Abstract
The aim of this study was to investigate the effects of intra-articular injection of polydeoxyribonucleotide (PDRN), compared with intraarticular triamcinolone (TA) injection, in subacute stroke patients with hemiplegic shoulder pain (HSP).
Participants were subacute stroke patients with HSP who had undergone 2 consecutive intra-articular injections of TA or PDRN.
Numeric rating scale (NRS) and passive range of motion (PROM) of hemiplegic shoulder were evaluated until 4 weeks after 2nd injection.
In the results, there were significant improvements in all PROM measures 2 weeks after the second injection, compared with pre-injection results, in both groups (P < .05). In the PDRN group, however, none of the PROM measures were significantly improved at 3 and 4 weeks after the second injection, compared with pre-injection results (P ≥ .05). When comparing pre-injection results with those at 4 weeks after the second injection, all PROM and NRS measures in the TA group were more improved than in the PDRN group, but this was not statistically significant (P ≥ .05).
In conclusion, considering the systemic side effects of steroids, especially among patients with diabetes or metabolic syndrome, PDRN seems to be a worthwhile treatment option for HSP, although PDRN does not seem to have an equivalent persistence effects when compared with TA.
Keywords: adenosine, hemiplegic shoulder pain, polydeoxyribonucleotide, steroid side effects, triamcinolone
1. Introduction
Hemiplegic shoulder pain (HSP) is one of the most common musculoskeletal complications after acute stroke.[1] Moreover, it can interfere with rehabilitative treatment and has been associated with poorer functional outcomes and prolonged hospital stays.[2–4] With regard to treatment, nothing has yet been proven effective, although different treatment methods, such as physical therapy,[5] functional electrical stimulation,[6,7] and intra-articular steroid injection[8,9] are employed. In clinical practice, physicians frequently treat HSP using steroid injections,[9] although their effects remain controversial.[8–10]
Although there are many causes of HSP, articular inflammation is one of the important pathophysiology of HSP.[11] From this perspective, intra-articular steroid injection can help relieve pain,[8,9] but the systemic side effects of steroid injection, such as suppression of the hypothalamus-pituitary-adrenal (HPA) axis and increased blood glucose,[12,13] as well as local side effects, such as tissue degeneration and tendon rupture[14–16] can also occur. In particular, the increased blood glucose level that can occur as a side effect of steroid injections limits their selection as a therapeutic agent, especially considering the fact that diabetes and metabolic syndrome are often combined with stroke.
Recently, there have been studies investigating the effects of polydeoxyribonucleotide (PDRN) in patients with plantar fasciitis,[17] lumbosacral radiculopathy,[18] supraspinatus tendinopathy,[19] and the effects of PDRN in rheumatoid arthritis animal models.[20] Unlike steroids, PDRN has anti-inflammatory effects without metabolic side effects such as elevated blood sugar levels, making it a possible alternative to steroids for the treatment of musculoskeletal disorders in those studies. To the best of our knowledge, however, there have been no studies on the effect of PDRN for HSP to date. Therefore, in this study, we tried to investigate the effects of intra-articular injections of PDRN, compared with intra-articular triamcinolone (TA) injections, in patients with HSP.
2. Method
2.1. Participants
This study received Institutional Review Board approval of Daegu Fatima Hospital. A written informed consent was not necessary for this retrospective study, and patient anonymity was preserved. Among the stroke patients who were admitted to our hospital, those with HSP were investigated retrospectively. We compared the effect of PDRN injection with TA injection instead of the control group due to ethical issues. Patients who had undergone 2 consecutive TA or PDRN intra-articular injections, and who had been clinically evaluated (passive ROM and NRS) after these 2 consecutive injections, were included. Patients who had limitation in passive external rotation of the hemiplegic shoulder of at least 20°, compared with the unaffected side, were included.[4] Patients who had any of the following were excluded: history of shoulder surgery, prior steroid injection, autoimmune diseases such as rheumatoid arthritis or ankylosing spondylitis, complex regional pain syndrome (CRPS), and chronic stroke patients (≥6 months). On the basis of these criteria, 64 subacute stroke patients, who were admitted to our hospital with HSP between March 2016 and March 2017, were initially included. Among them, 44 patients were excluded due to the exclusion criteria. Therefore, a total of 20 patients (10 patients with TA injections vs 10 patients with PDRN injections) were included for analysis in this study (Fig. 1A, B).
