rat hepatocarcinogenesis model. Int J Oncol 2008;32:603–8. [PubMed] [Google Scholar]
- [34].Masuzaki R, Tateishi R, Yoshida H, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 2009;49:1954–61. [DOI] [PubMed] [Google Scholar]
- [35].Wang HM, Hung CH, Lu SN, et al. Liver stiffness measurement as an alternative to fibrotic stage in risk assessment of hepatocellular carcinoma incidence for chronic hepatitis C patients. Liver Int 2013;33:756–61. [DOI] [PubMed] [Google Scholar]
- [36].Narita Y, Genda T, Tsuzura H, et al. Prediction of liver stiffness hepatocellular carcinoma in chronic hepatitis C patients on interferon-based anti-viral therapy. J Gastroenterol Hepatol 2014;29:137–43. [DOI] [PubMed] [Google Scholar]


