Abstract
Rationale:
Pegaspargase has been used in the treatment of acute lymphoblastic leukemia with promising results. However, it has also been associated with several potentially serious complications, including thrombosis. Pegaspargase-induced cerebral venous thrombosis and bone marrow necrosis are very rare.
Patient concerns:
A 50-year-old female developed headache, weakness of the right lower extremity, fever, and bone pain after chemotherapy including pegaspargase for the treatment of acute lymphoblastic leukemia.
Diagnoses:
Her imaging studies and bone marrow examinations were compatible with cerebral venous thrombosis and bone marrow necrosis.
Interventions:
The patient received anticoagulation therapy with rivaroxaban.
Outcomes:
After treatment with rivaroxaban, she had a good outcome without major or minor bleeding.
Lessons:
Clinicians should be aware of the very rare but possible induction of bone marrow necrosis during pegaspargase treatment when there is necrosis in other organs. Because of its greater safety and convenience, rivaroxaban gains popularity over traditional anticoagulant drugs.
Keywords: acute lymphoblastic leukemia, asparaginase, drug-related side effects and adverse reactions, rivaroxaban, venous thromboembolism
1. Introduction
Asparaginase (Asp) is an effective chemotherapy drug used in the treatment of acute lymphoblastic leukemia (ALL).[1] Despite the clinical benefits of Asp therapy in ALL, it has also been associated with various adverse reactions, including thrombosis. Cerebral venous thrombosis (CVT) is an uncommon form of stroke, in which the thrombosis affects the cerebral veins and sinus. CVT is associated with hypercoagulable states, infections, inflammatory disease, malignancy, oral contraceptives, and pregnancy.[2]
Bone marrow necrosis (BMN) is thought to be rare.[3] It is best defined as “necrosis of myeloid tissue and medullary stroma in large areas of the hematopoietic bone marrow”.[4] CVT has been reported in ALL patients who received therapies including Asp, but to our knowledge, there has been no reported case of pegaspargase-induced CVT accompanied by BMN. The present study presents the case of a patient with common B-ALL who developed CVT and BMN during maintenance therapy with pegaspargase. After anticoagulant treatment with rivaroxaban, our patient has had a good outcome. Written informed consent was obtained from the patient.
2. Case report
A 50-year-old female presented to Yantai Yuhuangding Hospital (Shandong, China) with complaints of fever and fatigue during the previous week on May 3, 2015. On physical examination, there were petechial rashes on the lower limbs. Pulse and blood pressure were normal. The lungs and heart were normal on auscultation, and there were no murmurs. No lymphadenopathy or hepatosplenomegaly were identified. The neurologic examination was unremarkable. A complete blood count revealed a hemoglobin (HGB) count of 99 g/L, white blood cell (WBC) count of 31.3 × 109 L−1 with 3% blast cells, and platelet count (PLT) of 48 × 109 L−1. Furthermore, a bone marrow smear identified active hyperplasia and a blast cell count of 84%, while the patient was peroxidase-negative. The immunophenotype of the leukemic blasts was analyzed with flow cytometry, and they were CD19, CD10, CD22, CD123, TdT, cCD79a, CD34, and HLA-DR positive, while cytoplasmic μ and surface membrane immunoglobulin were negative. Cytogenetic analysis revealed 46, XX [20], and a molecular biology examination demonstrated that the patient was E2A-HLF and HOX11 positive. Therefore, a diagnosis of common B-ALL was made.
During her initial diagnosis, standard induction chemotherapy with a cyclophosphamide, vindesine, daunorubicin, pegaspargase, and dexamethasone regimen was started.[5] At day 28, common B-ALL minimal residual disease was undetectable by immunophenotyping. Complete remission was achieved.