Figure 1.
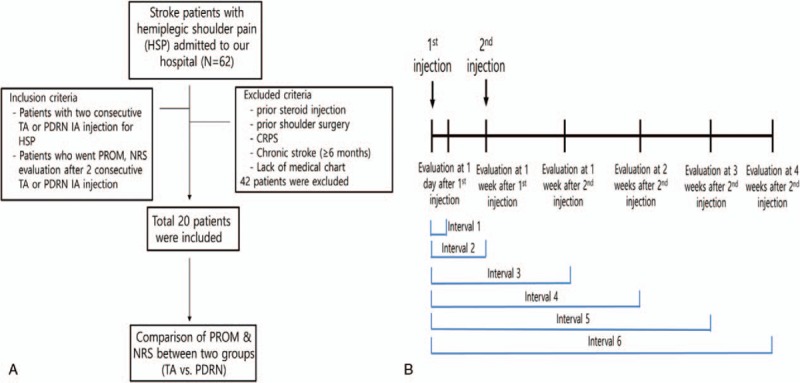
(A) Flowchart of this study. (B) Diagram of this study.
2.2. Intervention
All patients included in this study underwent ultrasound-guided intra-articular TA or PDRN injections in the hemiplegic shoulder. The TA group received intra-articular injections of TA 40 mg/1 mL (Dong Kwang Pharm., Seoul, Korea) and normal saline (N/S) 14 mL (total 15 mL). The PDRN group received intra-articular injections of PDRN (Rejuvenex, PharmaResearch Products, South Korea) 1 ampoule (PDRN sodium 5.625 mg/3 mL) and N/S 12 mL (total 15 mL). In this study, patients with diabetes mellitus (DM) and HSP were excluded, because 20 mg of TA was usually injected in DM patients with HSP in our department. Success of intra-articular shoulder injections was judged by checking drug flow into the articular cavity using color Doppler (Fig. 2).[21]
Figure 2.
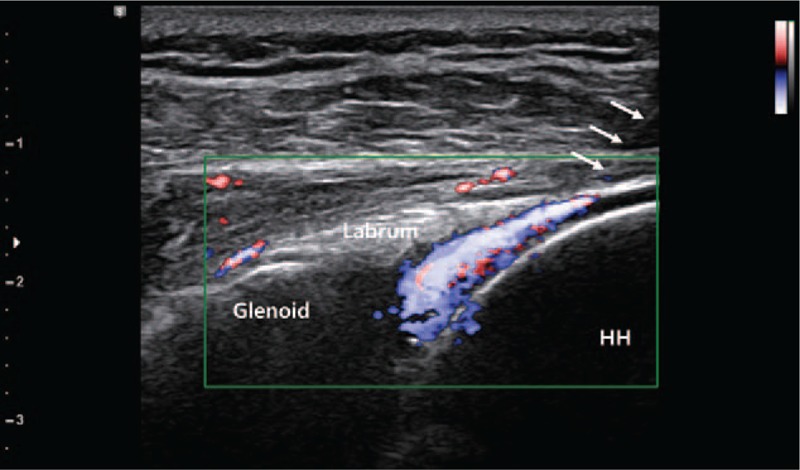
Intra-articular shoulder injection was confirmed using color Doppler. Arrow, needle. HH = humerus head.
2.3. Outcome measurement
The primary outcome measures were pain measured using a numeric rating scale[22] (NRS; on a scale of 0–10, where 0 = no pain and 10 = highest level of pain) during passive ROM of the shoulder in 4 planes (forward flexion, abduction, external, and internal rotation); and passive ROM of the shoulder in four planes (forward flexion, abduction, external rotation, and internal rotation) using goniometry.[4] All ROMs were measured in the seated position. Assessment was performed just before the first injection, 1 day after the first injection, 1 week after the first injection, 1 week after the second injection, 2 weeks after the second injection, 3 weeks after the second injection, and 4 weeks after the second injection (Fig. 1B).
2.4. Statistical analysis
Statistical analyses were performed using SPSS for Windows and R package for Windows (version 2.15.2; R Foundation for Statistical Computing, Vienna, Austria). The initial statistical analysis was carried out using a one-way analysis of variance (ANOVA) with a Tukey post-hoc test to compare the passive ROM and NRS measures across the time of assessments, and to evaluate the effectiveness of the treatments in each group. An independent t test was used to compare between-group differences in the degree of improvement in NRS and passive ROM after treatment. The results are presented as the mean ± standard deviation. Chi-square tests were used to compare categorical variables (e.g., sex ratio, hemi-side) between the groups. P values of < .05 were considered statistically significant.
3. Results
3.1. Characteristics of patients
There were no statistically significant differences in patients’ age, gender, hemi-side, duration since stroke, stroke type (infarction or hemorrhage), Brunnstrom motor recovery stage, NRS, and passive ROMs (flexion, abduction, external rotation, and internal rotation) between the 2 groups, before the injections (P ≥ .05) (Table 1).
Table 1.
Clinical characteristics of the stroke patients in the TA and PDRN groups.
3.2. Changes in NRS and passive ROM
In both groups, a significant improvement in NRS was observed 1 day after the first injection (Table 2). Both groups also showed significant improvement at 1 week, 2 weeks, and 3 weeks after the second injection, as compared with their initial status. However, in both groups, there was no statistically significant improvement 4 weeks after the second injection, as compared with their initial status (Fig. 3A–E).
Table 2.
Comparison of the physical findings in the TA and PDRN groups.
Figure 3.
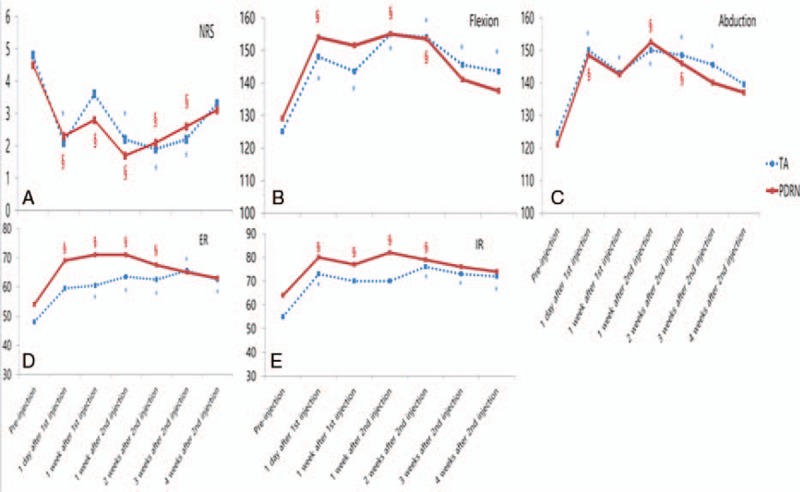
Changes in physical findings in both TA and PDRN groups. (A) Changes in NRS in both TA and PDRN groups. (B) Changes in passive flexion ROM in both TA and PDRN groups. (C) Changes in passive abduction ROM in both TA and PDRN groups. (D) Changes in passive external rotation ROM in both TA and PDRN groups. (E) Changes of passive internal rotation ROM in both TA and PDRN groups. NRS = numeric rating scale, PDRN = polydeoxyribonucleotide, ROM = range of motion, TA = triamcinolone. ∗P < .05 compared with pre-injection in the TA group; †P < .05 compared with pre-injection in the PDRN group.