Consolidation chemotherapy with cyclophosphamide, vindesine, daunorubicin, pegaspargase, and dexamethasone was administered. At day 4 of the therapy, the patient received 3750 IU of pegaspargase. On day 10, the patient developed headache and weakness of the right lower extremity. Coagulation tests showed a prothrombin time of 11.8 seconds; prothrombin activity of 85.5%; an activated partial thromboplastin time of 49 seconds; thrombin time of 18.6 seconds; fibrinogen, 0.86 mg/L; and d-dimer, 4.6 mg/L. Contrast-enhanced magnetic resonance imaging was performed, which showed a thrombus occluding the superior sagittal sinus, right transverse sinus, sigmoid sinus, and internal jugular vein (Fig. 1). Based on the contrast-enhanced magnetic resonance imaging, she was diagnosed as CVT. On day 12, the patient developed bone pains and fever. The hemogram showed HGB, 71 g/L; WBC, 1.78 × 109 L−1; and PLT, 31 × 109 L−1. Bone marrow aspiration from the iliac crest showed an amorphous extracellular eosinophilic proteinaceous material surrounding cells with bare nuclei (Wright stain, Fig. 2). A bone marrow biopsy was conducted, which showed disruption of the normal architecture, necrosis of the myeloid tissue, and medullary stroma (hematoxylin and eosin, Fig. 3). A diagnosis of BMN was established. Anticoagulation therapy with rivaroxaban at a dosage of 10 mg once daily was started. No complications were detected after treatment. Eight days after anticoagulation treatment, symptoms improved remarkably, including the relief of headache and recovery of right lower limb strength. Four weeks after treatment, blood counts were as follows: HGB, 95 g/L; WBC, 3.25 × 109 L−1; and PLT, 87 × 109 L−1. Seven weeks after rivaroxaban treatment, the hemogram was normal and a bone marrow aspiration showed no signs of necrosis. MRI of the brain showed dissolution of the thrombosis (Fig. 4).
Figure 1.
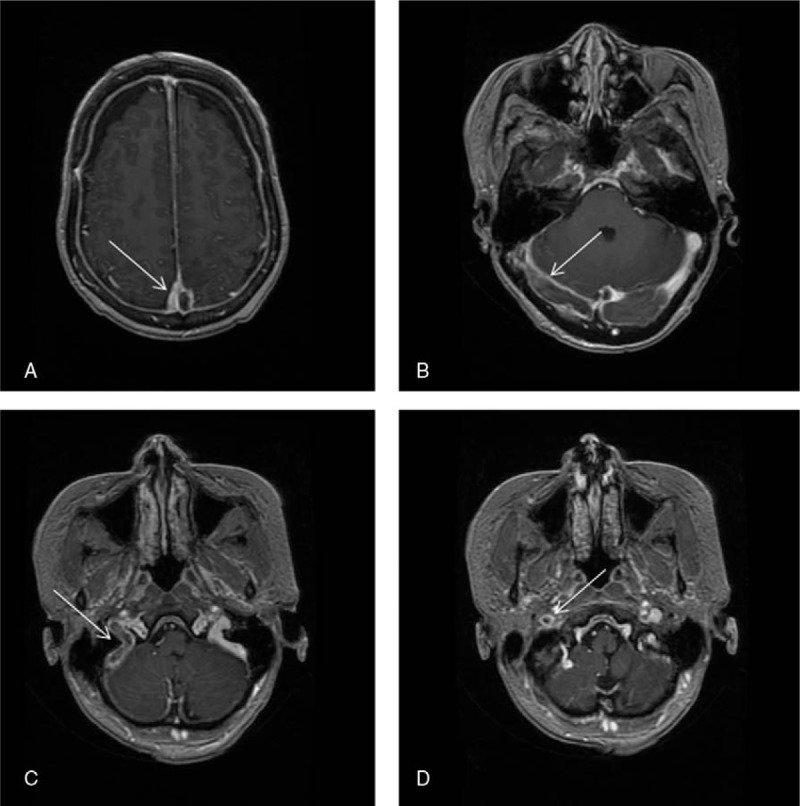
Contrast-enhanced magnetic resonance imaging showed sinus thrombosis (arrows).
Figure 2.