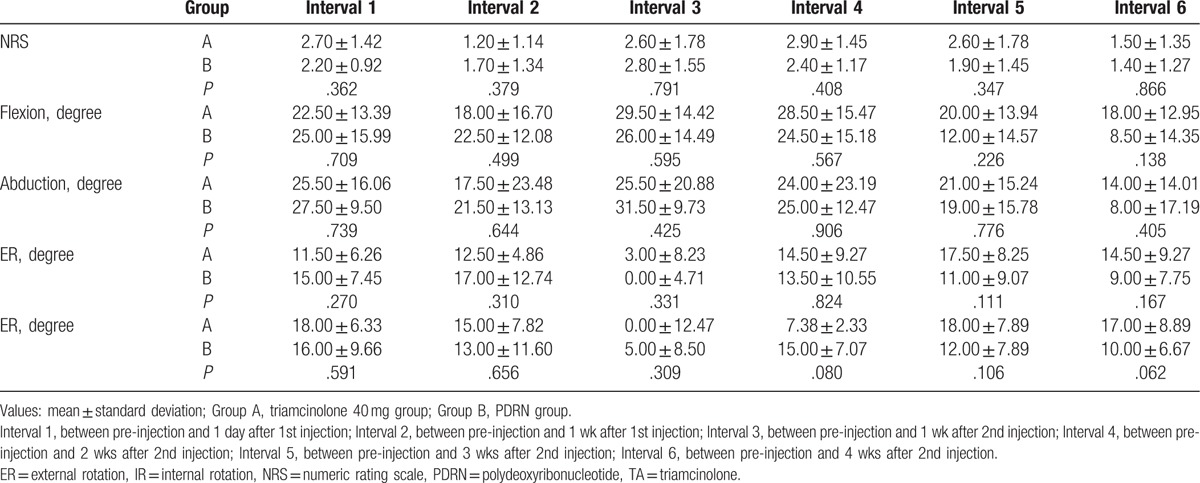
Both groups showed statistically significant improvements in terms of flexion, abduction, and internal rotation 1 day after the first injection, compared with their pre-injection results (P < .05). In addition, both groups showed statistically significant improvement in terms of all passive ROM measures 2 weeks after the second injection, compared with their pre-injection results (P < .05). However, at 3 and 4 weeks after the second injection, only the TA group showed significant improvements in terms of flexion, external rotation, and internal rotation, compared with their pre-injection results (P < .05). In the PDRN group, all passive ROM measures were not significantly improved 3 and 4 weeks after the second injection, compared with their pre-injection results (P ≥ .05) (Table 2).
3.3. Degree of improvement in NRS and passive ROM
We analyzed the degree of improvement in 6 intervals (interval 1–) (Fig. 1B). In all intervals, there was no significant difference between the TA and PDRN groups. When comparing 4 weeks after the second injection with the pre-injection results (interval 6), all passive ROM and NRS measures in the TA group were more improved than the PDRN group, but these differences were not statistically significant (P ≥ .05) (Fig. 4) (Table 3).
Figure 4.
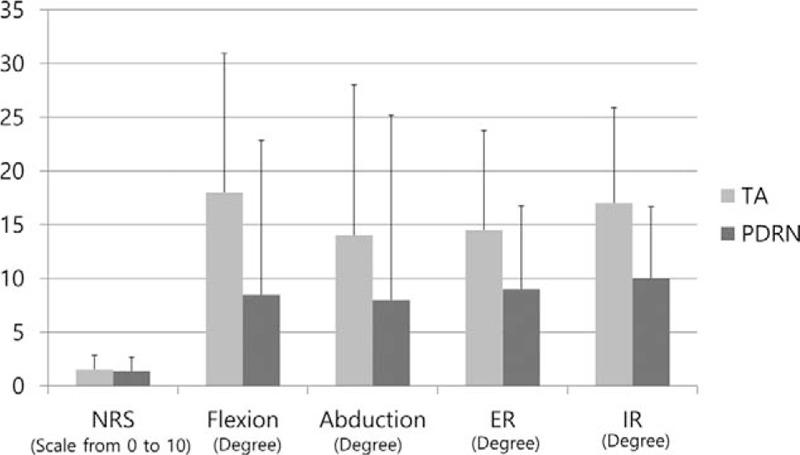
The degree of improvement in physical findings of both TA and PDRN groups. NRS = numeric rating scale, PDRN = polydeoxyribonucleotide, TA = triamcinolone.
Table 3.
Comparison of the differences in improvement of physical findings between the TA and PDRN groups.
4. Discussion
To the best of our knowledge, there has been no published study investigating the effects of intra-articular inject of PDRN in musculoskeletal disorders. Recently, studies of PDRN in patients with musculoskeletal pain have suggested the possibility of use of PDRN for musculoskeletal disorders, but to date, there has been no study of its effects on HSP.[17,19] PDRN is obtained from sperm trout through an extraction process.[23] The compound holds a mixture of deoxyribonucleotide polymers with chain lengths ranging from 50 to 2000 bp.[23] PDRN acts through stimulation of the A2A receptor under pathologic conditions of low tissue perfusion.[23] Adenosine is a purine nucleoside that is released from a variety of cells in response to several types of stress.[24,25] It has been suggested that adenosine regulates inflammation via interaction with 1 or more of its 4 known receptors (A1, A2A, A2B, and A3). Although adenosine receptor stimulation has been shown to have a differential effect on the release of pro-inflammatory cytokines, stimulation of the adenosine A2A receptor has been shown to inhibit tumor necrosis factor (TNF)-α production in human peripheral blood mononuclear cells (PBMCs).[19] Moreover, in a previous study, PDRN lowered the circulating levels and cartilage expression of the inflammatory cytokines TNF-α and interleukin-6 in a rheumatoid arthritis animal model.[20] These effects of PDRN, in markedly reducing the production of inflammatory cytokines, point to its potential as an alternative treatment option to steroids.
The findings of the current study indicated that both the TA group and the PDRN group experienced improvements immediately, from the first day after the injection. This immediate effect of the TA group was similar to previous studies.[26,27] In addition, there was no significant difference in the degree of improvement between the TA and PDRN groups until 4 weeks after the second injection, compared with their pre-injection results. These results may indicate that PDRN and TA have similar onset time and duration of therapeutic effects for HSP, until at least 4 weeks after 2 consecutive injections.
However, for passive ROMs, the PDRN group did not show a significant improvement in all passive ROM measures from the third week after the second injection, unlike the TA group. This is presumably due to the following 2 reasons. First, the potency of the anti-inflammatory effect of PDRN (PDRN sodium 5.625 mg) may be smaller than that of TA 40 mg. Second, it may be due to the difference in the form of PDRN and TA. Particulate TA may remain in the joint space for a longer time than soluble PDRN. In summary, our results suggest that there was no significant difference in the degree of improvement in the TA and PDRN groups until 4 weeks after the 2 consecutive injections, though there may be some differences thereafter. Further studies with various treatment doses, as well as long-term studies, will be necessary for a better understanding of these differences.
There are some limitations of our study. First, the number of patients was small and the study period was not long. To understand more about long-term therapeutic effects of PDRN, further studies with more patients and a longer period of time for follow-up will be necessary in the future. Second, this study is limited by its retrospective design. However, to date, there is no published study investigating the effects of PDRN on HSP. Moreover, in this study, PDRN was shown to have similar therapeutic effects to TA (which is widely used as a treatment for shoulder pain in clinical practice) for HSP. Considering these 2 points, this study seems to have sufficient significance as a preliminary study. Considering the anti-inflammatory effect of PDRN, the possibility of its use as an alternative therapy seems to be justified, especially in patients with diabetes or metabolic syndrome who are expected to have systemic side effects from frequent steroid injections. In addition, it is worth considering the effect of various doses of PDRN in future prospective, randomized controlled studies, and comparing the effects of TA and PDRN over a longer time period.
5. Conclusion
Even though PDRN seems not to have an equivalent persistence effect compared with TA, considering the systemic side effects of steroids, especially in patients with diabetes or metabolic syndrome, it appears that PDRN is worthwhile to be used as an option for treatment of HSP. As it is known that PDRN has a dose-dependent effect,[20] it is also necessary to study the therapeutic effects of various doses of PDRN in the future.
Footnotes
Abbreviations: CRPS = complex regional pain syndrome, HSP = hemiplegic shoulder pain, NRS = Numeric rating scale, PDRN = polydeoxyribonucleotide, PROM = passive range of motion, TA = triamcinolone.
Funding/support: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF- 2017R1D1A1B03033127)
The authors report no conflict of interest or financial support.