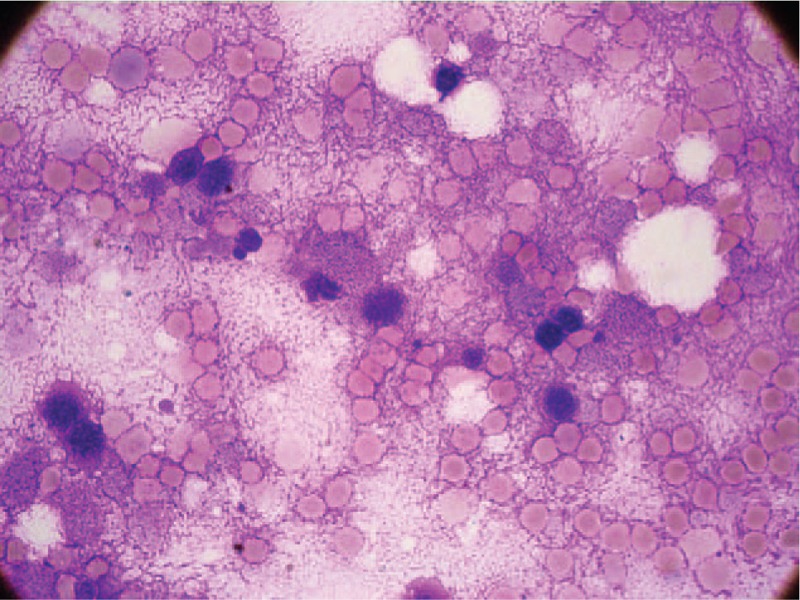
Bone marrow smears (Wright stain, ×1000) showed necrotic cells with indistinct or irregular cell membrane in a background of amorphous proteinaceous material.
Figure 3.
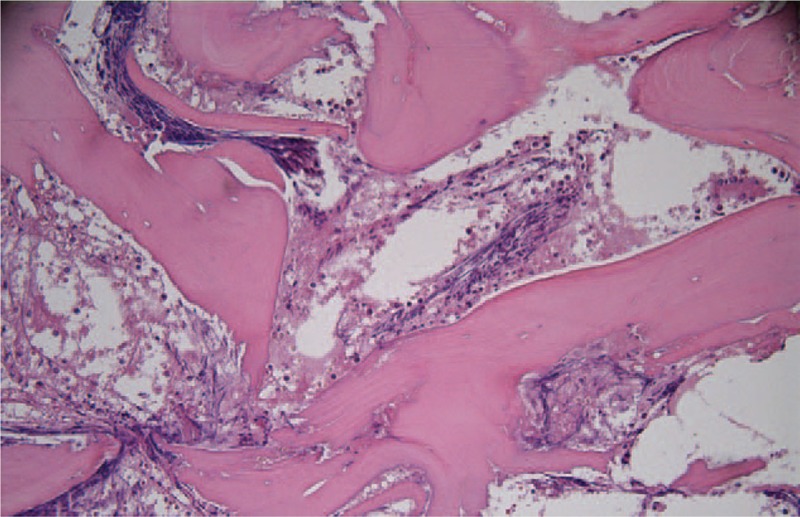
Bone marrow biopsy (hematoxylin and eosin, ×200) showed sheets of cells in an amorphous eosinophilic background.
Figure 4.
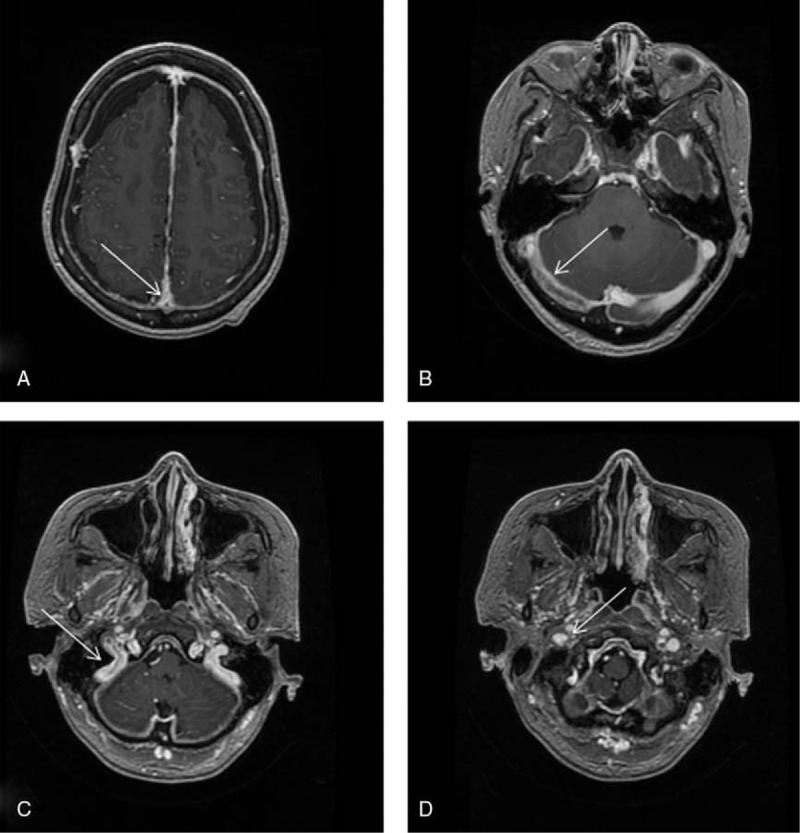
Contrast-enhanced magnetic resonance imaging after treatment with rivaroxaban, showing resolution of the thrombosis (arrows).