References
- [1].Huang YC, Leong CP, Wang L, et al. The effects of hyaluronic acid on hemiplegic shoulder injury and pain in patients with subacute stroke: a randomized controlled pilot study. Medicine 2016;95:e5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bender L, McKenna K. Hemiplegic shoulder pain: defining the problem and its management. Disabil Rehabil 2001;23:698–705. [DOI] [PubMed] [Google Scholar]
- [3].Wanklyn P, Forster A, Young J. Hemiplegic shoulder pain (HSP): natural history and investigation of associated features. Disabil Rehabil 1996;18:497–501. [DOI] [PubMed] [Google Scholar]
- [4].Lim JY, Koh JH, Paik NJ. Intramuscular botulinum toxin-A reduces hemiplegic shoulder pain: a randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke 2008;39:126–31. [DOI] [PubMed] [Google Scholar]
- [5].Geurts AC, Visschers BA, van Limbeek J, et al. Systematic review of aetiology and treatment of post-stroke hand oedema and shoulder-hand syndrome. Scand J Rehab Med 2000;32:4–10. [DOI] [PubMed] [Google Scholar]
- [6].Chantraine A, Baribeault A, Uebelhart D, et al. Shoulder pain and dysfunction in hemiplegia: effects of functional electrical stimulation. Arch Phys Med Rehab 1999;80:328–31. [DOI] [PubMed] [Google Scholar]
- [7].Renzenbrink GJ, IJzerman MJ. Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehab 2004;18:359–65. [DOI] [PubMed] [Google Scholar]
- [8].Snels IA, Beckerman H, Twisk JW, et al. Effect of triamcinolone acetonide injections on hemiplegic shoulder pain: a randomized clinical trial. Stroke 2000;31:2396–401. [DOI] [PubMed] [Google Scholar]
- [9].Dekker JH, Wagenaar RC, Lankhorst GJ, et al. The painful hemiplegic shoulder: effects of intra-articular triamcinolone acetonide. Am J Phys Med Rehab 1997;76:43–8. [DOI] [PubMed] [Google Scholar]
- [10].Snels IA, Dekker JH, van der Lee JH, et al. Treating patients with hemiplegic shoulder pain. Am J Phys Med Rehab 2002;81:150–60. [DOI] [PubMed] [Google Scholar]
- [11].Teasell RW, Foley NC, Bhogal SK, et al. An evidence-based review of stroke rehabilitation. Topics Stroke Rehab 2003;10:29–58. [DOI] [PubMed] [Google Scholar]
- [12].Lazarevic MB, Skosey JL, Djordjevic-Denic G, et al. Reduction of cortisol levels after single intra-articular and intramuscular steroid injection. Am J Med 1995;99:370–3. [DOI] [PubMed] [Google Scholar]
- [13].Maillefert JF, Aho S, Piroth-Chatard C, et al. Cortisol levels after single local steroid injection. Am J Med 1996;100:586–7. [DOI] [PubMed] [Google Scholar]
- [14].Halpern AA, Horowitz BG, Nagel DA. Tendon ruptures associated with corticosteroid therapy. West J Med 1977;127:378–82. [PMC free article] [PubMed] [Google Scholar]
- [15].Byun SD, Park DH, Hong YH, et al. The additive effects of hyaluronidase in subacromial bursa injections administered to patients with peri-articular shoulder disorder. Ann Rehab Med 2012;36:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Byun SD, Park DH, Choi WD, et al. Subacromial bursa injection of hyaluronate with steroid in patients with peri-articular shoulder disorders. Ann Rehab Med 2011;35:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim JK, Chung JY. Effectiveness of polydeoxyribonucleotide injection versus normal saline injection for treatment of chronic plantar fasciitis: a prospective randomised clinical trial. Int Orthop 2015;39:1329–34. [DOI] [PubMed] [Google Scholar]
- [18].Kang KN, Kim TW, Koh JW, et al. Effect of transforaminal epidural polydeoxyribonucleotide injections on lumbosacral radiculopathy: a case report. Medicine 2017;96:e7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yoon YC, Lee DH, Lee MY, et al. Polydeoxyribonucleotide injection in the treatment of chronic supraspinatus tendinopathy: a case-controlled, retrospective, comparative study with 6-month follow-up. Arch Phys Med Rehab 2017;98:874–80. [DOI] [PubMed] [Google Scholar]
- [20].Bitto A, Polito F, Irrera N, et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A((2)A) receptor. Arthritis Rheum 2011;63:3364–71. [DOI] [PubMed] [Google Scholar]
- [21].Park D. Evaluation of posterosuperior labral tear with shoulder sonography after intra-articular injection: a case series. Am J Phys Med Rehab 2017;96:e48–51. [DOI] [PubMed] [Google Scholar]
- [22].Gagliese L, Weizblit N, Ellis W, et al. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain 2005;117:412–20. [DOI] [PubMed] [Google Scholar]
- [23].Altavilla D, Bitto A, Polito F, et al. Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem 2009;7:313–21. [DOI] [PubMed] [Google Scholar]
- [24].Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 2006;5:247–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chan ES, Fernandez P, Cronstein BN. Adenosine in inflammatory joint diseases. Purinergic Signal 2007;3:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jang MH, Lee CH, Shin YI, et al. Effect of intra-articular hyaluronic acid injection on hemiplegic shoulder pain after stroke. Ann Rehab Med 2016;40:835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jeon WH, Park GW, Jeong HJ, et al. The comparison of effects of suprascapular nerve block, intra-articular steroid injection, and a combination therapy on hemiplegic shoulder pain: pilot study. Ann Rehab Med 2014;38:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