3. Discussion
Asp is a major drug used in therapies for ALL. There are 2 forms of commercial Asp available: l-Asparaginase (l-Asp) and pegaspargase. l-Asp is an Escherichia coli-derived Asp. Pegaspargase is a PEGylated form of Asp, which retains its antileukemic effect. Because pegaspargase shows fewer incidences of anaphylactic reaction and a long half-life, it is gaining popularity over l-Asp. Asp hydrolyses l-asparagine, leading to a deficiency in asparagine. Asparagine deficiency inhibits protein synthesis and causes apoptosis.[6] The asparagine synthetase activity of human lymphoblasts is low.[6] When exogenous asparagine is deficient, cytotoxicity occurs.[7]
Asp can improve long-term event-free survival in ALL patients considerably.[8] However, we cannot neglect one of its potential side effects, venous thromboembolism (VTE). The VTE incidence rate ranges from 1.7% to 36.7% in patients with ALL during treatment.[9] Use of Asp is an important therapy-related factor associated with increased incidence of VTE.[10] A recent meta-analysis demonstrated that the incidence of symptomatic thrombosis was 5.2%, and most events occur during induction.[11]
Age is the only factor predicting higher risk of VTE in ALL patients receiving therapy including Asp.[10] Patients aged >30 years are at higher risk for VTE. Our patient was 50 years old, which is within the vulnerable window for developing VTE. Typical presentations of VTE include CVT, deep-vein thrombosis of the legs, and pulmonary embolism.[10–12] Only a very small percentage, 1.64%, of ALL patients treated with Asp experienced CVT in a recent study.[10]
The mechanisms for thrombosis during Asp treatment are complicated. Asp can impair the synthesis of antithrombin, heparin cofactor II, protein C system, and plasminogen,[12,13] which are important factors required for anticoagulation and fibrinolysis. Levels of P-selectin, plasminogen activator inhibitor-1, tissue factor activity, and vWF antigen also increase during induction of Asp.[14,15] Besides, the disruption and activation of endothelium may be another mechanism by which Asp causes thrombosis. Consequently, the balance between the hemostatic and the anticoagulant system is disrupted, leading to hypercoagulability and higher risk of VTE.[12]BMN is an uncommon observation in bone marrow biopsy. The incidence of BMN is very low, ranging from 0.3% to 2.2%.[3,16] BMN is often associated with malignancies, infection, autoimmune diseases, disseminated intravascular coagulation, and sickle cell disease.[3] Pharmaceutical agents including trans retinoic acid, fludarabine, and granulocyte colony-stimulating factor are also known causes of BMN.[3,4] The mechanisms of BMN are not well understood, but microcirculatory failure of the marrow may play a critical role in its pathophysiology.[16] The microcirculation cannot supply the oxygen demanded by the bone marrow cells, which leads to ischemic necrosis, of which thrombosis is an important factor.[17,18]
ALL is a disease associated with VTE and BMN. Nonetheless, the patient was in a state of complete remission, and no other probable cause was recognized to be associated with these complications. Thus, we conclude that pegaspargase-induced hypercoagulability was the main reason for the thrombosis, rather than the ALL itself. After anticoagulation therapy, BMN disappeared. We theorized that thrombosis induced by pegaspargase led to microvascular occlusion and consequent BMN. We conclude that if there is necrosis in other organs, the presence of BMN is not extraordinary and routine bone marrow aspiration and biopsy should be conducted.
Traditional treatment of Asp-related VTE has been with heparin and low molecular weight heparin. Rivaroxaban is a direct oral Factor Xa inhibitor. When our patient required anticoagulant therapy, her platelets are at the level of subnormality. Given the low bleeding risk and the efficacy of rivaroxaban in treating venous thromboses, we opted to treat our patient with rivaroxaban.[19] Our patient had a good outcome with neurologic signs resolved, imaging studies showed dissolution of thrombosis and bone marrow smears no signs of necrosis. Also this lays a basis on further testing of rivaroxaban use in this setting.
Footnotes
Abbreviations: ALL = acute lymphoblastic leukemia, Asp = asparaginase, BMN = bone marrow necrosis, CVT = cerebral venous thrombosis, HGB = hemoglobin, l-Asp = l-asparaginase, PLT = platelet, VTE = venous thromboembolism, WBC = white blood cell.
The authors have no conflicts of interest.
References
- [1].Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med 2006;354:166–78. [DOI] [PubMed] [Google Scholar]
- [2].Kashyap AS, Anand KP, Kashyap S. Thrombosis of the cerebral veins and sinuses. N Engl J Med 2005;353:314–5. [PubMed] [Google Scholar]
- [3].Wool GD, Deucher A. Bone marrow necrosis: ten-year retrospective review of bone marrow biopsy specimens. Am J Clin Pathol 2015;143:201–13. [DOI] [PubMed] [Google Scholar]
- [4].Janssens AM, Offner FC, Van Hove WZ. Bone marrow necrosis. Cancer 2000;88:1769–80. [PubMed] [Google Scholar]
- [5].Larson RA, Dodge RK, Burns CP, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood 1995;85:2025–37. [PubMed] [Google Scholar]
- [6].Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol 2009;7:600–6. [PubMed] [Google Scholar]
- [7].Asselin BL, Ryan D, Frantz CN, et al. In vitro and in vivo killing of acute lymphoblastic leukemia cells by l-asparaginase. Cancer Res 1989;49:4363–8. [PubMed] [Google Scholar]
- [8].Pieters R, Hunger SP, Boos J, et al. l-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer 2011;117:238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br J Haematol 2007;138:430–45. [DOI] [PubMed] [Google Scholar]
- [10].Grace RF, Dahlberg SE, Neuberg D, et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol 2011;152:452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caruso V, Iacoviello L, Di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood 2006;108:2216–22. [DOI] [PubMed] [Google Scholar]
- [12].De Stefano V, Za T, Ciminello A, et al. Haemostatic alterations induced by treatment with asparaginases and clinical consequences. Thromb Haemost 2015;113:247–61. [DOI] [PubMed] [Google Scholar]
- [13].Mitchell L, Hoogendoorn H, Giles AR, et al. Increased endogenous thrombin generation in children with acute lymphoblastic leukemia: risk of thrombotic complications in L’Asparaginase-induced antithrombin III deficiency. Blood 1994;83:386–91. [PubMed] [Google Scholar]
- [14].Giordano P, Molinari AC, Del Vecchio GC, et al. Prospective study of hemostatic alterations in children with acute lymphoblastic leukemia. Am J Hematol 2010;85:325–30. [DOI] [PubMed] [Google Scholar]
- [15].Schneider P, Van Dreden P, Rousseau A, et al. Increased levels of tissue factor activity and procoagulant phospholipids during treatment of children with acute lymphoblastic leukaemia. Br J Haematol 2010;148:582–92. [DOI] [PubMed] [Google Scholar]
- [16].Paydas S, Ergin M, Baslamisli F, et al. Bone marrow necrosis: clinicopathologic analysis of 20 cases and review of the literature. Am J Hematol 2002;70:300–5. [DOI] [PubMed] [Google Scholar]
- [17].Markovic SN, Phyliky RL, Li CY. Pancytopenia due to bone marrow necrosis in acute myelogenous leukemia: role of reactive CD8 cells. Am J Hematol 1998;59:74–8. [DOI] [PubMed] [Google Scholar]
- [18].Khalil YK, Pistoria MJ, Wright RE. Catastrophic antiphospholipid antibody syndrome with bone marrow necrosis: a rare complication. J Rheumatol 2011;38:2279–81. [DOI] [PubMed] [Google Scholar]
- [19].Antoniou S. Rivaroxaban for the treatment and prevention of thromboembolic disease. J Pharm Pharmacol 2015;67:1119–32. [DOI] [PubMed] [Google Scholar]


